Abstract
For patients with severe chronic pancreatitis refractory to medical interventions, total pancreatectomy can be considered to relieve the root cause of pain. The goal of a simultaneous islet autotransplant is to prevent or minimize the otherwise inevitable surgical diabetes. Islet autotransplant can successfully preserve some endogenous islet function in the majority of recipients, which mediates protection against brittle diabetes. Most maintain reasonably good glycemic control, while 30-40% successfully discontinue insulin therapy. With islet autotransplants reaching a wider clinical audience, refinements in islet isolation techniques and strategies to protect islet grafts post-transplant may further improve the success of this procedure.
Keywords: pancreatitis, chronic pancreatitis, islet transplant, autotransplant, C-peptide, pancreatectomy, diabetes mellitus, beta cell, alpha cel, surgery
Introduction
Chronic pancreatitis is a disease with many etiologies and many treatments. There often comes a time when therapy no longer relieves the chronic pain and discussion of pancreatic extirpation is undertaken. Total pancreatectomy alone results in complete insulin deficiency and associated sequelae. At the time of total pancreatectomy (TP), islet autotransplantation (IAT) can decrease the insulin insufficiency morbidity of the operation [1-8].
Patients afflicted with chronic pancreatitis present with abdominal pain, and have inflammation and fibrosis on pancreatic histopathology [9-11]. The disease is often progressive, with escalating pain, potential progression to endocrine and exocrine insufficiency, and an elevated lifetime risk of adenocarcinoma [12]. Initial treatments for chronic pancreatitis include avoidance of alcohol and pancreatic irritants, cholecystectomy for biliary stones or sludge, if present, endoscopic pancreaticobiliary duct decompression, narcotic analgesics, pancreatic enzymes to reduce pancreatic stimulation, antioxidants and celiac plexus or nerve blocks [13-15]. When medical management and endoscopic interventions fail or there is a clear anatomic etiology, surgical management is considered to relieve pain and restore quality of life [9]. However as the repertoire of surgical procedures is broad with quite erratic clinical responses, our philosophy has evolved such that total pancreatectomy and islet autotransplantation is offered to patients with chronic pancreatitis who do not respond to adequate endoscopic duct drainage and medical management. The University of Minnesota patient population includes a significant subset of patients that have failed prior surgical duct drainage or pancreatic resections. Total pancreatectomy results in complete insulin and glucagon deficiency, leaving the patient with surgery-induced insulin-dependent diabetes mellitus, often labile or difficult to safely achieve glycemic control. The goal of an islet autotransplant is to salvage as much of the beta cell mass as possible, in order to preserve endogenous insulin secretion, and either prevent surgical diabetes or minimize the magnitude of such diabetes. In this procedure—an autologous islet transplant-- the patient’s own islets are isolated and infused into a tributary of the portal vein, without any need for immunosuppression.
Most islet autotransplant procedures are performed in the context of total (or partial) pancreatectomy for chronic pancreatitis or familial/genetic pancreatitis. However, this procedure has been utilized less commonly for other benign pancreatic disease including trauma and benign pancreatic lesions requiring extensive (60-80%) pancreatectomy [16-21]. Because some acinar and ductal tissue is co-transplanted with the islets, islet autotransplant should be avoided if pancreatectomy is performed for malignancy of the pancreas.
Technical aspects of islet isolation
The pancreatectomy is most often performed by open laparotomy, although a robotic-assisted laparoscopic approach has been described [22, 23]. The major technical goal is to preserve the blood supply to the pancreas until removal, to minimize ischemia of the islet tissue. After devascularization, the pancreas is quickly removed placed in cold preservation solution. It is inspected and prepared (removal of duodenum and spleen, pancreatic duct assessed for integrity and gross blood flushed from vessels) and packaged for transport to the islet isolation lab in cold tissue preservation solution. The goal of islet isolation is to digest the pancreas and disrupt the exocrine pancreatic tissue [24], to release relatively pure islets in a small tissue volume which can be safely infused into the portal vein. The pancreas is first distended by intraductal injection of a collagenase enzyme, and then gentle mechanical dispersion using the semi-automated method of Ricordi frees the islets from the exocrine tissue as much as possible [25, 26]. Because high tissue volume increases the risk for portal pressure elevation during islet infusion [27, 28], if the tissue volume is large (>~0.25 mL/kg), the islets can be purified by gradient separation method to reduce volume [29]. Use of customized density gradients, rather than fixed standard purification gradients, can minimize the loss of islets during the purification step [24]. Often, tissue volume is small and the autologous islet preparation can be transplanted unpurified. The number of islets are quantified as islet equivalents (IEQ), which is islet mass standardized to an islet size of 150 micrometers diameter [30].
For autologous islet transplant, islets are most often returned to the patient fresh (not cultured) while the patient is in the operating room with an open incision. Alternatively, islets may be transplanted post-operatively by percutaneous approach [6]. The islets are infused into the portal system using a stump of the splenic vein, or alternatively direct puncture of the portal or cannulation of the umbilical vein can be used [31]. While the portal site for infusion is most common, islets can be transplanted elsewhere, including the peritoneal cavity, bowel subserosa or submucosa, omental, or intramuscular [1, 31, 32]. With significant tissue volume infused into the portal vein, elevation of portal pressure can be a limiting element. Significant elevations in portal pressures are associated with marked reduction in blood flow and risk for subsequent portal thrombosis [28]. Because of this risk, routine heparin anticoagulation is utilized which in combination of acute portal hypertension elevates the incidence of perioperative bleeding. Minimizing tissue volume infused and thereby minimizing portal pressure elevations reduces bleeding risk. At our institution, when tissue volume is reduced to <0.25 mL/kg and change in portal pressure does not exceed 25 cmH20, the risk of clinically significant bleeding is <8% [28].
It should be emphasized that patients are receiving their own islets. No immunosuppression is required during or after islet infusion. Thus, islet autotransplant recipients do not have the long-term risks of infection and other immunosuppression side effects that are a consideration in type 1 diabetic recipients receiving islet allografts from cadaveric pancreatic donors, and islet engraftment and function is not impacted by agents such as tacrolimus and rapamycin which are necessary for islet allografts but may be detrimental to graft vascularization and beta cell function [33, 34].
Islet isolation, engraftment, and the early post-operative period
At the time of islet isolation, islet vasculature is disrupted, the islets are exposed to mechanical, osmotic, and hypoxic stress, and pro-apoptotic pathways are upregulated [35, 36]. In the early post-transplant period, islets are reliant on diffusion of nutrients and oxygen to the islet core until neovascularization is complete, a process that takes at least 2-4 weeks [37, 38]. In animal models, rates of beta cell apoptosis in the first month post-transplant are very high, and worsened under hyperglycemia, so care is taken to maintain strict glycemic control during the early post-transplant period [39-42]. At our institution, we initiate an insulin drip in the immediate post-operative period to target blood sugar 100-120 mg/dL, followed by transition to subcutaneous insulin at ~1 week post-operatively to target blood sugars 80-125 mg/dL. Post-operative insulin management protocols vary by institution. At the University of Minnesota, we maintain patients on insulin therapy for at least 3 months post-transplant (unless hypoglycemia is present) and then wean as tolerated to maintain long-term goals of fasting blood sugar <126 mg/dL, post-prandial blood sugar <140-180 mg/dL, and hemoglobin A1c level <6.5% (and ideally in normal range for insulin independent patients). Near euglycemia is targeted, to minimize hyperglycemic stress on the beta cell mass which could otherwise contribute to attrition of the beta cell mass over time [43, 44].
Long-term metabolic benefits of preserving endogenous beta cell function
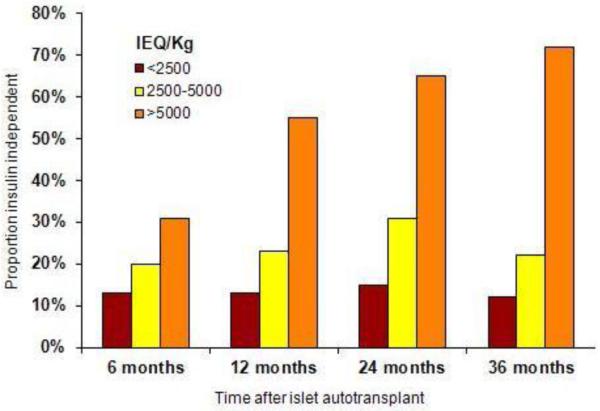
The largest series in islet autotransplantation published are from University of Minnesota, University of Cincinnati, and Leicester [2, 4, 8]. Overall, one-third of patients in the Minnesota series achieve insulin independence, but the majority have islet graft function, as documented by C-peptide positivity (Figure 1) [4, 8]. Cincinnati, Leicester, and other centers have published similar results, with 22%-40% of patients insulin independent [2, 5, 6]. The number of islets transplanted is an important prognostic factor, although there is much overlap between outcomes by islet mass--- some patients with high islet mass will never come off insulin while some with low islet mass do achieve insulin independence. There are many factors that likely mediate this difference—islet viability, beta cell functional capacity, and recipient characteristics, particularly the insulin sensitivity or insulin requirements of the recipient may all be important factors. Nevertheless, receiving a high islet mass generally conveys a favorable prognosis. Of those patients in the Minnesota series who received >5000 IEQ/kg at the time of transplant, 65-72% were insulin independent at 2-3 years post-transplant. This is in contrast to 12-15% of recipients with <2500 IEQ/kg transplanted [8]. Other factors associated with insulin independence include lack of a prior pancreatic surgery (particularly a partial pancreatectomy or lateral pancreaticojejunostomy) [8, 31] and higher C-peptide to glucose ratio at 1 month post-transplant [45]. The impact of prior pancreatic surgery can be quite dramatic, with an approximately 50% reduction in islet yield in patients who have had a lateral pancreaticojejunostomy (Puestow) or distal pancreatectomy (Table 1).
Figure 1.
Insulin independence by islet yield and duration after islet transplant in autograft recipients at the University of Minnesota (Data from Sutherland et al. [8].)
Table 1.
Relationship between the history of pancreatic surgery and islet yield in 413 isolation procedures
| Pancreatic Surgery | Islet Yield (IEQ/kg, mean ± SD) |
|---|---|
| No direct pancreatic surgery | 3794 ± 2242 |
| Whipple | 3647 ± 1672 |
| Berger/ Frey | 2654 ± 2151 |
| Distal pancreatectomy | 1973 ± 2028 |
| Puestow | 1883 ± 1853 |
Data from Sutherland et al. [8].
While attrition of insulin independence does occur with islet autografts, it is much less than that classically described for islet allografts for type 1 diabetes mellitus. In one analysis, of those islet autograft recipients who achieved insulin independence, three quarters maintained insulin independence for 2 or more years [46]. The rate of attrition relates to the initial islet mass; few patients resume insulin in the first several years post-transplant when the initial islet mass was high (>5000 IEQ/kg), but return to insulin use is much more rapid if islet mass was low (<2500 IEQ/kg) [47]. Insulin independence with euglycemia has been documented for 16 years, with graft function for over 2 decades [48] (and unpublished data).
It is important to emphasize that with islet autotransplant, the goal is good glycemic control without brittle diabetes. When insulin independence can be achieved, this is an important bonus, but minimizing the impact of post-surgical diabetes (short of insulin independence) is considered a success. By this standard, most pancreatectomy recipients benefit from an islet autograft. Ninety percent of patients in the Minnesota series and 100% of those in the Leicester series are C-peptide positive after the procedure [4, 8]. Most patients maintain glycemic control within a range recommended by the American Diabetes Association, with 82% of all recipients maintaining an average HbA1c level <7.0% [8]. When islet mass transplanted is high (>5000 IEQ/kg), 100% are C-peptide positive and 94% maintain a HbA1c <7.0% [8].
Ability to predict the number of islets before pancreatectomy is performed is currently limited. Preliminary evidence suggests that metabolic testing by intravenous glucose tolerance tests, arginine stimulation tests, or maximal stimulated arginine may provide a useful surrogate for islet count [48]. In children, body weight and fasting blood sugar were predictive in one small series [49]. When a prior distal pancreatectomy or a surgical drainage procedure such as Puestow or Frey has been performed, islet yield is frequently low, and history of such procedures confer a poor prognosis for the patient in regards both to islet number and insulin independence [8]. In one small series, greater evidence of chronic pancreatitis pathology on CT imaging, by endoscopic retrograde cholangiopancreatography (ERCP), or on endoscopic ultrasound (EUS) was associated with lower islet yield (<5000 IEQ/kg vs >5000 IEQ/kg) [50], and at the same center, body mass index (BMI) >23 kg/m2 is associated with better islet yield [51], although one would suspect that obese patients are also more likely to have high insulin demands/insulin resistance. Severe histopathologic changes on pancreatectomy biopsy characterized by severe fibrosis, acinar atrophy, and inflammation were associated with low islet yield in children and adults [10, 11]. However, it is important to note that islet isolation technique can also impact islet yield [52], and as we improve our isolation protocols, these relationships between predictors and islet isolation results may change.
Children and TPIAT
Although most islet autotransplant procedures have been performed in adults, children with severe chronic pancreatitis are also candidates for the procedure. Unlike adults who often have idiopathic pancreatitis, children frequently carry genetic mutations which predispose them to develop pancreatitis, including mutations in the cationic trypsinogen gene (PRSS1), thepancreatic secretory trypsin inhibitor (SPINK1), or the cystic fibrosis transmembrane conductance regulator (CFTR). While the procedure is essentially the same as in adults, children as a group have higher rates of insulin independence and normalization of quality of life. Overall 40-50% of children achieve insulin independence [31, 53]. This is largely driven by the youngest group of patients, <12 years of age, who more frequently come off insulin or require only once daily basal insulin therapy than their teenage counterparts [53]. The youngest two patients in the Minnesota series, both 5 years of age, have been off insulin for 4 and 7 years (Bellin, unpublished data).
Whether these outcomes are driven by the young age of the recipient, the intrinsic characteristics of the young beta cells, or both is unclear. The young children are smaller, often receive a greater islet number for body weight, and have lower insulin demands, all of which could be favorable for islet engraftment. However, beta cells from younger patients also have greater replicatory capacity than adult beta cells, particularly in children younger than 10 years of age, as found in autopsy studies of non-diabetic children [54]. In some young patients with severe chronic pancreatitis, islet neogenesis of ductal origin has been observed [55]. Since autologous preparations are relatively impure, some ductal tissue is frequently co-infused with the islets. Whether any beta cell replication or neogenesis can take place after transplant is entirely theoretical and has not been documented in clinical islet transplant.
Assessing beta and alpha cell function after TPIAT
Hemoglobin A1c, fasting glucose, and simple C-peptide levels can be routinely measured in clinic. We have observed higher stimulated C-peptide secretion in response to a mixed meal test with greater transplanted islet mass [8]. Mean stimulated C-peptide after 6 mL/kg (max 360 mL) Boost HP is significantly higher in patients with >5000 IEQ/kg vs those with 2500-5000 IEQ/kg or <2500 IEQ/kg (p<0.001, unpublished data). However, fasting C-peptide does not distinguish between these three groups.
More sophisticated measures of beta cell function and mass have been performed in a subset of islet autograft recipients enrolled in research trials. In 6 recipients studied longitudinally (at mean of 6 years post-transplant), half of islet autotransplant recipients exhibit a reduction over time in insulin secretion in response to intravenous glucose, while insulin secretory reserve as measured by glucose potentiated arginine stimulation generally remained stable [48]. In this study, glucose dispersal rate correlated with the number of islets transplanted. Glucose potentiated insulin response may be the best estimate of functional beta cell mass post-transplant, although the acute insulin response to glucose or arginine also correlate reasonably well with transplanted islet mass [56]. Intrahepatic islets secrete insulin in a normal pulsatile pattern, similar to that of the beta cells in the native pancreas although at a lesser magnitude [57]. Compared to islet allografts for type 1 diabetes-- which are subject to immunity and drug toxicity-- islet autografts exhibit about twice as much function per islet transplanted at 1-4 years post-transplant, as measured by insulin secretion on intravenous glucose tolerance tests and glycemic control by oral glucose tolerance test [58].
Whether alpha cells can mount a normal glucagon response to hypoglycemia following islet transplantation is less clear. Intraportal islet transplants produce glucagon normally in response to an injection of arginine, demonstrating the presence of alpha cells in the transplanted islet graft. However, Kendall et al demonstrated that glucagon responses were absent in response to hypoglycemia [59]. This may be mediated by the location of the islet graft. In diabetic dogs undergoing islet autotransplantation, glucagon production was similarly absent when islets were transplanted to the liver, but glucagon counter-regulation did occur when islets were transplanted into the peritoneal cavity [60]. Zhou et al. proposed that intrahepatic glucose flux related to glycogenolysis in the liver suppresses the ability of the alpha cell to recognize systemic hypoglycemia, thus resulting in defective glucagon counter-regulation [61]. Anecdotally, we have islet transplant recipients who report hypoglycemia even off insulin therapy, although the timing is most often post-prandial and less often fasting.
Opportunities for improvement, recent research, and future directions
While islet autotransplantation is successful in many cases, opportunities for improvement exist. Strategies that improve isolation outcomes, improve islet engraftment, prevent damage from innate immunity and inflammation, or minimize beta cell apoptosis represent potential strategies to increase the proportion of insulin independent patients or long-term survival of the islet graft. Refinements to collagenase and neutral protease solutions used for isolation and customization of pancreatic tissue density gradients for COBE purification have improved the yield and recovery during islet isolation [24, 52], and such improvements in islet isolation are likely to continue. Other ongoing studies are investigating agents that may protect the islets from stress or reduce beta cell apoptosis [62], or minimize the impact of innate inflammatory or thrombotic impact on the islet graft immediately post-transplant.
Additional research is ongoing with regards to the hepatic site for transplantation. Although intraportal islet transplantation is still the gold-standard for clinical islet autotransplantation, there are theoretical disadvantages including greater gluco-lipotoxicity and toxin exposure due to the direct contact with the portal blood. Intraportal islets may also elicit an instant blood mediated inflammatory reaction [63, 64], although the clinical significance in heparin treated clinical islet autotransplant recipients is unclear. Peritoneal transplants have been used as a second site in patients who could not receive all islets in the liver at our institution, and do function comparably in animal models. Recently, Rafeal et al reported successful outcome after forearm intramuscular islet transplant in a child [32], and mice data suggest superior revascularization, comparable to that seen in native pancreatic islets [65]. Thus, future studies may identify a site that has superior short and long-term outcomes compared to the liver. In the meantime, intraportal infusion continues to be the standard of care.
Conclusions
Total pancreatectomy is a major surgical intervention that should not be entertained lightly, but a spectrum of benign pancreatic diseases is best treated with this procedure. The goal of islet autotransplantation is to preserve as much beta cell mass as possible at the time of pancreatectomy, most often performed for chronic pancreatitis. Ninety percent or more of recipients have endogenous beta cell function (as documented by C-peptide positivity) after surgery and in 4 of 5 patients glycemic control is maintained within the goal range for diabetes mellitus. With a high islet mass transplanted, stimulated C-peptide is higher, HbA1c more frequently in goal range, and insulin independence more common; however, even some patients with a low mass islet graft will maintain normal blood sugars off insulin therapy, highlighting the potential for success with even a small number of islets under the right conditions. Current research is directed at improving the islet isolation process and the engraftment and long-term survival of the islet graft, directed to further improve our ability to prevent post-operative diabetes mellitus with this procedure.
Acknowledgments
Dr. Melena Bellin is supported by a career development award from the National Institute of Diabetes, Digestive, and Kidney Diseases (1K23DK084315-01A1). We thank Dr. David Radosevich for his contributions to the data analysis.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Melena Bellin, University of Minnesota Amplatz Children’s Hospital, East Building, Rm MB-671, 2450 Riverside Ave E, Minneapolis, MN 55454.
A.N. Balamurugan, University of Minnesota, Schulze Diabetes Institute, Department of Surgery, 420 Delaware St SE, Minneapolis, MN 55455, Phone 651-253-0656, Fax 612-626-5855, bala@umn.edu
Timothy L. Pruett, University of Minnesota, 420 Delaware St. S.E., Minneapolis, MN 55455, 612-626-7282 Phone, 612-624-7168 Fax, tlpreutt@umn.edu
David E.R. Sutherland, University of Minnesota, 420 Delaware St. S.E., Minneapolis, MN 55455, 612-625-7600 Phone, 612-624-7168 Fax, dsuther@umn.edu.
References
- [1].Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. The Surgical clinics of North America. 2007;87:1477–1501. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [2].Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. Journal of the American College of Surgeons. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- [3].Dixon J, DeLegge M, Morgan KA, Adams DB. Impact of total pancreatectomy with islet cell transplant on chronic pancreatitis management at a disease-based center. The American Surgeon. 2008;74:735–738. doi: 10.1177/000313480807400812. [DOI] [PubMed] [Google Scholar]
- [4].Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37:282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- [5].Sutton JM, Schmulewitz N, Sussman JJ, et al. Total pancreatectomy and islet cell autotransplantation as a means of treating patients with genetically linked pancreatitis. Surgery. 2010;148:676–685. doi: 10.1016/j.surg.2010.07.043. discussion 685-676. [DOI] [PubMed] [Google Scholar]
- [6].Morgan KA, Nishimura M, Uflacker R, Adams DB. Percutaneous transhepatic islet cell autotransplantation after pancreatectomy for chronic pancreatitis: a novel approach. HPB (Oxford) 2011;13:511–516. doi: 10.1111/j.1477-2574.2011.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morgan K, Owczarski SM, Borckardt J, et al. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:129–133. doi: 10.1007/s11605-011-1744-y. discussion 133-124. [DOI] [PubMed] [Google Scholar]
- [8]**.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409–424. doi: 10.1016/j.jamcollsurg.2011.12.040. This is the largest series of total pancretectomy and islet autotransplant to date, detailing outcomes in over 400 cases.
- [9].Ahmed SA, Wray C, Rilo HL, et al. Chronic pancreatitis: recent advances and ongoing challenges. Current problems in surgery. 2006;43:127–238. doi: 10.1067/j.cpsurg.2005.12.005. [DOI] [PubMed] [Google Scholar]
- [10].Kobayashi T, Manivel JC, Carlson AM, et al. Correlation of histopathology, islet yield, and islet graft function after islet autotransplantation in chronic pancreatitis. Pancreas. 2011;40:193–199. doi: 10.1097/mpa.0b013e3181fa4916. [DOI] [PubMed] [Google Scholar]
- [11].Kobayashi T, Manivel JC, Bellin MD, et al. Correlation of pancreatic histopathologic findings and islet yield in children with chronic pancreatitis undergoing total pancreatectomy and islet autotransplantation. Pancreas. 2010;39:57–63. doi: 10.1097/MPA.0b013e3181b8ff71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2004;2:252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- [13].Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss medical weekly : official journal of the Swiss Society of Infectious Diseases, the Swiss Society of Internal Medicine, the Swiss Society of Pneumology. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- [14].Bhardwaj P, Garg PK, Maulik SK, et al. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136:149–159. e142. doi: 10.1053/j.gastro.2008.09.028. [DOI] [PubMed] [Google Scholar]
- [15].Choudari CP, Nickl NJ, Fogel E, et al. Hereditary pancreatitis: clinical presentation, ERCP findings, and outcome of endoscopic therapy. Gastrointestinal endoscopy. 2002;56:66–71. doi: 10.1067/mge.2002.125103. [DOI] [PubMed] [Google Scholar]
- [16].Oberholzer J, Mathe Z, Bucher P, et al. Islet autotransplantation after left pancreatectomy for nonenucleable insulinoma. Am.J.Transplant. 2003;3:1302–1307. doi: 10.1046/j.1600-6143.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- [17].Berney T, Mathe Z, Bucher P, et al. Islet autotransplantation for the prevention of surgical diabetes after extended pancreatectomy for the resection of benign tumors of the pancreas. Transplantation proceedings. 2004;36:1123–1124. doi: 10.1016/j.transproceed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- [18].Lee BW, Jee JH, Heo JS, et al. The favorable outcome of human islet transplantation in Korea: experiences of 10 autologous transplantations. Transplantation. 2005;79:1568–1574. doi: 10.1097/01.tp.0000158427.07084.c5. [DOI] [PubMed] [Google Scholar]
- [19].Ris F, Niclauss N, Morel P, et al. Islet autotransplantation after extended pancreatectomy for focal benign disease of the pancreas. Transplantation. 2011;91:895–901. doi: 10.1097/TP.0b013e31820f0892. [DOI] [PubMed] [Google Scholar]
- [20].Jindal RM, Ricordi C, Shriver CD. Autologous pancreatic islet transplantation for severe trauma. N Engl J Med. 2010;362:1550. doi: 10.1056/NEJMc0912392. [DOI] [PubMed] [Google Scholar]
- [21].Khan A, Jindal RM, Shriver C, et al. REMOTE PROCESSING OF PANCREAS CAN RESTORE NORMAL GLUCOSE HOMEOSTASIS IN AUTOLOGOUS ISLET TRANSPLANTATION AFTER TRAUMATIC WHIPPLE PANCREATECTOMY: TECHNICAL CONSIDERATIONS. Cell Transplant. 2011 doi: 10.3727/096368911X600984. [DOI] [PubMed] [Google Scholar]
- [22].Giulianotti P, Gorodner V, Kinzer K, et al. Robot-assisted pancreatoduodenectomy with preservation of the vascular supply for autologous islet cell isolation and transplantation: a case report. Journal of medical case reports. 2012;6:74. doi: 10.1186/1752-1947-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marquez S, Marquez TT, Ikramuddin S, et al. Laparoscopic and da Vinci robot-assisted total pancreaticoduodenectomy and intraportal islet autotransplantation: case report of a definitive minimally invasive treatment of chronic pancreatitis. Pancreas. 2010;39:1109–1111. doi: 10.1097/MPA.0b013e3181df262c. [DOI] [PubMed] [Google Scholar]
- [24].Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation. 2011;91:508–514. doi: 10.1097/TP.0b013e3182066ecb. [DOI] [PubMed] [Google Scholar]
- [25].Lakey JR, Warnock GL, Shapiro AM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell transplantation. 1999;8:285–292. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- [26].Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- [27].Matsumoto S, Takita M, Shimoda M, et al. Impact of Tissue Volume and Purification on Clinical Autologous Islet Transplantation for the Treatment of Chronic Pancreatitis. Cell Transplant. 2012 doi: 10.3727/096368911X623899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wilhelm JJ, Bellin MD, Balamurugan AN, et al. A proposed threshold for dispersed-pancreatic tissue volume infused during intraportal islet autotransplantation after total pancreatectomy to treat chronic pancreatitis. Pancreas. 2011;40:1363. [Google Scholar]
- [29].Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 1989;38(Suppl 1):143–145. doi: 10.2337/diab.38.1.s143. [DOI] [PubMed] [Google Scholar]
- [30].Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetologica Latina. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- [31].Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:793–799. doi: 10.1016/j.cgh.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rafael E, Tibell A, Ryden M, et al. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:458–462. doi: 10.1111/j.1600-6143.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- [33].First MR, Gerber DA, Hariharan S, et al. Posttransplant diabetes mellitus in kidney allograft recipients: incidence, risk factors, and management. Transplantation. 2002;73:379–386. doi: 10.1097/00007890-200202150-00011. [DOI] [PubMed] [Google Scholar]
- [34].Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. The Journal of clinical investigation. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Noguchi H. Activation of c-Jun NH2-terminal kinase during islet isolation. Endocrine journal. 2007;54:169–176. doi: 10.1507/endocrj.kr-87. [DOI] [PubMed] [Google Scholar]
- [36].Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53:2815–2823. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- [37].Hathout E, Chan NK, Tan A, et al. In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatric transplantation. 2009;13:892–897. doi: 10.1111/j.1399-3046.2008.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature medicine. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Biarnes M, Montolio M, Nacher V, et al. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- [40].Finzi G, Davalli A, Placidi C, et al. Morphological and ultrastructural features of human islet grafts performed in diabetic nude mice. Ultrastructural pathology. 2005;29:525–533. doi: 10.1080/01913120500323563. [DOI] [PubMed] [Google Scholar]
- [41].Paraskevas S, Maysinger D, Wang R, et al. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20:270–276. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- [42].Davalli AM, Scaglia L, Zangen DH, et al. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- [43].Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bonora E. Protection of pancreatic beta-cells: is it feasible? Nutrition, metabolism, and cardiovascular diseases : NMCD. 2008;18:74–83. doi: 10.1016/j.numecd.2007.05.004. [DOI] [PubMed] [Google Scholar]
- [45].Matsumoto S, Takita M, Shimoda M, et al. Usefulness of the secretory unit of islet transplant objects (SUITO) index for evaluation of clinical autologous islet transplantation. Transplant Proc. 2011;43:3246–3249. doi: 10.1016/j.transproceed.2011.10.036. [DOI] [PubMed] [Google Scholar]
- [46].Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sutherland DE, Carlson AM, Blondet JJ, et al. Islet autotransplantation - long term results and lessons learned that could be applied to islet allotransplantation. Xenotransplantation. 2007;14:391. [Google Scholar]
- [48].Robertson RP, Lanz KJ, Sutherland DE, Kendall DM. Prevention of diabetes for up to 13 years by autoislet transplantation after pancreatectomy for chronic pancreatitis. Diabetes. 2001;50:47–50. doi: 10.2337/diabetes.50.1.47. [DOI] [PubMed] [Google Scholar]
- [49].Bellin MD, Blondet JJ, Beilman GJ, et al. Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis. Pediatric diabetes. 2010;11:227–234. doi: 10.1111/j.1399-5448.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Takita M, Naziruddin B, Matsumoto S, et al. Variables associated with islet yield in autologous islet cell transplantation for chronic pancreatitis. Proceedings (Baylor University. Medical Center) 2010;23:115–120. doi: 10.1080/08998280.2010.11928597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Takita M, Naziruddin B, Matsumoto S, et al. Body mass index reflects islet isolation outcome in islet autotransplantation for patients with chronic pancreatitis. Cell Transplant. 2011;20:313–322. doi: 10.3727/096368910X514611. [DOI] [PubMed] [Google Scholar]
- [52].Balamurugan AN, Loganathan G, Bellin MD, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93:693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. Journal of pediatric gastroenterology and nutrition. 2008;47:37–44. doi: 10.1097/MPG.0b013e31815cbaf9. [DOI] [PubMed] [Google Scholar]
- [54].Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Soltani SM, O’Brien TD, Loganathan G, et al. Severely fibrotic pancreases from young patients with chronic pancreatitis: evidence for a ductal origin of islet neogenesis. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Teuscher AU, Kendall DM, Smets YF, et al. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47:324–330. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]
- [57].Meier JJ, Hong-McAtee I, Galasso R, et al. Intrahepatic transplanted islets in humans secrete insulin in a coordinate pulsatile manner directly into the liver. Diabetes. 2006;55:2324–2332. doi: 10.2337/db06-0069. [DOI] [PubMed] [Google Scholar]
- [58].Bellin MD, Sutherland DE, Beilman GJ, et al. Similar islet function in islet allotransplant and autotransplant recipients, despite lower islet mass in autotransplants. Transplantation. 2011;91:367–372. doi: 10.1097/TP.0b013e318203fd09. [DOI] [PubMed] [Google Scholar]
- [59].Kendall DM, Teuscher AU, Robertson RP. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes. 1997;46:23–27. doi: 10.2337/diab.46.1.23. [DOI] [PubMed] [Google Scholar]
- [60].Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes. 1997;46:28–33. doi: 10.2337/diab.46.1.28. [DOI] [PubMed] [Google Scholar]
- [61].Zhou H, Zhang T, Bogdani M, et al. Intrahepatic glucose flux as a mechanism for defective intrahepatic islet alpha-cell response to hypoglycemia. Diabetes. 2008;57:1567–1574. doi: 10.2337/db08-0137. [DOI] [PubMed] [Google Scholar]
- [62].Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- [63].Moberg L. The role of the innate immunity in islet transplantation. Upsala journal of medical sciences. 2005;110:17–55. doi: 10.3109/2000-1967-181. [DOI] [PubMed] [Google Scholar]
- [64].Bennet W, Groth CG, Larsson R, et al. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala journal of medical sciences. 2000;105:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- [65].Christoffersson G, Henriksnas J, Johansson L, et al. Clinical and experimental pancreatic islet transplantation to striated muscle: establishment of a vascular system similar to that in native islets. Diabetes. 2010;59:2569–2578. doi: 10.2337/db10-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]