Abstract
Helicobacter pylori infection is associated with severe chronic inflammation, yet the host immune response is rarely able to clear the bacterium. Thymus derived lymphocyte populations such as T helper 1, T helper 17, and T regulatory cells are known to play important roles in the chronicity of H. pylori infection as well as contributing to ongoing gastric pathology. It is yet to be established how these immune cell populations interact in the gastric environment during H. pylori infection. Mouse models of infection offer an opportunity to investigate these interactions in detail. Flow cytometric analysis provides excellent lymphocyte characterization due to its high specificity, sensitivity and potential to perform multiple simultaneous measurements. However, this requires a viable enriched single cell suspension after adequate tissue dissociation, which poses a challenge due to the heterogeneity of gastric tissue. We have evaluated several isolation techniques and have optimized a protocol to isolate and enrich lymphocytes from the H. pylori-infected murine stomach. EDTA/DTT followed by collagenase IV digestion successfully dissociates an average of 1 × 107 cells per mouse. Further enrichment using lympholyte M gradient yields on average 4 × 106 CD45+ lymphocytes per stomach. Following isolation we compared lymphocyte stimulation by CD3/CD28, phorbol 12-myristate 13-acetate (PMA) and ionomycin or H. pylori lysate and determined that CD3/CD28 effectively induces stimulation of IFNγ and IL 17A, but impairs Foxp3 expression. Using an optimized protocol we observed a 2-fold increase of CD8+ IFNγ-expressing lymphocytes localized specifically to the gastric compartment during H. pylori infection. The mechanisms of H. pylori immunopathogenesis are still considered enigmatic, therefore this optimized protocol can help delineate further novel immune cell targets that mediate H. pylori-induced pathology and identify the correlates of immunity for vaccine development.
1. Introduction
Helicobacter pylori (H. pylori) is a gram negative bacterium that colonizes the gastric mucosa, eliciting a chronic gastric mucosal inflammatory response that contributes to the pathogenesis of several diseases, including gastric cancer (Kim et al., 2011). The immune response includes both innate and adaptive arms and multiple cellular populations. T helper lymphocytes, such as T-helper 1, T-helper 17 and T-regulatory cells are considered to be critical for both H. pylori clearance and in the development of the adverse sequelae of infection including chronic gastritis, peptic ulcer disease and gastric carcinogenesis (Wilson and Crabtree, 2007). Improved understanding of these cellular populations is dependent upon their successful isolation and identification from the gastric mucosa from suitable rodent models of H. pylori-infection.
Using multi-colored flow cytometry to immunophenotype a wide-range of leukocyte subsets is of considerable value when working with a limited number of cells. However, isolating the relatively small number of leukocytes from the murine gastric compartment is challenging due to the heterogeneity of the tissue and the abundance of connective and muscular fibers. Previous investigations have therefore employed a variety of techniques, including many types of mechanical or enzymatic dissociation, including the use of pronase, dispase II, and collagenase I, II, IV and VIII (Kurokawa et al., 1975; Algood et al., 2007). While mechanical processing may release many cells from the submucosa, intraepithelial lymphocytes may be missed (Alderuccio et al., 1995). Enzymatic treatments have been used extensively for tissues that are difficult to mechanically process, such as intestine, salivary glands and heart (Lefrancois and Lycke, 2001). However, vigorous enzymatic digestion substantially damages cellular surface proteins (Van Damme N et al., 2000). Thus, excessive digestion and mechanical processing may jeopardize the integrity and viability of the cell types of interest and as a result, incorrectly characterize gastric immune cell populations. Due to the heterogeneity of the tissue within the gastrointestinal tract, density gradients such as Percoll have been commonly employed to enrich for lymphocyte populations (Zavros et al., 2000). Other gradients are now available that may provide improved lymphocyte enrichment without sacrificing cell yields.
Ex vivo stimulation of lymphocytes to identify specific lymphoid subsets may also alter the cellular read-out. Consequently, the results obtained may not necessarily duplicate the immune cell phenotype in the environment of the host. Stimulation techniques commonly in use include T-cell receptor activation with anti-CD3 and anti-CD28 antibodies or chemical stimulation using the protein kinase C activator, phorbol 13-myristate 18-acetate (PMA) and the calcium ionopore, ionomycin (IONO). More biologically relevant stimuli applied to activate and phenotype lymphocytes include concanavalin A (ConA) and lipopolysaccharides (LPS), and in the context of H. pylori infection, H. pylori lysate. To better understand the immune environment during H. pylori infection it is essential to determine whether the cell phenotypes obtained in flow cytometric studies are modulated by the ex vivo stimulation protocol or whether they provide a true reflection of the cells present in the gastric niche.
We present a comparative analysis of isolation techniques used to extract immune cells from H. pylori-infected mouse stomachs and the effects of ex vivo stimulation on lymphocyte phenotypes. From these analyses, we have developed a protocol for successfully isolating a relatively large numbers of viable gastric lymphocytes that can be used for ex vivo experiments and subsequent immunophenotyping. This protocol is effective in investigating the cells types involved in H. pylori-mediated immunopathogenesis and determining how H. pylori infections alter gastric lymphocyte populations and functions.
2. Materials and methods
2.1 Bacteria
Helicobacter pylori (H. pylori) pre-mouse Sydney strain 1 (PMSS1) provided by Dr. M. R. Amieva (Department of Microbiology and Immunology and Pediatrics, Stanford University School of Medicine, Stanford, California) was used for this study due its increased pathogenic potential compared to the commonly used mouse-adapted H. pylori laboratory strain, SS1. H. pylori PMSS1 can successfully translocate the Cag A protein into epithelial cells and induce severe gastritis and pre-cancerous gastric lesions in mice as early as 3 months post-infection (Arnold et al., 2011). H. pylori PMSS1 was cultured on Brucella agar supplemented with 5% sheep blood in a microaerophilic humidified atmosphere at 37°C. H. pylori lysates were prepared as described (Sommer et al., 2004).
2.2 Animals and Infection
Six to eight week old male specific pathogen free C57BL/6 mice (Jackson Labs, Bar Harbor, ME) were gavaged with 109 colony forming units of PMSS1 H. pylori suspended in 100µl of Brucella Broth with 20% glycerol 3 times over a span of 5–7 days. Uninfected controls were gavaged with Brucella Broth with 20% glycerol alone. Animals were kept in microisolator cages and fed Harlan Teklan Global Diet 2018 (Indianapolis, IN) ad libitum. Experiments were conducted in accordance with institutional guidelines for animal care.
2.2 Harvesting of spleen and mesenteric lymph nodes
Mice were euthanized 10 ± 2 weeks post infection by isoflourane inhalation. The spleen, mesenteric lymph nodes and stomach were quickly removed. Splenic and mesenteric lymph node single cell suspensions were prepared by grinding and filtering tissues through a 70µm diameter nylon mesh (BD Bioscience). Splenic lymphocytes were treated with ammonium chloride to lyse erythrocytes and washed twice in RPMI 1640 complete medium (RPMI 1640 supplemented with L-Glutamine 200mM Solution (Lonza), 10% heat-inactivated FBS, 50 µM 0.2µm filter sterilized 2-Mercaptoethanol, 10,000 U/ml penicillin and 10,000 µg/ml streptomycin (Lonza). Cells isolated from the mesenteric lymph nodes were resuspended in RPMI complete medium after filtration. Viable cells were detected by trypan blue exclusion and counted with a hemocytometer.
2.3 Enzymatic processing of gastric tissue
Stomachs were excised and opened along the greater curvature. Gastric contents were removed by washing thoroughly with RPMI 1640 supplemented with L-glutamine and 5% FBS. The gastric tissues were then either left undigested or enzymatically processed using either one of three treatments (i) Dispase II (Sigma Aldrich), (ii) collagenase IV (Worthington), or (iii) EDTA/DTT plus collagenase IV. For Dispase digestion the tissue was incubated with 1 mg/ml dispase and 200 ug/ml DNase I (Sigma-Aldrich) in 10mls of RPMI for 20 minutes. For collagenase IV treatment, contents were digested in 0.5 mg/ml of collagenase IV and 200 ug/ml DNase I in 10 ml of RPMI. Samples that underwent EDTA/DTT and subsequent collagenase IV digestion were first placed in 10 ml of a 1mM EDTA and 1mM DTT Hank balanced salt solution without calcium and magnesium supplemented with 2% FBS and incubated for 30 minutes. Following EDTA/DTT digestion, contents in the digestion mix were passed through a 40µm filter and spun down at 1500 rpms to collect intraepithelial lymphocytes. Cells were washed twice with RPMI 1640 supplemented with L-glutamine and 5% FBS and place on ice. The remainder of the undigested gastric mucosa was scraped to separate the lamina propria from the muscular layer using a glass slide. Tissue that was still intact was cut into 1mm pieces. Both scraped contents and the tissue that was cut into smaller pieces was placed in collagenase IV/Dnase digestion mix (0.5 mg/ml of collagenase IV, 200ug/ml of DNase I suspended in wash buffer) for an additional 30 minutes. For each digestion procedure, samples were incubated at a temperature of 37°C in a volume of 5ml shaking at a speed of 250rpm. After each treatment, cellular isolates were filtered through 40µm diameter nylon mesh (BD Bioscience), washed twice in wash buffer and suspended in 5mls of RPMI supplemented with 5% FBS and kept on ice. Both intraepithelial and lamina propria lymphocyte populations were then combined for subsequent experimental steps. To test if these procedures diminished receptor detection efficiency, mesenteric lymph nodes underwent the same digestion treatments as described above. (Data not shown).
2.4 Enrichment of leukocyte population via density gradient centrifugation
For further purification of leukocytes, gastric cell suspensions were subjected to a 20% 40% 70% discontinuous Percoll (GE lifescience) or Lympholyte M gradient (Cedarlane). For the Percoll gradient a 1ml suspension of gastric cells was mixed with 1ml of 40% Percoll solution to obtain a 20% Percoll solution and overlayed on a 40%, 70% Percoll gradient. For Lympholyte M, cells were first resuspended in 5mls PBS and then placed on a lympholyte M overlay in a 1:1 ratio. Cells were spun at 2,200rpm for 20 minutes, collected from the 40% 70% Percoll or PBS/Lympholyte M interface, washed and suspended in RPMI complete media.
2.5 Primary culture preparation and lymphocytes staining
To induce cytokine production, gastric leukocytes were cultured for 10 hours in RPMI complete media containing a final concentration of 3.0 µg/ml brefeldin A (eBioscience) and one of the following stimuli: (1) 10µg/ml CD3/CD28 (BD Bioscience), (2) 50ng/ml phorbol 12-myristate 13-acetate (PMA) and 500ng/ml ionomyocin (Sigma-Aldrich) or (3) 50 µg/ml of H. pylori PMSS1 lysate. After stimulation, cells were washed in flow cytometery buffer (eBioscience) and immunophenotyped by flow cytometry using the following antibodies: CD8-APC-780, APC-CD25, PE-Foxp3, IFNγ-eflour450-IL-17A-Percp Cy5.5, IL-17A –PE (all from eBioscience), CD45-FITC, CD3-V500 (both from BD Biosciences) and pacific orange-CD4 (Invitrogen). For surface and intracellular staining, cells were first incubated with surface antibodies for 25 minutes at 4 °C and subsequently fixed with fix/perm and stained in perm/wash buffer for 25 minutes at 4°C (eBioscience), according to the manufacturer’s instructions.
2.6 Analysis of lymphocyte subsets
All cells were acquired on a FACSAria (BD Bioscience) and analyzed using FlowJo software (version 9.3.2; Tree Star Inc.).
3. Results and Discussion
3.1 Quantification of gastric leukocytes after enzymatic treatment
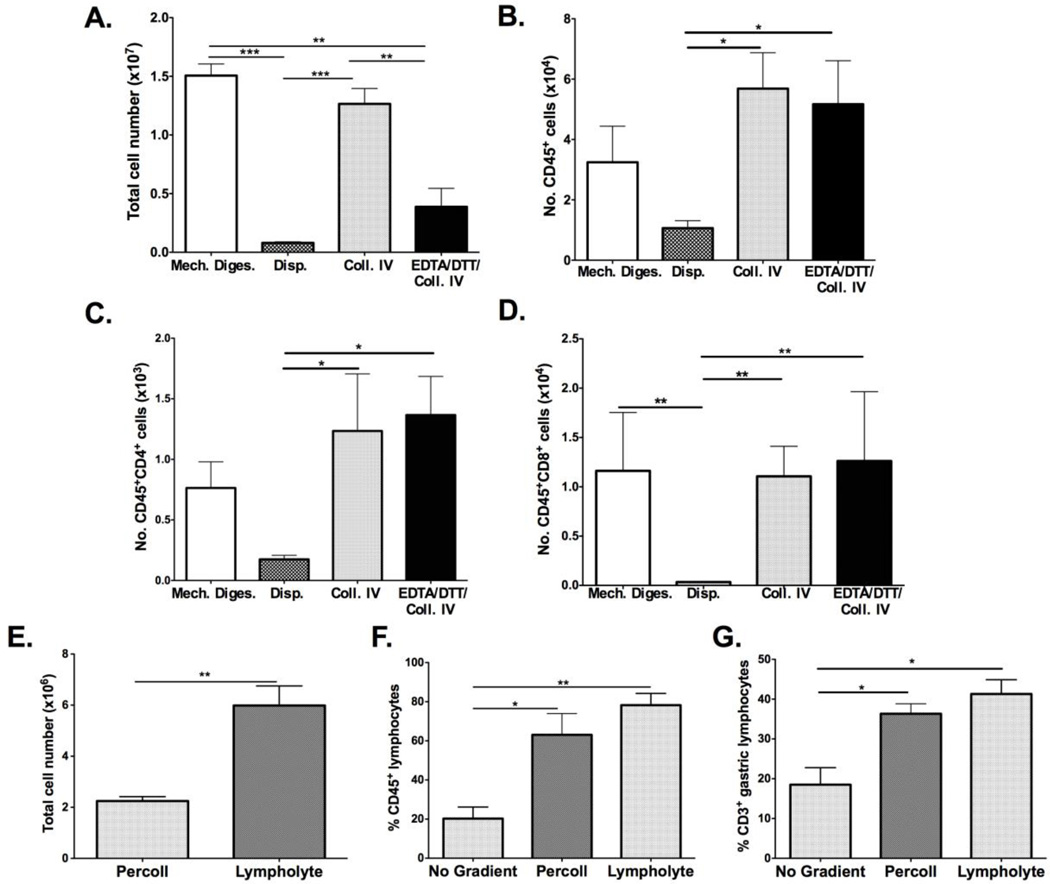
Several types of enzymatic treatments have been used to dissociate gastric tissue and liberate resident leukocytes, but the optimal methodology remains to be defined. To compare among the previous methods of isolating gastric leukocytes, stomachs of H. pylori PMSS1-infected mice were subjected to dispase II, collagenase IV, EDTA/DTT/collagenase IV dissociation or solely mechanical processing. Mechanical processing yielded the most total cells with a mean of 1.50 × 107 ± 0.17 × 107 per stomach while the number of cells isolated by enzymatic digestion ranged from 8.0 × 105 to 1.25 × 107. (Figure 1A) Mean yields using Dispase II, collagenase IV and collagenase IV/EDTA/DTT yields were 8.0 × 105 cells, 1.25 × 107 cells and 3.9 × 106 respectively. (Figure 1A) These numbers are consistent with previous reports of mechanical dissociation producing greater cell yields compared to enzymatic methods (Zavros et al., 2000). Enumerating specifically on the CD45high gate yielded a greater number of CD45+ cells after collagenase IV and collagenase IV/EDTA/DTT digestion compared to dispase II digestion or mechanical processing alone. (Figure 1B) Similarly, CD4+ and CD8+ lymphocyte numbers were greater with collagenase IV and collagenase IV/EDTA/DTT treatments compared to tissue digested with dispase II. (Figures 1C–D)
Figure 1. Comparison of strategies to extract and enrich gastric leukocytes.
Gastric tissue from H. pylori infected mice were subjected to mechanical processing, Dispase II (Disp.), Collagenase IV (Coll. IV) or Collagenase IV plus EDTA/DTT digestion (EDTA/DTT/Coll. IV). Mechanically dissociated tissue and collagenase IV digested tissue released the greatest number of total cells, while dispase treatment resulted in the fewest (A). The total number of CD45+ cells was highest after collagenase IV and collagenase IV/EDTA/DTT treatment (B). CD4+ cell numbers are significantly higher with collagenase IV and collagenase IV/EDTA/DTT treatment (C). CD8+ cell number was greatly decreased with dispase digestion while collagenase IV with EDTA/DTT digestion yielded the greatest amount of CD8+ cells (D). Lymphocyte enrichment was performed using a discontinuous Percoll or lympholyte M gradient (E). More cells were obtained using lympholyte M enrichment and greater percentages of CD45+ and CD3+ cells were also obtained using this gradient. Graphs shown represent 3 mice per group and are presented as mean ± standard error. Groups were compared using one-way analysis of variance (ANOVA) followed post-hoc by Tukey multiple comparison tests on log-transformed data. Significant differences are represented by the following *p<0.05, **p<0.01, ***p<0.001. Data shown are representative of 3 independent experiments performed on 3 occasions in total.
In order to determine if enzymatic treatment decreased yields of CD4+ and CD8+ cells through receptor cleavage, leukocytes were isolated from mesenteric lymph nodes and subjected to dispase II, collagenase IV, or collagenase IV/EDTA/DTT treatment and compared to cells derived from mesenteric lymph nodes that were not enzymatically processed. CD45+ leukocyte cell number and percentage remained unchanged in mesenteric lymph nodes cells treated with all three digestions. However, there was nearly a 2-fold decrease in CD4+ and CD8+ percentages and absolute cell numbers with dispase treatment compared to other enzymatic treatments and mechanical processing (data not shown).
These results show that although total cell yields were greatest after mechanical dissociation, the numbers of CD45+ leukocytes were higher following enzymatic digestion. The data also demonstrate that dispase treatment is not advisable for isolating gastric T-lymphocytes, due to the decreased yields of CD4+ and CD8+ populations. The use of EDTA/DTT prior to collagenase IV digestion increases the CD8+ population, most likely through releasing CD8+ cells from the intra-epithelial compartment, and thus should be used prior to collagenase IV digestion to successfully extract all lymphocyte populations resident in the H. pylori-infected murine stomach.
3.2 Enrichment of gastric lymphocytes using Percoll and Lympholyte M gradients
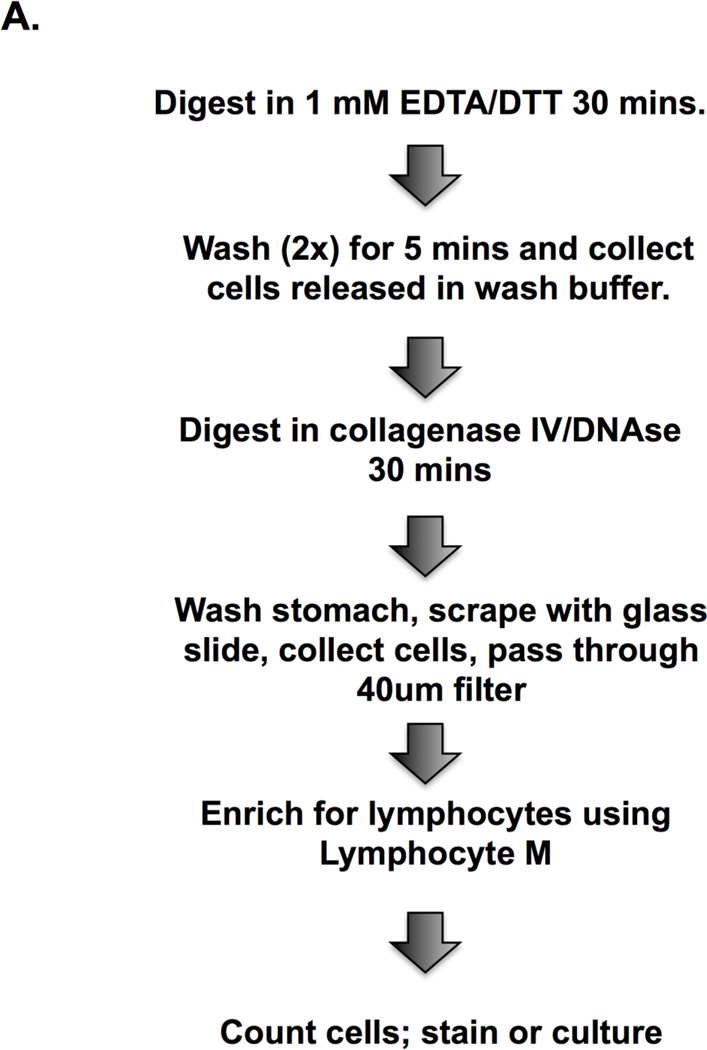
Gradients are used to enrich for specific immune cell populations and to decrease the number of non-viable cells. Gastric lymphocytes have generally been enriched using a discontinuous Percoll gradient but in some instances gradients have been avoided to retain a sizable cell yield for downstream analysis (Hitzler et al., 2011). We compared a Percoll gradient and a density gradient composed of polysucrose 400 and sodium diatrizoate, (Lympholyte M), and assessed total cell yields and the yields of total number of CD4+ and CD8+ lymphocytes. These enrichment steps decrease total cell numbers, as shown previously (Zavros et al., 2000), but increase the frequency and number of CD45+ and CD3+ cells. (Figures 1E–G) The total number of cells obtained was greater with lympholyte M than with Percoll but the percentage of leukocytes retrieved was not significantly different between the two gradients. (Figures 1E–G) Therefore we favor lympholyte M as the gradient in our protocol to isolate total leukocyte populations from the stomachs of H. pylori-infected mice. (Figure 2A)
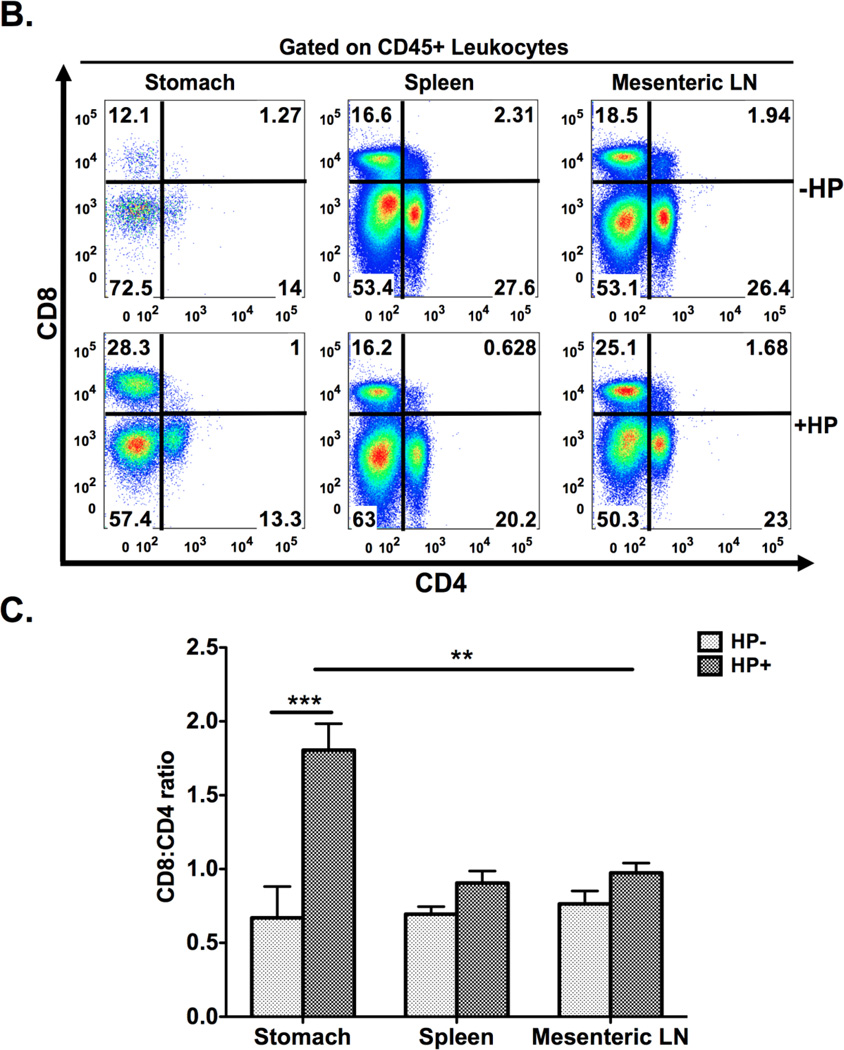
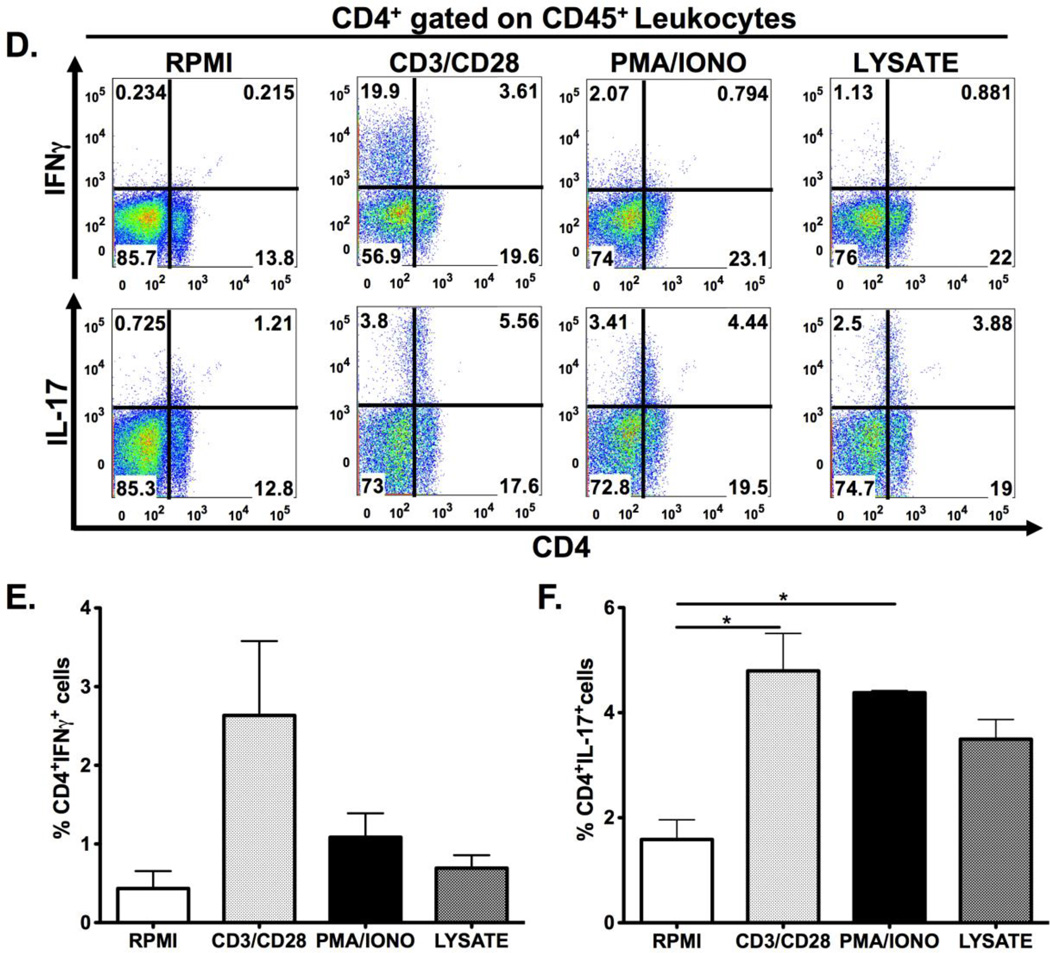
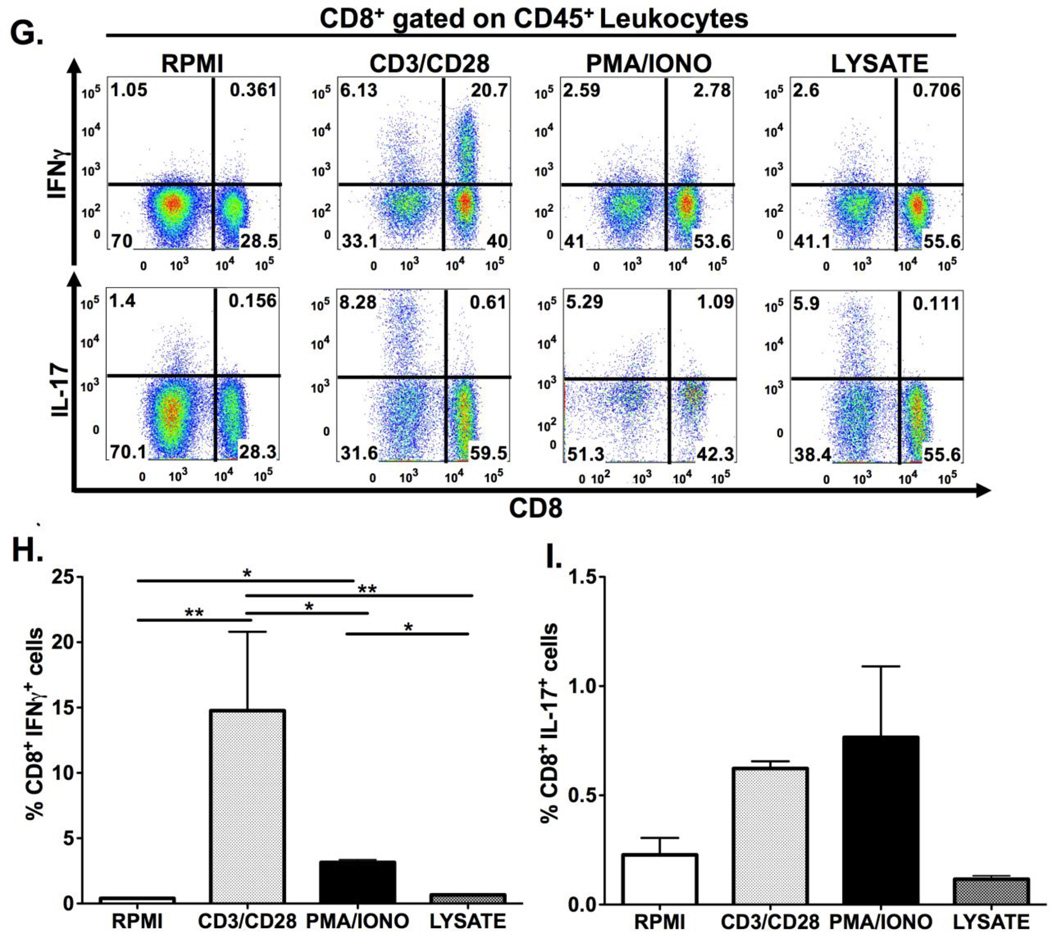
Figure 2. Schematic diagram of gastric isolation protocol (A) and immunophenotyping gastric CD4+ and CD8+ subsets during H. pylori infection. (B–I).
Infection with H. pylori (HP) strain PMSS1 increases the ratio of CD8+ to CD4+ lymphocytes altered within the gastric compartment (B, C). To phenotype CD4+ and CD8+ subsets, gastric lymphocytes were cultured with CD3/CD28, PMA/IONO and H. pylori lysate or RPMI as a control. CD4+ lymphocytes produce IL-17A with CD3/CD28, PMA/IONO and lysate stimulation. (D–F) IFNγ production is highest in CD4+ lymphocytes after CD3/CD28 stimulation. For CD8+ lymphocytes, IFNγ is significantly higher after CD3/CD28 stimulation compared to PMA/Ionomycin and lysate conditions. IL-17A expression is evident in a small percentage of CD4+ and, to a lesser extent, CD8+ cells after either indirect or direct stimulation. (G–I) Graphs represent the mean ± standard error of 4 mice pooled per experiment; results representative of 3 independent experiments. Groups were compared using one-way analysis of variance (ANOVA) followed post-hoc by Tukey multiple comparison tests on log-transformed data. Significant differences are represented by the following *p<0.05, **p<0.01, ***p<0.001.
3.3 Assessment of gastric lymphocytes after H. pylori PMSS1 infection
Following the sequential steps of Figure 2A, we enumerated gastric T lymphocyte populations in mice that had been infected with H. pylori strain PMSS1 for 4 months. In H. pylori-infected mice the proportion of gastric CD8+ to CD4+ cells nearly doubled when compared to the uninfected controls. (Figure 2B,C) To elucidate whether this was specific to the gastric compartment we also compared CD8+ and CD4+ populations in the spleens and mesenteric lymph nodes of these same mice. In those compartments the ratio of CD8+ to CD4+ expressing cells was not significantly altered by H. pylori infection, showing that the increased accumulation of CD8+ lymphocytes during H. pylori infection was specific to the stomach, the organ directly colonized by these extracellular bacteria. (Figures 2B,C)
3.4 Immunophenotyping gastric CD4+ and CD8+ gastric populations
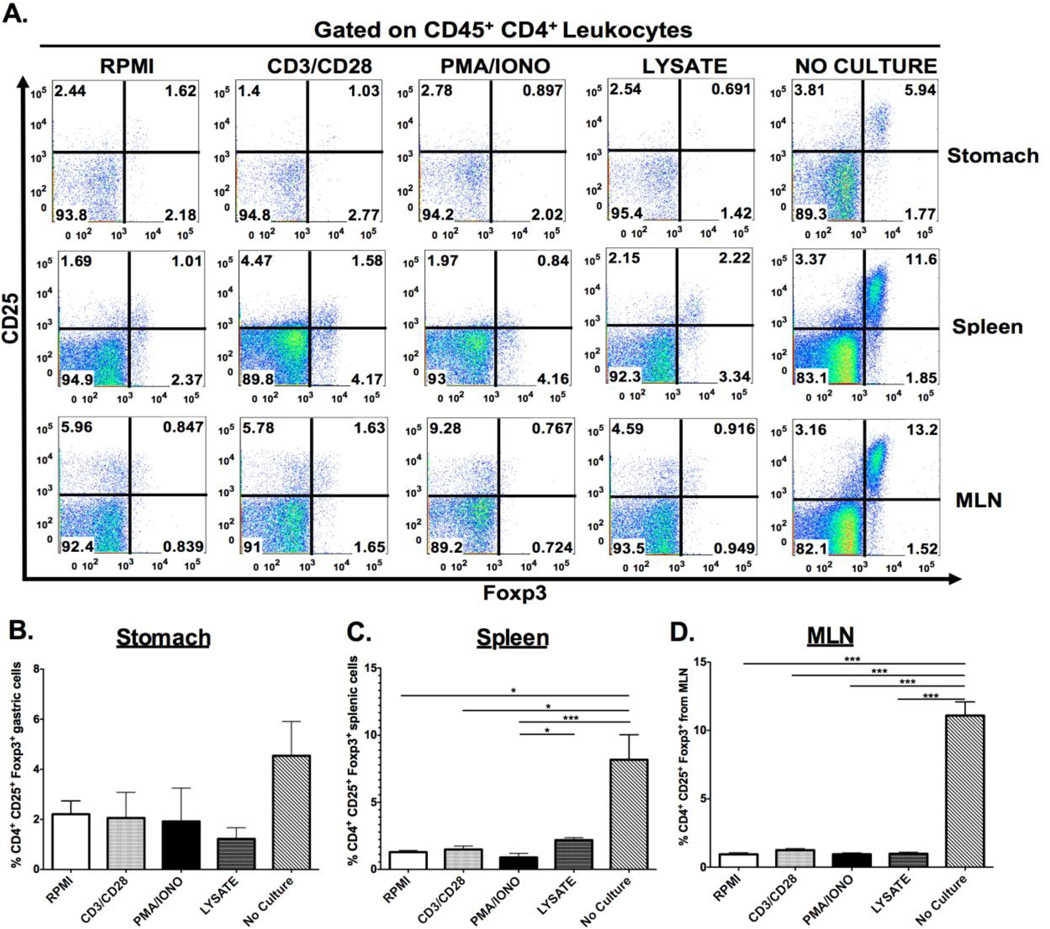
We next examined gastric CD4+ and CD8+ cell subsets after they had been subjected to ex vivo stimulation. Gastric CD4+ and CD8+ cells were phenotyped via production of 2 key cytokines known to mediate H. pylori-induced pathology, IFNγ and IL-17. Cytokine expression was induced through indirect stimulation (using crosslinking antibodies anti-CD3 and anti-CD28; or chemical agonists phorbol-13-mysterate-18 acetate [PMA]/Ionomycin [IONO]), or with a more direct biologically relevant stimulus, H. pylori lysate. Both indirect and direct stimulation elicited IL-17A expression in CD4+ lymphocytes, while the IFNγ response was detected only after CD3/CD28 stimulation. (Figures 2D–F) CD8+ IFNγ–expressing T cells were evident after CD3/CD28 stimulation, but not with the other stimuli. There were relatively few CD8+ T cells expressing IL-17 after PMA/IONO, CD3/CD28 or H. pylori lysate stimulation. (Figures 2G–I) Regulatory T cell numbers were evaluated through enumeration of cells expressing CD4+, CD25+ and the intracellular transcription factor Foxp3. As shown in Figure 3A, ex vivo culture may have detrimental effects on the identification of Foxp3 expressing regulatory T-cells. Indeed, all the ex vivo manipulations tested, including culture in RPMI medium alone, reduced the percentage of CD25+, Foxp3+ CD4+ lymphocytes compared with cells that had not been cultured. This was evident for gastric, splenic and mesenteric lymphocyte populations albeit not statistically significant for the gastric cells. (Figure 3B–D) These results suggest that gastric regulatory T cell numbers should be evaluated in freshly isolated lymphocyte populations, without ex-vivo manipulation.
Figure 3. Effect of ex vivo stimulation on detection of regulatory T cells.
Lymphocytes isolated from the gastric, splenic and mesenteric lymph node (MLN) compartments were cultured under either indirect (CD3/CD28, PMA/IONO) or direct stimulation (H. pylori lysate) and immunophenotype for CD25+ Foxp3+ regulatory T cells. Representative dot plots demonstrated that ex vivo stimulation yielded a decrease in the percentage of regulatory T cells regardless of the compartment the cells originated from. (A) This observation was quantified, and indicated that ex vivo stimulation significantly abrogates detection of CD4+ CD25+ Foxp3+ regulatory T-cells (B–D). Graphs represent the mean ± standard error of 4 mice pooled per experiment; results representative of 3 independent experiments. Groups were compared using one-way analysis of variance (ANOVA) followed post-hoc by Tukey multiple comparisons tests on log-transformed data. Significant differences are represented by the following *p<0.05, **p<0.01, ***p<0.001.
4. Conclusions
The isolation of gastric lymphocytes from H. pylori-infected mice for immunophenotyping is best achieved after digestion with EDTA/DTT and collagenase IV. This leads to the successful isolation of a large number of leukocytes from the stomach, with minimal damage to the cell surface receptors of interest. The addition of EDTA/DTT increases the yield of CD8+ lymphocytes, most likely by liberating intra-epithelial lymphocytes. We also observed that dispase digestion significantly decreased CD4 and CD8 expression. Regarding lymphocyte enrichment strategies, the lympholyte M sucrose gradient provided a greater cell yield, yet did not alter the percentage of CD4+ or CD8+ lymphocytes when compared to a Percoll gradient. Finally, we report that both indirect and direct ex vivo stimulation of the isolated cells induced IL-17 expression in CD4+ lymphocytes and IFNγ in both CD4+ and CD8+ lymphocytes. Overall, CD3/CD28 was the best stimulation with which to immunophenotype CD4+ and CD8+ cell populations. In contrast, any ex vivo manipulation decreased Foxp3 expression, leading to an underestimation of regulatory T cell numbers when employing this widely used intracellular marker. Thus to fully characterize the T cell populations of the gastric mucosa that best reflects the situation in the gastric mucosa, (enumerating both regulatory T cells and conventional T cell subsets), isolated gastric lymphocytes should be divided into 2 fractions - for both immediate enumeration and for ex vivo culture. In conclusion, we have established a protocol that can successfully isolate leukocytes from the stomach. Although, the protocol is broadly similar to procedures used to isolate murine intestinal leukocytes, it includes several significant differences, related to the gastric microanatomy, particularly concerning mucosal scraping and subsequent filtering steps. Additionally, we have demonstrated the comparative effects of a range of stimuli on the responses ex vivo of the extracted gastric lymphocytes. These experiments have led us to adopt the protocol outlined in Figure 2A for enumerating immune subsets from the stomachs of H. pylori-infected mice and subsequently using them ex vivo in functional studies.
Acknowledgements
We thank S. Terrizzi for technical assistance on the FACS Aria.
Funding Sources: NIH 1F31AI082948-01, Mander Research Pre-doctoral Fellowship Recipient, Brown University (VER), NIH R01CA111533 & NIH U19AI082642 (SFM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no financial conflicts of interest.
Contributor Information
Victoria E. Ruiz, Email: Victoria_ruiz@brown.edu.
Monisha Sachdev, Email: Monisha_Sachdev@brown.edu.
Songhua Zhang, Email: Songhua_Zhang@brown.edu.
Sicheng Wen, Email: Sicheng_Wen@brown.edu.
Steven F. Moss, Email: Steven_Moss@brown.edu.
References
- Alderuccio F, Toh BH, Gleeson PA, van Driel IR. A novel method for isolating mononuclear cells from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity. 1995;21:215–221. doi: 10.3109/08916939509008018. [DOI] [PubMed] [Google Scholar]
- Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr, Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol. Med. Microbiol. 2007;51:577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- Arnold I, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Müller A. Tolerance rather than immunity protects from Helicobacter pylori–induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzler I, Oertli M, Becher B, Agger EM, Muller A. Dendritic cells prevent rather than promote immunity conferred by a Helicobacter vaccine using a mycobacterial adjuvant. Gastroenterology. 2011;141:186–196.e1. doi: 10.1053/j.gastro.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305:228–238. doi: 10.1016/j.canlet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa Y, Saito S, Kanamaru R, Sato T, Sato H. Separation of gastric mucosal cells of rat with proteolytic enzymes, pronase and trypsin, with special reference to the collection, morphology and viability of the generative cells. Tohoku J. Exp. Med. 1975;116:241–252. doi: 10.1620/tjem.116.241. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr. Protoc. Immunol. 2001;Chapter 3(Unit 3.19) doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- Sommer F, Wilken H, Faller G, Lohoff M. Systemic Th1 Immunization of Mice against Helicobacter pylori Infection with CpG Oligodeoxynucleotides as Adjuvants Does Not Protect from Infection but Enhances Gastritis. Infect. Immun. 2004;72:1029–1035. doi: 10.1128/IAI.72.2.1029-1035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Baeten D, De Vos M, Demetter P, Elewaut D, Mielants H, Verbruggen G, Cuvelier C, Veys EM, De Keyser F. Chemical agents and enzymes used for the extraction of gut lymphocytes influence flow cytometric detection of T cell surface markers. J. Immunol. Methods. 2000;236:27–35. doi: 10.1016/s0022-1759(99)00243-4. [DOI] [PubMed] [Google Scholar]
- Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Zavros Y, Van Antwerp M, Merchant JL. Use of flow cytometry to quantify mouse gastric epithelial cell populations. Dig. Dis. Sci. 2000;45:1192–1199. doi: 10.1023/a:1005514422187. [DOI] [PubMed] [Google Scholar]