Abstract
E-selectin, expressed on inflamed endothelium, and sialyl Lewis x (sLex), present on the surface of leukocytes, play a key role in leukocyte–endothelial interactions during leukocyte recruitment to sites of inflammation. HECA-452 is a monoclonal antibody (mAb) that recognizes sLex and is routinely used by investigators from diverse fields who seek to unravel the mechanisms of leukocyte adhesion. The data regarding the ability of HECA-452 to inhibit carbohydrate-mediated leukocyte adhesion to E-selectin remains conflicted, in part due to the presence of a variety of potential E-selectin reactive moieties on leukocytes. Recognizing this, we utilized a complementary approach to gain insight into HECA-452 adhesion assays. Specifically, we used sLex microspheres to investigate the hypothesis that HECA-452 is a nonfunction blocking mAb for isolated sLex mediated adhesion to endothelial expressed E-selectin. Flow cytometric analysis revealed that HECA-452 recognizes and binds to the sLex microspheres. Perfusion of the sLex microspheres over human umbilical vein endothelial cells (HUVEC) at 1.5 dynes/cm2 revealed that the microspheres attach to 4 hr interleukin (IL)-1β activated HUVEC specifically via E-selectin. Pretreatment of the sLex microspheres with HECA-452 did not influence sLex microsphere initial tethering and accumulation on IL-1β activated HUVEC. Neuraminidase and fucosidase treatment of sLex microspheres revealed that sialic acid and fucose are required for E-selectin binding, whereas HECA-452 recognition of sLex does not depend on the fucose moiety to the extent required for E-selectin recognition. This latter finding suggests there are potential subtle differences between the sLex antigens for E-selectin and HECA-452. Combined, the data indicate that HECA-452 is a non-inhibitor of sLex-mediated adhesion to endothelial expressed E-selectin.
Keywords: sLex, adhesion, inflammation, leukocyte
1. Introduction
Leukocyte recruitment to a site of inflammation involves a series of adhesion events between the leukocytes and the vascular endothelium. These events include initial tethering and rolling that are mediated, in part, by the endothelial selectins (E and P-selectin) that bind to glycoproteins and glycolipids present on leukocytes (Lawrence and Springer, 1991; Bevilacqua and Nelson, 1993; Varki, 1994; McEver et al., 1995; Kansas, 1996; Luscinskas et al., 1996). E-selectin is expressed at sites of acute and chronic inflammation and on cultured endothelial cells treated with inflammatory cytokines such as interleukin (IL) -1β and tumor necrosis factor (TNF) – α (Bevilacqua and Nelson, 1993; Kansas, 1996; Dagia and Goetz, 2003). Both in vivo and in vitro studies have clearly established that E-selectin supports leukocyte tethering and rolling (Patel et al., 1995; Kulidjian et al., 2002).
Several glycoproteins can bind to E-selectin and thus could be considered ligands for E-selectin. These include P-selectin glycoprotein ligand-1 (PSGL-1) (Moore et al., 1994; Fuhlbrigge et al., 1997; Goetz et al., 1997; Zou et al., 2005), L-selectin (Patel et al., 1995; Zollner et al., 1997), CD11b/CD18 (Crutchfield et al., 2000), E-selectin ligand-1 (ESL-1) (Levinovitz et al., 1993; Steegmaier et al., 1995), CD44 (Dimitroff et al., 2001; Katayama et al., 2005; Hidalgo et al., 2007), and CD43 (Matsumoto et al., 2005; Fuhlbrigge et al., 2006). In addition, glycolipids can serve as ligands for E-selectin (Alon et al., 1995; Shirure et al., 2011). Although the potential ligands for E-selectin are numerous, it appears that for a molecule to have E-selectin binding activity it needs to be appropriately glycosylated. Indeed, only when the above-mentioned molecules are decorated with sialylated and fucosylated (sialofucosylated) oligosaccharides do they act as E-selectin ligands. These observations have given rise to the notion that the underlying lipids and proteins are scaffolds that present carbohydrates for binding to E-selectin (Sako et al., 1993). Perhaps the most well-studied carbohydrate epitope to which E-selectin binds is sLex, i.e. NeuAcα2–3Galβ1–4(Fucα1–3)GlcNAc (Tyrrell et al., 1991; Foxall et al., 1992). It is quite clear that sLex can mediate adhesive interactions with E-selectin since microspheres coated with sLex, alone, tether and roll on E-selectin (Brunk et al., 1996; Zou et al., 2005).
HECA-452 is a rat IgM monoclonal antibody (mAb) that is routinely used by investigators from diverse fields who seek to unravel the mechanisms of leukocyte adhesion (Duijvestijn et al., 1988; Berg et al., 1991a; Alon et al., 1994; De Boer et al., 1994; Wagers et al., 1996; Fuhlbrigge et al., 1997; Teraki and Picker, 1997; Knibbs et al., 1998; Wagers et al., 1998). HECA-452 was originally raised to detect antigens expressed on high endothelial venules of lymphoid organs that are supportive of peripheral blood lymphocyte invasion (Duijvestijn et al., 1988). Many subsequent investigations revealed that HECA-452 recognizes sLex and a broad class of sialofucosylated glycans [e.g. (Berg et al., 1991a)]. This recognition leads to the possibility that HECA-452 could inhibit sLex binding to E-selectin, i.e. HECA-452 could be a function-blocking mAb. The literature is conflicted regarding this issue. Several studies have shown that the presence of HECA-452 reactive epitopes on leukocytes correlates with the ability of leukocytes to adhere to E-selectin (Alon et al., 1994; De Boer et al., 1994; Fuhlbrigge et al., 1997; Teraki and Picker, 1997; Knibbs et al., 1998). In contrast, it has been observed that cells that lack the HECA-452 epitope can bind to E-selectin (Wagers et al., 1996; Wagers et al., 1998). HECA-452 does not inhibit HECA-452 positive T-lymphoblast adhesion to E-selectin under flow conditions (Knibbs et al., 1998). These observations have led to the hypothesis that HECA-452 is a sufficient, but not necessary, “marker” for the ability of a cell to bind to E-selectin (Wagers et al., 1998). Thus, the issue of whether HECA-452 is an actual function-blocking mAb for binding to E-selectin remains unclear. This is due, in part, to the fact that several E-selectin reactive moieties may be expressed on the same leukocyte leading to potentially extensive functional overlap.
Recognizing that results with leukocytes have been equivocal, we utilized a complementary approach to shed light on the above issue. Specifically, we used sLex microspheres to investigate the hypothesis that HECA-452 is a non-function blocking mAb for sLex-mediated adhesion to E-selectin under flow.
2. Materials and Methods
2.1. Reagents
Reagents for culturing human umbilical vein endothelial cells (HUVEC), bovine serum albumin (BSA), Hank’s balanced salt solution with Ca2+ and Mg2+ (HBSS+), blocking buffer (HBSS+, 1% BSA), formaldehyde and human interleukin-1β (IL-1β) have all been described previously (Dagia and Goetz, 2003). Biotinylated multivalent sLex, was purchased from Glycotech (Gaithersburg, MD). The 9.95 μm superAvidin microspheres, and Quantum FITC MESF medium level calibration beads were from Bangs Laboratories, Inc. (Fishers, IN). Vibrio cholera neuraminidase was obtained from Roche Diagnostics (Indianapolis, IN), and α (1–3, 4) fucosidase (almond meal) was obtained from Glyko (San Leandro, CA). Fluorescein isothiocyanate (FITC) conjugated biotin was obtained from Invitrogen (Carlsbad, CA).
2.2. Antibodies
Murine anti-human CD62E mAb HAE-1f was obtained from Ancell (Bayport, MN). Rat IgM HECA-452 mAb was purchased from BD Pharmingen (San Diego, CA). Purified rat IgM was obtained from Zymed (South San Francisco, CA). FITC affinity pure F(ab′)2 goat anti-rat IgM, μ chain specific was obtained from Jackson ImmunoResearch (West Grove, PA).
2.3. Cell Culture
HUVEC were obtained from Lonza (Walkersville, MD), cultured and activated with IL-1β for 4 h in 5 mm flexiPerm gaskets as described previously (Zou et al., 2005).
2.4. Generation of sLex microspheres and isolation of neutrophils
SLex microspheres were prepared as described previously (Zou et al., 2005). In brief, 9.95 μm superAvidin microspheres were washed, incubated for 30 min in blocking buffer and washed again. Subsequently, the microspheres (1×107/mL) were incubated in blocking buffer containing biotinylated sLex for 1 hour and then washed. The microspheres were resuspended to 5×105/mL in assay buffer (HBSS+, 0.5% BSA) immediately prior to perfusion through the parallel plate flow chamber. Human venous blood was obtained from normal healthy volunteers in accordance with a protocol approved by the Institutional Review Board Human Subjects Committee at Ohio University. Polymorphonuclear cells (PMNs) were isolated by Ficoll density gradient centrifugation (Sigma, St. Louis, MO) followed by hypotonic lysis of red blood cells (Zou et al., 2005). PMNs were 95.1% ± 3.9% pure as determined by forward scatter-side scatter plots in flow cytometry. PMNs were stored at 1×107/ml on ice, until use in the adhesion assays.
2.5. Flow cytometric analysis of sLex microspheres
Flow cytometric analysis of sLex microspheres was performed as described previously (Zou et al., 2005). In brief, sLex microspheres, or sLex microspheres pre-treated with enzymes, were treated with HECA-452 mAb for 20 min. Subsequently, the microspheres were washed once with blocking buffer and incubated for 20 min with an FITC-labeled anti-rat IgM polyclonal antibody. After incubation, the microspheres were washed with blocking buffer and then once with HBSS+. Finally, the microspheres were fixed in 1% formalin. All incubations were done at room temperature. A FACSort flow cytometer (BD Biosciences, San Jose, CA) or a FACSAria Special Order Research Product flow cytometer/sorter (BD Biosciences) was used to measure the FITC mean channel fluorescence (MCF) of the microspheres. The molecules of equivalent soluble fluorochrome (MESF) that corresponds to the MCF of the microspheres was determined from the calibration curve, MESF vs. MCF, developed using quantum FITC medium level calibration beads. The net MESF/microsphere was determined as described previously (Zou et al., 2005).
2.6. Flow adhesion assay
The adhesion assay, carried out at 37°C, using a parallel plate flow chamber (Glycotech, Gaithersburg, MD), was similar to that described previously (Zou et al., 2005). A 35 mm culture dish, whose center contained a confluent HUVEC monolayer, served as the bottom surface of the flow chamber. We perfused the sLex microspheres (5×105/mL) over 4 h. IL-1β activated HUVEC for 2.5 min at 1.5 dynes/cm2. Initial tethering, prior to rolling, was quantified as described previously (Zou et al., 2005). The number of microspheres or neutrophils present at the end of the perfusion period was determined for multiple fields of view and these numbers averaged to obtain the result for a given experiment. Microsphere rolling velocities were determined using Image J software (Bethesda, MD). The distance the microspheres traveled over a 5 second time period was determined and this distance used to calculate the rolling velocity. For a given experiment, the rolling velocities of 7–10 microspheres were determined and then averaged to obtain the result for that experiment.
2.7. Enzymatic treatment of sLex microspheres
For neuraminidase treatment, sLex microspheres were treated with 0.1 U/mL neuraminidase (diluted in HBSS+, 1% BSA, pH-7.4) and incubated at room temperature for 30 min. For fucosidase treatment, sLex microspheres were treated with 1 mU/mL fucosidase (diluted in HBSS+, 1% BSA, pH - 5.0) and incubated at 37°C for 24 h. In both cases, subsequent to treatment, the microspheres were washed and resuspended in blocking buffer to reach a final concentration of 1×108/mL.
2.8. Statistics
One way ANOVA with post-hoc Bonferroni multiple comparisons test was used to evaluate the difference between treatment levels. p values ≤ 0.05 were considered significantly different. All error bars represent ± standard error (SE).
3. Results
3.1. Generation and characterization of sLex microspheres
SLex microspheres were generated by conjugating biotinylated sLex to 10 μm superAvidin microspheres via avidin-biotin chemistry. The resulting sLex microspheres were characterized using HECA-452 and flow cytometric analysis. Flow cytometry revealed that HECA-452 recognized sLex microspheres, and the level of recognition increased with increasing concentrations of sLex used in the preparation of the microspheres (Fig. 1).
Figure 1.
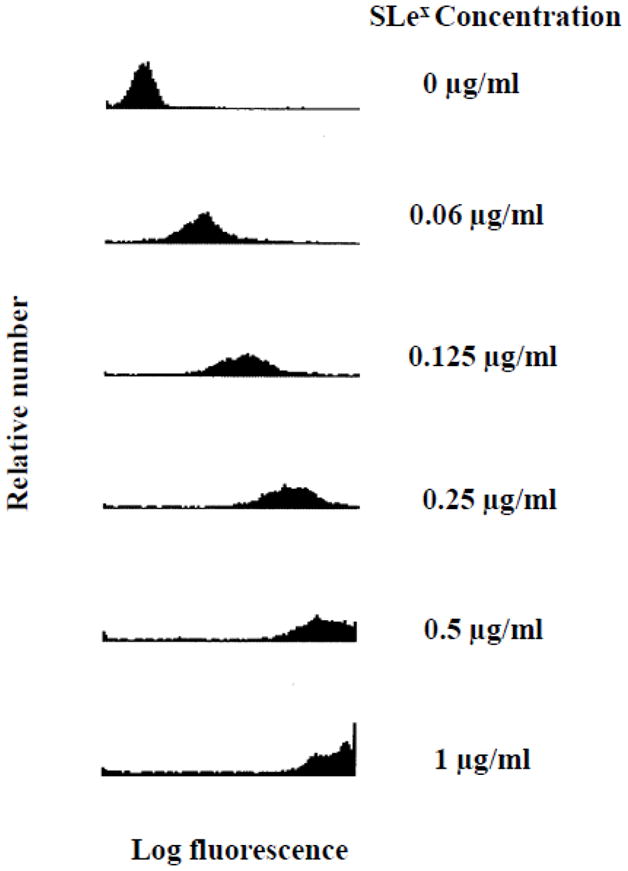
Analysis of sLex microspheres by flow cytometry. The sLex microspheres were first treated with HECA-452 and then a FITC labeled goat anti-rat IgM antibody. The histograms represent the fluorescence distribution of the population of microspheres analyzed. The log fluorescence correlates with the levels of HECA-452 epitopes bound to the sLex microspheres. The coating concentrations used to prepare the sLex microspheres are shown on the right side. The data presented represent n≥3 experiments.
To compare the sLex microspheres prepared in this work to our previous study (Zou et al., 2005), the net MESF/microsphere was estimated using a MESF vs. MCF calibration curve. Zou et al. have previously argued that sLex microspheres prepared with 0.125 μg/mL have similar HECA-452 reactivity as human neutrophils (Zou et al., 2005). In this study, we found that the HECA-452 net MESF/particle determined for sLex microspheres prepared with 0.125 μg/mL (1.1 × 105) is similar to the HECA-452 net MESF/particle determined for sLex microspheres prepared at 0.125 μg/mL in that previous study [1.2 × 105 (Zou et al., 2005)]. Additionally, a mathematical analysis of receptor – ligand binding indicated that a solution of 0.125 μg/ml sLex results in sLex microspheres that have 5 × 106 sLex molecules per microsphere which is near the surface density reported for neutrophils (107). Therefore for flow adhesion studies, unless otherwise specified, we used 0.125 μg/mL sLex to generate the sLex microspheres.
3.2. SLex microspheres tether to 4 h. IL-1β-treated HUVEC primarily through E-selectin
Zou et al. have previously shown that sLex microspheres tether to 4 h. IL-1β-treated HUVEC and the adhesion is inhibited by a mAb to E-selectin (Zou et al., 2005). We sought to determine if this result was also true for the sLex microspheres prepared for this study. Hence, we perfused sLex microspheres over unactivated HUVEC that express little, if any, E-selectin (Dagia and Goetz, 2003) and 4 h. IL-1β activated HUVEC that express significant levels of E-selectin (Dagia and Goetz, 2003). Consistent with our previous study (Zou et al., 2005), the sLex microspheres did not tether to unactivated HUVEC, but exhibited significant tethering to activated HUVEC that was inhibited by a mAb to E-selectin (Fig. 2). Zou et al. have previously shown that biotin microspheres, serving as a negative control for biotin-sLex microspheres, did not adhere to 4 h. IL-1β activated HUVEC (Zou et al., 2005). Thus, it appears that sLex microspheres adhere to 4 h. IL-1β activated HUVEC predominantly via E-selectin.
Figure 2.
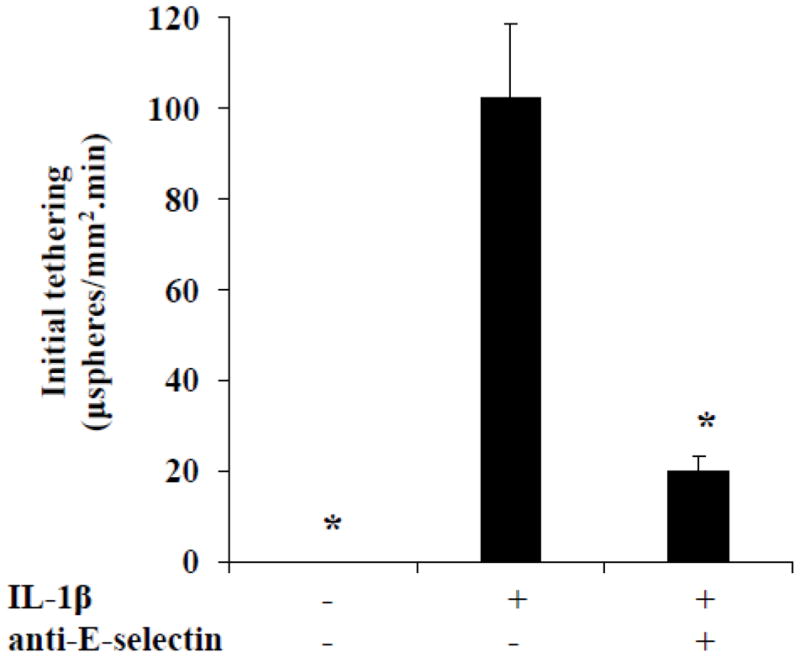
SLex microsphere initial tethering to 4 h. IL-1β activated HUVEC occurs primarily through E-selectin. Initial tethering of sLex microspheres to unactivated HUVEC, 4 h. IL-1β activated HUVEC and 4 h. IL-1β activated HUVEC pretreated with an anti-E-selectin mAb (HAE-1f) was determined. Shear stress = 1.5 dynes/cm2; *p ≤ 0.05 compared with the center bar.
3.3. Pretreatment of sLex microspheres with HECA-452 has no effect on adhesion to endothelial expressed E-selectin
With the above established, we next sought to determine the effect of HECA-452 on sLex microsphere adhesion. First, however, it was important to establish the range of HECA-452 binding to sLex microspheres using immunofluorescence and flow cytometry. We treated sLex microspheres with increasing concentrations of HECA-452. As shown in Fig. 3A, treatment of the sLex microspheres with 100 μg/ml HECA-452 resulted in saturation of the sLex microspheres. Thus, we pretreated the sLex microspheres with 100 μg/ml HECA-452 prior to the adhesion assay. As shown in Fig. 3B, 3C and 3D, HECA-452 failed to inhibit sLex microsphere initial tethering and accumulation on endothelial expressed E-selectin and did not significantly increase the rolling velocity. These results led us to try lower levels of sLex to determine if HECA-452 could block where a lesser amount of sLex was present, namely at or near the minimum sLex threshold level required to initiate tethering to HUVEC. We performed flow adhesion assays with different concentrations of sLex microspheres and determined that 0.03125 μg/mL is the minimum concentration required for the sLex microspheres to adhere to 4 h. IL-1β activated HUVEC used in this study (data not shown). We then used microspheres prepared with 0.0625 and 0.03125 μg/mL sLex and pretreated the microspheres with 200 μg/mL HECA-452. As shown in Fig. 4, HECA-452 does not inhibit the initial tethering or accumulation of sLex microspheres coated with relatively low levels of sLex and did not significantly increase the rolling velocity. This was true even at HECA-452 levels of 200 μg/mL. Combined, these results demonstrate that, although HECA-452 mAb recognizes and binds to sLex, it does not appear to block isolated sLex mediated adhesion to endothelial expressed E-selectin under flow conditions.
Figure 3.
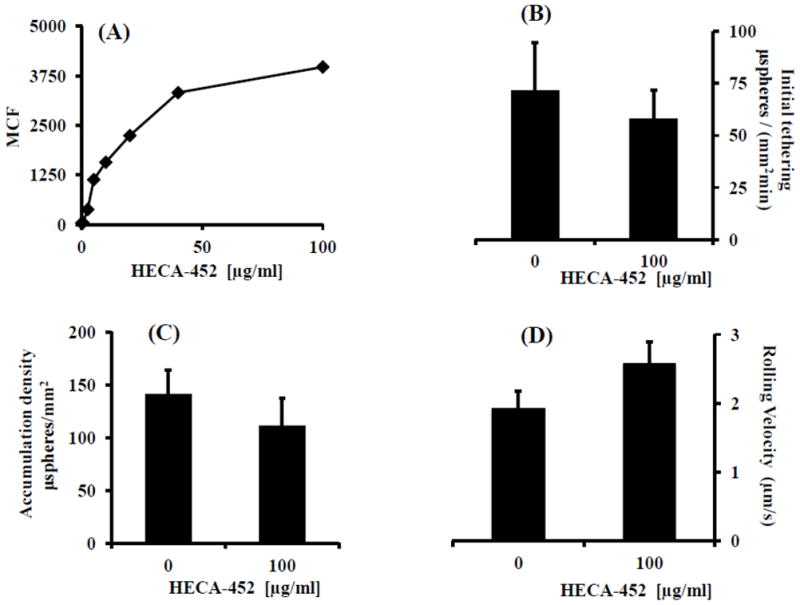
HECA-452 does not block sLex microsphere initial tethering and accumulation on IL-1β activated HUVEC and does not significantly increase the rolling velocity. (A) SLex microspheres were treated with various concentrations of HECA-452, as indicated, and then analyzed via flow cytometry. The Mean Channel Fluorescence (MCF) for the population of sLex microspheres is plotted vs. the concentration of HECA-452. (B, C and D) SLex microspheres were untreated or treated with 100 μg/ml HECA-452, as indicated, prior to the adhesion assay and perfused over 4 h. IL-1β activated HUVEC at a shear stress of 1.5 dynes/cm2. The initial tethering during the first 2.5 minutes of flow (B), the number of sLex microspheres present after the 2.5 minutes of flow (C), and the rolling velocity (D) was determined. A coating concentration of 0.125 μg/mL sLex was used for preparing the sLex microspheres; shear stress = 1.5 dynes/cm2; Results shown are the average of n=3.
Figure 4.
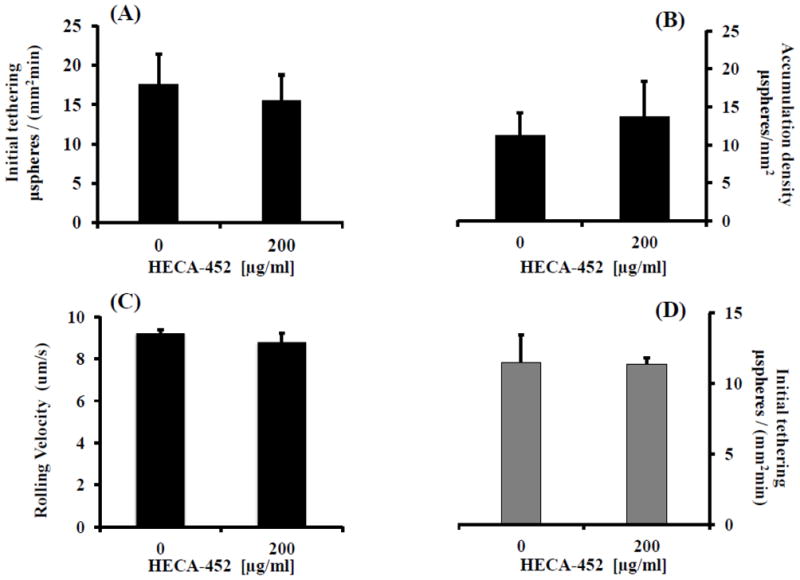
HECA-452 does not inhibit sLex microsphere, generated with a relatively low concentration of sLex, tethering and accumulation on IL-1β activated HUVEC and does not significantly increase the rolling velocity. (A) sLex microspheres generated with different concentrations of sLex [(A, B, C) 0.0625 μg/mL and (D) 0.03125 μg/mL] were either untreated or treated with HECA-452, as indicated, and perfused over 4 h. IL-1β activated HUVEC at a shear stress of 1.5 dynes/cm2. (A and D) The initial tethering during the first 2.5 minutes of flow. (B) The number of 0.0625 μg/mL sLex microspheres present after 2.5 minutes of flow. Note that accumulation data is not given for the sLex microspheres prepared with 0.03125 μg/mL sLex since there were an insignificant number of sLex microspheres present for both untreated and mAb treated conditions. (C) The rolling velocity of the 0.0625 μg/mL sLex microspheres. 0.03125 μg/mL sLex microspheres pretreated with HECA-452 did not exhibit an increase in the rolling velocity compared to untreated microspheres (data not shown). Data are the average of n ≥ 3 separate experiments.
3.4. Neuraminidase treatment of sLex microspheres abolishes both HECA-452 recognition and E-selectin binding
Since HECA-452 failed to inhibit sLex microsphere initial tethering to 4 h. IL-1β activated HUVEC, we next questioned whether the sLex binding epitopes for HECA-452 and E-selectin differ. To probe this issue, we first treated sLex microspheres with neuraminidase and then performed flow cytometric analysis to characterize HECA-452 recognition. Flow cytometric analysis revealed that treatment of sLex microspheres with neuraminidase abolishes the majority, if not all, of the HECA-452 recognition (Fig. 5 A). We then perfused sLex microspheres pretreated with neuraminidase over 4 h. IL-1β activated HUVEC. The neuraminidase treated sLex microspheres exhibited significantly diminished tethering to endothelial expressed E-selectin under flow conditions (Fig. 5 B). Combined, these results suggest that a sialic acid [or neuraminidase-sensitive site(s)] on sLex is necessary for both HECA-452 recognition and E-selectin binding.
Figure 5.
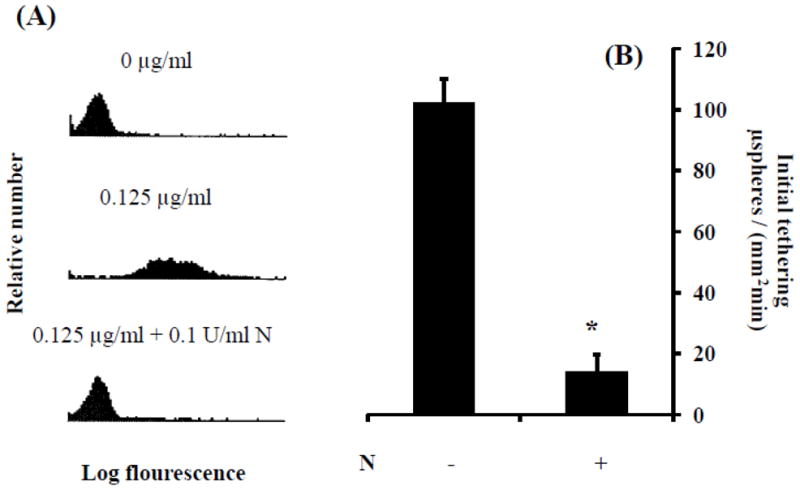
Neuraminidase treatment of sLex microspheres eliminated both HECA-452 recognition of sLex microspheres and adhesion to E-selectin. (A) Levels of HECA-452 reactive epitopes on sLex microspheres, with and without neuraminidase treatment, were analyzed by flow cytometry. Top panels - microspheres prepared with no sLex and not treated with neuraminidase; Middle panels-microspheres prepared with 0.125 μg/ml multivalent sLex and not treated with neuraminidase; Bottom panels-microspheres prepared with sLex and treated with neuraminidase (0.1 U/ml). (B) SLex microspheres, with and without neuraminidase treatment 30 min prior to the adhesion assay, were perfused over 4 h. IL-1β activated HUVEC at a shear stress of 1.5 dynes/cm2. N indicates neuraminidase treatment; *p≤0.05; n≥2.
3.5. HECA-452 recognition of sLex microspheres is less dependent on fucose than sLex microsphere adhesion to E-selectin
There is substantial evidence that sLex binding to E-selectin depends on the presence of fucose as well as sialic acid (Nelson et al., 1993). Thus, we next probed the role of fucose in HECA-452 recognition and E-selectin binding. Flow cytometric analysis of sLex microspheres treated with fucosidase revealed that fucose removal had a slight inhibitory effect on HECA-452 recognition (Fig. 6 A). In contrast, pretreatment of sLex microspheres with fucosidase had a relatively robust inhibitory effect on sLex microsphere initial tethering to endothelial expressed E-selectin (Fig. 6 B). Combined, these results suggest that E-selectin adhesion via sLex is more dependent on the presence of fucose than HECA-452 recognition of sLex.
Figure 6.
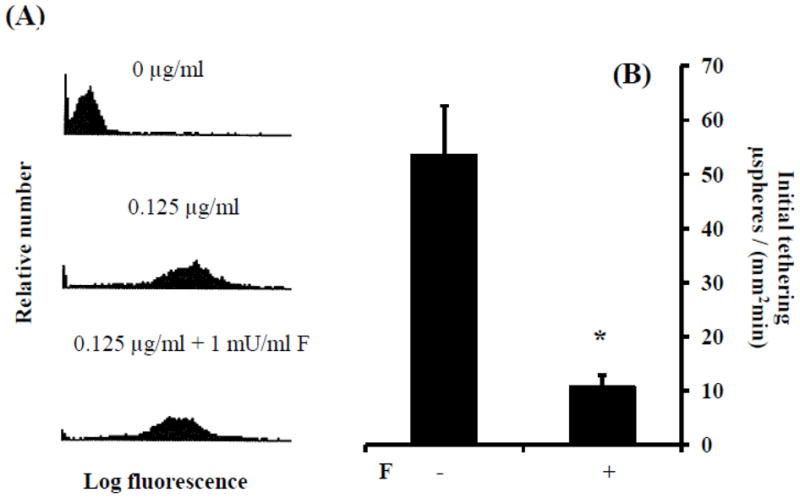
Fucosidase treatment of sLex microspheres had a slight inhibitory effect on HECA-452 recognition of sLex microspheres but a relatively robust inhibitory effect on sLex microsphere adhesion to E-selectin. (A) Levels of HECA-452 reactive epitopes on sLex microspheres, with and without fucosidase treatment, were analyzed by flow cytometry. Top panels - microspheres prepared with no sLex and not treated with fucosidase; Middle panels - microspheres prepared with 0.125 μg/ml sLex and not treated with fucosidase; Bottom panels - microspheres prepared with sLex and treated with fucosidase (0.1 U/ml). (B) SLex microspheres, treated with and without fucosidase 24 hr prior to the adhesion assay, were perfused over 4 hr. IL-1β activated HUVEC at a shear stress of 1.5 dynes/cm2. F indicates fucosidase treatment; *p≤0.05; n ≥4.
4. Discussion
In the present study we have demonstrated that HECA-452 mAb recognizes and binds to sLex microspheres (Fig. 1) but does not appear to block sLex microsphere initial tethering or accumulation on 4 h. IL-1β activated HUVEC under flow conditions and does not appear to significantly increase the rolling velocity (Figs. 3 and 4). Interestingly, for microspheres generated with a higher level of sLex (Fig. 3), each of our adhesion measurements (attachment, accumulation and rolling velocity), did reveal a slight, but insignificant, decrease in adhesion with treatment with HECA-452. That said, we did not observe this trend with the microspheres generated with a lower level of sLex (Fig. 4). Thus, in aggregate, our data support the hypothesis that HECA-452 is a non-function blocking antibody for isolated sLex adhesion to endothelial expressed E-selectin under flow conditions.
Our finding that HECA-452 does not block sLex microsphere adhesion is surprising since HECA-452 recognizes and binds to sLex on the microspheres and the size of sLex (820 Da) is small compared to the size of HECA-452 (~750 kDa). However, this observation is in line with previous studies which have shown that: (i) HECA-452 does not block HECA-452 (+) T cell binding to E-selectin (Teraki and Picker, 1997; Knibbs et al., 1998), (ii) HECA-452 does not block CHO-E cell binding to HECA-452 (+) PSGL-1 in an immunoblot flow assay (Fuhlbrigge et al., 2002) and (iii) HECA-452 recognizes PSGL-1 coated microspheres and does not block PSGL-1 microsphere initial tethering to E-selectin under similar conditions used in this study (Zou et al., 2005). In addition, we have found that pre-treatment of freshly isolated human neutrophils with HECA-452 does not inhibit neutrophil accumulation on 4 h. IL-1β activated HUVEC (Fig. 7).
Figure 7.
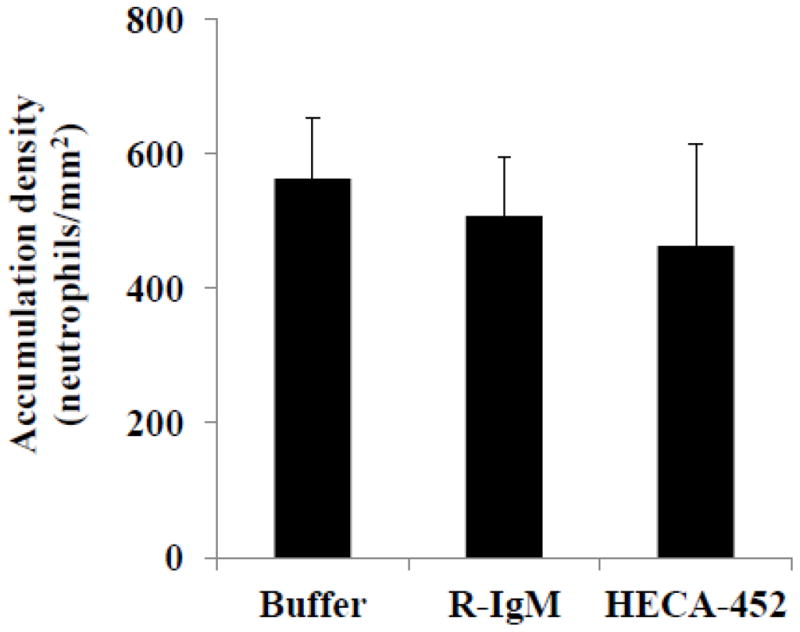
HECA-452 does not block neutrophil accumulation on 4 hr. IL-1β activated HUVEC. Freshly isolated human neutrophils were untreated, treated with rat IgM or treated with 100 μg/ml HECA-452, as indicated, prior to the adhesion assay and perfused over 4 h. IL-1β activated HUVEC for 2.5 minutes. The number of adherent neutrophils was determined after the perfusion period and normalized to the area of the field of view. Shear stress = 1.5 dynes/cm2; Results shown are the average of n=3.
The inability of HECA-452 to block adhesion raises the question of whether the HECA-452 and E-selectin binding sites differ. It is known that a critical characteristic feature of E-selectin ligands is the presence of sialofucosylated oligosaccharides (Varki, 1994). It is also known that HECA-452 recognizes sialofucosylated oligosaccharides such sLex and sLea (Berg et al., 1991a). Thus HECA-452 and E-selectin can bind to the same carbohydrate. However, they may not bind at the same epitope, and thus HECA-452 may not block E-selectin ligand function when used in adhesion assays. The results presented in Figs. 5 and 6 demonstrate that the sialic acid of sLex is required for both HECA-452 recognition and E-selectin binding, and that the fucose moiety is required for E-selectin binding but does not appear to be as important for HECA-452 recognition. The finding that sialic acid and fucose are required for E-selectin binding is consistent with several studies in which it has been reported that treatment of rat PMN (Misugi et al., 1995), HL-60 cells (Larsen et al., 1992) and PSGL-1 microspheres (Zou et al., 2005) with neuraminidase eliminates the binding of PMN, HL60 and PSGL-1 microsphere adhesion to E-selectin, and treatment of HL-60 cells (Larsen et al., 1992) and the HCELL glycoform of CD44 (Dimitroff et al., 2001) with fucosidase diminishes E-selectin binding activity. In regards to fucose, it has been shown that neutrophils in fucosyltransferase VII (Fuc T-VII) deficient mice, exhibit reduced E-selectin dependent rolling during inflammation in vivo (Sperandio, 2006). The finding that sialic acid is required for HECA-452 recognition is consistent with several studies in which it has been reported that HECA-452 does not bind to neuraminidase treated PSGL-1 microspheres (Zou et al., 2005) and CLA+ T cells (Berg et al., 1991b). Even though HECA-452 recognizes sialofucosylated oligosaccharides, it appears that HECA-452 recognition of sLex does not depend as much on fucose as E-selectin recognition of sLex (Fig. 6). This difference could underlie HECA-452’s inability to block sLex mediated adhesion to endothelial expressed E-selectin under flow conditions – our results suggest that the epitopes are not identical. Interestingly, computational models of sLex binding to E-selectin, show that the fucose of sLex forms stabilizing hydrogen bonds with the amino acids in the Ca2+ binding site of the carbohydrate recognition domain of E-selectin (Ishida, 2010).
The above thoughts aside, a second possible reason for the lack of inhibition by HECA-452 arises from physical considerations. HECA-452 is an IgM which is a pentamer of IgG. An IgG molecule covers a surface area of 60 nm2 (Crowther, 2000) and thus the surface area occupied by a HECA-452 molecule is 300 nm2. Approximately 1 × 106 HECA-452 molecules, occupying an area of ~300 nm2, could fit onto a 10 μm microsphere, assuming each HECA-452 lies flat to the microsphere surface. Since HECA-452 is a pentamer of IgG, and each IgG is divalent, there is the possibility of HECA-452 being decavalent. These considerations give a possible upper “steric” limit to the number of sLex moieties that can be bound by HECA-452. That limit is 1 × 106 for univalent to 1 × 107 for decavalent HECA-452 binding. It is informative to compare these numbers to the number of sLex moieties present on the microspheres which we estimate to be ~5 × 106 and ~1 × 106 for microspheres prepared with 0.125 and 0.03125 μg/ml sLex, respectively. Comparing the sLex numbers (~5 × 106 and ~1 × 106) to the “steric” limit numbers presented above (1 × 106 to 1 × 107) reveals that: (i) if HECA-452 binding is decavalent, “steric” limitations should be less of an issue, (ii) if HECA-452 binding is less than pentavalent, “steric” limitations would be an issue for the sLex microspheres prepared with 0.125 μg/ml, and (iii) if HECA-452 binding is univalent, “steric” limitations could be an issue for the sLex microspheres prepared with 0.03125 μg/ml sLex as well. Thus, there could be a physical effect, i.e. a “steric” effect, which limits the ability of HECA-452 to inhibit sLex-mediated adhesion to E-selectin.
5. Conclusion
In summary, our results demonstrate that the widely used mAb HECA-452 recognizes and binds to sLex but does not block isolated sLex-mediated adhesion to 4 h. IL-1β-activated HUVEC under flow conditions. We have identified two factors that may contribute to the lack of inhibition: (i) subtle differences in the binding epitopes of sLex for E-selectin and HECA-452 [a biochemical factor], and (ii) “steric” limitations on the number of HECA-452 molecules that can bind to a sLex particle [a physical factor].
Acknowledgments
This work was supported by The National Institutes of Health [Grant number GM57640 (DJG)] and The National Science Foundation [Grant number 1039869 (DJG, MMB, DFJT)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon R, Feizi T, Yuen CT, Fuhlbrigge RC, Springer TA. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J Immunol. 1995;154:5356–66. [PubMed] [Google Scholar]
- Alon R, Rossiter H, Wang X, Springer TA, Kupper TS. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J Cell Biol. 1994;127:1485–95. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991a;266:14869–72. [PubMed] [Google Scholar]
- Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991b;174:1461–6. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91:379–87. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk DK, Goetz DJ, Hammer DA. Sialyl Lewis(x)/E-selectin-mediated rolling in a cell-free system. Biophys J. 1996;71:2902–7. doi: 10.1016/S0006-3495(96)79487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther JR. The ELISA guidebook. Methods Mol Biol. 2000;149:III–IV. 1–413. doi: 10.1385/1592590497. [DOI] [PubMed] [Google Scholar]
- Crutchfield KL, Shinde Patil VR, Campbell CJ, Parkos CA, Allport JR, Goetz DJ. CD11b/CD18-coated microspheres attach to E-selectin under flow. J Leukoc Biol. 2000;67:196–205. doi: 10.1002/jlb.67.2.196. [DOI] [PubMed] [Google Scholar]
- Dagia NM, Goetz DJ. A proteasome inhibitor reduces concurrent, sequential, and long-term IL-1 beta- and TNF-alpha-induced ECAM expression and adhesion. Am J Physiol Cell Physiol. 2003;285:C813–22. doi: 10.1152/ajpcell.00102.2003. [DOI] [PubMed] [Google Scholar]
- De Boer OJ, Horst E, Pals ST, Bos JD, Das PK. Functional evidence that the HECA-452 antigen is involved in the adhesion of human neutrophils and lymphocytes to tumour necrosis factor-alpha-stimulated endothelial cells. Immunology. 1994;81:359–65. [PMC free article] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–86. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CJ, Butcher EC. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–55. [PMC free article] [PubMed] [Google Scholar]
- Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, Hasegawa A, Asa D, Brandley BK. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J Immunol. 2002;168:5645–51. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–6. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz DJ, Greif DM, Ding H, Camphausen RT, Howes S, Comess KM, Snapp KR, Kansas GS, Luscinskas FW. Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P- and E-selectin. J Cell Biol. 1997;137:509–19. doi: 10.1083/jcb.137.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–89. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T. Computational modeling of carbohydrate-recognition process in E-selectin complex: structural mapping of sialyl Lewis X onto ab initio QM/MM free energy surface. J Phys Chem B. 2010;114:3950–64. doi: 10.1021/jp905872t. [DOI] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–9. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–15. [PubMed] [Google Scholar]
- Kulidjian AA, Issekutz AC, Issekutz TB. Differential role of E-selectin and P-selectin in T lymphocyte migration to cutaneous inflammatory reactions induced by cytokines. Int Immunol. 2002;14:751–60. doi: 10.1093/intimm/dxf045. [DOI] [PubMed] [Google Scholar]
- Larsen GR, Sako D, Ahern TJ, Shaffer M, Erban J, Sajer SA, Gibson RM, Wagner DD, Furie BC, Furie B. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J Biol Chem. 1992;267:11104–10. [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Levinovitz A, Muhlhoff J, Isenmann S, Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993;121:449–59. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas FW, Ding H, Tan P, Cumming D, Tedder TF, Gerritsen ME. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J Immunol. 1996;157:326–35. [PubMed] [Google Scholar]
- Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042–50. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–8. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- Misugi E, Kawamura N, Imanishi N, Tojo SJ, Morooka S. Sialyl Lewis X moiety on rat polymorphonuclear leukocytes responsible for binding to rat E-selectin. Biochem Biophys Res Commun. 1995;215:547–54. doi: 10.1006/bbrc.1995.2499. [DOI] [PubMed] [Google Scholar]
- Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994;269:23318–27. [PubMed] [Google Scholar]
- Nelson RM, Dolich S, Aruffo A, Cecconi O, Bevilacqua MP. Higher-affinity oligosaccharide ligands for E-selectin. J Clin Invest. 1993;91:1157–66. doi: 10.1172/JCI116275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Moore KL, Nollert MU, McEver RP. Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest. 1995;96:1887–96. doi: 10.1172/JCI118234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–86. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Shirure VS, Henson KA, Schnaar RL, Nimrichter L, Burdick MM. Gangliosides expressed on breast cancer cells are E-selectin ligands. Biochem Biophys Res Commun. 2011;406:423–9. doi: 10.1016/j.bbrc.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. Febs J. 2006;273:4377–89. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–20. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- Teraki Y, Picker LJ. Independent regulation of cutaneous lymphocyte-associated antigen expression and cytokine synthesis phenotype during human CD4+ memory T cell differentiation. J Immunol. 1997;159:6018–29. [PubMed] [Google Scholar]
- Tyrrell D, James P, Rao N, Foxall C, Abbas S, Dasgupta F, Nashed M, Hasegawa A, Kiso M, Asa D, et al. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci U S A. 1991;88:10372–6. doi: 10.1073/pnas.88.22.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994;91:7390–7. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Lowe JB, Kansas GS. An important role for the alpha 1,3 fucosyltransferase, FucT-VII, in leukocyte adhesion to E-selectin. Blood. 1996;88:2125–32. [PubMed] [Google Scholar]
- Wagers AJ, Stoolman LM, Craig R, Knibbs RN, Kansas GS. An sLex-deficient variant of HL60 cells exhibits high levels of adhesion to vascular selectins: further evidence that HECA-452 and CSLEX1 monoclonal antibody epitopes are not essential for high avidity binding to vascular selectins. J Immunol. 1998;160:5122–9. [PubMed] [Google Scholar]
- Zollner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes HG, Vestweber D. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol. 1997;136:707–16. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Shinde Patil VR, Dagia NM, Smith LA, Wargo MJ, Interliggi KA, Lloyd CM, Tees DF, Walcheck B, Lawrence MB, Goetz DJ. PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. Am J Physiol Cell Physiol. 2005;289:C415–24. doi: 10.1152/ajpcell.00289.2004. [DOI] [PubMed] [Google Scholar]


