Abstract
The cell-mediated adaptive immune response depends upon the activation of T cells via recognition of antigen in the context of a major histocompatibility complex (MHC) molecule. Although studies have shown that alterations in T cell receptor glycosylation reduces the activation threshold, the data on MHC is far less definitive. Here, we discuss the data on MHC glycosylation and the role the glycans might play during the adaptive host response.
Keywords: glycosylation, MHC class I, MHC class II, HLA, N-glycan, antigen presentation
1. Introduction
The host response to infection is a multifaceted mechanism involving “innate” and “adaptive” immune activation in combinations that are only now becoming clear. From the adaptive side, the ability to present foreign antigen to T cells for recognition is a singular event that is required for the initiation of the downstream effector pathways, including CD4+ Th2 T cells that promote antibody class switching, proinflammatory CD4+ Th1 cells that promote microbial clearance, cytotoxic CD8+ T cells that are critical for clearing infected host cells, or a number of other variants (e.g., NKT cells). Indeed, the genetic variability of MHC molecules and the control of antigen presentation might be the two most important aspects of the adaptive immune response, and thus for protection against infectious disease.
It is not surprising that essentially every autoimmune disorder has some degree of linkage to at least one MHC allele. A rather famous example dating back to the mid-1970s is Ankylosing spondylitis which shows remarkably strong linkage to HLA-B27, although carrying that allele does not necessarily mean an individual will develop disease (or vice versa)(recently reviewed in [1,2]). While we will not directly address the linkages of MHC alleles with autoimmunity in this review, it is mentioned here for context in that MHC sits at the crossroads of the self-versus-non-self decision the immune system is faced with constantly. It is a delicate balance and when something alters that decision at the molecular level, trouble nearly always ensues.
2. The MHC Glycoprotein Family
The major histocompatibility complex (MHC), referred to as the human leukocyte antigen (HLA) in humans, is a family of structurally and genetically related glycoproteins [3] that present antigen from both exogenous (MHC class II; MHCII) and endogenous (MHC class I; MHCI) sources to T cells (recently reviewed in [4]). These glycoproteins are also key factors in lymphocyte development and assist in maintaining overall homeostasis [5,6]. The MHC family is highly polymorphic in some regions of the glycoprotein, while highly conserved in other domains, and are found as heterodimers at the cell surface [3]. Other members of the broad family include CD1, a MHCI homolog that is adapted to present glycolipids rather than the traditional peptide antigens presented by MHCI [7]. As with essentially every protein that travels the secretory pathway, MHC molecules are highly glycosylated. There are two major forms of protein glycosylation, O- and N-linked, but the dominant form found on MHC glycoproteins is N-linked [8]. What is perhaps most remarkable about the Asn-X-Thr/Ser acceptor sites for N-linked glycans in MHC molecules is their near absolute conservation across all animal species with MHC homologs. This suggests that there is strong selective pressure to maintain specific sites of glycosylation on MHC molecules, indicating that these glycans are critical for one or more aspects of MHC structure and/or function.
With respect to function, CD1 presents glycolipids such that the acyl chains are buried in long hydrophobic channels and the carbohydrate is protruding from the top of the molecule [7]. MHCI binds short peptides derived from proteins primarily localized in the cytoplasm of the cell, making MHCI the main pathway for presenting antigens from viruses and other intracellular pathogens [4]. MHCII, on the other hand, binds longer peptides derived from proteins released from lysed microbes in the endocytic pathway [4] as well as zwitterionic polysaccharides found within the capsules of some bacteria (e.g., Staphylococcus aureus and Bacteroides fragilis) [9–11]. Finally, MHCII is also responsible for superantigen (e.g., Staphylococcus enterotoxins) binding and the antigen presenting cell-T cell crosslinking [12] that initiates polyclonal and exaggerated T cell responses.
3. The N-Glycosylation Pathway
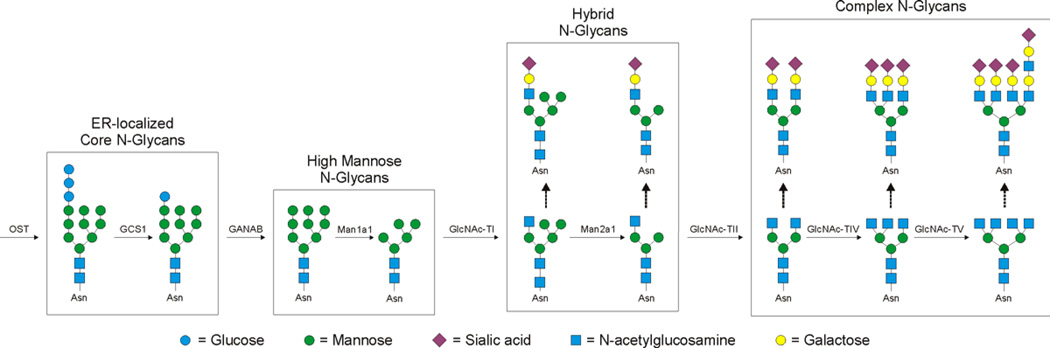
The N-glycosylation/secretory pathway in mammalian cells has been extensively reviewed in the past [13], but it is important for the reader to understand some basics for this review (summarized in Figure 1). Again, we will focus our discussion of the N-linked glycosylation pathway since it is the dominant glycan form on MHC molecules. Proteins destined to be glycoproteins are synthesized in the endoplasmic reticulum (ER), where the oligosaccharyltransferase (OST) enzyme catalyzes the attachment of the initial Glc3Man9GlcNAc2 glycan on target asparagine residues in a co-translational fashion. This core oligosaccharide binds to the ER chaperones calreticulin and calnexin, which assist in folding of the nascent glycoprotein [14,15]. The action of glucosidase enzymes then remove the terminal glucose molecules, thereby releasing the properly folded glycoprotein to proceed down the secretory pathway in the Golgi apparatus. As such, ER glycosylation can serve as a quality control step for the folding of glycoproteins.
Figure 1. Schematic of the mammalian N-glycosylation pathway.
The mammalian N-glycosylation pathway is a non-template driven pathway in the ER and Golgi apparatus. Within the ER, the core Glc3Man9GlcNAc2 N-glycan is added to asparagine residues within the Asn-X-Thr/Ser consensus sequence by the action of the oligosaccharyltransferase (OST). The ER resident glucosidase (GCS1) removes the two terminal glucose residues after proper folding is attained, thereby releasing the nascent glycoprotein to travel into the Golgi for further modification. In the ER, the remaining glucose is removed by the GANAB glucosidase, yielding a “high mannose” structure. Four of the outer-most mannose residues are cleaved by α-mannosidase 1A1 (Man1a1). Next, the first GlcNAc transferase (GlcNAcT-I; Mgat1) adds a GlcNAc to one of the mannose branches converting the “high mannose” structure into a “hybrid” N-glycan. This represents the first step in re-building the oligosaccharide. This GlcNAc can serve as a site for further modification, but much more commonly, two more mannose residues are removed by α-mannosidase IIA1 (Man2a1). This frees these termini for addition of more GlcNAc residues through the action of GlcNAc transferases II-V (Mgat2-5), thereby creating the traditional branched complex N-glycans. As with the first GlcNAc, these other GlcNAc sites serve as a basis for further modification, usually with the addition of galactose, GlcNAc, fucose, and sialic acid residues in various combinations and linkages.
Once the glycoprotein moves into the Golgi, the “high mannose” glycan attached to the asparagine site is further trimmed of most of its mannose residues by mannosidases [13]. At this point, the glycan receives its first additional GlcNAc residue by the GlcNAc transferase I enzyme (GlcNAcT-I), which is followed by further mannose trimming by the resident mannosidase. The result is termed a “hybrid” N-glycan, where a single branch of GlcNAc has been added but mannose residues remain at the termini [16]. The first step into the “complex-type” N-glycans is the addition of a second GlcNAc, this time by the GlcNAc transferase II (GlcNAcT-II) [17]. This critical step is then followed by the non-sequential modifications performed by the collective activity of other GlcNAcT enzymes (GlcNAcT-III through V)[18] as well as fucosyl-, sulfo-, galactosyl-, and sialyl-transferases [19].
To date, there are few known rules about how (or if) the nature of the glycan on a mature glycoprotein is regulated, but it is clear that metabolic activity of the cell, expression levels of the various enzymes, and the structure of the underlying protein backbone all contribute to the final result. Indeed, it is critical to understand that glycosylation is not a template-driven synthetic pathway, unlike protein and nucleic acid synthesis, and therefore provides complexity and heterogeneity even within the same molecule with multiple glycosylation sites.
4. Glycosylation of the MHC Class I Molecules
MHCI molecules are heterodimers comprised of a polymorphic transmembrane heavy chain and the non-glycosylated β2-microglobulin. Within the heavy chain, Asn86 is the single conserved site for Nglycosylation across all known alleles. As with essentially all glycoproteins, glycosylation of MHCI molecules can serve a number of fundamental roles. The ability of glycosylation to serve as a structural and folding check point has already been mentioned [20,21], but glycosylation could also act as a means to direct trafficking to the cell surface [20,21], to serve as a protective coat for the underlying protein against proteolytic cleavage via steric hindrance [22], and act as a mechanism by which optimal surface spacing is achieved within the plasma membrane for T cell recognition possibly through interactions with the galectin family of carbohydrate-binding proteins [23,24].
MHCI molecules serve as a key example of the importance of glycosylation in protein folding in ER [25]. The peptides that associate with MHCI molecules are derived primarily from the cytosol and are transported into the ER via the tapasin/TAP-dependent pathway [26,27]. As a result, peptide loading of MHCI occurs in the ER, prior to the N-glycan processing in the Golgi. In fact, the core Glc1Man9GlcNAc2 glycan binding to the calnexin/calreticulin chaperones is critical to promote association of the heavy chain with β2-microglobulin as well as loading of the antigenic peptide into the binding groove [15]. This can be seen when N-glycosylation is inhibited or in cells that express mutant MHCI lacking the conserved asparagine, where the amount of properly folded and loaded MHCI at the cell surface is dramatically reduced [25]. Although it appears that the conserved N-glycan site on MHCI does not directly interact with or impact the nature of the peptide that is presented, the N-glycan is critical for antigen binding to occur by virtue of its importance in recruiting the chaperones to assist in peptide loading.
The importance of N-glycosylation in peptide loading of MHCI in the ER is well established, but the role for the complex N-glycan on MHCI after moving through the secretory pathway and to the cell surface for T cell recognition is much less clear. One possibility is found in a theory born out of the crystal-packing properties of purified immune receptors for structural studies. For example, it was thought that the complex N-glycans on the T cell receptor (TCR) would act as physical spacers to prevent TCR self-clustering on the T cell surface, thereby reducing auto-activation [24,28]. This notion was generally correct, but the mechanism was not. It turns out that galectin molecules bind TCR glycans and prevent cis-interactions and auto-activation by forming a lattice of molecules at the cell surface [18]. Loss of galectin binding caused by galectin gene-knockout or changes in the TCR glycans causes a breakdown of the lattice and a dramatic decrease in activation threshold on the T cell. To date, it is unclear whether MHCI (or MHCII for that matter) bind galectins to form lattices, and if so, the degree to which proper recognition of MHCI is dependent upon those interactions; however, the crystal packing data on MHCI suggests the same spacing notion as it did with the TCR [24,28]. Indeed, it is possible that galectin interactions are important for stable MHCI cell surface localization and that loss of the N-glycans on MHCI could lead to enhanced surface loss by endocytosis, but future studies are needed to test these possibilities.
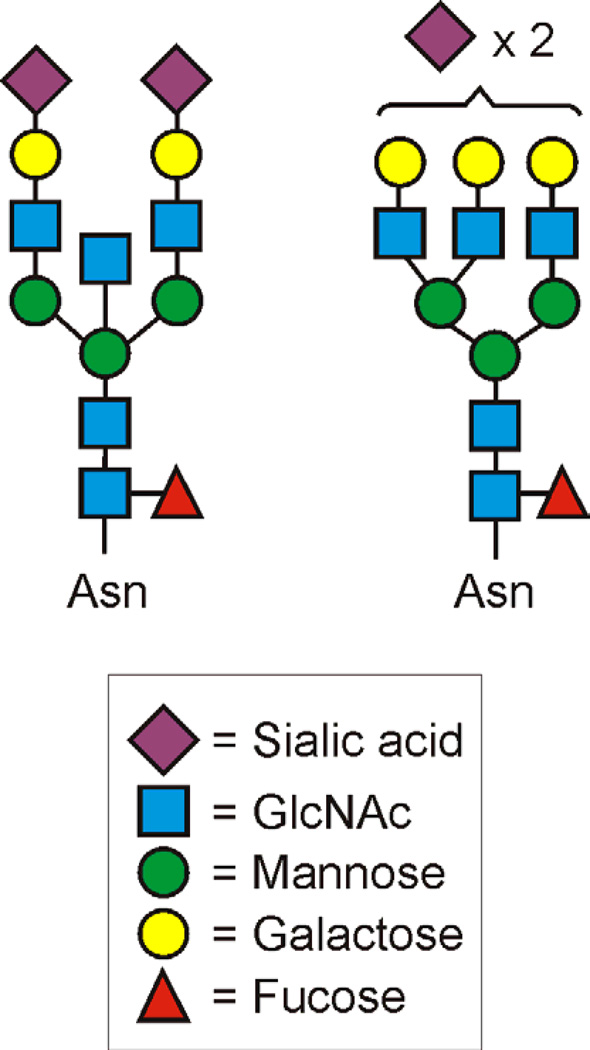
Perhaps what is more remarkable about MHCI glycosylation is the relatively limited diversity in the glycans that are attached at the Asn86 site. Different allotypes of HLA-A and -B from a variety of EBV-transformed B cell lines showed that the predominant glycan forms were either SA2Gal3Man3GlcNAc5Fuc1 or SA2Gal2Man3GlcNAc5Fuc1 (Figure 2), and this general observation held up in studies of MHCI isolated from peripheral blood lymphocytes of healthy volunteers [29]. Limited diversity of glycans on any given glycoprotein is more the exception than the rule [30], suggesting that these two complex structures could be functionally important, yet knowledge of the glycosylation composition of MHCI molecules from non-lymphocytes is lacking. The only data reported to date focused on the degree of terminal sialylation, which does vary between cells and tissues, but the differences in overall composition and branching was not studied [31]. It is possible that the limited diversity of glycans found on MHCI (HLA-A and HLA-B) play a role in signaling to T cells the difference between normal- and altered-self such that the two normal glycan forms represent normal-self, while the presence of other glycans on MHCI arising from infection or inflammation may signal altered-self. This possibility remains unexplored.
Figure 2. Schematic of the dominant HLA-A and HLA-B N-linked Glycans.
The MHCI molecules HLA-A and HLA-B carry unusually homogeneous N-glycan composition. Most glycoproteins tend to show high microheterogeneity at each N-glycosylation site, but for reasons that remain unclear, HLA-A and HLA-B molecules have been shown to contain one of these two structures most of the time. This is true for HLA-A and -B from cultured cells as well as in peripheral blood, demonstrating the universality of these findings. The functional consequences of this homogeneity is unknown.
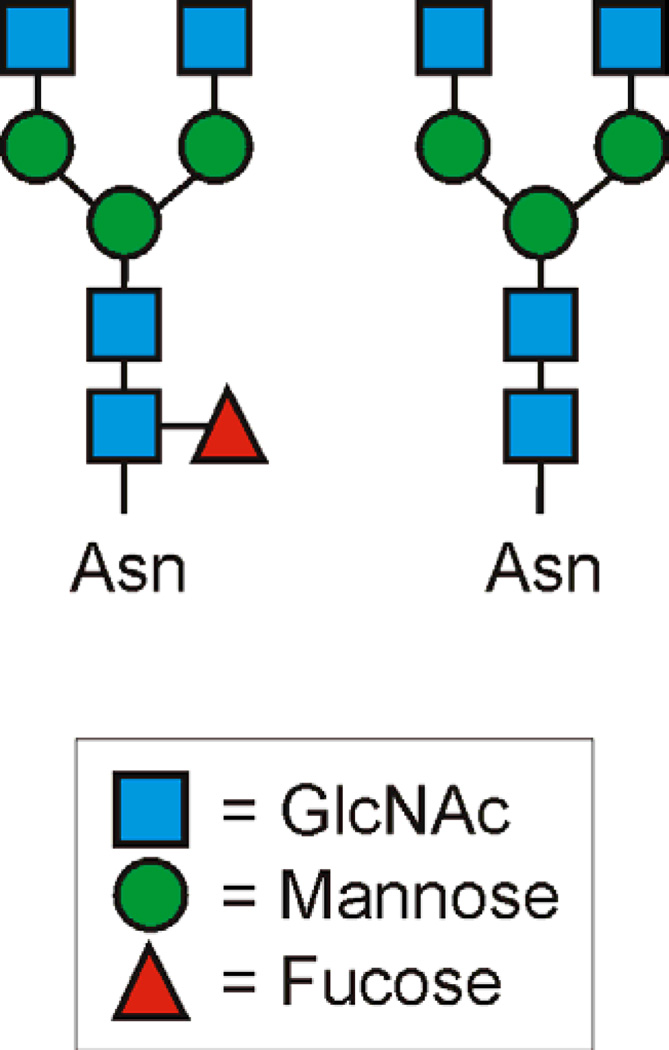
In contrast to HLA-A and -B, HLA-C commonly carries two different glycans at the conserved asparagine site that lack the galactose and sialic acid residues (Man3GlcNAc4Fuc1 or Man3GlcNAc4; Figure 3)[29]. HLA-C differs from HLA-A and -B in a number of ways, including lower surface expression and a possible role in natural killer (NK) cell activation. KIRs (killer cell immunoglobulin-like receptors) are surface molecules expressed by human NK cells that are responsible for sending either inhibitory or activating signals into the cell upon binding to HLA-C [32]. Interestingly, the glycan present at Asn86 of HLA-C appears to play an important role in the interaction between the inhibitory p58 KIRs, such as CD158a and CD158b, and HLA-C [32]. Ablation of the Asn86 glycosylation site on HLA-C to prevent N-glycosylation not only decreased binding between HLA-C and CD158a, but also led to a loss of NK cell lysis inhibition normally mediated by CD158a. Although modifying the composition of the N-glycan at the Asn86 site in HLA-C does not alter function, it is clear that the presence of an N-glycan is important for CD158a binding [32]. To date, it remains unclear whether this effect is direct in that the glycan participates in the binding event, or indirect via glycan-mediated conformational change of the binding site in HLA-C.
Figure 3. Schematic of the dominant HLA-C N-linked Glycans.
Like HLA-A and HLA-B, the MHCI molecule HLA-C shows a high level of homogeneity in terms of the N-glycans found, however, the structure is notably divergent from HLA-A and HLA-B. Although the functional meaning of this homogeneity is unknown, it is interesting to see the apparent conserved difference in the N-glycans on HLAC molecules compared to the other MHCI molecules. How this arises remains a mystery and is an obvious area in need of further investigation.
Although the Asn86 site is conserved across all species, there is a noteworthy difference between human and murine MHCI. Murine MHCI allotypes carry a second conserved N-glycosylation site at Asn176 in the heavy chain [33]. The functional significance of this site is not known, but a hint might be found in the cytomegalovirus (CMV) glycoprotein m154 which is a structural homolog to MHCI. Studies have shown that m154 appears to bind an inhibitory member of the Ly-49 family of NK cell receptor proteins in mice in order to evade immune detection [34]. Ly-49 receptors contain a carbohydrate recognition domain (CRD) reminiscent of the CRDs found in lectins and are sometimes classified as C-type lectin-like receptors [35]. Thus, it is not surprising that N-glycosylation of m154 appears to be required for binding to Ly-49 and this interaction significantly impacts NK cell function [34]. These data suggest that the Asn176 site in murine MHCI could mediate interactions with Ly-49 molecules to modulate NK cell activation. This interpretation is supported by the fact that human MHCI lacks this site and also lacks functional Ly-49 homologs, suggesting that the murine system may have evolved a specific mechanism to protect against murine-specific pathogens that is simply not required in other mammals.
In total, the role of glycosylation on MHCI molecules appears varied, but critically important to the function of the MHCI antigen presentation pathway. Transfer of the core N-glycan onto Asn86 of the heavy chain is required for proper folding, β2-microglobulin binding, and peptide loading of MHCI molecules. In addition, there is evidence that additional non-human sites of glycosylation may play roles in the protection against species-specific pathogens. Finally, the relative homogeneity of the glycans on human MHCI suggests that the glycan might play a role in differentiating self and non-self (or altered-self). These possibilities represent areas ready for further investigation.
5. Glycosylation of the MHC Class II Molecules
MHCI and MHCII molecules may share the same general antigen presentation function, but they are distinct structurally and mechanistically [3,36]. MHCII molecules are heterodimers of an α and β chain, each of which contains a transmembrane domain (compared to MHCI molecules that have one – on the heavy chain). MHCII molecules do not associate with β2-microglobulin and primarily present antigens derived from exogenous sources within the endocytic pathway. Antigen is encountered downstream of the secretory pathway, supporting the data which suggests that the role of MHCII glycosylation is remarkably different compared to MHCI.
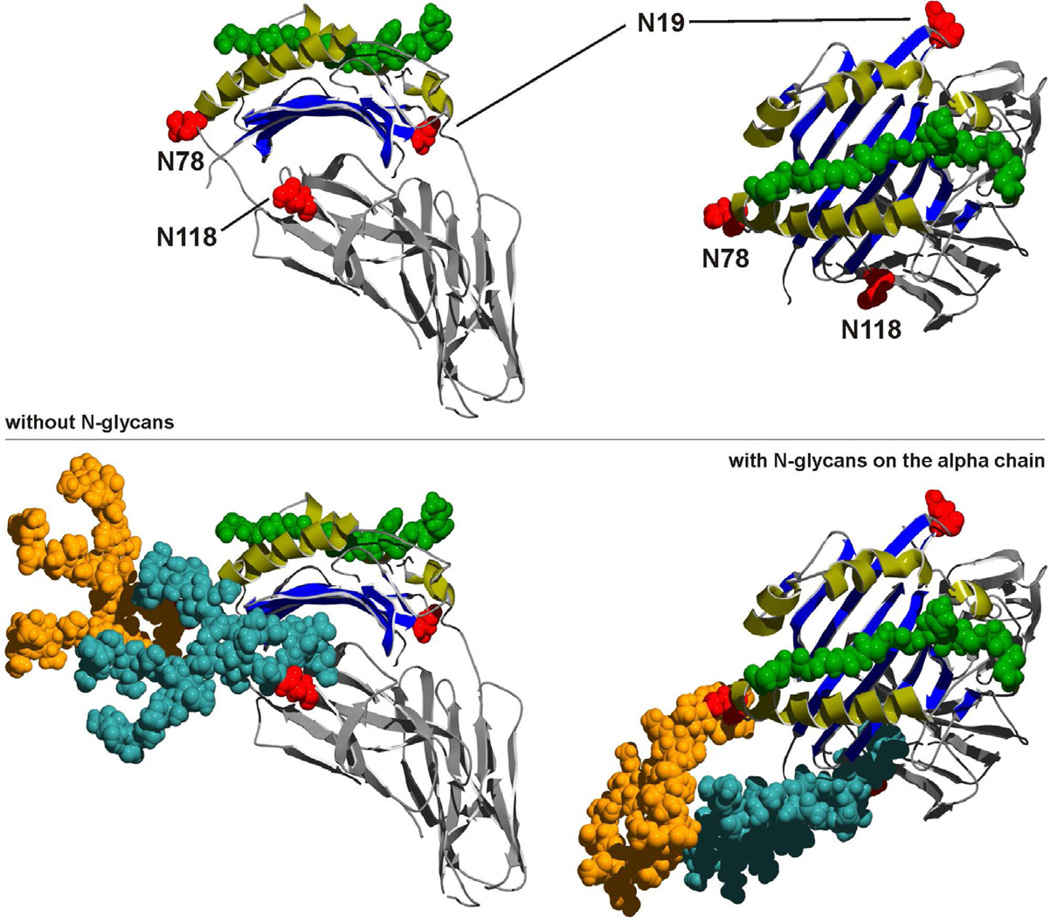
The first major difference between MHCI and MHCII with respect to glycosylation is that MHCII has three highly conserved sites N-glycosylation, two on the α chain (Asn78 and Asn118) and one on the β chain (Asn19) [37]. Interestingly, the Asn86 site in MHCI molecules (Figure 4) is in a homologous physical location as the universally conserved Asn78 site in the α chain of all MHCII alleles across species [38](Figure 5), although the significance of this fact remains completely unexplored. It is clear, however, that the N-glycans of MHCII are not required for proper folding or even trafficking of the MHCII dimer within the cell. This was shown with both asparagine mutants as well as treatment of antigen presenting cells with a N-glycosylation inhibitor (blocks the OST; Figure 1), both of which showed little to no effect on the surface localization or folding of MHCII molecules [39,40]. Instead, the invariant chain (Ii) binds to the nascent MHCII dimer in the ER, stabilizes the structure, and assists with trafficking through the Golgi and into the endocytic pathway [41,42]. Ii is glycosylated, but again, loss of N-glycosylation has no significant effect on MHCII trafficking [43–45], likely because the ability to direct MHCII movement in the cell is mediated by a signal sequence within the protein and not the calnexin/calreticulin chaperones.
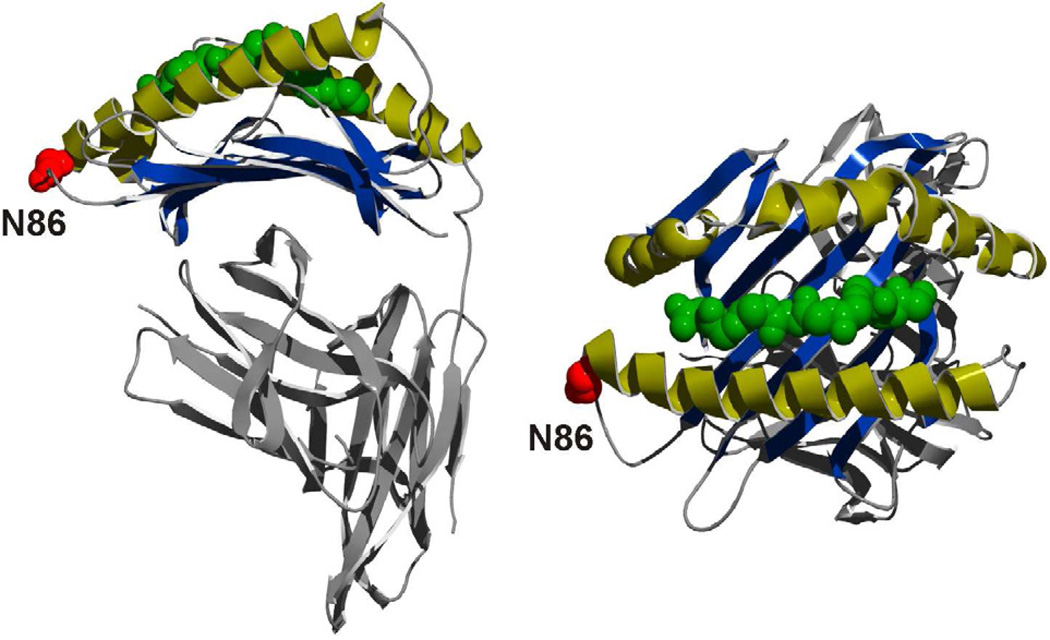
Figure 4. Structure of a Representative MHCI Molecule showing the conserved N-glycan Site.
The Asn78 in MHCII (Figure 5) and N86 in MHCI (HLA-A2; 3D25 in the PDB) sites of N-glycosylation (red) are localized in structurally analogous locations. This universally conserved N-glycosylation site is at one end of the peptide (green) binding groove. The function of the N-glycan at N86 promotes folding and chaperone interactions, but has not been shown to be directly involved with antigen binding or presentation.
Figure 5. Structures of Representative MHCII Molecules with and without complex N-glycans.
MHCII (HLA-DR2) contains three highly conserved sites for N-glycosylation, N78 and N118 in the alpha chain and N19 in the beta chain (top) that are occupied by large complex N-glycans (orange = N78 glycan; teal = N118 glycan; space filling model, bottom). The N78 position is structurally analogous to the N86 site in MHCI molecules (see Figure 4). As seen in the MHCI molecules, this universally conserved N-glycosylation site is at one end of the peptide (green) binding groove. Despite this similarity, the function of the N-glycans appear divergent between MHCI and MHCII, working to promote folding and chaperone interactions for MHCI and directly participating in antigen binding for MHCII. The mechanism for these (and likely other) roles remain poorly defined. Original protein structure coordinates were obtained in the PDB, code 1BX2.
To date, there is no direct evidence to suggest that the N-glycans protect MHCII from pre-mature proteolytic degradation, although glycosylation of Ii does appear to influence the lifetime of MHCII-Ii interactions in the cell [46]. Likewise, the relative homogeneity of MHCI N-glycans (Figures 2–3) is not seen on MHCII molecules. In fact, MHCII N-glycans can vary greatly as a function of cell type (i.e., macrophage vs. B cell vs. dendritic cell), and includes differences in branching patterns and even the degree of terminal sialylation [33]. Even within a clonal cell line, the N-glycans on MHCII are quite heterogeneous.
Heterogeneity is also seen in the presence of the Asn118 site on the α chain. Although this site is conserved among alleles within a species, there is species-to-species variability. For example, some monkey species (e.g., Rhesus) and chickens lack the Asn118 site that is present in humans [38]. In addition, some pigs carry a third asparagine site in the α chain. As exemplified by the observation that the Asn118 site in mouse MHCII (i.e., H2-E) seems to impact T cell repertoire selection (i.e., Vβ T cell receptor usage) [40], the site and compositional heterogeneity on the MHCII alleles within and across species suggests that glycosylation could lead to functional differences between MHCII molecules not seen for the MHCI system.
An early example of how MHCII glycosylation may impact antigen presentation was published in 1991. This study used asparagine mutants of MHCII to look for glycosylation effects on the specificity of T cell responses. The data revealed that removal of the α-Asn78 and β-Asn19 sites individually influenced the stimulation of antigen-specific T cell hybridoma lines [47]. These differences could not be attributed to surface expression or localization of MHCII within the antigen presenting cells. As with MHCI, it is possible that these sites are important for galectin interactions at the cell surface to form MHCII lattices on the APC for optimal TCR recognition by TCR-galectin lattices on the responding T cells, but this possibility remains unexplored.
Another study from over two decades ago also hinted that MHCII glycosylation may impact antigen presentation in B cells. Removal of terminal sialic acids from N-glycans by neuraminidase treatment of resting B cells resulted in a 25-fold increase in B cell antigen presentation [48]. This apparent difference in presentation was not accompanied by changes in the ability of the resting B cells to act as accessory cells in lectin-mediated T cell responses, suggesting that the effect might be localized to the MHC molecules themselves. Nonetheless, this particular study did not include direct analysis of MHCII antigen binding with and without neuraminidase treatment, limiting the impact of the finding.
These two examples suggest that glycosylation may play a role in MHCII-mediated antigen presentation, perhaps by fine-tuning the binding properties; however, the data was far from conclusive until recently. We discovered that the composition of N-glycans on MHCII molecules has a direct and significant impact on the ability of MHCII to associate with at least one class of antigen [49]. A number of years ago, it was found that zwitterionic polysaccharides derived from some bacteria had the ability to stimulate CD4+ T cells to produce IFNγ and other typical cytokines [50–52]. In 2004, this phenomenon was shown to be dependent upon MHCII-mediated binding and presentation of processed fragments of those “glycoantigens” and subsequent recognition by traditional αβ T cell receptors [9]. The polysaccharides known to fit into this glycoantigen family include serotype I Streptococcus pneumoniae capsule (Sp1) [51,53], serotypes V and VIII Staphylococcus aureus capsule [11], PSA and PSB from the capsule of Bacteroides fragilis [50,54,55], and the O-chain polysaccharide derived from the LPS of Morganella morganii [56]. The key link between these antigens and MHCII glycosylation was first revealed in the observation that bacterially-expressed recombinant MHCII protein (HLA-DR2) failed to bind the glycoantigen PSA, whereas the recombinant HLA-DR2 expressed in mammalian cells (i.e., Chinese hamster ovary; CHO) bound with nanomolar affinity [10,49]. The only significant difference between these two recombinant molecules was the presence or absence of N-linked glycosylation.
This initial observation was expanded both in vitro and in vivo to show that changes in the composition of the glycans on MHCII modulated antigen binding and presentation [49]. It turns out that inhibition of branched complex N-glycan synthesis in antigen presenting cells and even on recombinant CHO-expressed MHCII dramatically reduced the binding of glycoantigens. Cells treated with inhibitors of the mannosidases, for example, had decreased glycoantigen presentation at the cell surface and showed marked defects in T cell activation experiments in vitro. Conversely, the ability to present peptide antigen and to initiate peptide-specific T cell responses in vitro was unchanged. Furthermore, treatment of animals with these inhibitors likewise inhibited glycoantigen-specific T cell expansion in vivo, but this could be rescued by adoptive transfer of normally glycosylated antigen presenting cells. Finally, the GlcNAcT-II enzyme (Figure 1) was genetically ablated from dendritic cells (DCs) before being used for in vitro T cell activation experiments, and we found again that glycoantigen-driven T cell activation was deficient compared to normal DC controls, yet peptide antigen responses were indistinguishable. Importantly, for both antigen classes, endocytosis, processing, MHCII surface concentration, and cell viability were unaltered by the glycosylation changes. These data show conclusively that the composition of N-glycans on MHCII can directly impact the antigenic range of molecules that can be presented by an antigen presenting cell [49].
It remains unclear why the composition of N-glycans on MHCII has such a dramatic impact on glycoantigen binding and presentation, although two main possibilities are likely candidates. The first explanation could be that alterations in the N-glycans lead to conformational differences in MHCII that preclude glycoantigen binding. The problem with this idea is that the peptide binding is not changed, yet glycoantigen and peptide binding are competitive in nature. Any change in MHCII conformation must be relatively minor because of the overlapping binding domains for peptides and glycoantigens, thus it seems unlikely that such a change would have such a radical effect on glycoantigen binding. The second explanation, and one that we favor at the moment, is that the N-glycans themselves participate in the binding event through carbohydrate-carbohydrate interactions. Given the relative size of the presented form of glycoantigens (3–10 kDa) and the size of a typical complex N-glycan (approximately the size of an Ig domain; see Figure 5), it makes structural sense that the glycans serve to extend the binding platform of MHCII to accommodate the larger glycoantigen molecules. This second possibility fits with all of the data thus far and represents a working model to test in future studies. These findings are likely to hold significant biological impact because inflammation and disease are known to alter cellular glycosylation patterns in ways these data would predict to impact the nature and effectiveness of the adaptive immune response.
6. MHC Glycosylation and Disease
In general, the glycome (i.e., the array of possible glycan structures) of a cell is driven in part by the specific cohort of glycosylation enzymes present in the Golgi, including glycosyltransferases and various glycolytic enzymes, as well as other metabolic and structural attributes [57,58]. Under normal conditions, the glycome of a given cell tends to be fairly consistent, so why would glycosylation of MHC molecules impact the immune response? The simple answer is that disease, inflammation, and infection also impact host glycosylation, although the extent to which disease-associated changes affect MHC function has not yet been explored systematically. However, it is clear that many glycome changes are characteristic of specific disease mechanisms, suggesting a level of regulation of host glycosylation not presently understood or appreciated.
To date, most studies on disease-associated glycome changes have not focused on the MHC family of glycoproteins or their function. In fact, most of these types of studies have focused on merely cataloging the changes associated with a given state with only some mechanistic detail to explain the changes. An excellent example is Helicobacter pylori infection. Colonization with H. pylori alters mucin glycosylation in the gastrointestinal tract, apparently by increasing the expression of α-1,4-GlcNAc transferase (α4GnT) as a function of the inflammatory response [59–61]. It is presumed that the change in mucin glycosylation provides an advantage to the pathogen for infection, although that remains to be demonstrated. Interestingly, the level of α4GnT returns to baseline once the infection is cleared [62], again showing that the glycome is actively regulated.
A MHCI-specific application of this notion can be found in studies of the hepatitis C virus (HCV). MHCI expression at the cell surface of HCV-infected cells is dramatically reduced compared to normal, uninfected cells. The mechanism for this remains unclear, however, the amount of improperly folded MHCI molecules increased in the Golgi of infected cells, and this correlated with reduced glycosylation of all proteins in the cell [63]. This is likely an example of how a pathogen can manipulate the host glycosylation machinery to impact MHCI expression, thus evading the host.
Neither of these two examples provide any insight into what the signals are and how they are propagated in the cells that lead to glycome changes. However, work done in the context of inflammation may provide some clues. Inflammatory cytokines such as IL-1β, IL-6, and TNFα are all known to impact the glycome. N-glycan branching and the presence of sialyl-Lewisx structures are increased in cells exposed to either IL-1β or IL-6, while TNFα exposure results in increased sialotransferase and fucosyltransferase enzymes [64–68]. Given the fact that these cytokines are central to the inflammatory response that is common to cancer, autoimmunity, and infection, and that changes in cellular glycosylation affect all of the glycoproteins within that cell, these signals undoubtedly lead to changes in MHCI and MHCII glycosylation.
An example that may synthesize why we believe the glycosylation of MHC molecules is critical to understand comes from our recent work on glycoantigens already mentioned. In particular, the glycoantigens that are presented by MHCII in a N-glycosylation-dependent fashion [49] are thought to be key regulators of the immune system. The glycoantigen PSA is expressed by B. fragilis, and colonization with B. fragilis in the gut appears to down-regulate the inflammatory response and assist with maintaining immune homeostasis [69]. This phenomenon has been reported for both inflammatory bowel disease [70] as well as experimental autoimmune encephalomyelitis (EAE) [71], a model for multiple sclerosis. PSA induces a regulatory T cell response characterized by the expression and secretion of high concentrations of IL-10, which inhibits neighboring T cell responses [72]. Therefore, if a particular disease state alters the glycosylation pattern on antigen presenting cells within the gut such that PSA and other glycoantigens are no longer efficiently presented by MHCII, the inflammatory response may run unchecked, leading to disease exacerbation and increased morbidity.
7. Conclusions
The general theme of this review is to highlight MHC glycosylation and its important role in antigen presentation. For MHCI, the primary role of the N-glycans described to date involves the central interaction between MHCI and the calnexin/calreticulin chaperones. This key interaction is required for both folding as well as peptide loading and trafficking. In contrast, the composition of MHCII glycans appears to impact the cohort of antigens that can efficiently associate [49], thus directly impacting the downstream T cell responses. These data paint a picture where we might expect that disease-associated changes in host glycosylation may lead directly to MHC-mediate immune defects.
Perhaps the most remarkable attribute of MHC glycosylation is the complete conservation of one key site – Asn86 in the MHCI heavy chain (Figure 4) and Asn78 in the MHCII α chain (Figure 5). For both molecules, this site is in a structurally homologous location at the end of the peptide binding groove. While it is clear that this site is important for folding and trafficking of MHCI, the Asn78 site in MHCII does not impact either of these things. The N-glycan at Asn86 in MHCI plays a key role in antigen loading by virtue of its role in chaperone recruitment, yet the composition of MHCII N-glycans seems to impact antigen binding in a more direct way. Regardless, it is interesting that this one site has been evolutionarily selected across all species carrying MHC or MHC-like molecules and yet it still remains unclear why that particular site is so important. For MHCI, why not another site? For MHCII, it seems illogical to speculate that these glycosylation sites have been conserved only for the few glycoantigens known to benefit from complex N-glycans. As the field moves forward, it will be imperative to understand the selective pressure for maintaining these sites and what role the N-glycans play at diversifying and/or modulating the function of MHC and thus the adaptive immune response as a whole.
Acknowledgments
We wish to thank Lori S.C. Kreisman for critical evaluation of this manuscript. This work was supported by National Institutes of Health grants OD004225 and GM082916 to B.A. Cobb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interest.
Reference List
- 1.Braem K, Lories RJ. Insights into the pathophysiology of ankylosing spondylitis: Contributions from animal models. Joint Bone Spine. 2011 doi: 10.1016/j.jbspin.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Tam LS, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol. 2010;6:399–405. doi: 10.1038/nrrheum.2010.79. [DOI] [PubMed] [Google Scholar]
- 3.Kumánovics A, Takada T, Lindahl KF. Genomic organization of the mammalian MHC. Annu Rev Immunol. 2003;21:629–657. doi: 10.1146/annurev.immunol.21.090501.080116. [DOI] [PubMed] [Google Scholar]
- 4.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 5.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 6.Madsen L, Labrecque N, Engberg J, Dierich Ae, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 8.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb BA, Kasper DL. Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology. 2008;18:707–718. doi: 10.1093/glycob/cwn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzianabos AO, Wang JY, Lee JC. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9365–9370. doi: 10.1073/pnas.161175598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappler J, White J, Kozono H, Clements J, Marrack P. Binding of a soluble alpha beta T-cell receptor to superantigen/major histocompatibility complex ligands. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8462–8466. doi: 10.1073/pnas.91.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 14.Parham P. Functions for MHC class I carbohydrates inside and outside the cell. Trends Biochem Sci. 1996;21:427–433. doi: 10.1016/s0968-0004(96)10053-0. [DOI] [PubMed] [Google Scholar]
- 15.Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 16.Schachter H. Mgat1-dependent N-glycans are essential for the normal development of both vertebrate and invertebrate metazoans. Semin Cell Dev Biol. 2010;21:609–615. doi: 10.1016/j.semcdb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Tan J, Sutton-Smith M, Ditto D, Panico M, Campbell RM, Varki NM, Long JM, Jaeken J, Levinson SR, Wynshaw-Boris A, Morris HR, Le D, Dell A, Schachter H, Marth JD. Modeling human congenital disorder of glycosylation type IIa in the mouse: conservation of asparagine-linked glycan-dependent functions in mammalian physiology and insights into disease pathogenesis. Glycobiology. 2001;11:1051–1070. doi: 10.1093/glycob/11.12.1051. [DOI] [PubMed] [Google Scholar]
- 18.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 19.Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu. Rev. Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 20.Bergeron JJ, Zapun A, Ou WJ, Hemming R, Parlati F, Cameron PH, Thomas DY. The role of the lectin calnexin in conformation independent binding to N-linked glycoproteins and quality control. Adv. Exp. Med. Biol. 1998;435:105–116. doi: 10.1007/978-1-4615-5383-0_11. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009;98:1223–1245. doi: 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigorian A, Torossian S, Demetriou M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev. 2009;230:232–246. doi: 10.1111/j.1600-065X.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattsson N, Speir JA, DiGennaro JA, Fetrow JS, Dwek RA, Wilson IA. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J. Mol. Biol. 1999;293:351–366. doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa JA, Santos-Aguado J, Mentzer SJ, Strominger JL, Burakoff SJ, Biro PA. Site-directed mutagenesis of class I HLA genes. Role of glycosylation in surface expression and functional recognition. J Exp Med. 1987;166:1329–1350. doi: 10.1084/jem.166.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20:624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cresswell P, Bangia N, Dick T, Diedrich G. The nature of the MHC class I peptide loading complex. Immunol Rev. 1999;172:21–28. doi: 10.1111/j.1600-065x.1999.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 28.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 29.Barber LD, Patel TP, Percival L, Gumperz JE, Lanier LL, Phillips JH, Bigge JC, Wormwald MR, Parekh RB, Parham P. Unusual uniformity of the N-linked oligosaccharides of HLA-A, -B, and -C glycoproteins. J Immunol. 1996;156:3275–3284. [PubMed] [Google Scholar]
- 30.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol. 2009;13:421–426. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le AV, Doyle D. Differential regulation of mouse H-2 alloantigens. Biochemistry. 1982;21:5730–5738. doi: 10.1021/bi00266a001. [DOI] [PubMed] [Google Scholar]
- 32.Baba E, Erskine R, Boyson JE, Cohen GB, Davis DM, Malik P, Mandelboim O, Reyburn HT, Strominger JL. N-linked carbohydrate on human leukocyte antigen-C and recognition by natural killer cell inhibitory receptors. Hum Immunol. 2000;61:1202–1218. doi: 10.1016/s0198-8859(00)00184-1. [DOI] [PubMed] [Google Scholar]
- 33.Swiedler SJ, Freed JH, Tarentino AL, Plummer TH, Jr, Hart GW. Oligosaccharide microheterogeneity of the murine major histocompatibility antigens. Reproducible site-specific patterns of sialylation and branching in asparagine-linked oligosaccharides. J. Biol. Chem. 1985;260:4046–4054. [PubMed] [Google Scholar]
- 34.Abi-Rached L, Parham P. Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J Exp Med. 2005;201:1319–1332. doi: 10.1084/jem.20042558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parham P. NK cell receptors: Of missing sugar and missing self. Curr Biol. 2000;10:R195–R197. doi: 10.1016/s0960-9822(00)00350-x. [DOI] [PubMed] [Google Scholar]
- 36.Beck S, Trowsdale J. The human major histocompatability complex: lessons from the DNA sequence. Annu. Rev. Genomics Hum. Genet. 2000;1:117–137. doi: 10.1146/annurev.genom.1.1.117. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier L, Smith KJ, Pyrdol J, Kalandadze A, Strominger JL, Wiley DC, Wucherpfennig KW. Expression and crystallization of the complex of HLA-DR2 (DRA, DRB1*1501) and an immunodominant peptide of human myelin basic protein. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11828–11833. doi: 10.1073/pnas.95.20.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomonsen J, Marston D, Avila D, Bumstead N, Johansson B, Juul-Madsen H, Olesen GD, Riegert P, Skjodt K, Vainio O, Wiles MV, Kaufman J. The properties of the single chicken MHC classical class II a chain (B-LA) gene indicate an ancient origin for the DR/E-like isotype of class II molecules. Immunogenetics. 2003;55:605–614. doi: 10.1007/s00251-003-0620-7. [DOI] [PubMed] [Google Scholar]
- 39.Elliott WL, Stille CJ, Thomas LJ, Humphreys RE. An hypothesis on the binding of an amphipathic, alpha helical sequence in Ii to the desetope of class II antigens. J Immunol. 1987;138:2949–2952. [PubMed] [Google Scholar]
- 40.Ishikawa S, Kowal C, Cole B, Thomson C, Diamond B. Replacement of N-glycosylation sites on the MHC class II E alpha chain. Effect on thymic selection and peripheral T cell activation. J Immunol. 1995;154:5023–5029. [PubMed] [Google Scholar]
- 41.Neumann J, Koch N. A novel domain on HLA-DRá chain regulates the chaperone role of the invariant chain. J Cell Sci. 2006;119:4207–4214. doi: 10.1242/jcs.03177. [DOI] [PubMed] [Google Scholar]
- 42.Romagnoli P, Germain RN. The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport, and peptide occupancy. J Exp Med. 1994;180:1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 44.Bakke O, Nordeng TW. Intracellular traffic to compartments for MHC class II peptide loading: signals for endosomal and polarized sorting. Immunol Rev. 1999;172:171–187. doi: 10.1111/j.1600-065x.1999.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 45.Sevilla LM, Comstock SS, Swier K, Miller J. Endoplasmic reticulum-associated degradation-induced dissociation of class II invariant chain complexes containing a glycosylation-deficient form of p41. J Immunol. 2004;173:2586–2593. doi: 10.4049/jimmunol.173.4.2586. [DOI] [PubMed] [Google Scholar]
- 46.Neumann J, Schach N, Koch N. Glycosylation signals that separate the trimerization from the MHC class II-binding domain control intracellular degradation of invariant chain. J Biol Chem. 2001;276:13469–13475. doi: 10.1074/jbc.M010629200. [DOI] [PubMed] [Google Scholar]
- 47.Wei BY, Buerstedde JM, Bell M, Chase C, Nilson A, Browne A, Pease L, McKean DJ. Functional effects of N-linked oligosaccharides located on the external domain of murine class II molecules. J Immunol. 1991;146:2358–2366. [PubMed] [Google Scholar]
- 48.Frohman M, Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J. Immunol. 1985;134:2269–2275. [PubMed] [Google Scholar]
- 49.Ryan SO, Bonomo JA, Zhao F, Cobb BA. MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. J. Exp. Med. 2011;208:1041–1053. doi: 10.1084/jem.20100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brubaker JO, Li Q, Tzianabos AO, Kasper DL, Finberg RW. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J. Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- 51.Stingele F, Corthesy B, Kusy N, Porcelli SA, Kasper DL, Tzianabos AO. Zwitterionic polysaccharides stimulate T cells with no preferential Vbeta usage and promote anergy, resulting in protection against experimental abscess formation. J. Immunol. 2004;172:1483–1490. doi: 10.4049/jimmunol.172.3.1483. [DOI] [PubMed] [Google Scholar]
- 52.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 53.Velez CD, Lewis CJ, Kasper DL, Cobb BA. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology. 2009;127:73–82. doi: 10.1111/j.1365-2567.2008.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson FC, III, Onderdonk AB, Kasper DL, Tzianabos AO. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J. Immunol. 1998;160:5000–5006. [PubMed] [Google Scholar]
- 55.Tzianabos AO, Pantosti A, Baumann H, Brisson JR, Jennings HJ, Kasper DL. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J. Biol. Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- 56.Young NM, Kreisman LS, Stupak J, Maclean LL, Cobb BA, Richards JC. Structural characterization and MHCII-dependent immunological properties of the zwitterionic O-chain antigen of Morganella morganii. Glycobiology. 2011;21:1266–1276. doi: 10.1093/glycob/cwr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brockhausen I, Schachter H, Stanley P. O-GalNAc Glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd Edition ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 115–127. [Google Scholar]
- 58.Stanley P, Schachter H, Taniguchi N. N-Glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd Edition ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. pp. 101–114. [PubMed] [Google Scholar]
- 59.Cooke CL, An HJ, Kim J, Canfield DR, Torres J, Lebrilla CB, Solnick JV. Modification of gastric mucin oligosaccharide expression in rhesus macaques after infection with Helicobacter pylori. Gastroenterology. 2009;137:1061–1071. doi: 10.1053/j.gastro.2009.04.014. 1071. [DOI] [PubMed] [Google Scholar]
- 60.Linden S, Semino-Mora C, Liu H, Rick J, Dubois A. Role of mucin Lewis status in resistance to Helicobacter pylori infection in pediatric patients. Helicobacter. 2010;15:251–258. doi: 10.1111/j.1523-5378.2010.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadstrom T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarstrom L, Boren T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuzwa M, Ota H, Hayama M, Zhang MX, Sano K, Honda T, Ueno I, Akamatsu T, Nakayama J. Helicobacter pylori infection up-regulates gland mucous cell-type mucins in gastric pyloric mucosa. Helicobacter. 2003;8:594–600. doi: 10.1111/j.1523-5378.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 63.Tardif KD, Siddiqui A. Cell surface expression of major histocompatibility complex class I molecules is reduced in hepatitis C virus subgenomic replicon-expressing cells. J Virol. 2003;77:11644–11650. doi: 10.1128/JVI.77.21.11644-11650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azuma Y, Murata M, Matsumoto K. Alteration of sugar chains on alpha(1)-acid glycoprotein secreted following cytokine stimulation of HuH-7 cells in vitro. Clin. Chim. Acta. 2000;294:93–103. doi: 10.1016/s0009-8981(99)00248-x. [DOI] [PubMed] [Google Scholar]
- 65.Higai K, Miyazaki N, Azuma Y, Matsumoto K. Interleukin-1beta induces sialyl Lewis X on hepatocellular carcinoma HuH-7 cells via enhanced expression of ST3Gal IV and FUT VI gene. FEBS Lett. 2006;580:6069–6075. doi: 10.1016/j.febslet.2006.09.073. [DOI] [PubMed] [Google Scholar]
- 66.Ishibashi Y, Inouye Y, Okano T, Taniguchi A. Regulation of sialyl-Lewis x epitope expression by TNF-alpha and EGF in an airway carcinoma cell line. Glycoconj. J. 2005;22:53–62. doi: 10.1007/s10719-005-0292-7. [DOI] [PubMed] [Google Scholar]
- 67.Ishibashi Y, Imai S, Inouye Y, Okano T, Taniguchi A. Effects of carbocisteine on sialyl-Lewis x expression in an airway carcinoma cell line stimulated with tumor necrosis factor-alpha. Eur. J. Pharmacol. 2006;530:223–228. doi: 10.1016/j.ejphar.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Mackiewicz A, Rose-John S, Schooltink H, Laciak M, Gorny A, Heinrich PC. Soluble human interleukin-6-receptor modulates interleukin-6-dependent N-glycosylation of alpha 1-protease inhibitor secreted by HepG2 cells. FEBS Lett. 1992;306:257–261. doi: 10.1016/0014-5793(92)81012-b. [DOI] [PubMed] [Google Scholar]
- 69.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 71.Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal. Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 72.Kreisman LS, Cobb BA. Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem. 2011;286:8810–8818. doi: 10.1074/jbc.M110.206011. [DOI] [PMC free article] [PubMed] [Google Scholar]