Abstract
Lung surfactant secretion involves lamellar body docking and fusion with the plasma membrane in alveolar type II cells. Annexin A7 (A7) is postulated to play a role in membrane fusion during exocytosis. Our recent studies demonstrated increased co-localization of A7 with ABCA3 in lamellar bodies in type II cells stimulated with established secretagogues of lung surfactant. In this study, we investigated in vivo and in vitro interactions of A7 with the t-SNARE protein, SNAP23. Immuno-fluorescence studies showed time-dependent increases in co-localization of A7 with SNAP23 in PMA- and in A23187-stimulated cells. PMA and A23187 also caused a time-dependent increase in co-localization of ABCA3 with SNAP23. The relocation of A7 to SNAP23 domains was inhibited in the presence of PKC inhibitor, similar to that previously reported for co-localization of A7 with ABCA3. The interaction of A7 and SNAP23 was confirmed by affinity binding and by in vitro interaction of recombinant A7 and SNAP23 proteins. The in vitro binding of recombinant A7 (rA7) to GST-SNAP23 fusion protein was calcium-dependent. Phosphorylation of rA7 with PKC increased its in vitro binding to SNAP23 suggesting that a similar mechanism may operate during A7 relocation to t-SNARE domains. Thus, our studies demonstrate that annexin A7 may function in co-ordination with SNARE proteins and that protein kinase activation may be required for annexin A7 trafficking to the interacting membranes (lamellar bodies and plasma membrane) to facilitate membrane fusion during surfactant secretion.
Keywords: Lung surfactant secretion, t-SNARE proteins, exocytosis, membrane fusion, lamellar bodies, protein kinase C
Lung surfactant facilitates gas-exchange in the lung by lowering surface tension at air-liquid interface during end-expiration and prevents lung collapse. The principal component, phosphatidylcholine, together with surfactant proteins B and C is required for lowering of surface tension. All of these components are synthesized in alveolar type II cells. The surfactant storage organelles, lamellar bodies, must fuse with the apical plasma membrane in type II cells for surfactant secretion into the alveolar space. Although several agents promote lung surfactant secretion, and intracellular signaling mediating secretion has been extensively investigated [1-3], the mechanisms that regulate such membrane fusion have been relatively poorly investigated. We have previously shown that one of the annexin proteins, annexin A7 (A7), can facilitate membrane fusion between lamellar bodies and plasma membrane in vitro [4] and that A7 can promote surfactant secretion in semi-permeable alveolar type II cells [5]. More recently, our in vitro studies suggested that diacylglycerol could regulate A7 function during membrane fusion, since lamellar body enrichment with diacylglycerol increased the A7-mediated membrane fusion activity [6].
Intracellular membrane fusion studies have suggested involvement of soluble N-ethylmaleimide-sensitive fusion protein attachment receptors (SNARE) proteins (reviewed in [7-10]). Previous studies have defined the role of SNARE proteins complex, including the vesicle (v)-SNARE (synaptic vesicle-associated membrane protein, VAMP) and target (t)-SNARE (members of syntaxin and SNAP families), in synaptic transmission or during intracellular trafficking of proteins from the Golgi. These studies suggested an important role for the SNARE complex in docking of secretory vesicles on the target membrane. At the fusion site, cognate SNARE members can pair to allow ‘zippering’ for close apposition of the two fusing membranes [11]. Recent in vitro studies have demonstrated that proteins of SNARE complex are capable of causing vesicle fusion when incorporated into lipid vesicles [12]. However, studies in knockout animals lacking SNARE proteins SNAP25 [13] and VAMP2 [14] suggested that SNAREs may not be essential for fusion. Therefore, it is possible that other proteins may function during membrane fusion either independently or together with SNARE proteins. We and others have proposed a role for annexin proteins in membrane fusion during surfactant secretion in alveolar type II cells. Lipid vesicle fusion can also be facilitated by at least two of the annexin proteins, annexin A2 [15, 16] and A7 [17-19]. Some of the SNARE proteins are also postulated to play a role in lung surfactant secretion [20]. This study demonstrated that syntaxin2 is predominantly present in plasma membrane while SNAP23 was present in both the lamellar bodies and plasma membranes. Although this study proposed that annexin A2 and some of the SNARE proteins can interact as deduced from co-localization studies [21], the regulatory mechanisms controlling such interactions were not investigated.
We have previously postulated that A7 binding to lamellar bodies or plasma membrane would facilitate the membrane fusion [19]. We reported preferential binding of purified bovine A7 to isolated lamellar bodies and plasma membrane fractions [22]. The binding to plasma membrane or lamellar bodies from secretagogue-stimulated cells were higher in comparison to the fractions from untreated cells [22]. In our recent studies, we demonstrated that surfactant secretagogues promoted A7 relocation to the lamellar bodies in alveolar type II cells and that such relocation was regulated by protein kinase activation [23]. In the current study, we investigated if two of the surfactant secretagogues (phorbol myristate acetate, PMA, and calcium ionophore A23187) also increased relocation of A7 to the t-SNARE containing domains in type II cells. Our studies demonstrate both agents increase A7 co-localization with the t-SNARE protein SNAP23 in type II cells. The ATP-Binding Cassette A3 (ABCA3) protein in lamellar body membranes [24, 25] also showed co-localization with SNAP23, which was increased with cell-stimulation. As demonstrated previously for A7 and ABCA3 co-localization, BisI (a PKC inhibitor) blocked increased co-localization of cell A7 with SNAP23. In vitro binding showed that calcium and protein phosphorylation regulated binding of A7 to SNAP23. Thus, our studies support that increased membrane-association of A7 in domains containing t-SNARE proteins is facilitated by protein kinase activation, which may control membrane fusion during secretion.
METHODS
Isolation of alveolar type II cells
The use of animals for studies reported here was approved by the institutional animal care and use committee. Rat lung epithelial type II cells were isolated as described previously. Briefly, anesthetized (Nembutal, 50 mg/kg, ip) adult Sprague-Dawley male rats (200 – 300 g, body weight) were tracheostomized and ventilated with a rodent ventilator. Following sacrifice by exanguination, the lungs were visibly cleared of residual blood by perfusion through the pulmonary artery. Lungs along with the trachea were excised and intra-tracheally treated with porcine elastase (3units/ml, Worthington, Lakewood, NJ) for protease digestion of lungs for isolation of alveolar type II cells as described previously [26]. After elastase incubations, lung lobes were minced on a tissue-chopper, and the lung mince was shaken vigorously to release free cells that showed enrichment with alveolar type II cells. The contaminating macrophages were removed by adherence to rat serum IgG-coated bacteriological plates. The resulting free cells showing ~90% purity of type II cells (by acridine orange staining [27]) were suspended in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) and cultured for 20-22 h in tissue culture plastic dishes or on glass cover slips. The adherent cells at the end of this culture period show >95% purity with type II cells and >97% of the cells routinely exclude Trypan Blue. Adherent type II cells were used for all studies reported here.
Cell treatment with agents
Following culture of type II cells for 20-22h, the adherent cells were washed repeatedly and equilibrated in fresh MEM for 30 min before addition of secretagogues and incubated for specified periods. Stock solutions (×100) for phorbol myristate acetate (PMA) or calcium ionophore A23187 were prepared in ethanol and diluted in MEM before addition so that the incubation medium contained 0.1% ethanol. Following indicated incubations, the medium was removed and the cells harvested by scarping in homogenization buffer (50 mM Tris-100 mM NaCl-0.5 mM EDTA-0.5 mM EGTA, pH 7.4) containing protease and phosphatase inhibitors [1 mM PMSF, mixture of leupeptin, pepstatin and aprotinin (each 5 μg/ml) and 1 mM Na orthovanadate]. The cells were disrupted by sonication on ice (3 × 10sec) followed by passage through 271/2G needle (×5) and the membrane and the cytosol fractions were isolated by differential centrifugation (100,000g for 60 min) where applicable.
Immunoprecipitation of SNAP23
Adherent cells were harvested by scraping in ice-cold non-denaturing lysis buffer (50 mM Tris-HCl, pH 7.4, 0.3 M NaCl, 5 mM EDTA, 0.02% Na azide and 1% Triton X-100) to which 10mM iodoacetamide, 1 mM PMSF and 2 g/ml leupeptin were added before use. All processing was done at or below 4° C unless indicated. The suspension was transferred to pre-cooled centrifuged tubes, vortexed gently and allowed to further lyse on ice for at least 30 min. The suspension was cleared by centrifugation (16000g × 15min) and the supernatant used for immuno-precipitation with SNAP23 antibodies to bacterially expressed recombinant rat SNAP23 (see below) bound to protein A-Sepharose beads (Sigma-Aldrich, St. Louis, MO) that were pre-treated with preimmune serum before antibody binding. The antibody-bound beads (10 μl) were incubated with bovine serum albumin (10 μl of 10%) to block non-specific binding sites and then incubated for overnight with end-over-end mixing after addition of the clarified supernatant from cell lysate. The beads were centrifuged (100g for 150 sec), washed with lysis buffer (×4) and once with ice-cold phosphate-buffered saline. The proteins bound to the beads were eluted and analyzed by immuno-blotting using appropriate primary and secondary antibodies. To avoid interference with the IgGs in the immuno-precipitate, the blots were treated with (1: 500) Clean-Blot IP Detection Reagent (Pierce), which binds only to native (non-denatured) IgGs and allows their detection. The western blots were probed with antibodies to recombinant rat A7 and SNAP23 proteins. The commercially available antibodies used for immuno-staining of SNAP23, although suitable for IP experiments were not suitable for western blots possibly because of poor reactivity with denatured SNAP23.
Immuno-staining of cells
Studies for co-localization of two proteins by immunofluorescence staining were performed with type II cells cultured for 20-22h on glass cover slips. The cells adherent to cover slips were incubated for indicated periods, washed and fixed for 10 min in 4% paraformaldehyde. The cells were washed with phosphate-buffered saline (PBS) and stored at 4° C in PBS until processing. Prior to immuno-staining, simultaneous blocking and permeabilization was performed for 30min at room temperature in PBS containing 0.1% Triton X-100, 0.05% Tween20, 1% BSA and 5% donkey or goat serum. For staining with antibodies in different species (A7 and ABCA3 or SNAP23 and ABCA3), the cells were then incubated for overnight at 4° C with antibodies for A7 (1:500, custom-prepared) or SNAP23 (1:100, Synaptic Systems, Goettingen, Germany), washed in PBS and incubated for 1h at room temperature with anti-rabbit Alexa488-conjugated secondary antibodies (Invitrogen, Grand Island, NY).
The staining for ABCA3 was then performed by incubation for 1h with monoclonal anti-ABCA3 antibodies (1:500, Covance, Emeryville, CA) followed by 1h incubation with Alexa568-conjugated anti-mouse antibodies (1:500, Invitrogen, Grand Island, NY). The immuno-stained cells were viewed in a confocal laser scanning microscope. The protocol for immuno-staining using rabbit polyclonal antibodies to SNAP23 and A7 was modified to avoid cross-reactivity. Briefly, the cells were fixed for 10 min in 4% PFA, washed, simultaneously blocked and permeabilized for 30 min. The staining for first antigen, SNAP23, was performed as discussed above. Thereafter, the cells were washed and incubated for 30min each with biotin blocking agents (Invitrogen, Grand Island, NY), washed and incubated for overnight with biotinylated rabbit anti-A7 antibodies (1:500). Biotinylation of purified rabbit anti-A7 antibodies was performed with ChromaLink Biotin One Shot antibody labeling kit according to instructions (SoluLink, San Diego, CA). The cells were washed and incubated for 1h at room temperature with streptavidin-AlexaFluor568 conjugated anti-rabbit antibodies (1:500). The nuclei were counter-stained with DAPI for 1 min. The confocal scanning microscopy for immunofluorescence was performed after mounting cover slips in anti-fade reagent (ProLong®, Invitrogen, Grand Island, NY).
Bacterial expression GST-SNAP23 fusion protein
Rat lung cDNA library Zap-II (>2×106 total recombinants) (Stratagene, La Jolla, CA) was used as a template to amplify the coding sequence for SNAP23 using the following primers. A sequential touchdown PCR was carried out as follows. First, a forward primer (5′-cttaagagagtccaagagcagc-3′, Rat SNAP23, accession number NM_022689) and T7 reverse primer (5′-gtaatacgactcactatagggc-3′) were used to maximize gene-specific amplification of SNAP23-cDNA insert (94° C for 5 min, 5 cycles 94° C for 45 sec, 70° C for 45sec, 72° C for 2 min). This was followed by 5 cycles 94° C for 45 sec, 68° C for 45 sec, 72° C for 2 min and 25 cycles 94° C for 45 sec, 66° C for 45 sec followed by 72° C for 2 min. The PCR product was gel-isolated and used as template for second PCR using both SNAP23-specific primers with BamHI (forward: 5′ggtggaccgatgatctatcaccagaagaaattc-3′) and EcoRI (reverse-5′-gtgaattcttagctgtcaatgagtttctttgctc-3′) extensions, respectively. The PCR product was cloned into pGEX-2T vector (Amersham Biosciences, GE Healthcare, Piscataway, NJ) at the BamHI and EcoRI sites. The sequence and in frame insertion was verified by sequencing. The construct was transformed into E.coli ER2566 and GST-SNAP23 fusion protein was purified as previously described for GST-A7 fusion protein [28] and used for purification of recombinant GST-SNAP23 by binding to glutathione Sepharose-4B beads (GE Healthcare, Piscataway, NJ). The expression of SNAP23 protein was confirmed by SDS-PAGE after thrombin (Roche Diagnostics, Boehringer Mahnheim, Germany) cleavage of the fusion protein. The fusion protein was used for affinity binding of A7 in type II cell lysate or for in vitro binding of purified recombinant A7.
Purification of recombinant SNAP23 and preparation of polyclonal antibodies
The glutathione-Sepaharose4B bound GST-SNAP23 was treated for 90 min at room temperature with thrombin (5 units/ml) in cleavage buffer (20 mM Tris, pH 7.2, 150 mM NaCl, 0.05% NP-40, 1 mM CaCl2 and 0.01%β-mercaptoethanol). The reaction was stopped with the addition of PMSF (1mM, final concentration), the beads were separated by filtration and the filtrate was passed through columns (HiTrap Benzamidine FF-high sub 1ml column, GE Healthcare) for removal of residual thrombin. The protein containing fractions were pooled and dialyzed against PBS at 4° C with three changes of buffer after 12, 15 and 18 h). Thus purified SNAP23, which showed one major protein on followed by staining with Coomassie Blue-stained SDS-PAGE, was used for preparation of rabbit polyclonal antibodies through a commercial service (Covance).
In Vitro GST Pull-down Assay
For affinity binding studies, the glutathione Sepharose-4B beads were washed (×3) with cold PBS and incubated overnight with end-over-end rotation at 4° C with GST-SNAP23 or GST (0.1 μM) in a total volume of 500 μl of binding buffer (40 mM Hepes, pH 7.0, 100 mM KCl, 1 mM EGTA, 2 mM MgCl2, 1% NP40 and 1 mM CaCl2) for protein(s) binding.
Equal amounts of protein in cell lysates were precleared using GST-bound Sepharose4B beads. The GST-SNAP23-bound beads were washed 6× with the binding buffer and incubated for overnight with rotation with pre-cleared type II cell lysates (300-500 μg protein) in presence of indicated Ca2+ concentration buffered with 1 mM EGTA at 4° C. The Ca2+ concentrations in the binding reaction mixture were determined after taking into consideration buffering with EGTA or other ions and the effects of pH and the reaction temperature for the binding assay, as originally described [29]. The calculations used an open source program WinmaxC32 (http://maxchelator.stanford.edu). Following binding, the beads were washed 6x with the binding buffer, centrifuged and boiled for 5 min in SDS sample buffer. The beads were removed by centrifugation and the supernatant was analyzed by western blot using A7 antibody (1:1000) and HRP-conjugated secondary antibody (1:10,000 dilution).
In vitro binding of recombinant annexin A7 to GST-SNAP23
Direct interactions between SNAP23 and annexin A7 were investigated in vitro in the absence or presence of Ca2+ (buffered with 1mM EGTA). The binding reaction was carried out for 2h at 4° C in binding buffer (40 mM Hepes, pH 7.0, 100 mM KCl, 1 mM EGTA, 2 mM MgCl2, 1% NP-40 and protease and phosphatase inhibitors) containing 100 nM GST-SNAP23 bound to Sepharose4B beads and 200 nM recombinant A7 and indicated concentrations of free calcium. The reaction was stopped by centrifugation of beads to sequester bound proteins. The beads were washed and the amounts of proteins quantified by western blot analysis.
Annexin A7 phosphorylation and interactions with SNAP23
In vitro phosphorylation of recombinant annexin A7 with commercially available PKC (EMD Biosciences, Philadelphia, PA) was carried out as described previously [23]. Briefly, the phosphorylation mixture was incubated for 2h at 30° C (1 μg recombinant A7 in 10 μl reaction volume) in presence of 0.05 mUnits PKC, 4 μg phosphatidylserine, 100 nM PMA, 1 mM Na orthovanadate, 1 mM Ca2+ and 0.1 mM ATP.MgCl2. The phosphorylation was stopped by placing tubes on ice and with the addition of 1 mM EGTA to sequester Ca2+. In parallel reaction, control phosphorylation reaction was conducted in the absence of PKC or ATP. The in vitro binding to GST-SNAP23 was conducted for 2h at 4° C following 3-folds dilution of untreated annexin A7, non-phosphorylated A7 or phosphorylated A7 according to the above-described protocol.
Western blot analysis
Equal proportions of proteins eluted from beads or immunoprecipitated proteins were resolved on SDS-PAGE (4-20%), transferred to PVDF membranes, blocked with blocking solution containing 5% bovine serum albumin or fat-free dry milk, and reacted with primary antibodies (1:1000 dilution unless indicated). The membranes were then reacted with species-specific secondary antibodies (1:10,000 dilution) and the antigen(s) detected by chemiluminiscence using Super-Signal® reagent (Pierce). Blots were imaged with a photo-imager (Syngene, Fredrick, MD).
Other analyses
Proteins were quantified according to Bradford [30] using protein-binding dye reagent (Bio-Rad, Hercules, CA) and bovine-γ-globulin as standard. Results of immuno-fluorescence were analyzed after adjustment of the fluorescence intensities to the same range for individual fluorophore and the co-localization coefficients (CC) were obtained using the Zeiss LSM510, version 4.2 Program (Carl Zeiss Inc., Chester, VA). The program analyzes each image to determine total number of co-localizing pixels above the threshold level in each channel to calculate the CC. The weighted CC is calculated by normalizing the intensity of co-localizing pixels in each channel with total pixel intensity for that channel. Thus, the weighted CC is not influenced by variations in total fluorescence intensity. Although these calculations are reliable indicators of co-localization, further analysis of weighted CC from several experiments was analyzed by ANOVA (for multiple groups) or Student’s t-test for independent observations (for two groups) with GraphPad Prism (Version 5.00 for Windows, GraphPad Sofware, San Diego, CA). For some of the studies, western blots were analyzed by densitometry scanning of images (GeneTools software, Version 4.00, SynGene, USA, Frederick, MD). The results from several experiments were analyzed for statistical significance by ANOVA (for multiple groups) or by Student’s t-test (for two groups). In each case, P < 0.05 was considered significant.
RESULTS
A7 antibody
We have previously demonstrated that our purified antibody to recombinant annexin A7 recognizes a single band at ~47 kDa in lung, 24 h-cultured type II cells or in isolated lung lamellar bodies [23]. The specificity of antibody was demonstrated by loss of reactivity following pre-incubation with recombinant A7.
Immuno-localization studies
Annexin A7 co-localization with SNAP23
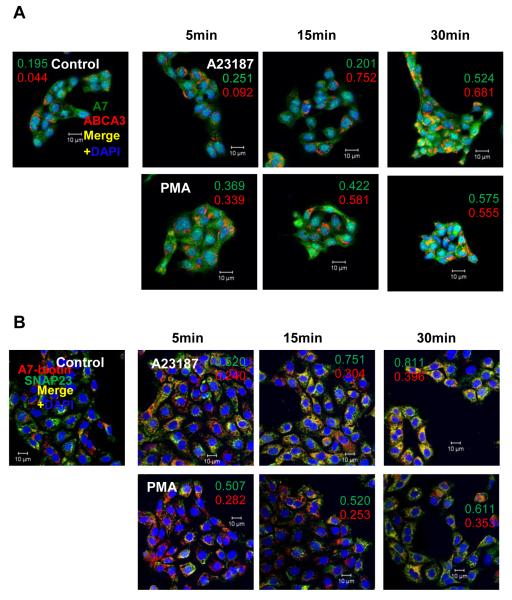
We have previously demonstrated that stimulation of type II cells with PMA, ATP, calcium ionophore or terbutaline, all established surfactant secretagogues [2, 3], increased membrane-association of cellular A7. One of these membranes was determined to be lamellar bodies as indicated by A7 co-localization with the marker protein, ABCA3 [23]. In current study, we first confirmed that both PMA and A23187 increased the co-localization of A7 and ABCA3 in a time-dependent manner (Figure 1A). In each case, the co-localization coefficient (CC) increased with the incubation period. The values for weighted CC values (i.e., normalized for total intensity) for each fluorophore are shown in individual panels. In parallel studies, isolated type II cells were incubated for up to 30min with or without 100nM PMA or 250nM A23187 and the cells immuno-stained for SNAP23 and biotinylated A7 (Figure 1B). The CC (0 = no and 1 = 100% co-localization) for A7 in control cells was 0.103, which increased to 0.121, 0.200 and 0.295 at 5, 15 and 30min, respectively, in A23187-treated cells. Similarly, the corresponding CC values were 0.129, 0.118 and 0.245 in PMA-treated cells. In this case also, the CC values weighted for the total intensity for each fluorophore are shown in each panel. Thus, the A7 co-localization with SNAP23 was clearly elevated at 30 min. Since SNAP23 is also present in the plasma membrane and lamellar bodies [20], our studies showing co-localization of A7 and SNAP23 suggest that A7 trafficking to plasma membrane is also associated with surfactant secretion. This observation is in agreement with previously reported in vitro binding of purified bovine A7 to the plasma membrane and lamellar body fractions from cells stimulated with A23187 or PMA [22].
Figure 1.
The PMA- and A23187-treated type II cells show increased co-localization of annexin A7 (A7) with ABCA3 or with SNAP23. Adherent cells were treated for indicated periods without or with 250 nM A23187 or 80 nM PMA, fixed and stained A7 and ABCA3 (A) or for SNAP23 and biotinylated anti-A7 (B). The pixel intensities for individual fluorophore for laser confocal microscope (LSM) images were adjusted to the same range for all groups. Cell treatment with PMA or A23187 increases co-localization of A7 with ABCA3 and with SNAP23 in a time-dependent manner. Weighted co-localization coefficients (fractional intensity of co-localizing pixels relative to total pixel intensity) for individual fluorophore are shown for each condition. Representative images are shown from experiments that were repeated at least two times.
Lamellar bodies targeted to t-SNARE domains
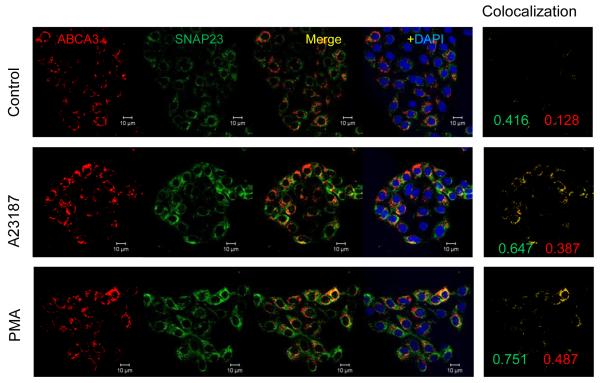
In the SNARE scheme of membrane fusion, syntaxin and SNAP, the two t-SNARE proteins that primarily reside in the plasma membrane and together may form the acceptor site for the v-SNARE synaptobrevin [10]. Our attempts to immuno-stain type II cells for synaptobrevin did not show any staining (not shown). Therefore, we elected to determine co-localization of ABCA3 with SNAP23 in control and stimulated cells (Figure 2). The cells were incubated for 30min without or with 100 nM PMA or 250 nM A23187, washed, fixed and immuno-stained for ABCA3 and SNAP23. In the control cells, SNAP23 was mostly localized to the extra-vesicular region and ABCA3 was mostly vesicular, presumably representing the lamellar body compartment. There was some co-localization of the two proteins in the control cells, possibly because of the presence of SNAP23 in lamellar bodies or because of lamellar body docking at the plasma membrane during surfactant secretion. In secretagouge-stimulated cells, the co-localization of two proteins was greater because greater number of lamellar bodies would localize close to the plasma membrane. Since cell-stimulation is not likely to increase SNAP23 in lamellar bodies, the increased co-localization of ABCA3 with the SNAP23 was likely related to exocytosis of lamellar bodies. These findings are in agreement with a previous report suggesting a role for SNAP23 in lung surfactant secretion [20].
Figure 2.
PMA and A23187 increase association of lamellar bodies with SNAP23. Adherents type II cells were incubated for 30min in the absence (Control) or presence of 250 nM A23187 or 80 nM PMA. Cells were fixed and stained for ABCA3 and SNAP23. Images are shown after intensities adjustment to the same range for individual fluorophore in all groups. The co-localization pattern of SNAP23 and ABCA3 is shown in the right panel for each group. Representative images are shown from experiments that were repeated at least three times with similar results. The weighted co-localization coefficients are shown for each fluorophore in the co-localization panel.
PKC-inhibitor studies
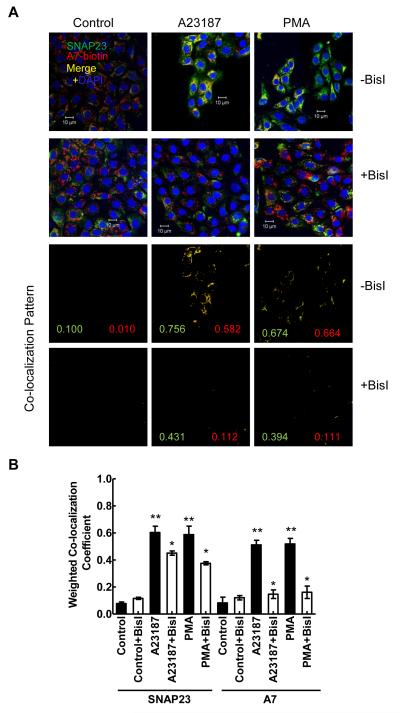
Next, we investigated if PKC activation is required for A7 association with SNAP23, similar to our previous observations showing A7 association with the lamellar bodies [23]. For these studies, type II cells were pre-incubated for 30min with PKC inhibitor BisI (100 nM) and then incubated for 30 min without or with 100 nM PMA or 250 nM A23187. In cells pre-treated for 30 min with 100 nM BisI prior to 30 min incubation with indicated secretagogues, the co-localization of annexin A7 with SNAP23 was diminished in comparison to those without the inhibitor (Figure 3). As described above, further statistical comparison of the weighted CC from several experiments (Figure 3B) showed increased co-localization of SNAP23 and A7 in A23187- and PMA-stimulated cells. Further, PKC inhibition diminished the increased co-localization of these two proteins in stimulated cells. In our previous studies also, BisI similarly diminished the increased co-localization of annexin A7 and ABCA3 in stimulated type II cells [23].
Figure 3.
Increased co-localization of annexin A7 and SNAP23 in PMA- or A23187-treated cells is diminished by PKC inhibitor. Isolated rat type II cells were pre-incubated for 30 min without or with 100 nM BisI before incubation for 30min without or with 250 nM A23187 or 80 nM PMA. A. Fluorescence images show that prior treatment with BisI diminished the increase in co-localization of A7 and SNAP23. The co-localization patterns for each group are shown in the lower panels and the numbers denote the weighted co-localization coefficient (CC) for individual fluorophore in each image. A value close to zero indicates lack of co-localization. B. Graphic representation of weighted CC (mean ± SE, n = 3 – 5) with statistical analysis (ANOVA) shows that both PMA and A23187 increased co-localization of two proteins in comparison to controls. Pretreatment with BisI caused diminished increase in co-localization of both proteins in comparison to without BisI (Student’s t-test). ** P < 0.05 vs. control; * P < 0.05 vs. without BisI.
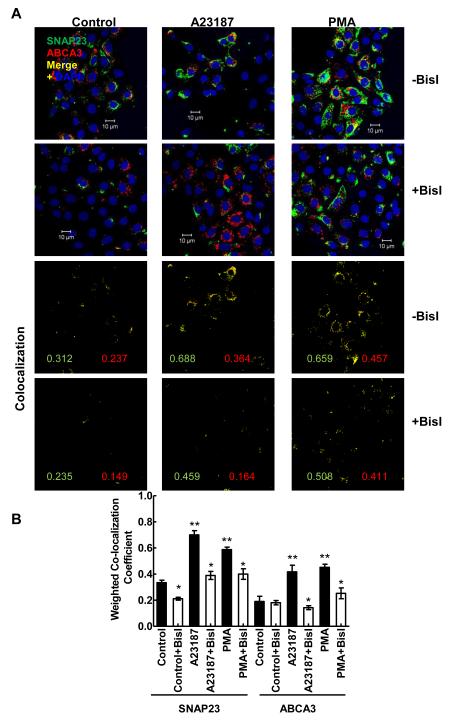
In parallel, we also investigated PKC regulation of SNAP23 and ABCA3 co-localization in stimulated type II cells (Figure 4). In these studies also, type II cells were pre-incubated for 30 min with 100 nM Bis I before stimulation with PMA or A23187 for 30 min. The immuno-fluorescence analysis of cells showed increased co-localization of SNAP23 and ABCA3 in stimulated cells, which was diminished in cells pre-treated with BisI (Figure 4A). Thus, the co-localization of ABCA3 and SNAP23 was also dependent on PKC-stimulated protein phosphorylation. The co-localization patterns with weighted CC for each condition are shown in the lower panels. Further statistical analysis of weighted CC from several experiments showed that both PMA and A23187 increased the co-localization of the two proteins in comparison to the controls (Figure 4B). The increase in co-localization was lower in cells were pre-treated for 30 min with BisI.
Figure 4.
Secretagogue-mediated increase in co-localization of ABCA3 with SNAP23 is blocked by PKC inhibitor. Isolated type II cells were treated for 30 min without or with 100 nM BisI prior to 30 min incubation with 80 nM PMA or 250 nM A23187. The cells were fixed and stained for SNAP23 and ABCA3 and the nuclei were counter-stained with DAPI. A. Normalized images for green (SNAP23) and red (ABCA3) fluorophores show that BisI pretreatment diminishes secretagogue-mediated increases in co-localization of two proteins. The co-localization patterns and weighted co-localization co-efficient (CC) for individual fluorophore are shown in the lower panels for each image. B. Weighted CC from 4 - 6 experiments are plotted as mean ± SE. ** P < 0.05 in comparison to the controls (ANOVA) and * P < 0.05 in comparison to without BisI (Student’s t-test).
In vitro and in vivo interactions of A7 and SNAP23
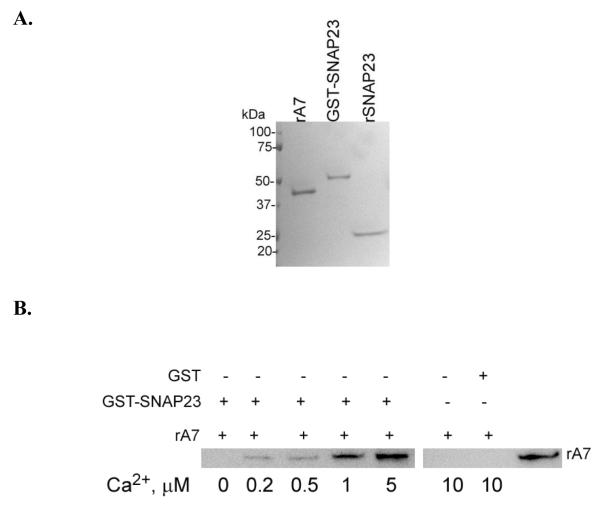
Next, we determined direct interaction of A7 interactions with SNAP23. In vitro affinity binding of bacterially expressed A7 (rA7) with GST-SNAP23 fusion protein (Figure 5A) bound to Sepharose 4B was measured in the presence of indicated concentrations of Ca2+ (Figure 5B). The binding increased with increasing Ca2+. The negative control (A7 binding to GST-beads) was conducted at slightly higher Ca2+ concentration (10 M) to rule out even small amounts of binding. In comparison to control (<30 nM, nominally zero Ca2+), the increase in binding was clearly observed at 0.5 μM and higher Ca2+ concentrations. However, the binding did not reach a maximum even at 5 μM Ca2+ (not shown).
Figure 5.
A7 binds to SNAP23 in calcium-dependent manner. A. Coomassie blue-stained SDS-PAGE of recombinant A7 (rA7), GST-SNAP23 fusion protein and purified SNAP23 demonstrating the presence of a single major protein in each preparation. B. For in vitro binding studies, rA7 was incubated for 2h at 4° C with Sepharose4B-bound recombinant GST-SNAP23 fusion protein or with GST in presence of indicated Ca2+ concentrations buffered with 1 mM EGTA. The beads were washed extensively and rA7 binding to beads detected by western blot analysis. The binding of A7 to SNAP23 was dependent on calcium concentrations. The last lane shows reaction with 45 ng rA7 as a positive control.
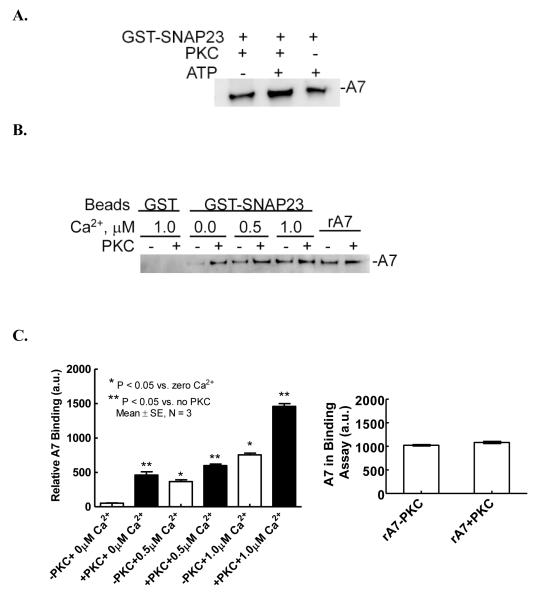
The effect of PKC phosphorylation of rA7 on binding to SNAP23 was investigated using affinity binding with by GST-SNAP23-Sepharose4B beads. We and others have previously demonstrated that PMA and ATP cause PKC activation in type II cells [31, 32] and PMA causes PKC-mediated phosphorylation of A7 in vitro and in vivo [18, 23]. For pilot studies, incubations of rA7 for phosphorylation reaction were conducted for 2 h at 30° C in the absence or presence of PKC or ATP. The phosphorylated A7 was not purified from this incubation mixture. Equal aliquots of phosphorylation incubation mixture were used to measure A7 binding to beads (Figure 6A). The levels of bound A7 were analyzed by western blot analysis. The binding was unaffected by the presence of only ATP or PKC, but was higher when rA7 was incubated in complete phosphorylation mixture. Thus, phosphorylation of rA7 increased its binding to SNAP23. The increased binding following PKC phosphorylation of A7 was unlikely due to subsequent phosphorylation of SNAP23, since the PKC enzyme and the co-factors were diluted 3-folds, the Ca2+ concentration was lowered from 1 mM to that indicated for the binding reaction by buffering with EGTA and, finally, the binding reaction was conducted at 4° C and not at 30° C as routinely used for PKC phosphorylation of A7. Next, we evaluated the binding of phosphorylated rA7 to SNAP23 at different Ca2+ concentrations (Figure 6B). The binding results from multiple experiments were evaluated by densitometry scanning of western blots, which showed that PKC-mediated phosphorylation increased A7 binding to SNAP 23 at all concentrations of Ca2+ in this experiment (Figure 6C) with similar amounts of total A7 in the two reaction mixtures (Figure 6C, right panel).
Figure 6.
Phosphorylation of A7 increases its binding to SNAP23. A. Recombinant A7 was incubated for 2 h at 30° C without or with PKC or ATP and MgCl2 and binding to Sepharose4B-bound GST-SNAP23 fusion protein was evaluated in presence of 100 μM Ca2+. The binding was increased for A7 incubated with PKC and ATP. B. In separate experiments, A7 was phosphorylated for 2 h without or with PKC and binding to Sepharose4B-bound GST-SNAP23 fusion protein was measured following two hours incubation at 4° C in presence of indicated Ca2+ concentrations. The binding of A7 was evaluated by western blot analysis. C. Left Panel. Densitometry analysis of western blots from three experiments is shown as mean ± SE of protein (a.u., arbitrary units) on the blot. * P < 0.05 for non-phosphorylated A7 in comparison to zero Ca2+, ** P < 0.05 for phosphorylated in comparison to non-phosphorylated A7 at each Ca2+ concentration. Right Panel. Densitometry analysis shows equivalent levels of A7 in the assay mixture in the absence or presence of PKC (last two lanes in B).
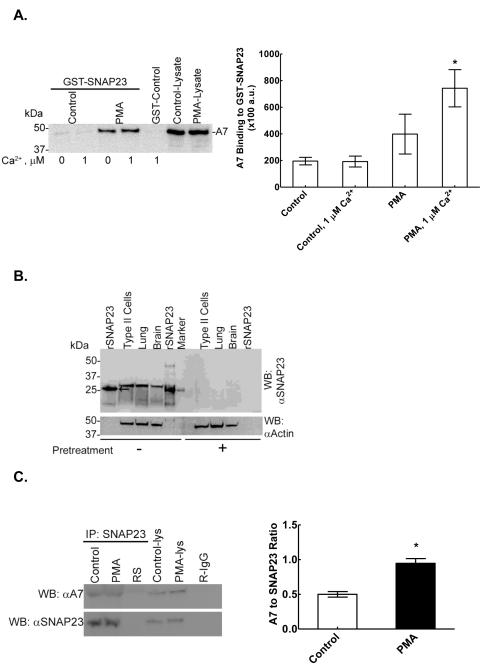
We also investigated the binding of cellular A7 to SNAP23, as measured by affinity binding to GST-SNAP23-Sepharose 4B beads and by immuno-precipitation (Figure 7). For these studies, type II cells were incubated for 30min without or with 100 nM PMA. The cells were washed and harvested by scraping in non-denaturing lysis buffer to prepare cell lysate for immuno-precipitation. Affinity binding using Sepahrose 4B-GST-SNAP23 showed increased binding of A7 from PMA-treated cells in comparison to the control cells (Figure 7A). The A7 binding in PMA-treated cells was higher in presence of 1 μM Ca2+ than in the absence of Ca2+.
Figure 7.
PMA increases in vitro and in vivo binding of A7 to SNAP23. A. Left Panel. Affinity binding of cell A7 to GST-SNAP23 is higher in PMA treated type II cells. Following incubation of cells for 30 min without or with 80 nM PMA, the cell lysates were prepared and equal amounts of cell proteins were analyzed for A7 binding to Sepharose4B-bound GST-SNAP23 by pull-down assay without or with 1 μM Ca2+. The beads were washed and analyzed for bound A7 by western blot analysis. Right Panel. Results are shown as mean ± SE (n = 3 separate experiments) of densitometry analysis (arbitrary units, a.u.) of western blots. Statistical analysis (Student’s t-test) was performed to analyze for the effects of PMA-treatment and for the presence of Ca2+. * P < 0.05 in comparison to PMA-treated cells with no Ca2+ or control cells without or with 1 μM Ca2+. B. Specificity of SNAP23 antibodies was demonstrated by western blot analysis of samples of homogenates (30 μg protein) from rat type II cells, lung and brain with 10 μl antiserum against recombinant (r) SNAP23 pretreated for 24h without (−) or with (+) 100 μg purified rSNAP23. The recombinant protein (arrow) migrated slightly faster in comparison to the tissue SNAP23. The western blot was then probed for actin. Pre-treatment with rSNAP23 protein diminished the reactivity of antiserum for SNAP23 in all samples. C. Left Panel. In separate experiments, lysates from control and PMA-treated cells (30 min incubation) or 5 μl rabbit serum (RS) were immuno-precipitated using SNAP23 antibodies and analyzed for A7 binding by western blot analysis. Rabbit IgG (R-IgG, 2 μg) was also included as a control. The blots were then probed with SNAP23 antibodies to demonstrate relative loading of SNAP23. Control-lys and PMA-lys denote cell lysates (40 μg protein) from control and PMA-stimulated cells, respectively. Right Panel. Western blots of immuno-precipitates from three separate experiments were analyzed by densitometry scanning and the ratios of A7 and SNAP23 (mean ± SE, n = 3) were compared between two groups. * P < 0.05 by Student’s t-test. In comparison to controls, the PMA-stimulated cells showed increased binding of A7 to SNAP23.
Because of poor western blot reactivity of commercially available SNAP23 antibodies used above for immuno-staining studies (see above), antibodies to recombinant SNAP23 antibodies (custom-prepared) were employed for the studies described below. Western blot analysis of homogenates from brain, lung and type II cells demonstrated the presence of a single major band similar to that seen for the recombinant SNAP23 (Figure 7B). The specificity of antibodies was verified by overnight pre-treatment of antiserum (10 μl) with purified recombinant protein (100 μg), which largely abolished the signal on western blot (Figure 7B, right section) of recombinant SNAP23 (30 ng) or samples of alveolar type II cells, lung or brain (50 μg protein, each). The relative abundance, determined from SNAP23 to actin ratio, was brain > type II cells > lung. The bacterially expressed recombinant protein showed slightly smaller size in comparison to the tissue protein (Figure 7B, arrow). These antibodies were then used for immuno-precipitation studies with type II cells stimulated for 30 min without or with 100 nM PMA. Immuno-precipitates with SNAP23 antibodies showed increased levels of A7 in PMA-treated cells in comparison to the control cells (Figure 7C). Since A7 levels are normalized to SNAP23 levels, equal loading of SNAP23 on gels is not relevant. The densitometry results from three different experiments are presented as the A7 to SNAP23 ratio, which showed increased A7 co-precipitation with SNAP23 (Figure 7C, right panel) Thus, in vitro and in vivo evidence demonstrates direct interaction between A7 and SNAP23, which is regulated by Ca2+ and PKC-dependent phosphorylation of A7.
DISCUSSION
In this study, we provide new information on annexin A7 trafficking to SNAP23 domains in alveolar type II cells. The immuno-fluorescence co-localization studies showed that relocation of this membrane fusion-promoting protein to SNAP23 domains increased following cell-stimulation with PMA and A23187, two of the established secretagogues of lung surfactant in alveolar type II cells. In parallel, these agents also increased lamellar body trafficking to SNAP23 domains as demonstrated by increased co-localization with of ABCA3 and SNAP23. Together, these observations suggest that stimulation of surfactant secretion is associated with A7 trafficking and binding to SNAP23 domains.
The regulation of A7 or lamellar body trafficking to SNAP23 domains appears to be through protein phosphorylation. Our studies with BisI, a specific inhibitor of PKC, showed diminished co-localization of A7 with SNAP23 in secretagogue-stimulated cells (Figure 3). The co-localization coefficients, weighted for emission intensity for individual fluorophore, show PKC-dependence of increased co-localization for each protein in stimulated type II cells. The BisI decrease of co-localization was higher for A7 co-localization in PMA- and A23187-stimulated cells suggesting that A7 phosphorylation was the primary determinant for relocation. Activation and involvement of PKC in regulation of lung surfactant secretion is amply demonstrated in several previous investigations (reviewed in [1-3]. We have previously suggested a role for annexin A7 in membrane fusion during surfactant secretion since this protein increased surfactant secretion in permeabilized type II cells [5]. Our previous [23] and present studies demonstrating BisI inhibition of A7 co-localization with SNAP23 and with lamellar bodies further suggest PKC regulation of A7 function presumably in membrane fusion during surfactant secretion. Thus, our co-localization studies allude to increased trafficking of A7 to the fusion site at the plasma membrane in cells stimulated with secretagogues of lung surfactant.
The in vitro studies on binding of recombinant proteins suggest direct interaction of A7 with SNAP23 that is regulated by increase in Ca2+ and in PKC activation. Annexin proteins share the common property of calcium-dependent binding to phospholipids membranes [33, 34]. Therefore, calcium-dependence of A7 binding to SNAP23 (Figure 5B) may be expected. However, the binding is also regulated by phosphorylation of A7. Importantly, the binding of endogenous A7 (Figure 7A) was higher even in the absence of Ca2+, suggesting that other cellular factors could enhance A7 binding to SNAP23. These studies also indicated additional regulation of interaction by Ca2+. Thus, the Ca2+-dependence without or with phosphorylation is similar for the recombinant and endogenous A7 and SNAP23 interactions. Additionally, the affinity binding studies with cell lyase suggest the presence of excess pool of A7 that are not available for binding to SNAP23 or lamellar bodies in intact cell, since A7 binding was greater from lysates of PMA-stimulated cells and more A7 could be immunoprecipitated with SNAP23 in comparison to that from control cells. This pool of A7 is made available by cell lysis for in vitro binding.
Of the two members of annexin family, A2 and A7, reported to facilitate membrane fusion, the function of A7 is apparently regulated by protein phosphorylation. Previous studies have demonstrated in vitro and in vivo phosphorylation of A7 in isolated chromaffin cells [18] and in alveolar type II cells [23]. The phosphorylation of recombinant A7 can be achieved with several classes of commercially available protein kinase enzymes, but only phosphorylation with PKC increased its membrane aggregation property [18]. Although phosphorylation of annexin A2 with PKC diminishes its membrane aggregation properties [35], it is likely that in vivo regulation of A2 is modulated by additional factors to overcome such inhibition. In case of A7 also, the phosphorylation and membrane-association of A7 was demonstrated to increase with both PKC and PKA activation in alveolar type II cells [23].
The in vitro [22] and in vivo [23] membrane-association of A7 in stimulated cells implies the presence of specific A7 binding domains in the lamellar bodies and in the plasma membrane of type II cells. Our current studies suggest that SNAP23 and possibly other t-SNARE proteins could be present in these domains. The interaction of A7 with the proteins of plasma membrane domains is apparently augmented by A7 phosphorylation. However, a similar protein phosphorylation in the binding domains could also influence the observed A7 relocation to SNAP23 domains. Our previous studies of in vitro binding of purified bovine A7 to plasma membrane and lamellar bodies from PMA- or A23187-treated cells showing increased binding [22] support such possibility. The phosphorylation of SNAP25 is critical for NMDA receptor delivery and exocytosis at the cell surface [36]. Although SNAP 23 is implicated in surfactant secretion [20], the importance of its phosphorylation in type II cells is yet to be demonstrated. If so, then protein phosphorylation (of SNAP23 and A7) process would be even more critical in the regulation of lung surfactant secretion in type II cells.
We have previously suggested that some properties of A7 like interaction with target and vesicle membranes and action as a Ca2+ sensor may be similar to those reported for synaptotagminI, one [37] of the several SNARE-interacting proteins including calmodulin [38], CaMKII [39], ENaC [40] and synaptophysin [41] (reviewed in [42]). Except synaptotagminI, all other are reported to interact with either the t- or the v-SNARE proteins [37, 43]. In addition to interacting with both the t- and v-SNARE proteins [44, 45], synaptotagminI has a critical function as Ca2+ sensor with ability to insert into membranes [46]. Similarly, A7 interacts with SNAP23 (a t-SNARE protein) in the plasma membrane and with lamellar bodies. The binding to the two compartments is possibly at the exocytic sites to promote membrane fusion, in co-operation with SNARE proteins. This speculation is supported by the presence of Ca2+ and phospholipids binding domains in A7 and in synaptotagmins [47, 48] and because the two proteins show similar properties of Ca2+-dependent membrane aggregation and ability to interact with two types of biological or synthetic membranes [6, 49]. Also, similar to synaptotagminI action as a Ca2+ sensor in synaptic transmission [47], A7 is postulated also to act as Ca2+-dependent GTPase and could very likely function as a Ca2+/GTP sensor [50, 51] during lung surfactant secretion.
In conclusion, our in vivo and in vitro studies demonstrate the regulation of A7 trafficking to the plasma membrane compartment in alveolar type II cells by Ca2+ and PKC-dependent mechanisms. Since both processes are important in the regulation of lung surfactant secretion and because of our previous observation of importance of A7 in membrane fusion, we suggest that increased trafficking of A7 to plasma membrane is for augmentation of lung surfactant secretion. However, confirmation of precise role for A7 interactions with SNAP23 would require additional studies establishing regulatory structural or phosphorylation mutants of A7 and their in vivo effects on regulated secretion of lung surfactant.
Research Highlights.
Interactions of annexin A7 with SNARE protein SNAP23 are demonstrated.
Surfactant secretagogues increase annexin A7 and SNAP23 interactions in lung cells.
The intracellular co-localization and in vitro binding is PKC dependent.
The trafficking of lamellar body to SNAP23 domains is regulated by PKC.
ACKNOWLEDGMENTS
The authors are thankful for excellent technical assistance of Delon Callender and Kelly Holbrook. The confocal microscopy imaging and analysis were performed at the Confocal Microscopy Imaging Center of the Stony Brook University. These studies were supported by a grant (HL49959 to AC) from NHLBI, NIH, Bethesda, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:233–243. doi: 10.1016/s1095-6433(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 2.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. American journal of physiology. 2007;293:L259–271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 3.Chander A, Fisher AB. Regulation of lung surfactant secretion. The American journal of physiology. 1990;258:L241–253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 4.Chander A, Wu RD. In vitro fusion of lung lamellar bodies and plasma membrane is augmented by lung synexin. Biochimica et biophysica acta. 1991;1086:157–166. doi: 10.1016/0005-2760(91)90003-z. [DOI] [PubMed] [Google Scholar]
- 5.Chander A, Sen N, Spitzer AR. Synexin and GTP increase surfactant secretion in permeabilized alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L991–998. doi: 10.1152/ajplung.2001.280.5.L991. [DOI] [PubMed] [Google Scholar]
- 6.Chander A, Chen XL, Naidu DG. A role for diacylglycerol in annexin A7-mediated fusion of lung lamellar bodies. Biochimica et biophysica acta. 2007;1771:1308–1318. doi: 10.1016/j.bbalip.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich LE, Boeddinghaus C, LaGrassa TJ, Ungermann C. Control of eukaryotic membrane fusion by N-terminal domains of SNARE proteins. Biochimica et biophysica acta. 2003;1641:111–119. doi: 10.1016/s0167-4889(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 8.Fasshauer D. Structural insights into the SNARE mechanism. Biochimica et biophysica acta. 2003;1641:87–97. doi: 10.1016/s0167-4889(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 9.Gerst JE. SNARE regulators: matchmakers and matchbreakers. Biochimica et biophysica acta. 2003;1641:99–110. doi: 10.1016/s0167-4889(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 10.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 11.Mayer A. Membrane fusion in eukaryotic cells. Annual review of cell and developmental biology. 2002;18:289–314. doi: 10.1146/annurev.cellbio.18.032202.114809. [DOI] [PubMed] [Google Scholar]
- 12.Paumet F, Rahimian V, Rothman JE. The specificity of SNARE-dependent fusion is encoded in the SNARE motif. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3376–3380. doi: 10.1073/pnas.0400271101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nature neuroscience. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 14.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science (New York, N.Y. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Wang M, Fisher AB, Zimmerman UJ. Involvement of annexin II in exocytosis of lamellar bodies from alveolar epithelial type II cells. The American journal of physiology. 1996;270:L668–676. doi: 10.1152/ajplung.1996.270.4.L668. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay S, Sun P, Wang P, Abonyo B, Cross NL, Liu L. Fusion of lamellar body with plasma membrane is driven by the dual action of annexin II tetramer and arachidonic acid. J Biol Chem. 2003;278:39675–39683. doi: 10.1074/jbc.M212594200. [DOI] [PubMed] [Google Scholar]
- 17.Hong K, Duzgunes N, Ekerdt R, Papahadjopoulos D. Synexin facilitates fusion of specific phospholipid membranes at divalent cation concentrations found intracellularly. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:4642–4644. doi: 10.1073/pnas.79.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caohuy H, Pollard HB. Activation of annexin 7 by protein kinase C in vitro and in vivo. J Biol Chem. 2001;276:12813–12821. doi: 10.1074/jbc.M008482200. [DOI] [PubMed] [Google Scholar]
- 19.Sen N, Spitzer AR, Chander A. Calcium-dependence of synexin binding may determine aggregation and fusion of lamellar bodies. The Biochemical journal. 1997;322(Pt 1):103–109. doi: 10.1042/bj3220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abonyo BO, Gou D, Wang P, Narasaraju T, Wang Z, Liu L. Syntaxin 2 and SNAP-23 are required for regulated surfactant secretion. Biochemistry. 2004;43:3499–3506. doi: 10.1021/bi036338y. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Chintagari NR, Gou D, Su L, Liu L. Physical and functional interactions of SNAP-23 with annexin A2. American journal of respiratory cell and molecular biology. 2007;37:467–476. doi: 10.1165/rcmb.2006-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chander A, Sen N, Naidu DG, Spitzer AR. Calcium ionophore and phorbol ester increase membrane binding of annexin a7 in alveolar type II cells. Cell calcium. 2003;33:11–17. doi: 10.1016/s0143-4160(02)00177-x. [DOI] [PubMed] [Google Scholar]
- 23.Gerelsaikhan T, Chen XL, Chander A. Secretagogues of lung surfactant increase annexin A7 localization with ABCA3 in alveolar type II cells. Biochimica et biophysica acta. 2011;1813:2017–2025. doi: 10.1016/j.bbamcr.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, Morohoshi T, Ogawa J, Shioda S, Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS letters. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 25.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- 26.Chander A, Sen N. Inhibition of phosphatidylcholine secretion by stilbene disulfonates in alveolar type II cells. Biochemical pharmacology. 1993;45:1905–1912. doi: 10.1016/0006-2952(93)90450-b. [DOI] [PubMed] [Google Scholar]
- 27.Chander A, Johnson RG, Reicherter J, Fisher AB. Lung lamellar bodies maintain an acidic internal pH. The Journal of biological chemistry. 1986;261:6126–6131. [PubMed] [Google Scholar]
- 28.Naidu DG, Raha A, Chen XL, Spitzer AR, Chander A. Partial truncation of the NH2-terminus affects physical characteristics and membrane binding, aggregation, and fusion properties of annexin A7. Biochimica et biophysica acta. 2005;1734:152–168. doi: 10.1016/j.bbalip.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. The American journal of physiology. 1982;242:C404–408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 31.Chander A, Sen N, Wu AM, Spitzer AR. Protein kinase C in ATP regulation of lung surfactant secretion in type II cells. The American journal of physiology. 1995;268:L108–116. doi: 10.1152/ajplung.1995.268.1.L108. [DOI] [PubMed] [Google Scholar]
- 32.Linke MJ, Burton FM, Fiedeldey DT, Rice WR. Surfactant phospholipid secretion from rat alveolar type II cells: possible role of PKC isozymes. The American journal of physiology. 1997;272:L171–177. doi: 10.1152/ajplung.1997.272.2.L171. [DOI] [PubMed] [Google Scholar]
- 33.Gerke V, Moss SE. Annexins and membrane dynamics. Biochimica et biophysica acta. 1997;1357:129–154. doi: 10.1016/s0167-4889(97)00038-4. [DOI] [PubMed] [Google Scholar]
- 34.Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochimica et biophysica acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 35.Johnstone SA, Hubaishy I, Waisman DM. Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. J Biol Chem. 1992;267:25976–25981. [PubMed] [Google Scholar]
- 36.Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rickman C, Davletov B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J Biol Chem. 2003;278:5501–5504. doi: 10.1074/jbc.C200692200. [DOI] [PubMed] [Google Scholar]
- 38.Quetglas S, Iborra C, Sasakawa N, De Haro L, Kumakura K, Sato K, Leveque C, Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. The EMBO journal. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohyama A, Hosaka K, Komiya Y, Akagawa K, Yamauchi E, Taniguchi H, Sasagawa N, Kumakura K, Mochida S, Yamauchi T, Igarashi M. Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J Neurosci. 2002;22:3342–3351. doi: 10.1523/JNEUROSCI.22-09-03342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condliffe SB, Zhang H, Frizzell RA. Syntaxin 1A regulates ENaC channel activity. J Biol Chem. 2004;279:10085–10092. doi: 10.1074/jbc.M313592200. [DOI] [PubMed] [Google Scholar]
- 41.Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. The EMBO journal. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duman JG, Forte JG. What is the role of SNARE proteins in membrane fusion? Am J Physiol Cell Physiol. 2003;285:C237–249. doi: 10.1152/ajpcell.00091.2003. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda M. Vesicle-associated membrane protein-2/synaptobrevin binding to synaptotagmin I promotes O-glycosylation of synaptotagmin I. J Biol Chem. 2002;277:30351–30358. doi: 10.1074/jbc.M204056200. [DOI] [PubMed] [Google Scholar]
- 44.Chapman ER, An S, Edwardson JM, Jahn R. A novel function for the second C2 domain of synaptotagmin. Ca2+-triggered dimerization. J Biol Chem. 1996;271:5844–5849. doi: 10.1074/jbc.271.10.5844. [DOI] [PubMed] [Google Scholar]
- 45.Sutton RB, Ernst JA, Brunger AT. Crystal structure of the cytosolic C2A-C2B domains of synaptotagmin III. Implications for Ca(+2)-independent snare complex interaction. The Journal of cell biology. 1999;147:589–598. doi: 10.1083/jcb.147.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nature structural & molecular biology. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 48.Rhee JS, Li LY, Shin OH, Rah JC, Rizo J, Sudhof TC, Rosenmund C. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrick DZ, Kuo W, Huang H, Schwieters CD, Ellena JF, Cafiso DS. Solution and membrane-bound conformations of the tandem C2A and C2B domains of synaptotagmin 1: Evidence for bilayer bridging. Journal of molecular biology. 2009;390:913–923. doi: 10.1016/j.jmb.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caohuy H, Pollard HB. Protein kinase C and guanosine triphosphate combine to potentiate calcium-dependent membrane fusion driven by annexin 7. J Biol Chem. 2002;277:25217–25225. doi: 10.1074/jbc.M202452200. [DOI] [PubMed] [Google Scholar]
- 51.Caohuy H, Srivastava M, Pollard HB. Membrane fusion protein synexin (annexin VII) as a Ca2+/GTP sensor in exocytotic secretion. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10797–10802. doi: 10.1073/pnas.93.20.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]