Abstract
Background
SLOB binds to and modulates the activity of the Drosophila Slowpoke (dSlo) calcium activated potassium channel. Recent microarray analyses demonstrated circadian cycling of slob mRNA.
Results
We report the mRNA and protein expression pattern of slob in Drosophila heads. slob transcript is present in the photoreceptors, optic lobe, pars intercerebralis (PI) neurons and surrounding brain cortex. SLOB protein exhibits a similar distribution pattern, and we show that it cycles in Drosophila heads, in photoreceptor cells and in neurosecretory cells of the PI. The cycling of SLOB is altered in various clock gene mutants, and SLOB is expressed in ectopic locations in tim01 flies. We also demonstrate that SLOB no longer cycles in the PI neurons of Clkjrk flies, and that SLOB expression is reduced in the PI neurons of flies that lack pigment dispersing factor (PDF), a neuropeptide secreted by clock cells.
Conclusions
These data are consistent with the idea that SLOB may participate in one or more circadian pathways in Drosophila.
Background
Five independent groups [1-5] recently conducted genome-wide microarray analyses to identify Drosophila transcripts that display circadian oscillations. Each group uncovered slob as a robustly cycling RNA transcript. SLOB, Slowpoke binding protein, is a key component of the Drosophila Slowpoke/SLOB/Leonardo dynamic protein complex [6]. This complex is thought to affect membrane excitability, as electrophysiological recordings reveal that SLOB binding to the channel results in an increase in channel activity, whereas the addition of Leonardo to the SLOB/dSlo complex dramatically shifts the channel voltage range of activation to more depolarized potentials [6].
The circadian system consists of an input pathway, a central clock, and an output pathway [7]. The clock itself is comprised of the transcription factors CLOCK (CLK) and CYCLE (CYC), which bind to the promoters of period (per) and timeless (tim), inducing their expression. The PER and TIM proteins heterodimerize and feed back to repress activity of CLK/CYC. Although the expression patterns of CLK and CYC are not known, the localization of PER and TIM has been important with respect to identifying neurons relevant for circadian behavioral rhythms [8]. This molecular loop must transduce signals to surrounding cells to generate rhythmic behavior [9]. Altering membrane excitability is a key mechanism for transducing neuronal information, and thus it is reasonable to suspect that the molecular feedback loop might communicate with ion channels.
Ion channels may be under direct transcriptional control of the clock genes or modulated by clock-controlled genes [10,11]. Mounting evidence supports the importance of electrical activity for the propagation of circadian oscillations. For example, electrical silencing of clock neurons through targeted expression of potassium channels stops the oscillation of PER and TIM proteins and causes arrhythmicity in flies [12]. The cyclic release of neuropeptides from clock cells [13] may be a direct consequence of a rhythmic fluctuation in membrane potential [14]. Diurnal modulation of pacemaker potentials and calcium current, intracellular calcium levels and NMDA-evoked calcium currents have all been observed within a mammalian central clock, the suprachiasmatic nucleus (SCN) [11,15,16]. In addition, microarray screens have detected the cycling transcripts of ion channels such as Shaker, trpl and slowpoke [1,3], and flies mutant in slowpoke have weak locomotor rhythms [1]. These observations suggest that ion channels and their modulators may participate in circadian regulation.
We explored a role for SLOB in the circadian system and found that SLOB protein cycles in Drosophila heads during both light/dark and constant darkness conditions. SLOB oscillates in at least two discrete areas of the fly head, the photoreceptor cells and the PI neurons. The photoreceptors have their own peripheral circadian oscillator [17], whereas the PI neurons, large neurosecretory neurons, are suspected to play a role in the output pathway that drives rest:activity rhythms (Kaneko and Hall, 2000). Our results reveal differential effects of clock mutations on SLOB expression and cycling in these two regions. There is a significant decrease in SLOB levels in the PI neurons of Pdf01 flies, thus implicating PDF as an upstream regulator of SLOB. SLOB also no longer cycles in the PI neurons of Clkjrk flies, supporting the idea that SLOB is a clock controlled protein. Together with the observation that flies overexpressing SLOB exhibit a breakdown of rest:activity patterns, these data are consistent with the idea that SLOB participates in circadian rhythms.
Results
SLOB protein cycles in Drosophila heads
To determine whether SLOB protein cycles, we performed western blot analysis on Drosophila head lysates from wild type Canton S (CS) or yellow-white (y w) flies entrained for three days in 12:12 hour light/dark cycles (LD). We found that SLOB protein cycles (Figure 1A) with a peak at Zeitgeber Time (ZT) 10–14, and a trough around ZT 2 (ZT 0 = lights on; ZT 12 = lights off in a 12 hour:12 hour light/dark cycle).
Figure 1.
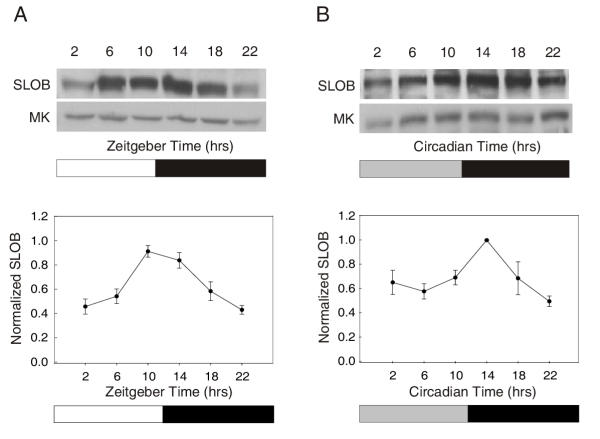
SLOB protein cycles in both LD and DD. (A) Canton S flies were entrained in LD for three days and collected at six time points over a 24 hour period. Fly head lysates were prepared and an anti-SLOB antibody was used to identify the 58 kDa band representing SLOB on the top western blot. The bottom blot was probed for MAPK, the protein level of which does not cycle [37] and was used as a loading control. SLOB oscillation has a trough at ZT 2 and a peak between ZT 10–14. The graph below represents the mean ± SEM for each time point from a minimum of three independent experiments. (B) Canton S flies were entrained in LD for three days and transferred to constant darkness. They were collected on the second day of DD at six time points, and SLOB cycling was determined as in (A).
To ensure that rhythms of SLOB expression are circadian and not driven only by external LD cycling we analyzed SLOB protein cycling in conditions of constant darkness. Flies were entrained for three days in LD, and then placed in constant darkness (DD) for two days. SLOB protein still cycles under these conditions, with a slightly dampened amplitude, but with the same overall phase (Figure 1B). A similar decrease in amplitude has been described for oscillations of other clock genes in DD [18,19].
Expression of SLOB protein is influenced by clock genes
To determine if the clock genes control the cycling of SLOB, we assayed its expression in different mutant backgrounds. Clkjrk flies, flies mutant for CLK, were entrained in LD cycles for three days and then collected at six time points. Some flies were transferred from LD to DD for two additional days and likewise collected at six time points. Western blot analysis of Drosophila heads shows that SLOB protein continues to cycle in Clkjrk adults in LD, with a phase and amplitude similar to wild type (Figure 2A). In DD, the western blot analysis shows SLOB expression to have no discernable peak indicating that it does not cycle (Figure 2B). Claridge-Chang et al. [2] noted that slob RNA is down regulated in the Clkjrk mutant, suggesting that CLK acts as a transcriptional activator of slob expression. We did not observe a dramatic decrease in protein levels in Clkjrk flies; the level was between the trough and peak levels seen in wild type flies.
Figure 2.
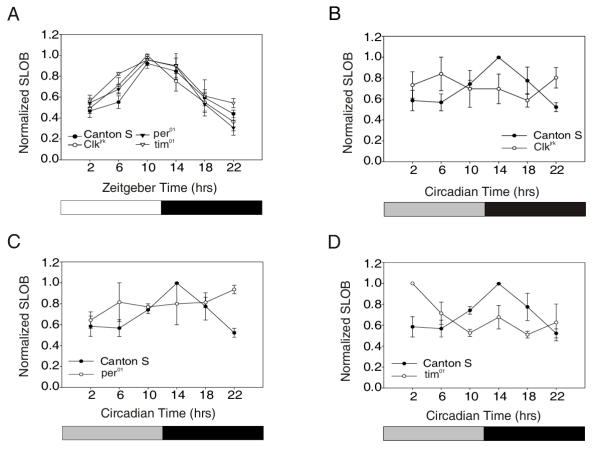
SLOB cycling is altered under DD conditions in clock mutants. (A) Western blot analysis of SLOB cycling in Canton S, per01, tim01 and Clkjrk flies under LD conditions. Oscillations in all flies are in phase with one another. The graphs represent the mean ± SEM for each time point from a minimum of three experiments for each genotype. (B, C) Western blot analysis of Canton S, Clkjrk and per01 flies in DD conditions. There is no obvious cycling of SLOB in Clkjrk and per01 flies. (D) Western blot analysis of Canton S and tim01 flies in DD conditions. There is a shift in the phase of SLOB cycling in tim01 flies. The peak is no longer at CT 14 but at CT 2. The same Canton S data are illustrated in panels (B-D).
The cycling of SLOB was also analyzed in per01 and tim01 mutants during LD (Figure 2A) and DD (Figure 2C,2D). In both mutant lines, SLOB continues to cycle in LD with a phase similar to that of wild type (Figure 2A). SLOB may be regulated by a direct light-dependent mechanism, obviating the need for these clock genes in LD. As in the case of Clkjrk, there appears to be either dampened or no protein cycling in per01 flies under DD conditions (Figure 2C). However, in tim01 flies SLOB still cycles, but there is a shift in the phase of the oscillation in DD (Figure 2D). Instead of peaking at Circadian Time (CT) 10–14, SLOB now peaks at CT 2. This suggests that the regulation of SLOB by TIM may be different from that by PER, which is not unprecedented [20].
Expression pattern of slob transcript in Drosophila heads
To determine the expression pattern of slob transcript, we performed in situ hybridizations on cryosections of Drosophila yellow-white (y w) fly heads. Digoxygenin-labeled antisense and sense RNA probes of slob were used on frontal sections and the antisense revealed a widespread distribution of slob. Expression of slob was detected in the brain cortex (Figure 3A), photoreceptors, lamina and medulla (Figure 3B) and the PI region of the brain (Figure 3C). A similar pattern of transcript distribution, particularly in the photoreceptors, optic lobes and brain cortex, is seen for timeless, clock and cycle [21]. Figure 3D,3E,3F shows the respective areas of Figures 3A,3B,3C using the sense RNA probe for slob.
Figure 3.
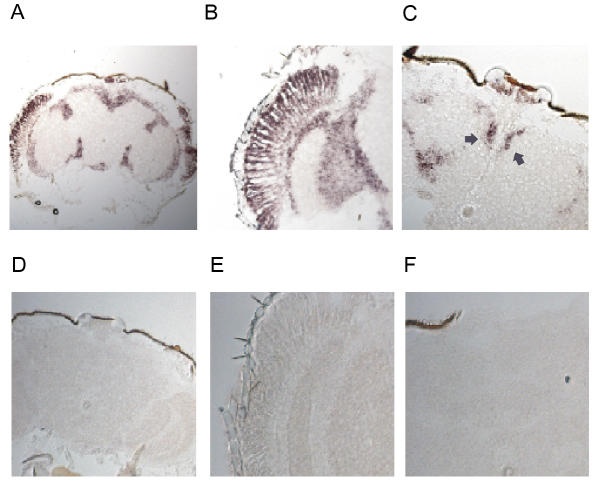
Distribution of slob mRNA. Frontal sections of Drosophila heads were probed with digoxigenin labeled RNA probes for slob. (A) Low magnification to survey entire head section. Antisense slob probes hybridized to the photoreceptors, optic lobe, PI neurons and the surrounding brain cortex. (B) High levels of expression detected in the photoreceptors, lamina and medulla. (C) slob message appears in the PI region. The arrows point to the dorsal medial PI neurons. (D-F) Digoxigenin labeled sense probes of slob reveal no significant labeling. The three panels correspond to the head sections of (A-C).
SLOB is expressed in the photoreceptors and the brain
Previously, we demonstrated that SLOB protein is present at the neuromuscular junction of Drosophila larvae [6]. In order to locate SLOB protein in the adult fly, frontal head sections and wholemounts of the brain were prepared from Canton S and y w flies. Immunostaining of sections reveals intense staining in the nuclei of the photoreceptor cells and the basal cells of the eye (Figure 4A). The brain wholemounts reveal very bright and discrete cytoplasmic staining of 6–8 PI neurons, with widespread but less intense staining elsewhere in the optic lobe and brain cortex (Figure 4B,4C). Although the overall patterns of distribution of mRNA and protein are comparable, there are apparent differences in the abundance of protein relative to that of mRNA in some regions. This may simply be due to the histological differences between head sections (Figure 3) and brain wholemounts (Figure 4B), or to the different detection methods used. It is also possible that SLOB protein does not accumulate everywhere the mRNA is found. SLOB protein is also expressed in other head tissues such as the antenna and proboscis (data not shown).
Figure 4.
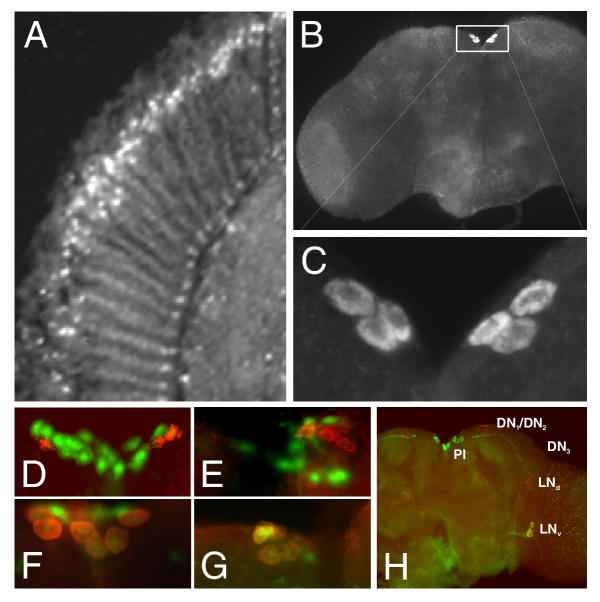
SLOB is expressed prominently in photoreceptor cells and PI neurons. (A) Head frontal section from y w (wild type) flies immunostained for SLOB protein. SLOB is expressed in the nuclei of the photoreceptor cells. (B) Brain wholemounts from y w flies immunostained for SLOB protein. SLOB is expressed prominently in the PI neurons of the protocerebrum (box), as well as elsewhere in the brain. (C) Enlargement of the boxed area in (B) to illustrate the cytoplasmic localization of SLOB in the PI neurons. Typically 6–8 PI neurons are SLOB positive. (D-G) GAL4 flies, specific for the three subsets of PI neurons, were crossed to UAS-GFP flies and immunostained for SLOB. (D) Mai 301 GAL4 is expressed in PI-1 neurons [22], shown by GFP expression. UAS-GFP tends to have a nuclear localization. SLOB positive PI neurons are immunostained with Texas Red, and SLOB has a cytoplasmic expression pattern. SLOB does not localize within PI-1 neurons. (E) Kurs 58 GAL4 is expressed in PI-2 neurons. SLOB is not found within the PI-2 neurons. (F) Mai 281 GAL4 is expressed in two of the three subsets of PI neurons (PI-2 and PI-3). SLOB is localized within some of these PI neurons and because SLOB was not found within PI-2 this suggests that the SLOB positive subset is PI-3. (G) Kurs 45 GAL4 is expressed only in PI-3 neurons. SLOB positive cells colocalize with the GFP expressing PI-3 cells. (H) Brain wholemount from y w flies highlighting components of the circadian system – the small ventral lateral neurons (LNv), the dorsal lateral neurons (LNd), the dorsal neurons (DN1–3) and the pars intercerebralis neurons (PI). The wholemount was immunostained for SLOB, PER and PDF. The red component is PER staining; the green staining in the PI neurons corresponds to SLOB, and the green staining elsewhere is PDF. Previous staining in Pdf01 flies reveals no PDF in the PI neurons (data not shown). PDF filled projections end close to, but appear to stop short of, the PI neurons. The projections of the DN (not visible) may synapse on the PI neurons.
Siegmund and Korge [22] performed a large scale analysis of peptidergic neurons of Drosophila larvae. Their study identified three distinct subsets of PI neurons (PI 1–3) that innervate the corpora cardiaca (a glandular tissue) and the aorta. To determine which subset of PI neurons is SLOB positive we used four of their enhancer trap lines. Mai 301, Kurs 58 and Kurs 45 GAL4 lines express GAL4 exclusively in subsets PI-1, PI-2 and PI-3, respectively. Mai 281 GAL4 expresses GAL4 in two subsets, PI-2 and PI-3. We crossed these GAL4 flies to a UAS-GFP transgenic line, and found by immunostaining that SLOB is expressed exclusively in subset PI-3 (Figure 4F,4G). SLOB does not colocalize with GFP from the enhancer fly lines that express GFP only in subsets PI-1 or -2 (Figure 4D,4E).
Figure 4H highlights components of the circadian system in order to illustrate the location of the PI neurons in relation to other clock gene expressing cells. The lateral neurons (LNs) as mentioned before consist of three clusters, a cluster of cells located dorsally (LNds), and two other ventrally located clusters differing in the size of their somata, large LNvs and small LNvs. Another group of clock gene expressing cells are the dorsal neurons (DNs), which consists of three subsets as well, DN1–3. This wholemount was immunostained for SLOB, PER and PDF. The LNs and DNs are immunostained red indicating the presence of PER. Projections from the LNvs are green due to PDF immunostaining. The PI neurons are the SLOB positive green cells.
The phase of SLOB protein cycling is different in the photoreceptor and PI neurons
Immunohistochemistry was used to determine whether SLOB cycles in the photoreceptor and PI neurons. Flies were entrained for three days in LD and collected at four time points; others were then transferred to DD for two additional days and likewise collected at four time points. Antibody against SLOB was used on whole head sections and wholemounts of the brain. We find that SLOB protein cycles in the nuclei of the photoreceptor neurons with a trough at ZT 2 and a peak at ZT 14–21 (Figure 5A). During constant darkness there is a peak at around CT 14 (Figure 5A). This correlates with western blot data collected from the entire Drosophila head (see Figure 2). Intriguingly, in the PI neurons, SLOB cycles with a peak at ZT 3 and a trough at ZT 15–21 (Figure 5B), out of phase to the oscillation in the photoreceptor neurons. SLOB continues to oscillate during constant darkness conditions in both the photoreceptor and PI neurons (Figure 5A,5B).
Figure 5.
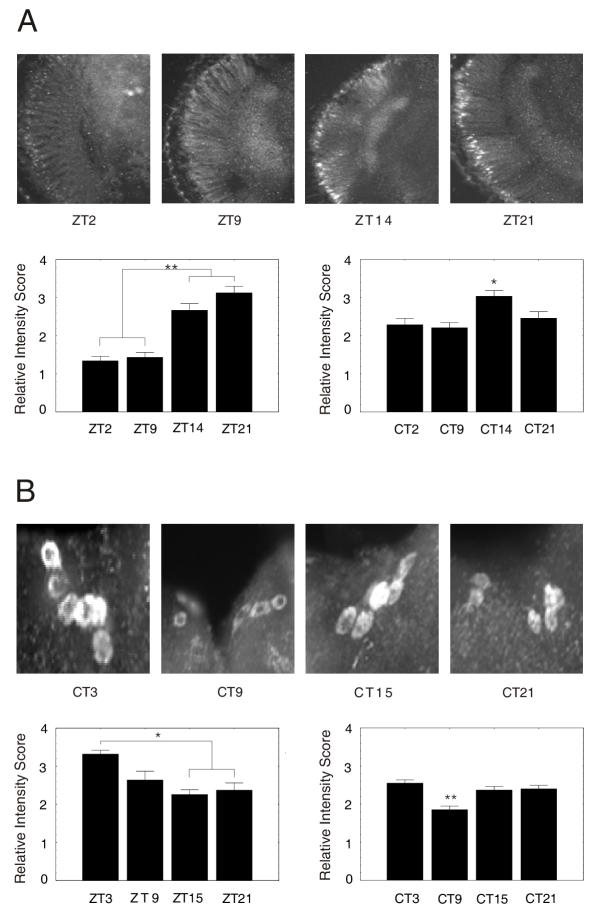
SLOB cycles in the photoreceptor cell and the PI neurons. y w flies were entrained in LD for three days and collected at four time points over a 24 hour period. Some flies, after three days entrainment, were transferred to DD for two additional days. At least 5 heads were assayed per time point. (A) Head frontal sections were immunostained for SLOB. The bars depict mean ± SEM staining intensity scores (*p < 0.05, ** p < 0.001). The left bar graph is for LD and the right is for DD. SLOB cycling in the photoreceptors closely follows that of the western blots for both LD and DD. (B) Brain wholemounts from y w flies immunostained for SLOB. SLOB cycles in the PI neurons with an altered phase compared to the photoreceptors. The left bar graph is for LD and the right is for DD. Statistical analysis was done as in (A).
Ectopic expression of SLOB in tim01 flies
We noticed an increase in SLOB protein levels, at all time points examined by western blot, in tim01 flies. To locate where the increase in SLOB occurs, we stained tim01 heads with anti-SLOB antibody, and found considerable SLOB expression in the optic lobe, specifically in the outer edges of the lamina (Figure 6A). Samples shown here are at ZT 14. Expression in these regions is much more limited in the wild type controls. No increases in SLOB expression were detectable in the PI neurons of tim01 flies (Figure 6B). Since SLOB protein normally appears to be expressed at relatively low levels in the optic lobe, we infer that the loss of TIM leads to elevated or ectopic expression of SLOB in specific cells. We cannot exclude the possibility that the SLOB signal from tim01 flies seen on western blots represents other head tissues as well. This elevated expression of SLOB in tim01 flies further demonstrates the regulation of SLOB expression by the clock genes.
Figure 6.
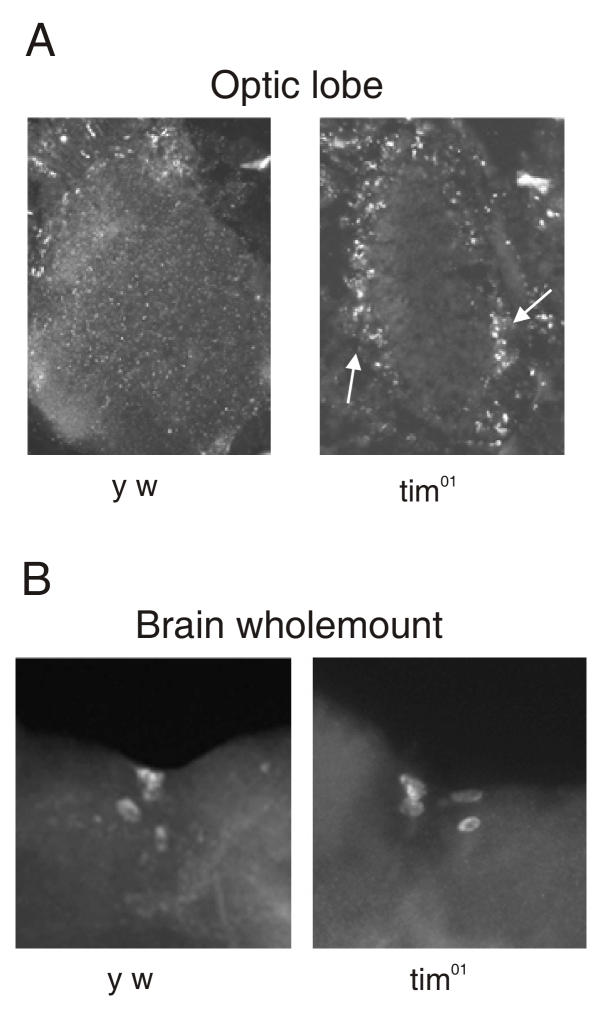
Elevated or ectopic expression of SLOB in tim01 flies. (A) Head sections from y w and tim01 flies immunostained for SLOB. There is greater expression of SLOB in the optic lobe in tim01 flies. The arrowheads point to the outer edges of the lamina. (B) Brain wholemounts from y w and tim01 flies immunostained for SLOB. There is no change in SLOB expression within the PI neurons of the tim01 flies.
SLOB is regulated by a circadian output molecule, PDF
The neuropeptide, PDF, accumulates at dorsal axon terminals of the small LNvs in a cyclic fashion, indicative of regulated release from these terminals [13]. After two to three days in constant darkness, the majority of Pdf null flies are behaviorally arrhythmic supporting a role for PDF in clock output [23]. The location of SLOB in PI neurons makes it a candidate for an output molecule. To determine whether SLOB is a component of the output pathway, we analyzed SLOB expression in the PI neurons of a clock mutant, Clkjrk, and the output mutant, Pdf01. Flies were entrained and collected at four time points. Western analysis of head lysates during DD indicated that SLOB cycling is dampened or absent in Clkjrk flies (Figure 3B). Similarly, SLOB cycling in DD is diminished in the PI neurons in Clkjrk flies (Figure 7A). This suggests that CLK is at least indirectly required for SLOB oscillation in the PI neurons. Wholemounts of brains from Pdf01 flies reveal a decrease in SLOB expression and dampened cycling in the PI neurons (Figure 7B), consistent with a role for SLOB downstream of the PDF-secreting neurons.
Figure 7.
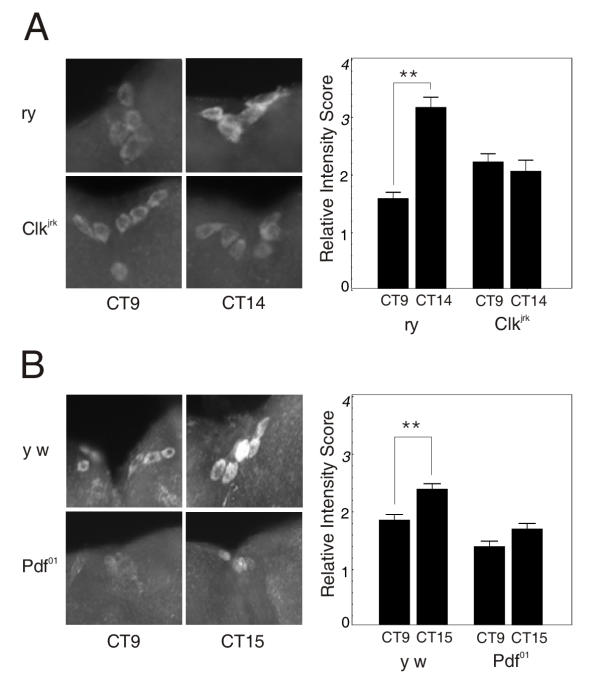
SLOB expression is altered in Clkjrk and Pdf01 flies. (A) Brain wholemount from wild type rosy (ry) and Clkjrk flies. Two time points, taken on the second day of DD, show that SLOB does not cycle in the PI neurons of the Clkjrk flies. The accompanying bar graph illustrates relative intensity scores. Data were analyzed as described in Figure 5. (B) Brain wholemount from wild type y w and Pdf01 flies. Two time points, taken on the second day of DD, demonstrate the decrease in SLOB expression and dampened cycling in the PI neurons of Pdf01 flies. PDF appears to positively regulate SLOB protein. The bar graph represents relative intensity scores.
Overexpression of SLOB alters locomotor activity
To determine whether manipulation of SLOB levels can alter behavioral rhythms during constant darkness conditions, we created two transgenic UAS (upstream activational sequence) lines. One line, UAS-slob, expresses a wild type version of the SLOB protein whereas the second, UAS-mtslob, expresses a mutant form of SLOB that renders it unable to bind Leonardo [6]. The UAS lines were crossed to elavC155 (panneuronal), GMR (eye) and Kurs 45 (PI-3) GAL4 drivers. Flies were entrained for three days in LD cycles and then monitored for rest:activity in DD for 14 days. Progeny of the crosses between UAS-slob or UAS-mtslob and elavc155GAL4 are robustly rhythmic for the first seven days, followed by a breakdown in their rhythms during the last seven days (Figure 8A). This breakdown is not seen in control lines. Thus, the lines that overexpress SLOB exhibit a decrease in the strength of rhythmicity determined by their Fast Fourier Transform (FFT) value during the last seven days, as compared to the control lines (Figure 8B). Overexpression of either form of SLOB protein results in the breakdown of rhythms suggesting that Leonardo binding is not required for this phenomenon. There is some breakdown in rhythmicity in UAS-slob1/+ flies (Figure 8B), but this is most likely due to leaky expression of the UAS-slob transgene (inset in Figure 8B). However, breakdown in rhythmicity does not occur in the UAS-mtslob/+ flies (Figure 8B), and western blot analysis indicates no such leaky expression of the UAS-mtslob transgene (inset in Figure 8B). Fly lines carrying either the GMR or K45 driver along with UAS-slob show no decrease in rhythmicity (Figure 8C), suggesting that SLOB overexpression in the eye or PI-3 may not be the cause of the behavioral breakdown. These data indicate that ectopic SLOB expression is capable of altering a behavioral rhythm produced by the circadian system.
Figure 8.
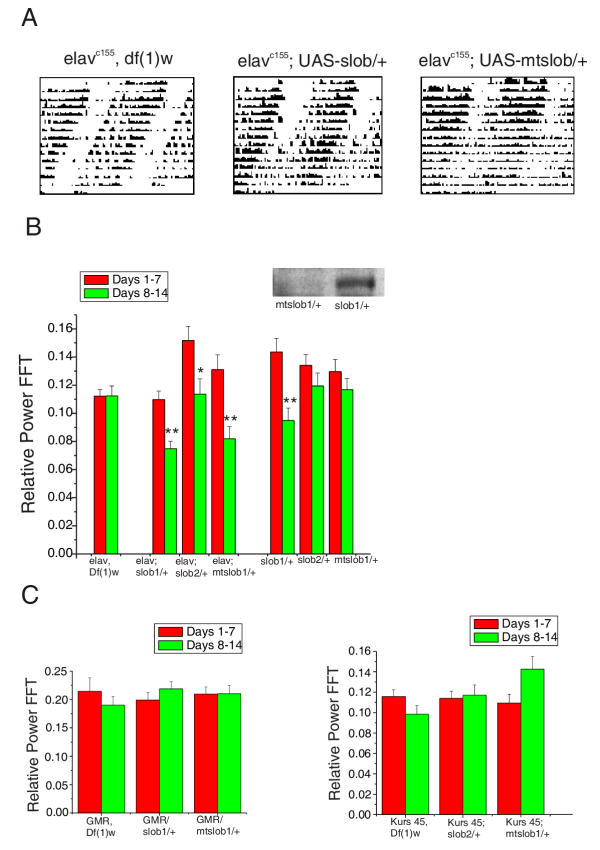
Overexpression of SLOB alters behavioral rhythms. Elavc155, GMR and Kurs 45 GAL4 flies were crossed to UAS-slob, UAS-mtslob and Df(1)w flies. Progeny were entrained for three days in LD cycles and then transferred to a locomotor monitoring device for 14 days in DD. (A) Actograms representing control, UAS-slob and UAS-mtslob lines crossed to elavc155. There is an apparent breakdown of rhythmicity after 7 days in flies overexpressing wild type and mutant SLOBs (middle and right panels). (B) Summary of behavioral data for elavc155 flies crossed to control and UAS lines. The FFT value is a measure of rhythmic strength and is plotted in the bar graph for the first and second week periods per fly line. Using Student's t test, all overexpressing SLOB lines exhibit significant breakdown of rhythms during the second seven days (*p < 0.05 and **p < 0.001, respectively). Controls show no significant breakdown except for one line, UAS-slob1/+. However, as demonstrated by the inserted western blot, this line expresses transgenic SLOB in the absence of a GAL4 driver, whereas the UAS-mtslob1/+ line does not. The blot was probed with an anti-HA antibody that detects only transgenic SLOB. Levels of MAPK were used as loading controls (data not shown). (C) Summary of behavioral data for GMR and Kurs 45 GAL4 flies crossed to various lines. No change in rhythmicity is evident when SLOB is overexpressed only in the Drosophila eye or PI neurons.
Discussion
We report here that the dSlo binding protein, SLOB, cycles in Drosophila heads. Microarray analyses reported slob transcript cycling with a peak at either ZT 15 [2] or at CT 11 [3]. We show by western analysis that SLOB protein peaks at ZT 10–14/CT 14, consistent with an earlier peak for the RNA. Under LD conditions, SLOB continues to cycle in per01, tim01 and Clkjrk flies in phase with the oscillation of wild type flies. It was noted in one of the recent microarray studies [2] that there is a cluster of genes, named the apterous cluster, that shows rhythmicity in these three mutants during LD. This cluster has a characteristic peak at ZT 17 and includes such proteins as transcription factors, synaptic regulators and transporters. The genes in this group may be regulated not only by the circadian clock, but also by a light-dependent mechanism. However in DD, where light is no longer a factor, we find that SLOB does not cycle in Clkjrk and per01 and exhibits an altered phase in tim01 flies. One might expect tim01 and per01 flies to give similar results, but tim01 flies may not be true genetic nulls [24]. Consistent with the observation that SLOB does not cycle in Clkjrk flies during DD (Figure 2B) are microarray data from Ueda et al. [5] indicating that slob levels do not change in Clkjrk flies, and other microarray data from McDonald and Rosbash [3] demonstrating that slob levels are at mid-point in the Clkjrk mutants. Taken together these data demonstrate clearly that clock genes regulate slob mRNA and protein expression.
Within Drosophila adult heads, slob mRNA is present in the photoreceptors, optic lobe, the neurosecretory cells of the PI and the surrounding brain cortex. We also find prominent immunostaining for SLOB protein in the photoreceptor cells and the PI neurons. In situ hybridization experiments with larval brain revealed slob RNA in an area of the brain close to PDF-filled projections of the lateral neurons [2]. This is consistent with our findings of slob transcript in the PI neurons and surrounding cortex.
The Drosophila eye expresses many of the major circadian genes, and is thought to contain an autonomous oscillator that presumably regulates an eye-specific function [17]. In addition, the eye contributes to photic entrainment of the pacemaker LN cells [25]. Interestingly, the SLOB binding partner, Slo is also expressed in the visual system including the eye, lamina, medulla and lobula [26]. Ceriani et al. [1] have demonstrated that the RNA levels of both slob and slo cycle in phase in both LD and DD. The dSlo protein was also shown by western blot to cycle and peak at ZT 20 [1]. This correlates with the cycling of SLOB in the photoreceptors, where SLOB peaks at ZT 14–21 (Figure 5A).
The PI region lies directly beneath the root of the ocellar nerve. The PI neurons have large, 15 μm diameter, cell bodies, and their axons project along the median bundle and then bifurcate [27]. One of the branches proceeds ventrally and arborises in the dorsal tritocerebrum region, below the oesophagus. The other branch moves in a posterior direction and enters the cardiac recurrent nerve in the oesophageal canal. The PI neurons have an extensive network of endoplasmic reticulum and contain secretory granules, suggesting that they are neurosecretory cells [27]. In insects, peptidergic neurons of the central nervous system regulate the synthesis of developmental hormones. The PI neurons, in particular, have been implicated in hormone production and release in various insects [28,29]. Three subsets of PI neurons have been identified. We have identified the subgroup PI-3 to be the SLOB positive subset of PI neurons. Among the hormones identified in the PI neurons is insulin, and it has been proposed that the release of insulin into the hemolymph is essential for growth control and carbohydrate homeostasis [30]. We have confirmed that the SLOB positive PI neurons are also insulin positive (data not shown).
The photoreceptors and the PI neurons express oscillating SLOB protein and intriguingly, the rhythms in the two neuronal types are not in phase with each other. The mechanisms responsible for these phase differences are not known. PER and TIM are expressed in the lateral neurons in the central brain, in glial cells of the optic lobes, and in the photoreceptor cells [7]. PER and TIM protein have not been shown to be expressed in the PI neurons, although Kaneko and Hall [31] found that there is expression of GAL4 driven by the per promoter in PI neurons. The photoreceptors in contrast have all the traditional clock genes that might contribute to SLOB cycling [32-34]. The presence of these genes in the eye, but not in the PI, may contribute to the phase differences. In addition, it is reasonable to expect that there will be molecular differences in circadian regulation in different cell types. Furthermore, the subcellular localization of SLOB is different between the two areas (Figure 4A,4C). SLOB appears to be primarily cytoplasmic in the PI neurons, and nuclear in the photoreceptors.
How is SLOB regulated in the PI neurons? Interestingly, the PI neurons have been associated with behavioral rhythmicity and it has been hypothesized that this involves hormone release [35]. In fact, the PI neurons of Teleogryllus commodus (crickets) have long been hypothesized to serve as a region of coupling between the circadian pacemakers and behavioral rhythms [36]. A pathway for Drosophila proposed by Kaneko and Hall [31] suggests that oscillatory signals of the "master pacemaker" in the small LNvs first modulate the oscillatory mechanism or neuronal activity operating within neurons in the dorsal region. The DNs send their oscillatory signals to the PI, which may lead to rhythmic neurosecretory peptide release. It has been observed in the larval CNS that projections of the DNs terminate near the midline in the PI region [31]. These DNs express both PER and TIM and hence may send robust oscillatory signals to downstream targets such as PI neurons. Interestingly, two neurons of the DN group express PER and TIM cycling antiphase to the other DNs and LNvs [31]. Regardless of the precise role of the DNs, it is clear that this dorsal region of the brain is important for rest:activity rhythms. For example, PDF release and MAPK activity cycle specifically in this region, and both participate in behavioral rhythmicity [13,37].
Clock mutants alter either SLOB protein oscillation or levels in both the eye and PI neurons. One striking observation is the ectopic or elevated expression of SLOB in the lamina of tim01 flies. This suggests that TIM negatively regulates SLOB. Western analyses show that upregulation of SLOB does not occur in per01 flies. Ectopic expression of PER occurs in double-time (dbt) flies that are mutant for a casein kinase 1ε involved in PER turnover [38]. The interpretation in that case is that PER is synthesized in many cell types where its expression is normally undetectable due to destabilization by the kinase. A similar mechanism may account for elevated expression of SLOB in tim01 flies. The observation that tim01 alters SLOB expression while per01 does not could suggest a pathway for slob regulation that is independent of PER. We also found that SLOB fails to cycle in the PI neurons of Clkjrk flies.
Perhaps most intriguing is the decrease of SLOB in the PI neurons of Pdf01 flies. The oscillation of PDF is restricted to the dorsal projections emanating from the lateral neurons [13]. Dorsal terminals of the LNs express abundant PDF early in the morning, which is indicative of a block in its release. Thus, PDF release is low during the day while SLOB is at its trough, consistent with PDF being a positive regulator of SLOB. In Figure 4H we see that PDF expressing terminals do not appear to contact the PI neurons. As discussed above, we hypothesize that PDF termini affect the DN, or alternatively, other neurons of the dorsal region, which in turn communicate with the PI neurons. Clkjrk flies lack PDF in the small ventral LNs, but still express it in the large LNs [13]. This may account for the difference in the phenotype of Clkjrk and Pdf01 mutants and would suggest a role for the large LNs in SLOB regulation.
The molecular oscillations of the circadian clock proteins result ultimately in behavioral rhythmicity [24]. Our data demonstrate that the panneuronal expression of SLOB causes a delayed breakdown of rhythms. Breakdown after several days is characteristic of some circadian output mutants such as Pdf01 flies [13]. Likewise, panneuronal overexpression of PDF results in a delayed disruption of rhythms, and overexpression of PDF in the PI neurons results in a shortened period and an advance of the morning peak [39]. disconnected (disco) flies, which lack LNs, also become arrhythmic only after several days in DD [40].
Using GAL4 drivers that direct overexpression of SLOB specifically to the eye or PI neurons, we found no obvious circadian locomotion phenotype. It is possible that these drivers are not strong enough, compared to the panneuronal driver elavc155. We note that the overexpression of PER and TIM with the tim-GAL4 driver results in a more severe phenotype than with per-GAL4, even though the expression pattern of the two drivers is similar [24], possibly because the per promoter is weaker [41]. Alternatively, the specific drivers we used may not target all the behaviorally-relevant SLOB positive neurons in the eye and PI region. Any SLOB positive neurons that are not overexpressing SLOB are therefore wild type, and this may prevent rhythmicity breakdown. Similar explanations have been proposed for the lack of a phenotype when PER and TIM are overexpressed by the pdf-GAL4 driver although both genes cause arrhythmia when their overexpression is driven by the more widely expressed tim and per-GAL4 drivers [24,42].
The widespread distribution of SLOB in the eye and brain suggests that other cells, in addition to or instead of the photoreceptor and PI neurons, may account for SLOB's apparent role in behavioral rhythms. The panneuronal overexpression of SLOB in other SLOB-expressing cells in the brain cortex may explain the rhythmic breakdown. Alternatively, ectopic expression of SLOB in neurons involved in locomotor rhythms might also account for the altered rhythmicity.
Conclusions
In this study, we have demonstrated that SLOB protein cycles in a circadian fashion in Drosophila heads. SLOB oscillates in two discrete areas of the fly head, the photoreceptor cells and the PI neurons. Our results reveal differential effects of clock mutations on SLOB expression and cycling in these two regions. There is a significant decrease in SLOB levels in the PI neurons of Pdf01 flies, thus implicating PDF as an upstream regulator of SLOB. Along with the observation that flies overexpressing SLOB exhibit a breakdown of rest:activity patterns, these data suggest that SLOB is a clock controlled protein.
We showed previously that SLOB, along with dSlo and Leonardo, participates in a dynamic regulatory complex in presynaptic nerve terminals. Leonardo binding is dynamically regulated by phosphorylation of SLOB by the calcium/calmodulin-dependent protein kinase II (CAMKII) [6]. Intriguingly, CAMKII has recently been implicated in circadian rhythmicity in vertebrates [43], and it is tempting to speculate that this regulatory complex participates in the fly circadian output pathway. Not only do dslo mutants have weak rhythms [1,44], but leonardo mutants have defects in behavior, synaptic transmission and plasticity [45,46]. Our data are consistent with the hypothesis that SLOB participates in circadian rhythmicity by regulating synaptic function and membrane excitability.
Methods
Fly stocks and germ line transformation
D. melanogaster strains Canton S (wild type), y w, ry, tim01, per01, Clkjrk, and Pdf01 and transgenic fly strains were raised at 25°C on standard Drosophila medium. slob cDNA was cloned into a pUAST vector and P-element-mediated transformation was performed as described previously [47]. The transformed lines were crossed to either elavC155 (provided by Leslie Griffith), GMR (provided by Konrad Zinsmaier) or Mai 281, Mai 301, Kurs 45, and Kurs 58 GAL4 (provided by Gunter Korge).
In situ hybridizations
The slob RNA antisense and sense probes were synthesized using the DIG RNA Labeling Mix (Boehringer Manheim). The sequence used for the RNA probes was made from basepairs 1142–1441 of the slob transcript. In situ hybridization on adult 12 μm head sections were done according to the protocols found at http://www.rockefeller.edu/labheads/vosshall/protocols.php with slight modifications. All hybridizations and washes were done at 55°C. Sections are developed in the dark for 3 days.
SLOB antibody purification
A GST-SLOB fusion protein was used to immunize rabbits as described previously [48]. Polyclonal antibodies specific to SLOB were generated by purifying the serum using a combination of CNBr conjugated GST and CNBr conjugated GST-SLOB columns.
Western blotting and quantitation
Flies were entrained and collected in LD and DD conditions at four hour intervals. Fly heads were lysed in 1% CHAPS, 20 mM Tris-HCl (pH 7.5), 10 mM EDTA, 120 mM NaCl, 50 mM KCl, 2 mM DTT and protease inhibitors (1 mM PMSF, 1 μg/mL each aprotonin, leupeptin, and pepstatin A (SIGMA)). Protein concentration was determined using the BioRad DC Protein Assay. 100 μg of protein was loaded on 4–15% polyacrylamide gradient gels and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk in TBST (0.1% Tween 20 in Tris-buffered saline), the blots were probed with the appropriate primary and secondary antibodies. Enhanced chemiluminescence detection system (Amersham) was used to visualize the proteins. Film exposures of western blots were scanned using Bio Rad Molecular Analyst. The level of SLOB at each time point was calculated as the SLOB signal minus the background in each lane. The blots were stripped and reprobed with anti-MAP Kinase (Sigma). The ratio of SLOB to MAP Kinase was normalized and averaged between several westerns.
Immunohistochemistry
Brain whole mount: Flies were entrained in LD for three days and then transferred to DD. Fly heads were collected at given time points, fixed in 4% paraformaldehyde (PFA), and the brains were dissected and kept in cold PBS, and subsequently blocked with 6% normal donkey serum in phosphate-buffered saline (PBS)/0.3% Triton X-100 for one hour. Samples were then incubated with primary antibody at a dilution of 1:400 overnight at 4°C. After washing in PBS/0.3% Triton X-100 three times for 30 min each at room temperature, samples were incubated with the appropriate secondary antibody (Fluorescein (FITC)-conjugated AffiniPure Donkey Anti-Rabbit IgG and Texas Red dye-conjugated AffiniPure Donkey Anti-Rabbit IgG from Jackson ImmunoResearch) at a dilution of 1:500 in 3% normal donkey serum in PBS/0.3% Triton X-100 for 1 hour at room temperature, and washed in PBS three times for 30 minutes each. Brains were mounted onto slides with mounting medium (Vector H-1200). Wholemounts were visualized using fluorescence microscopy on a Leica DMIRE2.
Section: Flies were collected at given time points, mounted with mounting medium, and sectioned at 12 μm using a cryostat (Leica 3450). Sections were fixed with 4% PFA, washed in PBS/0.3% Triton X-100, and blocked with 6% normal donkey serum in PBS/0.3% Triton X-100 for 1 hour, and subsequently incubated with primary antibody in 6% normal donkey serum in PBS/0.3% Triton X-100 overnight at 4°C. The sections were then washed and incubated with secondary antibodies as described above.
The staining intensity of brain whole mounts and sections was assessed by blind scoring. A subjective intensity scale from zero to four was used, with zero being undetectable and four being maximal. Statistical analysis of average staining intensity scores was done using ANOVA and the Tukey HSD test.
Behavioral analysis
Flies aged from 1–5 days were entrained for three days in 12 hr light/dark cycles at 25°C and then kept in constant darkness for 14 days. Activity was monitored by using the Trikinetics system. Individual flies were analyzed for rhythmicity based on their by chi-square periodogram and Fast Fourier Transform (FFT) values [24]. The analyses were performed using ClockLab software.
Authors' contributions
AMJ performed the SLOB antibody purification, western blotting and quantitation, immunohistochemistry, in situ hybridization and behavioral analysis. XZ carried out immunohistochemistry and scoring analysis. DAA and AS participated in the immunohistochemistry and western blotting. AMJ and IL drafted the manuscript. AMJ, XZ, YZ, IL and AS conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by grants from the National Institutes of Health to I.B.L. and A.S., an NRSA to A.M.J. and the US Army Medical Research Command to A.S. We are grateful to Hua Wen, Konrad Zinsmaier, Leslie Griffith and Joan Hendricks for helpful discussions and to Gunter Korge for his GAL4 lines.
Contributor Information
Angela M Jaramillo, Email: jaramill@mail.med.upenn.edu.
Xiangzhong Zheng, Email: zhengx@mail.med.upenn.edu.
Yi Zhou, Email: yzhou@nrc.uab.edu.
Defne A Amado, Email: defne@mail.med.upenn.edu.
Amanda Sheldon, Email: alsheldo@mail.med.upenn.edu.
Amita Sehgal, Email: amita@mail.med.upenn.edu.
Irwin B Levitan, Email: levitani@mail.med.upenn.edu.
References
- Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- Lin Y, Han M, Shimada B, Wang L, Gibler TM, Amarakone A, Awad TA, Stormo GD, Van Gelder RN, Taghert PH. Influence of the period-dependent circadian clock on diurnal, circadian, and aperiodic gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:9562–9567. doi: 10.1073/pnas.132269699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J Biol Chem. 2002;277:14048–14052. doi: 10.1074/jbc.C100765200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schopperle WM, Murrey H, Jaramillo A, Dagan D, Griffith LC, Levitan IB. A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 1999;22:809–818. doi: 10.1016/s0896-6273(00)80739-4. [DOI] [PubMed] [Google Scholar]
- Williams JA, Sehgal A. Molecular components of the circadian system in Drosophila. Annu Rev Physiol. 2001;63:729–755. doi: 10.1146/annurev.physiol.63.1.729. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Helfrich-Forster C, Hall JC. Spatial and temporal expression of the period and timeless genes in the developing nervous system of Drosophila: newly identified pacemaker candidates and novel features of clock gene product cycling. J Neurosci. 1997;17:6745–6760. doi: 10.1523/JNEUROSCI.17-17-06745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science. 1993;259:239–241. doi: 10.1126/science.8421785. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system. Eur J Neurosci. 2001;13:1420–1428. doi: 10.1046/j.0953-816x.2001.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Hardin PE. Drosophila photoreceptors contain an autonomous circadian oscillator that can function without period mRNA cycling. J Neurosci. 1998;18:741–750. doi: 10.1523/JNEUROSCI.18-02-00741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery I, Zwiebel LJ, Dembinska ME, Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci U S A. 1994;91:2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Rothenfluh-Hilfiker A, Hunter-Ensor M, Chen Y, Myers MP, Young MW. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science. 1995;270:808–810. doi: 10.1126/science.270.5237.808. [DOI] [PubMed] [Google Scholar]
- Stempfl T, Vogel M, Szabo G, Wulbeck C, Liu J, Hall JC, Stanewsky R. Identification of circadian-clock-regulated enhancers and genes of Drosophila melanogaster by transposon mobilization and luciferase reporting of cyclical gene expression. Genetics. 2002;160:571–593. doi: 10.1093/genetics/160.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So WV, Sarov-Blat L, Kotarski CK, McDonald MJ, Allada R, Rosbash M. takeout, a novel Drosophila gene under circadian clock transcriptional regulation. Mol Cell Biol. 2000;20:6935–6944. doi: 10.1128/MCB.20.18.6935-6944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund T, Korge G. Innervation of the ring gland of Drosophila melanogaster. J Comp Neurol. 2001;431:481–491. doi: 10.1002/1096-9861(20010319)431:4<481::AID-CNE1084>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Becker MN, Brenner R, Atkinson NS. Tissue-specific expression of a Drosophila calcium-activated potassium channel. J Neurosci. 1995;15:6250–6259. doi: 10.1523/JNEUROSCI.15-09-06250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekhar KP, Singh RN. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. The Journal of Comparative Neurology. 1994;349:633–645. doi: 10.1002/cne.903490410. [DOI] [PubMed] [Google Scholar]
- Nassel DR. Neuropeptides in the insect brain: a review. Cell Tissue Res. 1993;273:1–29. doi: 10.1007/BF00304608. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Shiga S, Mohrherr CJ, Rao KR. Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol. 1993;331:183–198. doi: 10.1002/cne.903310204. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol. 2000;422:66–94. doi: 10.1002/(SICI)1096-9861(20000619)422:1<66::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Eastman C, Petersen G, Rosbash M, Hall JC. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988;1:141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Gatti S, Ferveur J, Martin J. Genetic identification of neurons controlling a sexually dimorphic behavior. Curr Biol. 2000;10:667–670. doi: 10.1016/S0960-9822(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Loher W. Role of eyes, optic lobes, and pars interecerebralis in locomotory and stridulatory circadian rhythms of Teleogryllus commodus. J Insect Physiol. 1975;21:785–799. doi: 10.1016/0022-1910(75)90009-8. [DOI] [PubMed] [Google Scholar]
- Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Tauber M, Park JH, Muhlig-Versen M, Schneuwly S, Hofbauer A. Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J Neurosci. 2000;20:3339–3353. doi: 10.1523/JNEUROSCI.20-09-03339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay MS, Rosbash M, Hall JC. The disconnected visual system mutations in Drosophila melanogaster drastically disrupt circadian rhythms. J Biol Rhythms. 1989;4:1–27. doi: 10.1177/074873048900400101. [DOI] [PubMed] [Google Scholar]
- So WV, Rosbash M. Post-transcriptional regulation contributes to Drosophila clock gene mRNA cycling. EMBO J. 1997;16:7146–7155. doi: 10.1093/emboj/16.23.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol. 2000;43:207–233. doi: 10.1002/(SICI)1097-4695(20000605)43:3<207::AID-NEU1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Broadie K, Rushton E, Skoulakis EM, Davis RL. Leonardo, a Drosophila 14-3-3 protein involved in learning, regulates presynaptic function. Neuron. 1997;19:391–402. doi: 10.1016/s0896-6273(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Skoulakis EM, Davis RL. Olfactory learning deficits in mutants for leonardo, a Drosophila gene encoding a 14-3-3 protein. Neuron. 1996;17:931–944. doi: 10.1016/s0896-6273(00)80224-x. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Schopperle WM, Holmqvist MH, Zhou Y, Wang J, Wang Z, Griffith LC, Keselman I, Kusinitz F, Dagan D, Levitan IB. Slob, a novel protein that interacts with the Slowpoke calcium- dependent potassium channel. Neuron. 1998;20:565–573. doi: 10.1016/s0896-6273(00)80995-2. [DOI] [PubMed] [Google Scholar]


