Abstract
Class-A Scavenger receptor (SR-A) is expressed by microglia, and we show here that it is also expressed by astrocytes, where it participates on their inflammatory activation. Astrocytes play a key role on the inflammatory response of the central nervous system, secreting several soluble mediators like cytokines and radical species. Exposure to SRs ligands activated MAPKs and NF-κB signaling and increase production of IL1β and nitric oxide (NO). IL1β classically an inflammatory cytokine surprisingly did not increase but inhibited LPS+IFNγ-induced NO production by astrocytes. Our results suggest that SRs expressed by astrocytes participate in the modulation of inflammatory activation.
Keywords: ERK, glia, IL1β, JNK, MAPK, Neuroinflammation, NFκB, NO, SR-A
1. Introduction
Neuroinflammation is a complex pathophysiological response involving soluble factors and glial cell activation, in response to infection and tissue damage induced by ischemic, traumatic, immune or other injuries, such as toxic, and heat/cold, radiation. The inflammatory process normally leads to recovery and healing. However, inflammation can lose its repair function and enhance tissue damage if the process is not properly regulated (Nathan, 2002). Inflammation is recognized as a major contributor to many acute and chronic central nervous system (CNS) disorders, playing an important role in their pathogenesis. For chronic neurodegenerative disorders like Alzheimer’s disease and Parkinson’s disease, neuroinflammation depends on the activation of the brain innate immune response (Rivest, 2009; Glass et al., 2010; von Bernhardi et al., 2010) involving dysregulation of glial cells (von Bernhardi, 2007).
Astrocytes and microglia participate in the immune inflammatory response, representing the first line of defense of the CNS. They produce inflammatory cytokines like IL1β and release short-lived cytotoxic mediators such as nitric oxide (NO) and reactive oxygen species (ROS) (Griffiths et al., 2009; von Bernhardi and Eugenín, 2012). In fact, astrocytes stimulated with brain-derived neurotrophic factor (BDNF) release NO, and conditioned medium from activated astrocytes can be detrimental for neurons. A process that is amplified by NO (Colombo et al., 2012) and could be relevant for the neuroinflammatory response in neurodegenerative diseases like multiple sclerosis (Liu et al., 2001; Colombo et al., 2012). On the other hand, astrocytes provide trophic support to the CNS and establish an intimate interaction with neurons, microglia and endothelial cells. They also play a regulatory and protective role by modulating microglial cell activity by secreting modulating cytokines like transforming growth factor-β (TGFβ) (von Bernhardi and Eugenín, 2004; Ramírez et al., 2005; Herrera-Molina and von Bernhardi, 2005).
Astrocytes and microglia express several receptors that participate in the inflammatory response. One of these receptor groups are the Scavenger Receptors (SRs). Scavenger Receptor A (SR-A) was described for the first time in 1979 by Goldstein et al., who described a binding site mediating the uptake and degradation of acetylated LDL (acLDL) by macrophages (Goldstein et al., 1979; de Winther et al., 2000), which was initially referred to as the acLDL receptor. Currently, it is known that SR-A belongs to a large family of SR denominated Class A SR, which also includes the Scavenger Macrophage Receptor with Collagenous Structure (SR-MARCO) (Krieger, 1997; de Winther et al., 2000).
SRs recognize apoptotic cells, secreted pattern recognition molecules and several different microbial structures including lipopolysaccharide (LPS) (Mukhopadhyay and Gordon, 2004; Areschoug and Gordon, 2009). In addition to binding and uptake of ligands, SRs also could play an important role in innate immune defense acting as regulators of cytokines production (Ozeki et al., 2006). Macrophages stimulated with SR-A ligands increase production of inflammatory cytokines, like tumor necrosis factor-α (TNFα), in a time- and dose-dependent manner. In the macrophage cell line J774A.1, SR-A ligands also induce TNFα and Interleukin-1β (IL1β) production, effects that appear to be mediated by the activation of mitogen activated protein kinases (MAPKs) JNK, p38 and ERK1/2 signaling pathways (Hsu et al., 2001). Similarly, activation of SR-A ligands mediated signaling induce NO production on RAW 264.7 cells (a mouse leukaemic monocyte macrophage cell line) as well as activation of JNK, p38 and ERK1/2 MAPK, and NF-κB (Campa et al., 2005). Furthermore, IL1β release induced by the activation of p38 and ERK1/2 pathways in murine peritoneal macrophages depended on the presence of SR-A ligands (Kwon et al., 2007).
Up to now, the expression of SR-A was thought to be mainly confined to cells belonging to the monocyte macrophage family (Naito et al., 1991; Hughes et al., 1995). In the CNS, SR-A has been observed in resident macrophage cells, the microglia (Alarcón et al., 2005, Farina et al., 2007). In contrast, only a few SRs have been described in astrocytes, which include SR-B1, SR-CL and SR-MARCO, recently described by our group (Alarcón et al., 2005; Farina et al., 2007).
Expression of SR-A and its capability for inducing signaling pathways and release of inflammatory molecules has not been described in astrocytes. Because astrocytes are a major player in the regulation of cytotoxic neuroinflammatory activation, we are interested in evaluating the presence of SR-A in astrocytes, and the effect of SRs ligands like fucoidan and Poly I on the activation of ERK1/2, JNK1/2 and IκB/NFκB signaling pathways, all of which are associated with inflammatory cellular responses including IL1β synthesis and NO secretion. Furthermore, we evaluated if IL1β plays a role on the regulation of NO secretion by inflammatory activation of astrocytes.
2. Materials and methods
Materials
Lipopolysaccharide (LPS; from Escherichia coli 0111:B4), fucoidan, polyinosinic acid (Poly I) and polycytidylic acid (Poly C) were purchased from Sigma (USA); IFNγ and IL1β were purchased from R&D (USA). Cell culture media, antibiotics and serum were purchase from Gibco (Life Technologies, USA). Animals were obtained from the institutional animal facility. All procedures were performed following the animal handling and bioethical requirements defined by the Pontificia Universidad Católica de Chile School of Medicine Ethics Committee. All animals were anaesthetized before sacrifice.
Glial Cultures
Mixed glial cell cultures, containing astrocytes and microglia, were obtained from the cerebral cortex of 1–2 day old Sprague-Dawley rats as described by Giulian and Baker (1986). Cortices were rinsed with Ca2+/Mg2+- free Hank’s balanced salt solution (HBSS); meninges were removed, and tissue was minced and incubated with 0.25% trypsin-EDTA in HBSS at 37°C for 10 min. The tissue was mechanically dissociated and cells were seeded in 75 cm2 cell culture flasks (one brain per flask) in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. Cultures were incubated in water saturated, 5% CO2 atmosphere at 37°C.
After 14 days in culture, microglial cells were obtained by shaking the mixed glial culture at 110 rpm in an orbital shaker (Unimax 1010, Heidolph, Germany) at 37°C for 15–20 min. Astrocytes were purified after microglial cell purification by trypsinization of attached cells. This procedure yields a highly enriched astrocyte (95% or more astrocytes) and microglial cell (over 99% microglia) cultures. Cell identity was evaluated on cultures by labeling with fluorescein isothiocyanate (FITC)-conjugated lectin from Griffonnia simplicifolia (1:200; Sigma), which recognizes microglia, and glial fibrillary acidic protein immunocitochemistry (antibodies α-GFAP; 1:200; Dako, Denmark) to identify astrocytes (data not shown). Glial cells were seeded in 96-well plates at a density of 3X104 cells per well for nitrites (NO2−) determination and plated at a density of 5X105 cells per 35 mm diameters Petri dishes for western blot assays.
Cell line culture
The DI-TNC1 (ATCC® Number: CRL-2005™) rat astrocytes cell line, was cultured in DMEM/F12 supplemented with 10% FBS, and antibiotics. The mouse microglia N9 cell line was cultured in DMEM/F12 supplemented with 5% FBS and antibiotics. Cultures were maintained in a water saturated, 5% CO2 atmosphere at 37°C.
Determination of Nitrites (NO2−)
Nitrites (NO2−), is a stable downstream product of the NO released by cells, was determined by the Griess assay (Pfeiffer et al., 1997). For NO2− determination, cells were maintained under control culture conditions or were incubated with either inflammatory molecules (1 μg/mL LPS + 10 ng/mL INFγ) or with various concentrations of SRs ligands (1 to 100 μg/ml fucoidan; 10 to 200 μg/ml Poly I; 10 to 200 μg/ml Dextran Sulphate; 200 μg/ml Poly C). For the determination of NO2−, 50 μl of medium was mixed with 10 μl EDTA:H2O 1:1 (0.5 M, pH 8.0) and 60 μl of freshly prepared Griess reagent (20 mg N-[1-naphtyl]-ethylendiamine and 0.2 g sulphanilamide dissolved in 20 ml of 5% phosphoric acid, w/v). Standard curves were established with 1–80 μM NaNO2. Absorbency was measured at 570 nm in a microplate auto reader (ANTHOS 2010, Anthos Labtec Instrument).
Western Blot Analysis
Astrocytes (5x105 cells per 35 mm diameter petri dish), after incubation with the various experimental conditions, were lysed in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and protease inhibitors). Protein concentration was determined by the BCA assay. Cell samples (70 μg protein) were electrophoretically separated on 12% polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was blocked with 0.1% Tween 20, 5% milk in phosphate buffer saline (PBS) for 1 h, and then incubated with the primary antibody in blocking buffer: goat α-IL1β (1:1,000; R&D), rabbit αpERK and α-ERK (1:500; Santa Cruz Biotechnology), rabbit α-pJNK and α-JNK (1:500; Cell signal), rabbit α-IκB 1:500; Santa Cruz Biotechnology) or mouse α-βTub I+II (1:1,000; Chemicon). Primary antibodies were rinsed and membranes were incubated with horseradish peroxidase-labeled donkey α-goat, α-rabbit or α-mouse depending on the primary antibody previously used, as secondary antibody. Signals were detected by enhanced chemiluminescence (Amersham Biosciences) in accordance with the manufacturer’s instructions. Densitometry was done with the ImageJ program.
Statistical Analysis
In vitro data correspond to at least 3–5 independent experiments in triplicate and were expressed as mean ± SEM. Statistical analysis was performed with the Kruskal Wallis one-way ANOVA and the Wilcoxon Rank Sum/Mann-Whitney U-test. A Dunn’s comparison was used for multiple comparisons. Evaluation was performed using the GBstat statistical software (Dynamic Microsystems, Inc). For all statistical analysis, a value of p <0.05 was considered significant.
3. Results
Astrocytes express SR-MARCO and SR-A
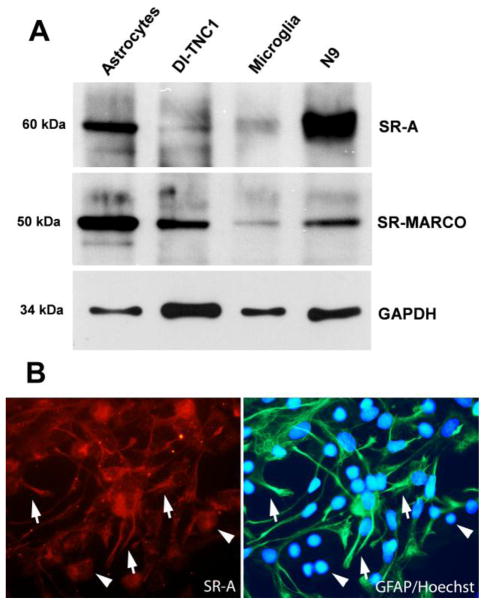
Expression of SR-A at the protein level by astrocytes in culture was evaluated by western blot assay using reductive conditions (Fig. 1A) and by immunofluorescence (Fig. 1B). The blot showed a band of 60 kDa corresponding to the SR-A monomer, which was observed both in astrocytes and microglial cells. Furthermore, expression of SR-A by the astrocyte cell line DI-TNC1 (Fig. 1) demonstrated that SR-A was effectively present in astrocytes and it was not the result of the microglial cell contamination that is unavoidable in astrocyte cultures. SR-MARCO monomeric isoform (50 kDa) was expressed both by microglia and astrocytes, as previously described (Alarcón et al., 2005). Figure 1B shows that SR-A was expressed by both astrocytes (arrows) and microglia (arrow heads).
Figure 1. Presence of SR-A in primary astroglial and microglia cells.
A) Western blot of monomeric SR-A (60 kDa) and SR-MARCO (50 kDa). Both proteins were detected in rat astrocytes, the astrocytes cell line DI-TNC1 of rat origin, microglia and the mice microglial cell line N9. Immune detection of GAPDH was used as loading control. B) Immunofluorescence labeling of SR-A (Rho) and GFAP as astrocyte identity marker (Alexa 488), and Hoechst stain for nuclea (blue). Both astrocytes (arrows) and microglia (arrow heads: GFAP(-) cells) are labeled by anti-SR-A antibodies.
SR ligands induced NO production by astrocytes and microglia
Once the presence of SR-A and SR-MARCO on astrocytes and microglia was demonstrated, we evaluated the stimulation of glial cell cultures with different SRs ligands, assessing the production of NO, a marker of inflammatory cell activation (Table 1). We used classically described SRs ligands, fucoidan and Polyinosinic acid (Poly I) (Goldstein et al., 1979; Krieger, 1997; Shechter et al., 1981; Zhang et al., 1993; Nishikawa et al., 1990; de Winther et al., 2000). Polycytidylic acid C (Poly C) a polyanion that does not bind to SR class A, and Dextran sulfate (DS) a ligand described as not being able to bind to SRs but unable to induce a transduction signaling response (Nakamura et al., 2006), were used as negative controls. As positive control we used inflammatory activation with LPS plus INFγ (LI).
Table 1.
Astrocytes - Inflammatory activation
| Exp. stimuli | Fucoidan | Poly I | LPS+IFNγ |
|---|---|---|---|
| IL1β | ↑ | ↑ ↑ | ↑ ↑ |
| NO | - | ↑ ↑ | ↑ ↑ |
| ERK activ | - | ↑ | ↑ ↑ |
| JNK activ | - | ↑ | ↑ |
| NFκB activ | - | - | ↑ |
Effect of SR-A ligands on the Inflammatory activation of astrocytes in culture.
ERKactiv, JNKactiv and NFκB active indicates the activation of the signaling pathways.
(↑) increases, and (-) no change.
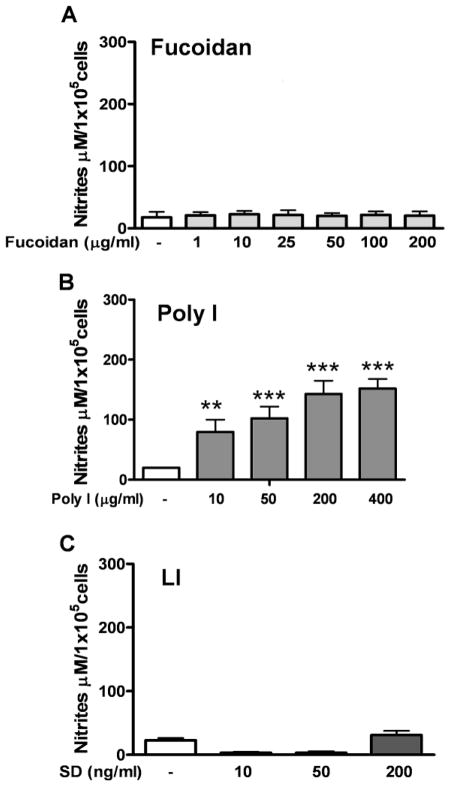
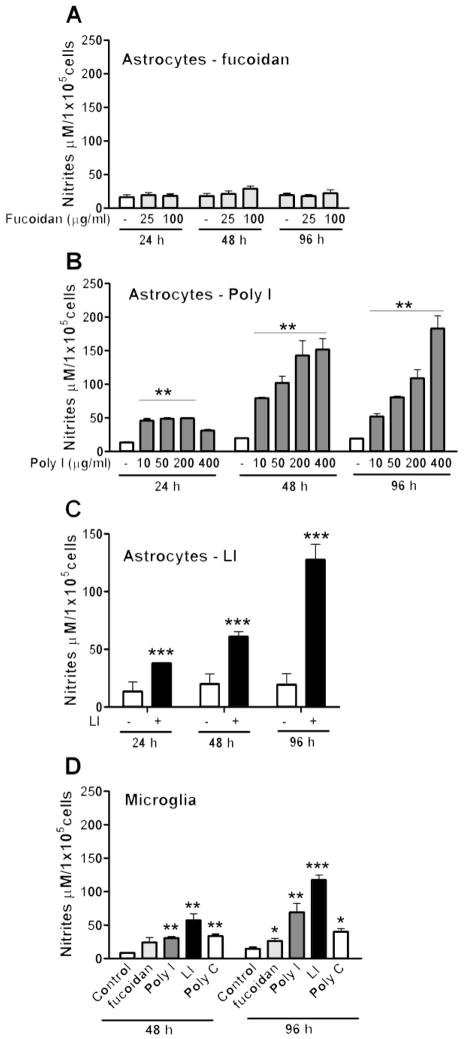
Increasing concentrations of fucoidan (1 to 200 μg/ml) (Fig. 2A) and increasing duration (24 to 96 h) of stimulation (Fig. 3A) were unable to induce NO production by astrocytes (Fig. 2A and Fig. 3A, respectively). On the other hand, astrocytes that were exposed to Poly I showed induction of NO production in a concentration- (10 to 400 μg/ml) and time-dependent manner (Dunn’s Multiple comparisons, p<0.001). Treatment with 200 μg/ml of Poly I (Fig. 2B) for 48 h and 96 h (Fig. 3B) were the concentration and time at which production of NO reached a plateau (180 μM NO2−). Higher concentration of Poly I and longer exposition maintained this same level of NO2−. In contrast, astrocytes exposed to increasing concentrations of DS (10, 50 and 200 ng/ml) (Fig. 2C), did not produce detectable levels of NO. However, we observed that DS was cytotoxic for astrocytes at all of the concentrations tested (data not shown). Astrocytes exposed to LI (Fig. 3C) for increasing times (24 to 96 h) produced high amounts of NO in a time dependent manner (Fig. 3C), which was similar to that obtained by Poly I. Astrocytes exposed to Poly C at increasing times (24 to 96 h) failed showing induction of NO production (Fig. 2C).
Figure 2. Astrocytes exposed to SR ligands increased production of NO.
Production of NO by astrocytes exposed for 48 h to increasing concentrations of A) fucoidan, B) Poly I and C) Dextran Sulphate (DS). The results correspond to the mean ± SEM of 2–4 independent experiments performed in quadruplicate. * p<0.05; ** p<0.01; *** p<0.001, of SR-A ligand stimulated astrocytes vs. the control condition.
Figure 3. NO production by astrocytes exposed to Poly I and LI increased with time.
Production of NO by astrocytes (A-C) exposed for increasing concentrations and times to A) fucoidan, B) Poly I and C) LI; and microglial cells exposed to various stimulus D), 200 μg/ml fucoidan, 200 μg/ml Poly I, 1μg/ml LPS plus 10 ng/ml INFγ (LI) and 200 μg/ml Poly C for 48 and 96 h of stimulation. Results correspond to the mean ± SEM of 3–5 independent experiments, performed in quadruplicate. * p<0.05; ** p<0.01; *** p<0.001 of SR-A ligand stimulated cell vs. the control condition.
Microglia exposed to fucoidan increased NO production by 2.2-fold at 48 h and 2.0-fold at 96 h compared with the control condition (Fig. 3D). Similarly Poly I induced a 3.0-fold increase of NO production at 48 h and a 6.0-fold increase at 96 h compared with control cells. LI induced a 9.6-fold increase of NO production at 48 h and a 10-fold increase at 96 h compared with its control.
Our results indicate that SRs ligands induced a differential effect on astrocytes and microglia. Only Poly I and LI were capable to inducing NO production by astrocytes (Table 1), whereas microglia also showed activation when exposed to fucoidan.
SRs ligands activated MAPKs and NFκB signaling pathways of astrocytes
Knowing that binding of specific SR-A and SR-MARCO ligands can induce production of NO by astrocytes, as a next step we evaluated the activation of downstream signaling pathways, JNK, ERK and IκB/NF-κB, which could be involved on the induction of inducible NO synthase (iNOS) by fucoidan, Poly I or LI (Fig. 4; Table 1). Astrocytes exposed to Poly I induced a 3.0-fold increase of the phosphorylation of ERK; induction that reached a 5.0-fold increase after stimulation with LI (Fig. 4A). In contrast, fucoidan was unable to induce activation of ERK signaling at 24 h (Fig. 4A). Similarly, treatment with Poly I and LI, but not fucoidan, induced a 2.5-fold and 4.3-fold increment of JNK phosphorylation respectively (Fig. 4B). For the assessment of the activation of IκB/NFκB signaling pathway, we evaluated the levels of IκB. Astrocytes exposed to fucoidan and Poly I ligands maintained unaffected IκB levels (Fig. 4C). In contrast, LI induced a 2.0-fold decrease of IκB compared with the control (Fig. 4C) indicating the activation of this pathway at 24 h of stimulation.
Figure 4. SR ligands differentially activated ERK, JNK and IκB/NFκB signaling pathways in astrocytes.
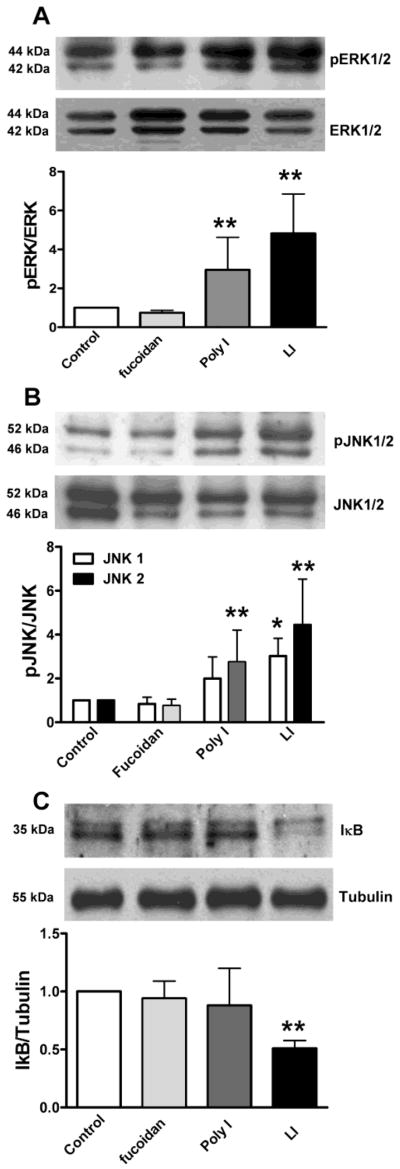
Astrocytes were stimulated with 200 μg/ml fucoidan, 200 μg/ml Poly I and 1μg/ml LPS and 10 ng/ml INFγ (LI) for 24 h. The activation of A) ERK, and B) JNK, expressed as the increased ratio of phosphorylated to total ERK or JNK (pERK/ERK and pJNK/JNK respectively) and C) decrease of IκB as a measure of the activation of NFκB pathway was evaluated. Results correspond to the mean ± SEM of 3–4 independent experiments performed in quadruplicate. *p<0.05, for SR-A ligand stimulated cells vs. the control condition.
SRs ligands induced IL1β production by astrocytes
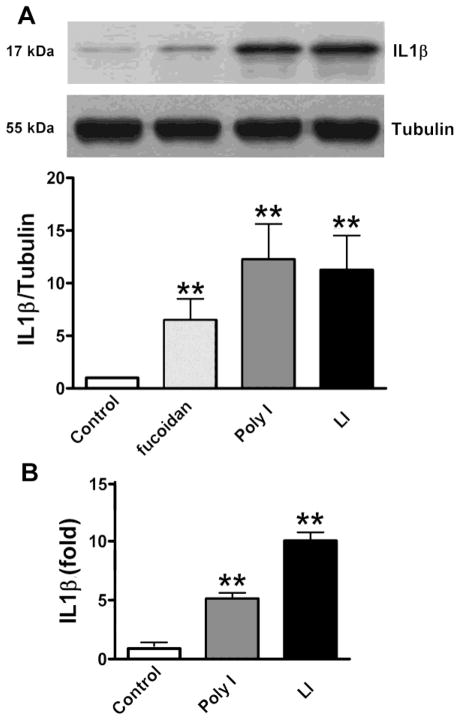
IL1β is a cytokine secreted early during the inflammatory response of glial cells. We evaluated by western blot the de novo production and by ELISA the secretion of this cytokine by astrocytes stimulated with SRs ligands (Fig. 5, Table 1). Astrocytes treated with fucoidan, Poly I and LI for 24 h, showed an increment on the presence of IL1β by 8.2-fold, 11.4-fold, and 17.7-fold, respectively, compared with astrocytes under control condition (Fig. 5, Table 1). These results indicate that regardless of the different patterns of inflammatory activation induced by SRs ligands, both fucoidan and Poly I induced the production of IL1β by astrocytes.
Figure 5. Astrocytes exposed to SRs ligands produced increased levels of IL1β.
Astrocytes were stimulated with 200 μg/ml fucoidan, 200 μg/ml Poly I and LI (1μg/ml LPS and 10 ng/ml INFγ) for 24 h. IL1β was evaluated by A) Western blot and quantification by densitometry, which was normalized by tubulin and B) ELISA. Data represent the mean ± SEM of 3–4 independent experiments performed in quadruplicate.*p<0.05, **p<0.01, for stimulated cells vs. the control condition.
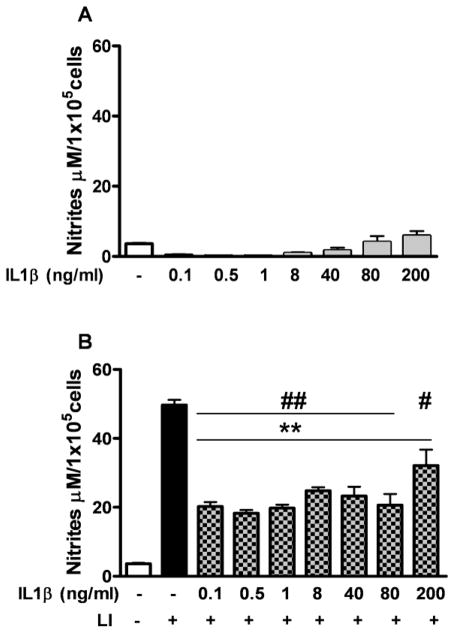
IL1β inhibited NO production in astrocytes
Astrocytes stimulated with increasing concentrations of IL1β (0.1 to 200 ng/ml) did not increase their production of NO (Fig. 6A). Furthermore, IL1β inhibited LI-induced production of NO by astrocytes. We stimulated astrocytes with IL1β at increasing concentrations (0.1-to-200 ng/ml), with and without co-stimulation with LI for 96 h (Fig. 6B). Astrocytes simultaneously stimulated with IL1β and LI produced levels of NO that were approximately 2.2-fold lower than astrocytes exposed to LI, which showed a 20-fold induction of NO production compared with the control conditions. However, IL1β at high concentration (200 ng/ml) was less effective reducing NO induction.
Figure 6. IL1β inhibits LI-induced NO production by astrocytes.
NO production by astrocytes exposed for 24 h to A) increasing concentrations of IL1β and B) LI with or without co-stimulation with increasing concentration of IL1β. The results correspond to the mean ± SEM of 4 independent experiments performed in quadruplicate. ** p<0.001, for LI or LI+IL1β stimulated cells vs. the control condition. # p<0.05, ## p<0.001 for LI+IL1β stimulated cells vs. LI stimulated cells.
These data suggest that IL1β, depending on the stimulation condition, can both potentiate and decrease inflammatory response on astrocytes.
4. Discussion
One of the main findings of this work is that it shows for the first time the presence of SR-A in rat astrocytes, indicating that in addition to the SRs previously described, including SR-MARCO (Alarcón et al., 2005; Farina et al., 2007), astrocytes also express SR-A, capable of binding ligands like fucoidan, Poly I, and LPS. Stimulation with these SRs-ligand showed a differential effect on the activation of ERK, JNK and IκB/NF-κB signaling pathways, as well as on the induction of NO and IL1β production, being both markers of inflammatory activation for glial cells.
SRs ligand binding is characterized by high affinity and broad specificity (Krieger and Herz, 1994). Class A SRs contain a cluster of positive charged residues in their collagenous domain that are highly conserved and appears to provide a sticky surface that function as “molecular flypaper” for several different ligands (Krieger and Herz, 1994). The signaling determinants that allow different SR ligands to induce different cell responses have not been identified. SR-A and SR-MARCO appear to be unable to initiate inflammatory signaling pathways by themselves (Fong and Le., 1999; Kosswig et al., 2003). Therefore, the existence of multiple binding sites on a single SR, or the interaction of SR, with other SRs or with other types of receptors, could differentially activate signaling pathways and allow for the diversity of cellular responses.
There are also differences on ligand binding depending on cell type. Microglia, in contrast to astrocytes, responded to all ligands including Poly C showing an increased NO production. Microglia could have more SRs at their surface or express other co-receptors like TLRs (Farina et al., 2007), a group of pattern recognition receptors that as mentioned, have ligands shared with class A SRs, for example LPS, which also binds to TLR-4, or Poly I and Poly C, which also bind to TLR-3 (Chang, 2010). Our data supports the existence of differences on SR-A ligand differences between microglia and astrocytes. Microglial cell activation appears to be more robust and broader in terms of ligands.
The presence of SR-A on astrocytes is especially relevant because SR-A is involved in inflammatory diseases (Sun et al., 2007; Hickman et al., 2008; Manning-Tobin et al., 2009) and astrocytes are key regulator of the inflammatory response in the CNS. Astrocytes modulate the inflammatory response of microglial cells, inhibiting NO production in presence of ligands such as IL1β and TNFα (Tichauer et al., 2007). Furthermore, binding of SR-A ligands induce the production of cytokines such as IL1β, a master regulator of neuroinflammation, which is produced by astrocytes in the CNS (Campa et al., 2005; Basu et al., 2004; Hsu et el., 2001; Palkama, 1991). Therefore, the presence of this scavenger receptor on astrocytes is an exciting premise.
In this work, we used the classical ligands fucoidan and Poly I (Takakura et al., 1999), and LPS, a ligand binding both Toll like receptor-4 (TLR-4) and SRs (Amiel et al., 2009). The various ligands generated different responses on astrocytes. Poly I and LI but not fucoidan induced production of NO in a concentration- and time-dependent manner. Coherently, Poly I and LI, but not fucoidan induced MAPKs and NFκB signaling pathways, classical activators of iNOS (Saha and Pahan, 2006). In contrast, all three ligands induced IL1β production by astrocytes.
SR-A can internalize Aβ peptide in animal models of AD (Husemann et al., 2002; Hickman et al., 2008). Association of Aβ with SR-A, have also been associated with inflammatory activation in the CNS (Hsu et al., 2001; Kim et al., 2003) and potentiation of the activation induced other SR ligands in vitro (Murgas et al., 2012). Increased inflammatory activation can facilitate cytotoxicity and neurodegeneration (von Bernhardi et al., 2007; Ramírez et al., 2008). There are reports on the detrimental effects of absence of both SR-MARCO and SR-A on antibacterial defenses in mice. A defect in the uptake or killing of bacteria as well as dysregulation of inflammatory responses may contribute to defective antibacterial defense in both SR-MARCO- and SR-A-deficient mice, being one of the mechanisms responsible for decreased survival of receptor KO mice during bacterial infections (Józefowski et al., 2005; Chen et al., 2010). However, although there is an important wealth of information that SR-A and SR-MARCO trigger intracellular signaling, modulating inflammatory, phagocytic and microbicidal activities of macrophages (Józefowski et al., 2005; Chen et al., 2010), it is less known their participation in brain parenchyma processes.
IL1β is a classical inflammatory cytokine that promotes glial activation, inducing production of other inflammatory cytokines (Viviani et al., 2004; Mrak and Griffin, 2005). However, we have previously found that acting with different timing, both TGF-β1 and IL-1β modulate the amplitude and duration of glial activation in response to inflammatory activation, suggesting they can be key for the understanding of inflammatory mechanisms involved in the pathogenesis of neurodegenerative diseases (Ramírez et al., 2005). Here, we detected that IL1β did not induce NO production by astrocytes. Furthermore, co-stimulation with LI and IL1β resulted in the inhibition of LI-induced NO production, suggesting that IL1β has a modulatory function on astrocytes exposed to inflammatory conditions. Production of IL1β is induced by the stimulation of glial cells by LPS (Rankine et al., 2006; Harvath et al., 2008), as well as by the binding of SR ligands (Palkama, 1991). IL1β is an early cytokine on the neuroinflammatory process (Basu et al., 2004). It is upregulated in several neurological diseases and neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (Blum-Degen et al., 1995; Basu et al., 2004). Increase of IL1β plus TNFα has been related to the induction of iNOS in astrocytes (Marcus et al., 2003). Furthermore, IL1β induces the activation of the transcriptional factor NFκB in astrocytes, promoting transcription of genes encoding for adhesion molecules, chemokines and cytokines (John et al., 1986; John et al., 2004; Xia and Zhai, 2010). However, as mentioned, we have also found that IL1β can also decrease NO production by glial cells exposed to inflammatory molecules, inhibiting ERK phosphorylation (Saud et al., 2005; Tichauer et al., 2007). There are also reports that IL1β can generate tolerance to LPS in vivo, and down regulate the presence of TLR-4, a receptor that is crucial in the signaling of LPS (Alves-Rosa et al., 2002). IL1β appears to exert beneficial effects, particularly when released at low concentrations. Astrocytes activated by IL1β have an enhanced capacity to sustain neuronal survival, reestablishing CNS homeostasis and blood brain barrier function (Liberto et al., 2004). Astroglial activation is delayed in mice lacking IL1β as well as in mice lacking the IL1β type 1 receptor (Herx et al., 2000). Altogether, these findings suggest that IL1β promotes the adaptive responses of astrocytes to injury, and probably could exert a protector effect regulatory the inflammatory response.
Conclusion
We demonstrated the presence of SR-A in astrocytes. We showed that SRs ligands can induce the activation of MAPKs and IκB/NFκB signaling pathways inducing NO and IL1β production, indicating that astrocytes expressed functional SRs. On the other hand, although IL1β can be a potent inflammatory cytokine, it inhibited NO production by astrocytes stimulated with LI at the present stimulation conditions; indicating that IL1β appears to exercise a novel effect on astrocytes response as a modulator of neuroinflammation.
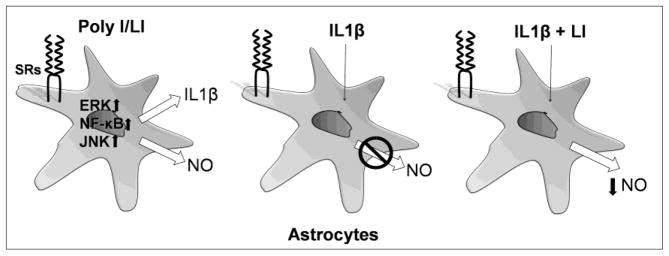
Figure 7. Scavenger receptors class A ligands induce secretion of IL1β and exert a modulatory effect on the inflammatory activation of astrocytes in culture.
Astrocytes exposed to Poly I and LI release NO and produced increased levels of IL1β in a concentration and time dependent manner. Astrocytes exposed to increased concentrations of IL1β did not produce detectable levels of NO. Furthermore, astrocytes exposed to the co-stimulation with LI and IL1β LI produced at least 50% less NO compared with astrocytes stimulated solely with LI, indicating that IL1β inhibited NO production induced by inflammatory stimuli.
Acknowledgments
This work was supported by grant Fondecyt 1090353 (RvB) and grant NIH R03 TW008019. We thank Dr. Lisette Leyton for providing the cell line DI-TNC1. We thank Gigliola Ramírez for excellent technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcón R, Fuenzalida C, Santibáñez M, von Bernhardi R. Expression of Scavenger Receptors in Glial Cells. J Biol Chem. 2005;280:30406–30415. doi: 10.1074/jbc.M414686200. [DOI] [PubMed] [Google Scholar]
- Alves-Rosa F, Vulcano M, Beigier-Bompadre M, Fernández G, Palermo M, Isturiz MA. Interleukin-1beta induces in vivo tolerance to lipopolysaccharide in mice. Clin Exp Immunol. 2002;128:221–228. doi: 10.1046/j.1365-2249.2002.01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel E, Alonso A, Uematsu S. Pivotal Advance: Toll like receptor regulation of scavenger receptor-A-mediated phagocytosis. J Leukoc Biol. 2009;85:595–605. doi: 10.1189/jlb.1008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol. 2009;11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: A master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995;29:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Campa VM, Iglesias JM, Carcedo MT, Rodriguez R, Riera J, Ramos S, Lazo PS. Polyinositic acid induces TNF and NO production as well as NF-kappaB and AP-1 transcripcional activation in the monocyte/macrophage cell line RAW264.7. Inflamm Res. 2005;54:328–337. doi: 10.1007/s00011-005-1359-4. [DOI] [PubMed] [Google Scholar]
- Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791–808. doi: 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wermeling F, Sundqvist J, Jonsson AB, Tryggvason K, Pikkarainen T, Karlsson MC. A regulatory role for macrophage class A scavenger receptors in TLR4-mediated LPS responses. Eur J Immunol. 2010;40:1451–1460. doi: 10.1002/eji.200939891. [DOI] [PubMed] [Google Scholar]
- Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, Medico E, Hohlfeld R, Meinl E, Farina C. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med. 2012;209:521–535. doi: 10.1084/jem.20110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fong LG, Le D. The processing of ligands by the class A scavenger receptor is dependent on signal information located in the cytoplasmic domain. J Biol Chem. 1999;274:36808–36816. doi: 10.1074/jbc.274.51.36808. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;8:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. J Neropathol Exp Neurol. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- Herrera-Molina R, von Bernhardi R. Transforming growth factor-beta 1 produced by hippocampal cells modulates microglial reactivity in culture. Neurobiol Dis. 2005;19:229–236. doi: 10.1016/j.nbd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Herx LM, Rivest S, Yong VW. Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1 beta is required for the production of ciliary neurotrophic factor. J Immunol. 2000;165:2232–2239. doi: 10.4049/jimmunol.165.4.2232. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DA, Fraser IP, Gordon S. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol. 1995;25:466–473. doi: 10.1002/eji.1830250224. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Chiu SL, Wen MH, Chen KY, Hua KF. Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J Biol Chem. 2001;276:28719–28730. doi: 10.1074/jbc.M011117200. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulted responses in astrocytes mammalian brain. J Neurosci. 1986;6:2163–2178. [Google Scholar]
- John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;17:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefowski S, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–8041. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- Kim WS, Ordija CM, Freeman MW. Activation of signaling pathways by putative scavenger receptor class A (SR-A) ligands requires CD14 but not SR-A. Biochem Biophys Res Commun. 2003;310:542–549. doi: 10.1016/j.bbrc.2003.09.049. [DOI] [PubMed] [Google Scholar]
- Kosswig N, Rice S, Daugherty A, Post SR. Class A scavenger receptor-mediated adhesion and internalization require distinct cytoplasmic domains. J Biol Chem. 2003;278:34219–34225. doi: 10.1074/jbc.M303465200. [DOI] [PubMed] [Google Scholar]
- Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Kwon KH, Ohigashi H, Murakami A. Dextran sulfate sodium enhances interleukin-1b release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life Sci. 2007;81:362–371. doi: 10.1016/j.lfs.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JS, Karackattu SL, Fleegal MA, Summers C. Cytokine-simulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-kappaB. Glia. 2003;41:152–160. doi: 10.1002/glia.10168. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Gordon S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunology. 2004;209:39–49. doi: 10.1016/j.imbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Murgas P, Godoy B, von Bernhardi R. Aβ potentiates inflammatory activation of glial cells induced by Scavenger receptor ligands in culture. Neurotoxicity Res. 2012 doi: 10.1007/s12640-011-9306-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Suzuki H, Wada Y, Kodama T, Doi T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-kappaB-dependent signaling pathways through macrophage scavenger receptors. Biochem Biophys Res Commun. 2006;343:286–294. doi: 10.1016/j.bbrc.2006.02.146. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Naito M, Kodama T, Matsumoto A, Doi T, Takahashi K. Tissue distribution, intracellular localization, and in vitro expression of bovine macrophage scavenger receptors. Am J Pathol. 1991;139:1411–1423. [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Arai H, Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages. J Biol Chem. 1990;265:5226–5231. [PubMed] [Google Scholar]
- Ozeki Y, Tsutsui H, Kawada N. Macrophage scavenger receptor downregulates mycobacterial cord factor-induced proinflammatory cytokines production by alveolar and hepatic macrophages. Microbial Pathogenesis. 2006;40:171–176. doi: 10.1016/j.micpath.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Palkama T. Induction of Interleukin-1 production by ligans to the scavenger receptor in human monocytes and the THP-1 cell line. Immunology. 1991;74:432–438. [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S, Gorren AC, Schmidt K, Werner ER, Hansert B, Bohle DS, Mayer B. Metabolic fate of peroxynitrite in aqueous solution. Reaction with nitric oxide and pH-dependent decomposition to nitrite and oxygen in a 2:1 stoichiometry. J Biol Chem. 1997;272:3465–3470. doi: 10.1074/jbc.272.6.3465. [DOI] [PubMed] [Google Scholar]
- Ramírez G, Toro R, Döbeli H, von Bernhardi R. Protection of rat primary hippocampal cultures from Aβ cytotoxicity by pro-inflammatory molecules is mediated by astrocytes. Neurobiol Disease. 2005;19:243–254. doi: 10.1016/j.nbd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Ramírez G, Rey S, von Bernhardi R. Proinflammatory stimuli induce microglial cell- mediated APP- and Aβ-neurotoxicity in hippocampal cultures. J Alzheimer Dis. 2008;15:45–59. doi: 10.3233/jad-2008-15104. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rankine EL, Hughes PM, Botham MS, Perry VH, Felton LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1deficient mice prior to leucocyte recruitment. Eur J Neurosci. 2006;24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saud K, Herrera-Molina R, von Bernhardi R. Pro- and anti-inflammatory cytokines regulate the ERK pathway: implication of the timing for the activation of microglial cells. Neurotox Research. 2005;8:277–287. doi: 10.1007/BF03033981. [DOI] [PubMed] [Google Scholar]
- Shechter I, Fogelman AM, Haberland ME, Seager J, Hokom M, Edwards PA. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981;22:63–71. [PubMed] [Google Scholar]
- Sun J, Turner A, Xu J, Grönberg H, Isaacs W. Genetic variability in inflammation pathways and prostate cancer risk. Urol Oncol. 2007;25:250–259. doi: 10.1016/j.urolonc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Takagi T, Hashiguchi M, Nishikawa M, Yamashita F, Doi T, Imanishi T, Suzuki H, Kodama T, Hashida M. Characterization of plasmid DNA binding and uptake by peritoneal macrophages from class A scavenger receptor knockout mice. Pharm Res. 1999;16:503–508. doi: 10.1023/a:1018842210588. [DOI] [PubMed] [Google Scholar]
- Tichauer J, Saud K, von Bernhardi R. Modulation by astrocytes of microglial cell-mediated neuroinflammation: effect on the activation of microglial signaling pathways. Neuroimmunomodulation. 2007;14:168–174. doi: 10.1159/000110642. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Corsini E, Galli CL, Marinovich M. Cytokines role in neurodegenerative events. Toxicol Lett. 2004;149:85–89. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R. Glial cell dysregulation: a new perspective on Alzheimer disease. Neurotoxicity Res. 2007;12:215–232. doi: 10.1007/BF03033906. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Eugenín J. Microglia – astrocyte interaction in Alzheimer’s disease: modulation of cell reactivity to Aβ. Brain Res. 2004;1025:186–193. [Google Scholar]
- von Bernhardi R, Eugenín J. Alzheimer’s Disease: Redox Dysregulation as a Common Denominator for Diverse Pathogenic Mechanisms. Antiox Redox Signal. 2012 doi: 10.1089/ars.2011.4082. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Ramírez G, Toro R, Eugenín J. Pro-inflammatory conditions promote neuronal damage mediated by Amyloid Precursor Protein and decrease its phagocytosis and degradation by microglial cells in culture. Neurobiol Disease. 2007;26:153–164. doi: 10.1016/j.nbd.2006.12.006. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Tichauer J, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zhai Q. IL-1beta enhances the antibacterial activity of astrocytes by activation of NF-KappaB. Glia. 2010;58:244–252. doi: 10.1002/glia.20921. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang Y, Steinbrecher UP. Structural requirements for the binding of modified proteins to the scavenger receptor of macrophages. J Biol Chem. 1993;268:5535–5542. [PubMed] [Google Scholar]