Abstract
Visual experience is known to guide ocular growth. We tested the hypothesis that vision-guided ocular growth is disrupted in a model system with diminished visual acuity. We examine whether ocular elongation is influenced by form-deprivation (FD) and lens-imposed defocus in the Retinopathy, Globe Enlarged (RGE) chicken. Young RGE chicks have poor visual acuity, without significant retinal pathology, resulting from a mutation in guanine nucleotide-binding protein β3 (GNB3), also known as transducin β3 or Gβ3. The mutation in GNB3 destabilizes the protein and causes a loss of Gβ3 from photoreceptors and ON-bipolar cells. (Ritchey et al. 2010)FD increased ocular elongation in RGE eyes in a manner similar to that seen in wild-type (WT) eyes. By comparison, the excessive ocular elongation that results from hyperopic defocus was increased, whereas myopic defocus failed to significantly decrease ocular elongation in RGE eyes. Brief daily periods of unrestricted vision interrupting FD prevented ocular elongation in RGE chicks in a manner similar to that seen in WT chicks. Glucagonergic amacrine cells differentially expressed the immediate early gene Egr1 in response to growth-guiding stimuli in RGE retinas, but the defocus-dependent up-regulation of Egr1 was lesser in RGE retinas compared to that of WT retinas. We conclude that high visual acuity, and the retinal signaling mediated by Gβ3, is not required for emmetropization and the excessive ocular elongation caused by FD and hyperopic defocus. However, the loss of acuity and Gβ3 from RGE retinas causes enhanced responses to hyperopic defocus and diminished responses to myopic defocus.
1. Introduction
As the eye grows, the combined refractive powers of the lens and cornea must be matched ocular elongation to avoid the development of significant refractive error. The process of minimization of refractive error while the eye is growing is known as emmetropia. Unobstructed vision is required to achieve emmetropia. Vision-dependent ocular growth has been demonstrated by the phenomena of form-deprivation myopia and lens-induced ametropias. Deprivation of form-vision results in excessive ocular growth, vitreous chamber elongation and myopia (Norton 1990; O'Leary and Millodot 1979; Sherman et al. 1977; Tejedor and de la Villa 2003; Troilo and Judge 1993; Wallman et al. 1978; Wiesel and Raviola 1977). Lens-imposed defocus also influences rates of ocular growth, with minus lens-wear accelerating ocular growth and plus lens-wear slowing ocular growth (Nickla et al. 1997; Wallman et al. 1995; Wildsoet and Wallman 1995). The mechanisms responsible for vision-guided ocular growth are confined to the eye and are derived locally from the retina (McBrien et al. 1995; Rada et al. 1994; Wallman et al. 1987); (McBrien et al. 1995; Norton 1990; Norton et al. 1994).
It is generally accepted that retinal processing of visual information is required for emmetropization. Studies involving the ablation of different populations of retinal neurons have suggested that amacrine cells are needed to regulate ocular growth. In the chicken retina, for example, damage to glucagonergic amacrine cells causes excessive ocular growth and alters the response of the eye to visual stimuli that influence growth (Fischer et al. 1999b; Fischer et al. 2008). Additional studies have demonstrated that widespread retinal damage, through the application of metabolic toxins or excitotoxins, tends to increase ocular size (Ehrlich et al. 1990; Fischer et al. 1999b; Fischer et al. 1998b; Wildsoet and Pettigrew 1988). The findings of these ablation studies suggest that retinal neurons normally act to slow rates of ocular growth. The interpretation of findings of the ablation studies, however, is confounded by the lack of cell-type specificity of the toxins; unintended collateral damage to neurons and glia makes interpretation of the data difficult. Therefore, an animal model with diminished visual function resulting from a well-defined genetic defect, without retinal damage, would be advantageous for the study of the retinal circuitry involved in regulating ocular growth.
In this study we investigate how vision-guided ocular growth is affected in an animal model, the Retinopathy, Globe Enlarged (RGE) chicken, with a genetic defect that causes diminished visual acuity. The RGE phenotype was first observed in commercial chicken broods in the United Kingdom (Curtis et al. 1987). The RGE chicks have diminished visual acuity, as assessed by optokinetic responses. The optokinetic responses of RGE chicks to higher spatial frequencies are diminished at the time of hatching (post-hatch day 1 – P1); these responses progressively worsen through to P30, despite no significant retinal pathology (Montiani-Ferreira et al. 2005; Montiani-Ferreira et al. 2003). Although the eyes of RGE chicks undergo some narrowing of the anterior chamber by 2 weeks after hatching, no significant differences in vitreous chamber depth (VCD), axial length, radial diameter, corneal thickness, corneal curvature or refraction are observed until after P33 (Montiani-Ferreira et al. 2003). Electroretinograms (ERGs) are abnormal in young RGE chicks, between P7 and P30, before the onset of retinal degeneration. The ERGs of light- and dark-adapted RGE eyes have a-waves (generated by photoreceptors) with significantly diminished amplitudes, elevated thresholds and shallower leading slopes, whereas b-waves (generated by inner retinal neurons and Müller glia) are supernormal with smoother and broader wave-forms compared to those of WT chicks (Montiani-Ferreira et al. 2007).
The phenotype of RGE chickens results from a mutation, a single codon deletion (D153del), in guanine nucleotide-binding protein beta-3 (GNB3), also known as transducin β3 or Gβ3 (Tummala et al. 2006). Gβ3 acts cooperatively with the γ-subunit of transducin to deactivate GTP-bound α-transducin which, in turn, deactives phospodiesterase to stop hydrolysis of cGMP and facilitate opening of cGMP-gated channels in photoreceptors and ON-bipolar cells (reviewed by Arshavsky et al. 2002). Gβ3 participates in signal transduction through G-protein-coupled receptors and normally functions to enhance the temporal fidelity of phototransduction and ON-center signaling in the retina (reviewed by Lagnado 2002; Makino et al. 2003; Shichida and Matsuyama 2009). The mutation responsible for the RGE phenotype is thought to cause improper post-translational folding and destabilization the Gβ3 protein (Tummala et al. 2006). In the chick retina, Gβ3 is normally expressed by ON-bipolar cells and rod and cone photoreceptors, and Gβ3 is completely lost from the RGE retina (Ritchey et al. 2010). An in-depth histological examination of young (<P30) RGE retinas indicated subtle changes in the outer nuclear layer (ONL) and disorganization of synaptic layers in the outer plexiform layer (OPL) (Montiani-Ferreira et al. 2005). With the exception of relatively minor abnormalities in photoreceptors and bipolar cells, the loss of Gβ3 from RGE retinas has no pathological effects upon retinal cells, including photoreceptors, bipolar cells, many different types of amacrine cells, ganglion cells and Müller glia (Montiani-Ferreira et al. 2005; Ritchey et al. 2010). However, beginning at about P90, progressive retinal degeneration occurs in RGE chicks, resulting in severe retinopathy and blindness (Montiani-Ferreira et al. 2005). The loss of visual acuity, retinopathy and globe enlargement in the RGE chick occur solely from the loss of Gβ3 from photoreceptors and ON-bipolar cells; Gβ3 is not expressed in extra-retinal ocular tissue nor in higher visual centers (Ritchey et al. 2010).
Given that the vision-loss observed in young RGE chicks occurs in the absence of gross retinal abnormalities or degeneration, the young RGE chick provides a unique opportunity to study vision-guided ocular growth in a model system with reduced visual acuity. The RGE model system permits us to test the hypothesis that high visual acuity is required for vision-guided ocular growth. Some of this work has been presented at the 13th International Myopia Conference (Ritchey et al. 2011) and the 2009 ARVO conference (Ritchey et al. 2009).
2. Methods and Materials
2.1 Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the Ohio State University. Homozygous RGE-/- chickens (a strain of Gallus gallus domesticus) were hatched from fertilized eggs obtained from an established colony at the Department of Small Animal Clinical Sciences, Michigan State University. All RGE chicks that were studied were between age P1 and P30, long before the onset of globe enlargement and retinal degeneration. Wild-type (WT) white leghorn chickens (Gallus gallus domesticus) were obtained at P0 from the Department of Poultry Science at The Ohio State University. Chicks were housed in a stainless steel brooder and kept on a cycle of 12 hours light, 12 hours dark (lights on at 8:00 AM). Chicks received water and Purina chick starter ad libitum. Daytime luminosity levels between 400 and 700Lux were maintained during the 12 hour light cycle with fluorescent luminaires (GE Ecolux F32T8SP35Eco).
2.2 Visual manipulations
Form-deprivation (FD) goggles, plano lenses, −7 or +7 diopter lenses (Anchor Optics, Barrington, NJ) were applied to the right eye only of RGE or WT chicks using cyanoacrylate adhesive and Velcrotm rings. The left eye of each animal was untreated. All visual manipulations were started at P7. For all lens experiments, lenses were cleaned approximately every 2.5 hours during the light on cycle for the duration of the experiment. The experimental paradigms, numbers of animals, visual manipulations and outcome measures are listed in table 1.
Table 1.
experimental paradigms
| Experiment # and Figure # |
Numbers of animals |
Treatment | Outcome measures |
|---|---|---|---|
| Experiment 1 Figure 1 |
10 WT 9 RGE |
6 consecutive days of FD | Digital biometry of enucleated eyes |
| Experiment 2 Figure 2 |
10 WT 10 RGE |
4.5 consecutive days of Plus-lens wear | Refraction, ultrasound, and digital biometry of eyes |
| 10 WT 10 RGE |
4.5 consecutive days of Minus-lens wear | Refraction, ultrasound, and digital biometry of eyes | |
| 6 WT 6 RGE |
4.5 consecutive days of Plano-lens wear | Refraction, ultrasound, and digital biometry of eyes | |
| Experiment 3 Figure 3 |
10 WT 12 RGE |
7 consecutive days of FD- uninterrupted | Refraction, ultrasound, and digital biometry of eyes |
| 12 WT 14 RGE |
7 days of FD interrupted daily by 130 minutes of unrestricted vision | Refraction, ultrasound, and digital biometry of eyes | |
| Experiment 4 Figures 4a-d |
5 WT 5 RGE |
6 consecutive days of FD followed by 2 hrs of unrestricted vision | Retinas processed for Egr1/glucagon immunofluoresence |
| 5 WT 5 RGE |
4.5 days of minus lens-wear followed by 2 hrs of unrestricted vision | Retinas processed for Egr1/glucagon immunofluoresence | |
| 5 WT 5 RGE |
4.5 days of plus lens-wear followed by 2 hrs of unrestricted vision | Retinas processed for Egr1/glucagon immunofluoresence | |
| Experiment 5 Figures 4e-h |
6 WT 5 RGE |
2 hrs of minus lens-wear | Retinas processed for Egr1/glucagon immunofluoresence |
| 6 WT 5 RGE |
2 hrs of plus lens-wear | Retinas processed for Egr1/glucagon immunofluoresence | |
| Experiment 6 Figure 5 |
5 WT 5 RGE |
4.5 days FD | Retinas individually processed for mRNA extraction, cDNA synthesis and qRT-PCR |
| 5 WT 5 RGE |
4.5 days of minus lens-wear | Retinas individually processed for mRNA extraction, cDNA synthesis and qRT-PCR | |
| 4 WT 4 RGE |
4.5 days of plus lens-wear | Retinas individually processed for mRNA extraction, cDNA synthesis and qRT-PCR |
Abbreviations: WT – wild type, RGE – retinopathy, globe enlargement, FD- form-deprivation, qRT-PCR - quantitative reverse transcriptase polymerase chain reaction.
2.3 Retinoscopy and Refraction
Non-cycloplegic streak retinoscopy to the nearest 0.25 diopter was performed using trial lenses to measure refractive error in control and treated eyes, similar to previous studies (Fischer et al. 1998a; Fischer et al. 1998b). Retinoscopy was performed by one individual to prevent inter-individual variability.
2.4 Ocular Measurements
High-resolution A-scan ultrasonography was used to measure ocular components along the optical axis in vivo. Corneal anesthesia was achieved by using one drop of topical 0.5% proparacaine hydrochloride ophthalmic solution. Given that corneal anesthesia can cause edema, we consistently obtained measurements with 2 minutes of application. After insertion of a 4mm-Barraquer pediatric lid speculum, a 20 MHz Panametrics-NDT (Waltham, MA) transducer with a polystyrene delay line offset (V208-RM) driven by a Panametrics-NDT 5072 pulser-receiver was coupled to the corneal apex using ultrasound coupling gel (Medline Industries, Inc.; Mundelein, IL). The acoustic reflections were collected and digitized using a PicoScope® 5203 USB-PC oscilloscope and PicoScope® 6 PC Oscilloscope software (version 6.3.43.0). Ultrasound signals were first filtered with a low-pass filter at the cutoff frequency of 80MHz to exclude high frequency noise. The envelope of the signals was then extracted using the analytic signal magnitude (Gammell 1981). Peaks corresponding to anterior cornea surface, anterior lens surface, posterior lens surface, and vitread surface of the retina were selected for measurements. Ultrasound reflections were converted to distance assuming the constant speed of sound of 1540m/s. It is noted that the speed of sound in cornea and lens may be slightly higher that the assumed value because of differences in density. Since the goal of the present study was to compare the thickness and depths between control and treated tissues, the assumption of a uniform speed of sound should have minimal impact on outcome measures. It is noted that increases in lens thickness that can occur with form-deprivation would shorten ultrasound measurements of axial length, the distance from the anterior surface of the cornea to the posterior pole of the sclera.
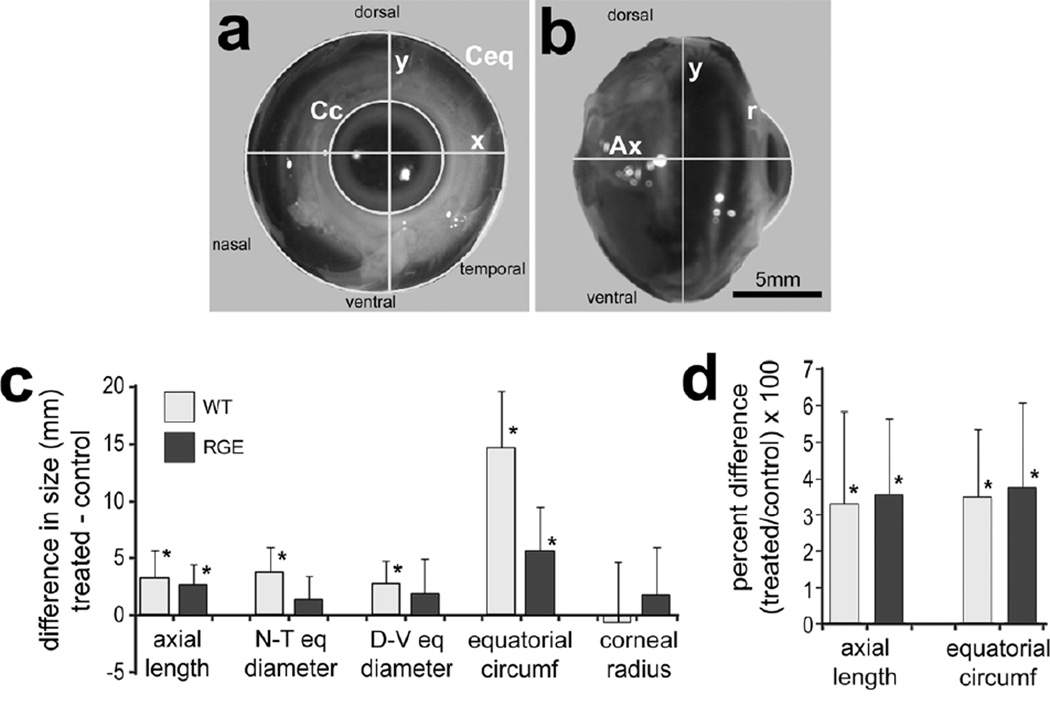
Photographs of enucleated eyes were taken with a Nikon D100 digital SLR camera. Biometric measurements were acquired from high resolution (>50 pixels/mm) digital photographs of enucleated eyes using ImagePro Plus v6.2 (Media Cybernetics, Bethesda, MD) (Figs. 1a and 1b). Digital biometry of axial length, equatorial circumference, corneal diameter and corneal arc provides accurate and reproducible measurements, as previously described (Fischer et al. 2008).
Figure 1.
Form deprivation-induced changes in ocular growth in WT and RGE eyes. WT (n=10) and RGE (n=9) eyes were form-deprived for 6 days beginning at P7. For each individual the difference in ocular dimension was determined between the right (treated) eye and the left (control) eye. Panels a and b are representative images of enucleated eyes with ImagePro demarcations of ocular dimensions including axial length (Ax), nasal-temporal diameter (x), dorsal-ventral diameter (y), corneal circumference (Cc), equatorial circumference (Ceq), and corneal arc/radius (r). The scale bar in panel b represent 5 mm and applies to panel a and b. Histograms in c and d illustrate the mean (mm or percentage change) and standard deviation (error bars) for different ocular parameters. Significance of difference (*p<0.05) was determined by using a paired two-tailed Student’s t-test.
2.5 Tissue Preparation for Immunohistochemistry
Ocular tissues were processed for immunohistochemistry as previously described (Fischer et al. 2005). Antibodies used for immunohistochemistry included; (1) mouse anti-glucagon at 1:100 (clone GG.236; Dr. M. Gregor, University of Tübingen via the Center for Ulcer Research and Education, University of California Los Angeles), (2) rabbit anti-Gβ3 antibody at 1:400 (HPA005645; lot RO7381, Sigma-Aldrich), and (3) goat anti- Egr1 at 1:200 (#AF2818; lot VMX01, R&D Systems). Primary Antibodies were diluted in PBS plus 0.2% Triton X-100 and 5% normal goat/donkey serum. Double-immunofluorescence was optimized for Egr1 and glucagon as follows: sections were washed in PBS, blocked for 30 minutes in antibody diluent plus 5% normal donkey serum, washed in PBS, and incubated for 1 hour with anti-Egr1 (diluted in PBS) overnight. The Egr1 antibody produced optimal labeling when Tx-100 was omitted from the diluent. Sections were then washed in PBS and secondary antibody donkey-anti-goat- Alexa488 (Invitrogen; Carlsbad, CA), diluted to 1:1000 in PBS plus 0.2% Triton X- 100. After washing in PBS, sections were incubated overnight with anti-glucagon diluted in PBS. After washing with PBS, sections were incubated for 1 hour with goat-anti-mouse-IgM-Alexa568 (Invitrogen) diluted to 1:1000. Immunolabeling for Egr1 and glucagon was processed in sequence to avoid cross-reactivity of the donkey-anti-goat-Alexa488 with the mouse monoclonal anti-glucagon antibody. After incubation with secondary antibodies, sections were washed in PBS and coverglass mounted on 4:1 (v:v) glycerol in water. We evaluated the specificity of primary antibodies by comparison with published examples of results and demonstrations of specificity (Fischer et al. 2005; Fischer et al. 2008; Fischer et al. 2009; Fischer et al. 2006; Ritchey et al. 2010). None of the observed labeling was due to non-specific labeling of secondary antibodies or autofluorescence because sections labeled with secondary antibodies alone were devoid of fluorescence.
2.6 Reverse transcriptase PCR
After enucleation, eyes were immediately placed in chilled Hank’s Balanced Salt Solution added with 3% D-glucose and 0.01 M HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ) buffer. Individual retinas were removed and immediately placed in RNAlater (Ambion, Austin Tx). After RNA stabilization, retinas were placed in 1.5 ml of Trizol Reagent (Invitrogen) and total RNA was isolated according to the Trizol protocol and resuspended in 50 µl RNAse free water. Genomic DNA was removed by using the DNA FREE kit provided by Ambion (Austin, TX). cDNA was synthesized from mRNA by using Superscripttm III First Strand Synthesis System (Invitrogen) and oligo dT primers according to the manufacturer’s protocol. Primers for preproglucagon and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed using the Primer-BLAST primer design tool at NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer sequences and predicted product sizes were: preproglucagon 87 bp – forward 5’ AAT GAC AAA TTC CCG GAT CA 3’, reverse 5’ GGT GTA GGT GCC TTC AGC AT 3’; GAPDH 218 bp – forward 5’ GGA ACA CTA TAA AGG CGA GAT 3’, reverse 5’ TCA CAA GTT TCC CGT TCT CA 3’.
qRT-PCR reactions were performed using SYBRGreen PCR mastermix and the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Control reactions were performed using all components with the exception of the reverse transcriptase to exclude the possibility that primers were amplifying genomic DNA. PCR products were run on a 2.0% agarose gel to verify the predicted product sizes.
2.7 Microscopy
Confocal images were obtained using a Zeiss LSM 510 imaging system at the Hunt-Curtis Imaging Facility at the Ohio State University. Images were optimized for color, brightness and contrast, multiple channels overlaid and figures constructed by using Adobe Photoshop™6.0. To avoid the possibility of region-specific differences within the retina, cell counts were consistently made from the central retina. Central retina was assessed within 20° of the posterior pole of the eye, with a radius of approximately 2 mm from the visual axis. Post-hatch chick retina is approximately 13 mm across.
2.8 Cell counts and statistics
To account for inter-individual variability in eye size, statistical analyses were performed for the differences between measurements of treated and control eyes for each individual. Thus, significance of difference (p-values) for the magnitude of interocular change was calculated for the mean differences from zero by using a Student’s t-test. To account for inter-strain variability in overall body size, statistical analyses were performed for the differences between the percentage change in eye size ([treated – control]/control X 100); determined for each individual at the completion of the experiment. Significance of difference was determined between two treatment groups by using a two-tailed, unpaired Student’s t-test. Significance of difference was determined between two treatment groups, accounting for inter-individual variability (means of treated-control values), by using a two-tailed, paired Student’s t-test. Significance of difference was assessed between multiple experimental groups was assessed by using two-way ANOVA and a post-hoc Bonferroni test.
3. Results
3.1 Young RGE chicks are emmetropic
Previous reports indicated that RGE chickens become hyperopic; however, the hyperopia develops after day P33 (Montiani-Ferreira et al. 2003). Prior to the initiation of experiments involving vision-guided ocular growth, we tested whether the eyes of RGE chickens developed refractive errors during the first 2 weeks of post-hatch development. We found that the eyes of RGE chicks had no significant refractive error at P12 (−0.03 ± 0.37D, n=17), and this refractive error was not significantly different from that of P12 WT chicks (0.31 ± 0.44D, n=15).
3.2 Form-deprivation (FD) stimulates eye growth in RGE chickens
We tested whether rates of ocular growth were stimulated by FD in RGE chicks in a manner similar to that seen in age-matched WT eyes. After 6 days of FD, axial length was increased by about 2.6 mm in treated RGE eyes compared to that of control eyes (Figs. 1a-c). As expected, axial length was increased by about 3.3 mm following deprivation of WT eyes, and this increase was not significantly different from that seen in form-deprived RGE eyes (Fig. 1c). Given that the overall size of RGE chickens tends to be smaller than WT chicks, we compared the axial length of eyes between the two cohorts. Comparison of the axial length and equatorial circumference of untreated control RGE eyes and age-matched WT eyes indicates that RGE animals have smaller eyes compared to those of WT animals (data not shown). Given the variation in normal overall body size and ocular size between RGE and WT chickens, we compared the percentage change between the treated and control eyes. We found that FD-induced increase in the percent change in axial length of RGE eyes was not significantly different compared to that of WT eyes (Fig. 1d).
Examination of circumferential equatorial growth revealed that RGE eyes grew significantly in response to FD compared to contralateral control eyes (Fig.1c). Significant differences in equatorial diameter across nasal-temporal and dorsal-ventral axes were seen in form-deprived WT eyes, but not in form-deprived RGE eyes (Fig. 1c). However, measurements of circumference around the ocular equator revealed significant FD-induced increases (Fig. 1c). WT eyes showed an average increase in equatorial circumference that was more than 2-fold larger than that seen in RGE eyes (Fig. 1c). After conversion to percentage change, we found that FD-induced increases in equatorial circumference of RGE eyes were not significantly different compared to those of WT eyes (Fig.1d). FD did not influence corneal arc, radius or circumference in either WT or RGE animals (Fig. 1c and data not shown).
3.3 Minus lens-wear stimulates ocular growth in WT and RGE eyes
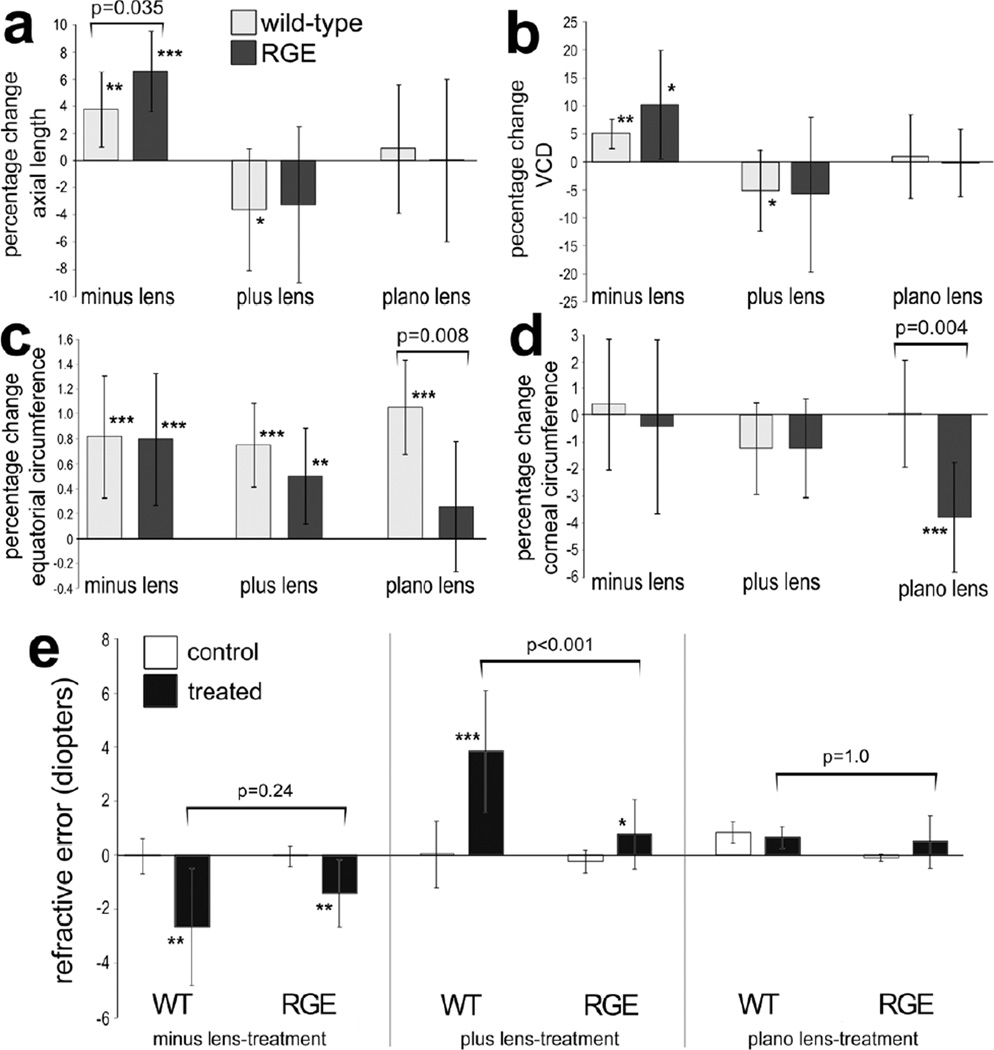
We next tested whether rates of growth in RGE eyes are affected by lens-imposed defocus. Minus 7 diopter lenses were applied at P7 and ocular dimensions examined at P11. The axial length of RGE eyes treated with minus lenses was significantly longer than that of RGE control eyes (Fig. 2a). Similarly, WT eyes treated with minus lenses were significantly longer in axial length than WT control eyes (Fig. 2a). We found that the percentage increase in axial elongation of minus lens-treated RGE eyes was significantly greater than that of minus lens-treated WT eyes (Fig. 2a). The axial elongation resulted from significant increases in VCD (vitreous chamber depth; Fig. 2b), whereas anterior chamber depth (ACD) and lens thickness had no significant contribution to changes in ocular length (data not shown). There was no significant difference between the VCD elongation seen in RGE and WT eyes treated with minus lens-wear (Fig. 2b). The equatorial circumference of RGE and WT eyes was increased in response to minus lens-treatment (Fig. 2c). Comparison of the percentage change in equatorial circumference revealed no significant difference between RGE and WT eyes treated with minus lens-wear (Fig. 2c). The corneal circumference, arc and radius did not change for either RGE or WT eyes treated with minus lens-wear (Fig. 2d and data not shown). Consistent with the observed increases in axial length, treatment of WT and RGE eyes with minus lenses caused significant refractive error compared to that of control WT and RGE eyes (Fig. 2e). The refractive error that resulted from minus lens-wear was not significantly different between treated WT and RGE eyes (Fig. 2e).
Figure 2.
Lens-induced changes in ocular growth in WT and RGE eyes. The right eyes of WT and RGE chickens were treated with minus 7 lenses (n=10), plus 7 lenses (n=10) or plano lenses (n=6) for 4.5 days beginning at P7, and left eyes were used as controls. Measurements of axial length (a) and vitreous chamber depth (b) were made using A-scan ultrasound. Measurements of equatorial circumference (c) and corneal circumference (d) were obtained by analysis of digital images with ImagePro 6.2. The mean percentage change between treated and control ocular parameters were calculated. Retinoscopy was used to determine refractive error in WT and RGE eyes treated minus lens-wear, plus lens-wear or plano lens-wear. Error bars represent standard deviation. Significance of difference (*p<0.05, **p<0.01, ***p<0.001) for percentage change of control vs treated within a group was determined by using a Student’s t-test. Significance of difference between treatment groups was determined by using a two-way ANOVA (p<0.001) and a post-hoc Bonferroni analysis. Asterisks indicate significant differences within a treatment group (percent change), and brackets and p-values indicate significant differences between treatment groups (WT vs RGE). Abbreviations: WT – wild type, RGE – retinopathy globe enlargement, VCD – vitreous chamber depth.
3.4 Plus-lens wear has little effect on RGE eyes
Plus lens-wear has been shown to reduce rates of axial ocular growth (Nickla et al. 1997; Wallman et al. 1995; Wildsoet and Wallman 1995). Accordingly, we examined whether RGE eyes respond to treatment with plus 7 diopter lenses in a manner similar to WT eyes. After plus lens-treatment, the axial length of RGE eyes was not significantly different from that of the contralateral control eyes (Fig. 2a). Although there was a trend with high variability toward shortening, there was no significant change in the VCD of RGE eyes treated with plus lenses (Fig. 2a and 2b). By comparison, WT eyes treated with plus lenses were significantly shorter in axial length compared to contra-lateral WT control eyes (Fig.2a). The change in the axial length of plus lens-treated WT eyes resulted from reduced VCD (Fig. 2b). Surprisingly, plus lens-wear increased circumferential eye growth in RGE and WT eyes (Fig. 2c). Plus lens-treatment did not affect corneal circumference, ACD or lens thickness in RGE or WT birds (Fig. 2d and data not shown). Application of plus lenses induced significant hyperopic refractive error in treated eyes when compared to the refractive state of control eyes in both WT and RGE chicks (Fig. 2e). However, the hyperopic shift in RGE eyes treated with plus lenses was not as significant as that seen in WT animals (Fig. 2e).
To test whether the growth-altering effects of lens-treatment were due to the application of lenses to the periorbital tissue, plano lenses without refractive power were applied to RGE and WT chicks as a control. Treatment with plano lenses resulted in no significant changes in axial length, VCD (Figs. 2a and 2b), ACD, lens thickness, or corneal radius (data not shown) in RGE or WT eyes. Examination of equatorial ocular growth revealed that WT eyes grew significantly with plano lens-wear, whereas RGE eyes did not show a significant increase (Fig. 2c). Corneal circumference was significantly smaller with plano lens-wear in RGE eyes, whereas corneal circumference was unaffected by plano lens-wear in WT eyes (Fig. 2d). Treatment with plano lenses did not cause significant refractive error in WT or RGE eyes (Fig. 2e).
3.5 Brief daily periods of unobstructed vision prevent form-deprivation myopia in RGE eyes
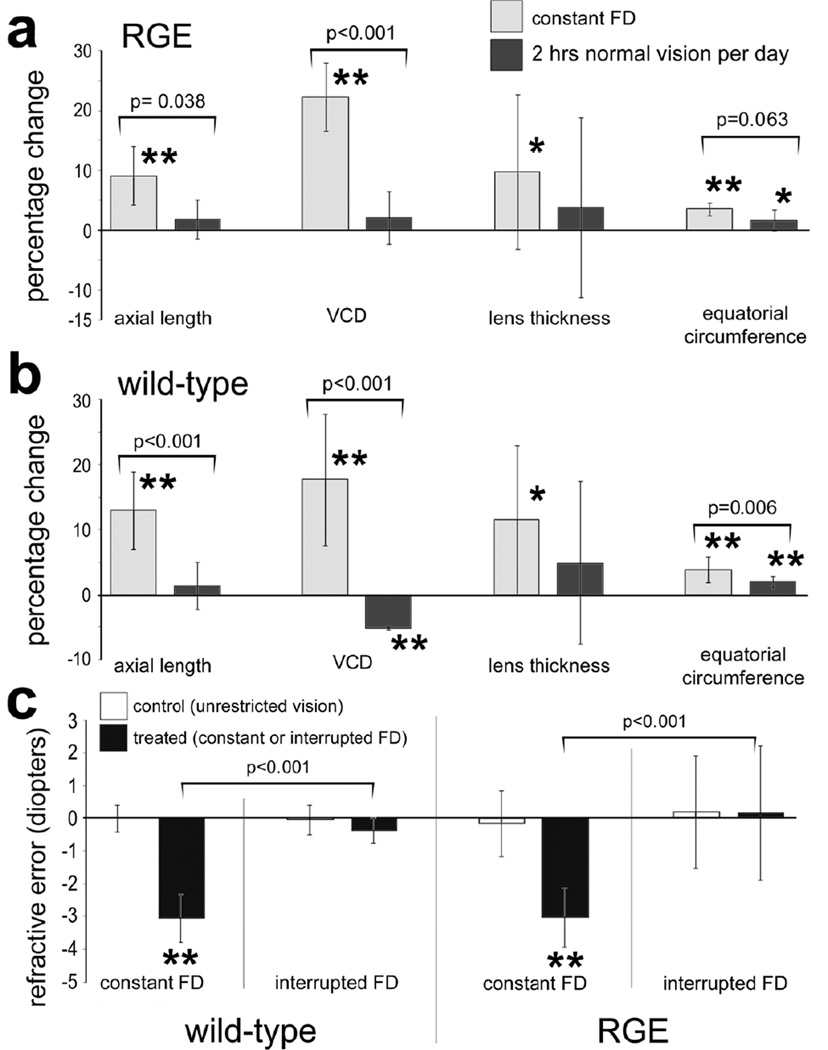
We tested whether RGE chicks respond to brief periods of unrestricted vision interrupting constant FD. Brief daily periods (≤130 minutes) of unrestricted vision interrupting FD are known to prevent excessive ocular elongation (Napper et al. 1995). Thus, we examined two cohorts of WT and RGE chicks; one treated with constant FD for 1 week and a second cohort treated with 130 minutes of unobstructed vision per day (interruption of FD). For both WT and RGE eyes, interruption of FD significantly reduced the axial length and VCD compared to eyes that were treated with continuous FD (Figs. 3a and 3b). The axial length of RGE and WT eyes treated with interruption of FD was no different from that of untreated contralateral eyes that remained open for the duration of the experiment (Figs. 3a and 3b). The percentage decrease in VCD in WT eyes was significantly (p<0.001) greater than that seen in RGE eyes treated with interruption of FD. In both WT and RGE eyes treated with constant FD we found a small, but significant, increase in lens thickness compared to contralateral control eyes (Figs. 3a and 3b). By comparison there was no significant increase in lens thickness in WT and RGE eyes treated with interruption of FD compared to contralateral control eyes (Figs. 3a and 3b). In both WT and RGE eyes treated with constant FD there was a significant increase in equatorial circumference compared to that of contralateral untreated eyes (Figs. 3a and 3b). In WT eyes, interruption of FD significantly inhibited the excessive equatorial ocular growth that resulted from continuous FD (Fig. 3b). By comparison, in RGE eyes, interruption of FD did not significantly inhibit the excessive equatorial ocular growth that resulted from continuous FD (Fig. 3a). Continuous and interruption of FD had no significant effect upon ACD or corneal parameters (data not shown). Interruption of FD effectively prevented the development of refractive error that normally occurs with continuous FD. RGE and WT eyes treated with interruption of FD did not develop myopic refractive error, nor did the refractive state differ significantly from control eyes that had unobstructed vision for the duration of the experiment (Fig. 3c).
Figure 3.
Brief daily periods of unobstructed vision interrupting FD prevent ocular enlargement and myopia in WT and RGE chicks. Measurements of axial length, vitreous chamber depth (VCD), lens thickness, and equatorial circumference were calculated as percentage change relative to contralateral control eyes (a and b). The mean refractive error for treated and control eyes was determined for each experimental condition; WT constant FD, WT interruption of FD, RGE constant FD and RGE interruption of FD. Significance of difference in percentage change or between control and treated eyes within a treatment group was determined by using a paired two tailed Student’s t-test (*p<0.05; **p<0.001). Significance of difference between treatment groups was determined by using a one-way ANOVA (p≤0.002) and a post-hoc Bonferroni analysis. Error bars represent standard deviation. Asterisks indicate significant differences within a treatment group (percent change), and brackets and p-values indicate significant differences between treatment groups (constant FD vs interrupted FD).
3.6 Egr1 expression changes in glucagon-positive amacrine cells with visual manipulation in RGE retinas
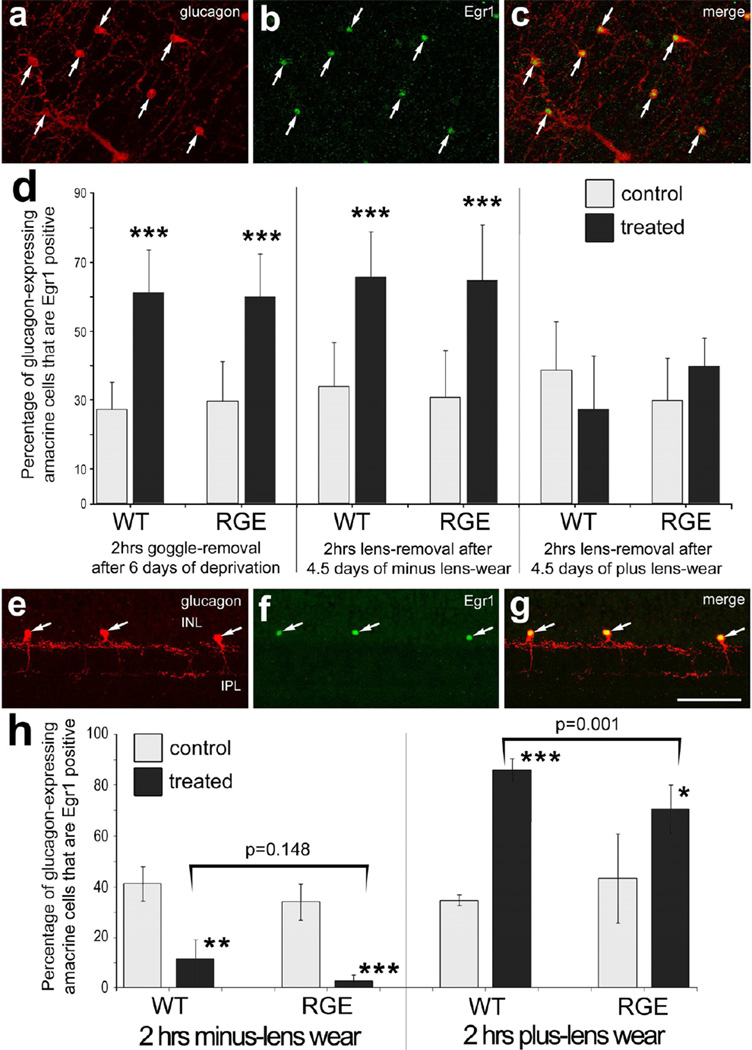
The immediate early gene Egr1 is differentially expressed by glucagonexpressing amacrine cells in response to growth-regulating visual cues (Fischer et al. 1999a). We tested whether Egr1 expression in glucagonergic amacrine cells was regulated by growth-regulating visual stimuli in RGE retinas. After undergoing 6 days of FD, goggles were removed, animals received 2 hours of unrestricted vision, and retinas were harvested for analysis. In RGE retinas, Egr1 was significantly up-regulated in glucagonergic amacrine cells in response to recovery from FD (Figs. 4a-d). The percentage increase in glucagonergic cells that up-regulate Egr1 in RGE retinas was not significantly different than that observed in WT retinas (Fig. 4d). After visual recovery from 4.5 days of minus lens-wear, there was a significant increase in the percentage of Egr1-positive glucagoneric cells observed compared to that seen in control eye (Fig. 4d). After visual recovery from 4.5 days of plus lens-wear, there was no significant change in the percentage of Egr1-positive glucagoneric cells compared to the percentage seen in control retinas (Fig. 4d). There were no significant differences between the percentages of Egr1-positive glucagoneric cells in control retinas of WT and RGE chicks with recovery from FD or lens-wear (Fig. 4d).
Figure 4.
The expression of Egr1 is differentially regulated by defocus in glucagonergic amacrine cells in both WT and RGE retinas. Whole-mount preparations (a-c) and vertical sections (e-g) of the retina were labeled with antibodies to Egr1 (green) and glucagon (red) (a-c and e-g). Arrows indicate amacrine cells double-labeled for Egr1 and glucagon. Representative images of immunolabeled retinas were obtained from RGE eyes that were treated with 2 hrs of visual recovery following 6 days of form-deprivation (a-c) or from RGE eyes that were treated with 2 hrs of plus lens-wear (e-g). The calibration bar (50 µm) in panel g applies to panels a-c and e-g. Histograms in panels d and h illustrate the mean and standard deviation for the percentage of glucagonergic amacrine cells that are Egr1-positive in WT and RGE retinas. Panel d; WT and RGE eyes were treated with 6 days of FD followed by 2 hrs of unobstructed vision, 4.5 days of minus lens-wear followed by 2 hrs of unobstructed vision, 4.5 days of plus lens-wear followed by 2 hrs of unobstructed vision, 2 hrs of minus lens-wear, or 2 hrs of plus lens-wear. Panel h; WT and RGE eyes were treated 2 hrs of minus lens-wear or 2hrs of plus lens-wear. Significance of difference (*p<0.05; **p<0.002; ***p<0.0001) between control and treated eyes within a treatment group was determined by using a paired two-tailed Student’s t-test. Significance of difference between treatment groups (lens-treated RGE vs lens-treated WT) was determined by using a one-way ANOVA (p<0.001) and a post-hoc Bonferroni analysis (brackets and p-values). Asterisks indicate significant differences within a treatment group (control vs treated), and brackets and p-values indicate significant differences between treatment groups (WT vs RGE).
We next tested whether short-term treatment with lens-imposed defocus influenced Egr1 expression in the glucagonergic cells in RGE retinas. In WT retinas, we found a significant decrease the percentage of glucagonergic amacrine cells that were Egr1-positive with 2 hours of minus lens-wear (Fig. 4h), consistent with previous reports (Bitzer and Schaeffel 2002; Fischer et al. 1999a). In RGE retinas, we found a significant decrease in the percentage of glucagonergic cells that expressed Egr1 in retinas from minus lens-wear (Fig. 4h). The percentage of glucagonergic cells that were Egr1-positive was not significantly less in RGE retinas compared to that in WT retinas in minus-lens treated eyes (Fig. 4h). In WT retinas, we found that 2 hours of plus lens-wear significantly increased the percentage, by more than 50%, of glucagonergic amacrine cells that were Egr1-positive (Figs. 4h), consistent with previous reports (Bitzer and Schaeffel 2002; Fischer et al. 1999a). In RGE retinas, we found that 2 hours of plus lens-wear significantly increased, by nearly 25%, the percentage of glucagonergic amacrine cells that were Egr1-positive (Figs. 4e-h). This increased in the percentage of Egr1-positive glucagonergic cells was significantly greater in WT retinas compared to that seen in RGE retinas (Fig. 4h). Further, there was more variability in the percentage of Egr1-expressing glucagonergic cells in RGE retinas compared to that seen in WT retinas (Fig. 4h). The standard deviation in control WT retinas was 2.08% compared to 19.28% in control RGE retinas, and the standard deviations in plus-lens treated WT retinas was 4.43% compared to 11.11% in treated RGE retinas.
3.7 Regulation of glucagon mRNA in RGE chickens
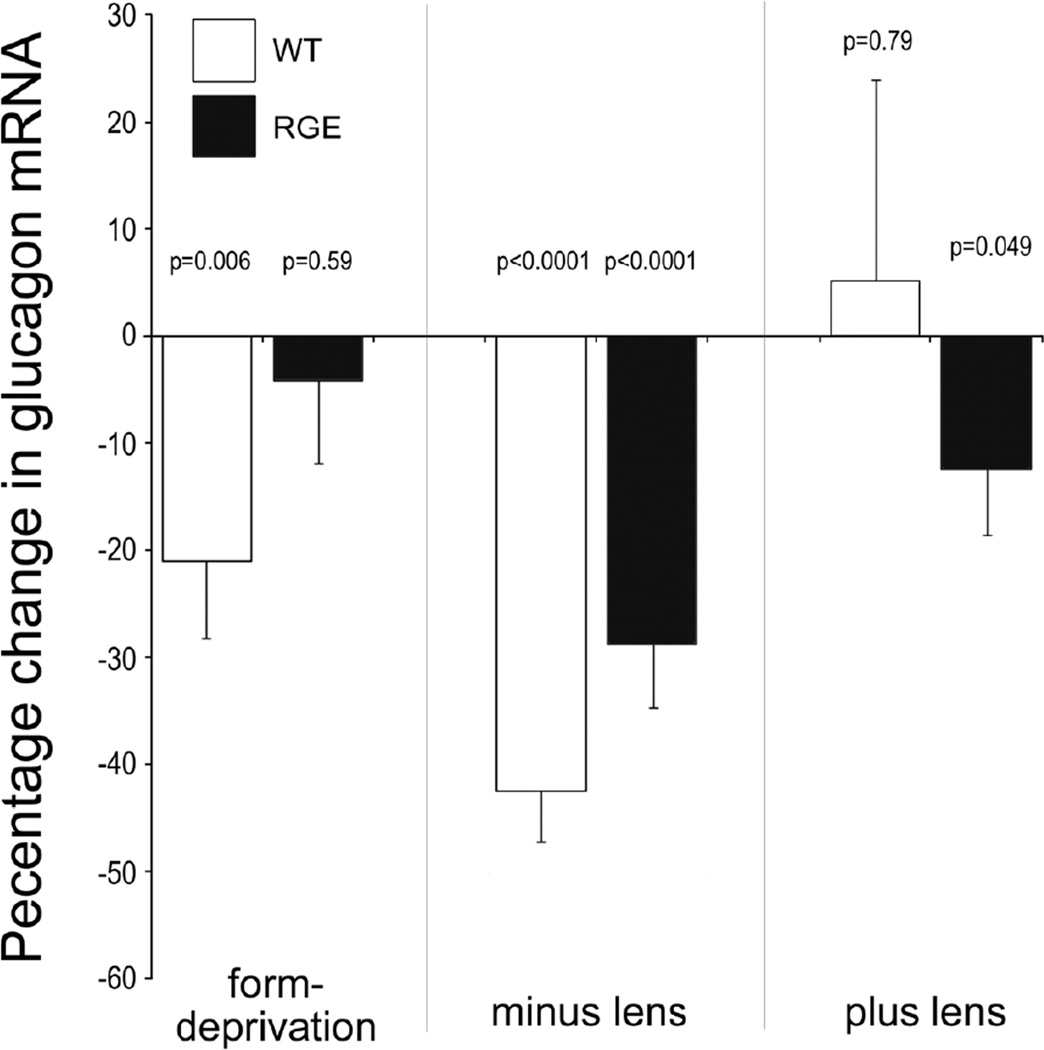
The expression of glucagon mRNA is believed to be down-stream of Egr1 expression (Buck et al. 2004). Thus, we examined whether expression of glucagon mRNA was altered in RGE retinas following FD or lens-imposed defocus. After 4.5 days of treatment, retinas were harvested without visual recovery. After 4.5 days of FD, WT retinas had decreased levels of glucagon mRNA (Fig. 5). By comparison, RGE retinas had no significant change in levels of glucagon mRNA (Fig. 5). Both RGE and WT retinas had decreased levels of glucagon mRNA in response to treatment with minus lenses (Fig. 5). Treatment with plus lenses for 4.5 days had no effect on levels of glucagon mRNA in WT retinas, whereas there was a small, but significant, decrease levels of glucagon mRNA in RGE retinas (Fig. 5).
Figure 5.
Levels of glucagon mRNA are affected by visual experience in WT and RGE retinas. Quantitative RT-PCR was used to measure total retinal levels of pro-glucagon mRNA. Levels were standardized to GAPDH. Retinas were harvested from eyes that were treated with 4.5 days of form-deprivation (n=5), minus lens-wear (n=5), or plus lens-wear (n=4). Significance of difference was determined by using a two-tailed Student’s t-test.
3.8 Glucagonergic amacrine cells and Gβ3-positive bipolar cells
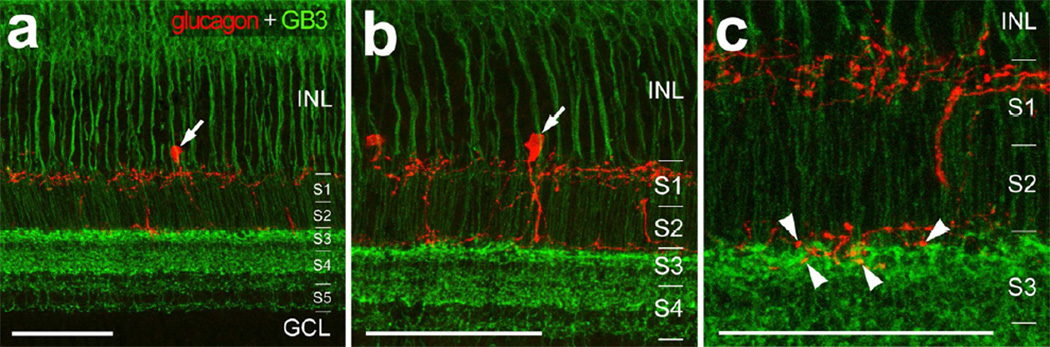
The loss of vision in RGE chicks results, at least in part, from a loss of Gβ3 from ON-bipolar cells (Ritchey et al. 2010). Since retina-guided ocular growth is believed to be a function of the glucagonergic amacrine cells (Bitzer and Schaeffel 2002; Fischer et al. 1999a; Vessey et al. 2005), we tested whether the growth-regulating glucagonergic amacrine cells might be directly downstream of the Gβ3-positive ON-bipolar cells. We used high-resolution confocal microscopy to examine whether the axon terminals of Gβ3-positive bipolar cells were in close proximity to the glucagonergic neurites in the IPL. The Gβ3-positive terminals were densely clustered near the middle of the inner plexiform layer (IPL) in sublamina 3 (Fig. 6), consistent with the findings of a previous report (Ritchey et al. 2010). Interestingly, the Gβ3-positive terminals in sublamina 3 were interspersed among the vitread dendritic endings of the glucagonergic amacrine cells (Fig. 6). This finding suggests that the synaptic output of Gβ3-positive bipolar cells could directly influence the glucagonergic amacrine cells.
Figure 6.
The processes of Gβ3-positive bipolar cells and glucagonergic amacrine cells are closely associated within the middle of the IPL. Vertical sections of the retina were labeled with antibodies to Gβ3 (green) and glucagon (red). Arrows indicate the somata of glucagonergic amacrine cells and arrowheads indicate glucagonergic processes that are closely apposed to the axon terminals of Gβ3-positive bipolar cells within sublamina 3 (S3) of the IPL. Confocal microscopy was used to obtain high-resolution images. Images were made by projecting 3 optical sections (a and b) or 2 optical sections (c). The calibration bar in each panel represents 50 µm.
4. Discussion
We report here that the eyes of RGE chickens respond to vision-guided ocular growth signals. Although young RGE chickens have a significant loss of visual acuity, including diminished ERG and optokinetic responses (Montiani-Ferreira et al. 2003), the RGE eyes respond to visual manipulations that influence rates of ocular growth. When treated with FD or hyperopic defocus, the eyes of RGE chicks developed increases in axial length and ocular circumference; responses that have been well-established in WT chickens (Gottlieb et al. 1987; Wallman et al. 1987; Wallman et al. 1978). When treated with myopic defocus, axial length in RGE chicks was not significantly reduced, unlike that observed in WT chicks (Feldkaemper and Schaeffel 2002; Kee et al. 2001; Wallman et al. 1995; Wildsoet and Wallman 1995). Collectively, our findings suggest that the RGE retina can detect FD-mediated image blur and respond with excessive axial elongation. Moreover, despite significant deficits in vision, RGE eyes detect and respond to myopic and hyperopic defocus. However, compared to WT eyes, RGE eyes have diminished responses to myopic defocus and enhanced responses to hyperopic defocus. Clearly, older (>P40) RGE animals lose the capacity for retina-guided ocular growth; these eyes are known to grow excessively despite becoming hyperopic from corneal flattening (Montiani-Ferreira et al. 2003; Montiani-Ferreira et al. 2007; Tummala et al. 2006). Thus, before the onset of corneal flattening and neuronal degeneration, RGE retinas retain the capacity to process visual cues to guide ocular growth despite significant losses in visual acuity.
Our data suggest that the growth-regulating glucagonergic amacrine cells may not function normally in RGE retinas with a bias toward permitting excessive axial elongation in response to hyperopic defocus and under-correcting in response to acute myopic defocus. Two hours of myopic defocus increased the percentage of Egr1- positive glucagoneric cells in RGE retinas, but this increase was significantly less than the up-regulation of Egr1 in glucagonergic cells in WT retinas. By comparison, 2 hours of hyperopic defocus decreased the percentage of Egr1-positive glucagoneric cells in both WT and RGE retinas, but the percentage decrease of Egr1 in RGE retinas was not significantly greater than that seen in WT retinas. Although RGE eyes had an increased elongation in response to minus-lens treatment, the refractive error of these eyes was less than that of minus lens-treated WT eyes. It is possible that the refractive components of RGE eyes may be differentially affected by minus lens-treatment to off-set the increased VCD elongation. Further studies are required to determine whether the refractive properties of the lens and cornea are affected by minus lens-wear in RGE eyes. Collectively, these findings suggest that the regulation of Egr1 in glucagonergic amacrine cells in RGE retinas may underlie the lens-induced growth-responses of RGE eyes that differ from the responses of WT eyes.
Changes in Egr1 expression are thought to affect changes in glucagon expression in amacrine cells (Feldkaemper et al. 2004; Feldkaemper and Schaeffel 2002; Vessey et al. 2005). Although short-term (<2hrs) and sustained (>24 hrs) FD is known to down-regulate Egr1 in the glucagonergic amacrine cells in WT retinas (Fischer et al. 1999), we failed to detect a significant down-regulation of glucagon mRNA in RGE retinas after 4.5 days of FD, unlike the decrease in glucagon mRNA seen in WT retinas. It remains uncertain whether FD-induced changes in Egr1 expression by glucagonergic amacrine cells in RGE retinas differ from those of WT retinas given that RGE retinas, unlike WT retinas, fail to down-regulate glucagon mRNA in eyes treated with FD. By comparison, hyperopic defocus decreased retinal levels of glucagon mRNA in both WT and RGE retinas. Myopic defocus in RGE retinas caused a significant decrease in glucagon mRNA; whereas the WT retinas showed no change, consistent with a previous report (Buck et al. 2004). Caution should be used in the interpretation of the PCR data given that confounding observations have been reported for assays of glucagon mRNA. For example, Buck and colleagues reported a short-term down-regulation of mRNA levels in the contralateral control eye with lens-treatment (Buck et al. 2004). Nevertheless, it remains possible that abnormal expression of Egr1 and glucagon by amacrine cells underlies the diminished response of RGE eyes to myopic defocus. Further studies are required to examine whether there are temporal differences in the vision-mediated expression of Egr1 and glucagon mRNA in RGE retinas compared to those of WT retinas.
Treatment with minus lenses caused a significant increase in circumferential eye growth and, surprisingly, treatment with plus and plano lenses also increased circumferential eye growth. Myopic defocus is a potent growth-slowing signal, thus it is expected that equatorial growth should be reduced. In addition, although 2 hrs of unrestricted vision interrupting constant FD effectively inhibited excessive axial elongation, excessive equatorial growth was reduced but not prevented in either WT or RGE chicks. It is possible that myopic defocus did not stimulate the bullwhip and mini-bullwhip cells in the retinas of these animals, thereby failing to elicit a growth-slowing signal to the ocular equator. Bullwhip and mini-bullwhip cells are a small subset (~1000 cells per retina) of glucagon-positive neurons in peripheral regions of the retina (Fischer et al. 2005; Fischer et al. 2006). These cells have been shown to differentially express Egr1 in response to growth-regulating visual stimuli and selectively influence circumferential eye growth (Fischer et al. 2008). Although bullwhip and mini-bullwhip cells appear to be normal in RGE retinas (data not shown), the response of these cells to growth-regulating visual stimuli in RGE retinas have not been characterized. Alternatively, the increased equatorial growth seen with lens-wear may have been caused by peripheral deprivation from the rings of Velcrotm that were used to apply the lenses. It is also possible that equatorial changes in ocular growth were slower to recover and require longer periods of unrestricted vision than the 2 hours provided.
RGE chicks have poor visual acuity resulting from a complete loss of Gβ3 from photoreceptors and ON-bipolar cells (Ritchey et al. 2010). Gβ3 is part of the heterotrimeric G-protein that regulates phosphodiesterase (PDE) activation; the PDE degrades cGMP, which, in turn, closes cGMP-gated channels resulting in photoreceptor hyperpolarization (Shichida and Matsuyama 2009). Thus, the loss of Gβ3 is expected to impair visual signal transduction by permitting sustained phosphodiesterase activity. In ON-bipolar cells, Gβ3 regulates signaling through metabotropic glutamate receptor 6 (mGluR6), and deficits in Gβ3 function should cause abnormal center-ON responses (Dhingra et al. 2011; Huang et al. 2003). The loss of Gβ3 in the RGE chick results in abnormal a- and b-waves from ERG recordings (Montiani-Ferreira et al. 2007). Collectively, the findings that Gβ3 is lost from RGE retinas and young RGE eyes are capable of emmetropization, and developing lens-induced ametropias, suggest that ON-bipolar cells and photoreceptors are not the primary neurons that detect defocus and execute the mechanisms that drive vision-guided ocular growth. While transduction of photons into electrical signals must occur to initiate vision, the processing of images to generate vision-guiding growth cues does not require Gβ3-mediated cell signaling. Previous studies have suggested that ON-bipolar cell function can be compromised without impairing the ability of eyes to respond to vision-dependent growth cues (Pardue et al. 2008). Collectively, our data combined with evidence that ganglion cell function is not required for retina-guided ocular growth (McBrien et al. 1995; Norton et al. 1994; Troilo et al. 1987), confirms the importance of amacrine cells, particularly glucagon-positive amacrine cells in chickens, for vision-guided ocular growth.
With respect to Egr1 expression, the glucagonergic amacrine cells in RGE retinas under-compensate for growth-slowing myopic defocus. We provide evidence that Gβ3-positive ON-bipolar cells could directly influence the glucagonergic amacrine cells; the axon terminals of the Gβ3-bipolar cells are closely apposed to the dendrites of the glucagonergic amacrine cells in the middle of the INL. Thus, the loss of Gβ3 from the ON-bipolar cells, and the impact of Gβ3 loss upon bipolar cell function, may directly impact the activity of the glucagonergic amacrine cells in the RGE retina. Our findings are consistent with the hypothesis that the glucagonergic amacrine cells are key regulators of vision-guided ocular growth in chicks (Bitzer and Schaeffel 2002; Feldkaemper and Schaeffel 2002; Fischer et al. 1999a; Vessey et al. 2005).
Conclusions
Our findings indicate that RGE chickens, despite significant losses in visual acuity, respond to growth-accelerating stimuli, including form-deprivation and hyperopic defocus, with increases in axial length and VCD. By comparison, the growth-responses of RGE eyes to myopic defocus are diminished when compared to those of WT eyes. Similar to WT eyes, RGE eyes fail to grow excessively when given brief daily interruptions in FD, suggesting sufficient visual function remains in the RGE retinas to promote emmetropization. In RGE retinas, the glucagonergic amacrine cells have a diminished up-regulation of Egr1 in response to myopic defocus compared to the responses of the amacrine cells in WT retinas. The diminished response of the glucagonergic amacrine cells in RGE retinas likely underlies the diminished growth-slowing response of RGE eyes to myopic defocus. Our findings indicate that the expression of Gβ3 by photoreceptors and ON-bipolar cells is not required for emmetropization. The aberrant activity of photoreceptors and bipolar cells in RGE retinas (Montiani-Ferreira et al. 2003) do not interfere with, and may enhance, the growth-accelerating effects of FD and hyperopic defocus. In contrast, the abnormal functions of photoreceptors and ON-bipolar cells in RGE retinas cause diminished responses to myopic defocus. We conclude that high visual acuity is not necessary for the growth-accelerating influence of FD or hyperopic defocus, whereas diminished visual acuity impairs the growth-slowing influence of myopic defocus.
-
➢
We study ocular growth in the RGE mutant chick which has diminished visual acuity
-
➢
The diminished visual acuity of RGE chicks permit emmetropization
-
➢
RGE eyes grow excessively in response to form-deprivation and hyperopic defocus
-
➢
Growth-slowing visual stimuli are effective, but diminished in RGE eyes compared to WT eyes
-
➢
Retinal expression of Egr1 in response to growth-guiding stimuli is nearly normal in RGE eyes
Acknowledgements
The authors thank Drs. D. Mutti and A. Hartwick for critical reading of the manuscript. Confocal microscopy was performed at the Hunt-Curtis Imaging Facility at the Department of Neuroscience of The Ohio State University. This work was supported by grants (AJF: EY016043-05; ERR: K12EY015447) from the National Institutes of Health, National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Commercial Relationships: E.R. Ritchey, Employed by VISTAKON® A Division of Johnson & Johnson Vision Care, Inc; C. Zelinka, None; Junhua Tang, None; Jun Liu, None; K.A. Code, None; S. Petersen-Jones, None; A.J. Fischer, None.
References
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43(1):246–252. [PubMed] [Google Scholar]
- Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest Ophthalmol Vis Sci. 2004;45(2):402–409. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- Curtis PE, Baker JR, Curtis R, Johnston A. Impaired vision in chickens associated with retinal defects. Vet Rec. 1987;120(5):113–114. doi: 10.1136/vr.120.5.113. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Fina M, Vardi N. Gβ3 is Expressed in Retinal ON Bipolar Neurons and is Required for Light ON Responses. Fort Lauderdale: Association for Research in Vision and Ophthalmology Annual Meeting. 2011 [Google Scholar]
- Ehrlich D, Sattayasai J, Zappia J, Barrington M. Effects of selective neurotoxins on eye growth in the young chick. Ciba Found Symp. 1990;155:63–84. doi: 10.1002/9780470514023.ch5. discussion 84-8. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Burkhardt E, Schaeffel F. Localization and regulation of glucagon receptors in the chick eye and preproglucagon and glucagon receptor expression in the mouse eye. Exp Eye Res. 2004;79(3):321–329. doi: 10.1016/j.exer.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19(6):755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999a;2(8):706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Miethke P, Morgan IG, Stell WK. Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Res. 1998a;794(1):48–60. doi: 10.1016/s0006-8993(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999b;39(4):685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci. 2005;25(44):10157–10166. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008;317(1):196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009;57(2):166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Stell WK. Opiate and N-methyl-D-aspartate receptors in form-deprivation myopia. Vis Neurosci. 1998b;15(6):1089–1096. doi: 10.1017/s0952523898156080. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Skorupa D, Schonberg DL, Walton NA. Characterization of glucagon-expressing neurons in the chicken retina. J Comp Neurol. 2006;496(4):479–494. doi: 10.1002/cne.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammell PM. Improved ultrasonic detection using the analytic signal magnitude. Ultrasonics. 1981;19(2):73–76. [Google Scholar]
- Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28(8):1225–1235. [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455(1):1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42(3):575–583. [PubMed] [Google Scholar]
- Lagnado L. Signal amplification: let's turn down the lights. Curr Biol. 2002;12(6):R215–R217. doi: 10.1016/s0960-9822(02)00754-6. [DOI] [PubMed] [Google Scholar]
- Makino CL, Wen XH, Lem J. Piecing together the timetable for visual transduction with transgenic animals. Curr Opin Neurobiol. 2003;13(4):404–412. doi: 10.1016/s0959-4388(03)00091-6. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, Cornell LM. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Res. 1995;35(9):1141–1152. doi: 10.1016/0042-6989(94)00237-g. [DOI] [PubMed] [Google Scholar]
- Montiani-Ferreira F, Fischer A, Cernuda-Cernuda R, Kiupel M, DeGrip WJ, Sherry D, Cho SS, Shaw GC, Evans MG, Hocking PM, et al. Detailed histopathologic characterization of the retinopathy, globe enlarged (rge) chick phenotype. Mol Vis. 2005;11:11–27. [PubMed] [Google Scholar]
- Montiani-Ferreira F, Li T, Kiupel M, Howland H, Hocking P, Curtis R, Petersen-Jones S. Clinical features of the retinopathy, globe enlarged (rge) chick phenotype. Vision Res. 2003;43(19):2009–2018. doi: 10.1016/s0042-6989(03)00303-1. [DOI] [PubMed] [Google Scholar]
- Montiani-Ferreira F, Shaw GC, Geller AM, Petersen-Jones SM. Electroretinographic features of the retinopathy, globe enlarged (rge) chick phenotype. Mol Vis. 2007;13:553–565. [PMC free article] [PubMed] [Google Scholar]
- Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Res. 1995;35(9):1337–1344. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16(4):320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- Norton TT. Experimental myopia in tree shrews. Ciba Found Symp. 1990;155:178–194. discussion 194-9. [PubMed] [Google Scholar]
- Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11(1):143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- O'Leary DJ, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979;35(11):1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49(2):706–712. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Matthews AL, Brenza H. Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp Eye Res. 1994;59(6):747–760. doi: 10.1006/exer.1994.1161. [DOI] [PubMed] [Google Scholar]
- Ritchey ER, Bongini RE, Code KA, Zelinka C, Petersen-Jones S, Fischer AJ. The pattern of expression of guanine nucleotide-binding protein beta3 in the retina is conserved across vertebrate species. Neuroscience. 2010;169(3):1376–1391. doi: 10.1016/j.neuroscience.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Code KA, Petersen-Jones S, Fischer AJ. Form-Deprivation and Eye Growth in the Retinopathy, Globe Enlarged (RGE) Chicken. ARVO. 2009;50(5):3935. [Google Scholar]
- Ritchey ER, Zelinka C, Petersen-Jones S, Fischer AJ. Vision-guided ocular growth in RGE mutant chickens with diminished visual acuity. Optom Vis Sci; 13th International Myopia Conference.2011. [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124(1):154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Shichida Y, Matsuyama T. Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2881–2895. doi: 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003;44(1):32–36. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6(8):993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33(10):1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Tummala H, Ali M, Getty P, Hocking PM, Burt DW, Inglehearn CF, Lester DH. Mutation in the guanine nucleotide-binding protein beta-3 causes retinal degeneration and embryonic mortality in chickens. Invest Ophthalmol Vis Sci. 2006;47(11):4714–4718. doi: 10.1167/iovs.06-0292. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005;46(11):3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237(4810):73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Pettigrew JD. Kainic acid-induced eye enlargement in chickens: differential effects on anterior and posterior segments. Invest Ophthalmol Vis Sci. 1988;29(2):311–319. [PubMed] [Google Scholar]