Abstract
Objective
We examined the association of second trimester maternal plasma 25-hydroxyvitamin D (25[OH]D) during pregnancy with gestational diabetes mellitus(GDM).
Study Design
Among 1314 pregnant women participating in Project Viva, a birth cohort study, we measured 25(OH)D levels at 26–28 weeks’ gestation during GDM screening using a 1-hour 50g glucose challenge test.
Results
We found 25(OH)D levels <25nmol/L in 44/1087(4.0%) women with normal glucose tolerance, 9/159(5.7%) women with impaired glucose tolerance and 9/68(13.2%) women with GDM. Analyses adjusted for sociodemographics, season, maternal BMI, gestational weight gain and dietary factors, suggested that women with 25(OH)D levels <25 vs. ≥25 nmol/L may have higher odds of GDM (2.2 [0.8, 5.5]). Glucose levels after the glucose challenge test were inversely associated with 25(OH)D levels(P <0.01).
Conclusion
Second trimester 25(OH)D levels were inversely associated with glucose levels after 1-hour 50g glucose challenge test and low 25(OH)D levels may be associated with increased risk of GDM.
Keywords: Vitamin D, Gestational Diabetes Mellitus, 25-hydroxyvitamin D, GDM, pregnancy
Introduction
Gestational diabetes mellitus (GDM) and impaired glucose intolerance (IGT) affect maternal, fetal and neonatal well-being. GDM complicates 14% of pregnancies in the United States1 and its incidence is rising.2 In mothers, GDM is associated with higher risk of cesarean section and the later development of Type 2 diabetes. For offspring, GDM is associated with macrosomia, birth trauma, respiratory distress syndrome, jaundice and hypoglycemia.3 The causes of GDM and IGT are an active area of investigation4 with growing interest in vitamin D deficiency as a potential cause.5 Although epidemiologic studies have shown a fairly consistent link between vitamin D deficiency and a higher risk of type 2 diabetes,6, 7 and obesity is strongly associated with both GDM 8, 9 and vitamin D deficiency,10–12 it remains unclear if vitamin D status affects a mother’s risk of developing GDM.
A few studies support an association between low 25(OH)D levels and an increased risk of GDM,13–16 but a recent prospective study found no evidence of an association between first trimester 25(OH)D and subsequent development of GDM.17 Another study did not find an association between 25(OH)D level and GDM, but did report an inverse relationship between 25(OH)D level and glucose concentrations 30 minutes after a 100g glucose load.18 These studies have various limitations including a lack of adjustment for maternal BMI,14 a low incidence of obesity and thus lack of generalizability to US and other Western populations,18 and no adjustment for dietary factors or physical activity.13–18 Physical activity could be an important confounder of the relationship between GDM and 25(OH)D as active women are less likely to have impaired glucose tolerance19 and may be more likely to have higher 25(OH)D levels than more sedentary women due to increased sunlight exposure.20
To study the relationship between vitamin D status during pregnancy and gestational diabetes, we analyzed data from a prospective prenatal cohort of more than 1300 mothers-infant pairs. We hypothesized that lower 25(OH)D levels would be associated with a increased odds of GDM and higher glucose concentrations. We also hypothesized that maternal physical activity would account for part of the association between lower 25(OH)D and higher risk of GDM and that adjustment for this factor would attenuate the observed association.
Materials and Methods
We studied participants from Project Viva, a prospective prenatal cohort study of gestational factors and offspring health.21 We recruited women attending their initial prenatal visit at 8 obstetrical offices of a multi-specialty group practice in Massachusetts. Eligibility criteria included maternal fluency in English, singleton pregnancy and gestational age less than 22 weeks. Details of cohort recruitment and retention processes have been previously published.21, 22 Participants provided written informed consent. Institutional review boards of participating institutions approved the study.
Of the 2128 participants who gave birth, 1314 mothers had both second trimester 25(OH)D levels and GDM status. Cohort participants included in this analysis were similar to those excluded except they were more likely to have graduated from college (68% vs. 59%), were slightly older (mean age 32 vs. 31 years), and were slightly less likely to have GDM (5.2% vs. 6.4%).
Measurement of Vitamin D status
We measured 25(OH)D levels, a combination of 25(OH)D2 and 25(OH)D3, in previously frozen maternal blood samples obtained during a routine non-fasting clinical blood draw between 26–28 weeks’ gestation. We refrigerated blood samples, centrifuged the samples and stored plasma aliquots at −80°C. We measured 25(OH)D level for each specimen twice, first with an automated chemiluminescence immunoassay (CLIA)23 and then with a manual radioimmunoassay (RIA).24 For quality control, the laboratory used US National Institute of Standards and Technology (NIST) level 1. Because the singlicate 25(OH)D results from the two assays were not identical (r=0.81) we averaged the two values for each specimen to obtain a more stable estimate of 25(OH)D level. In general, analyses using either the CLIA data only or RIA data only yielded similar results.
Ascertainment of gestational diabetes
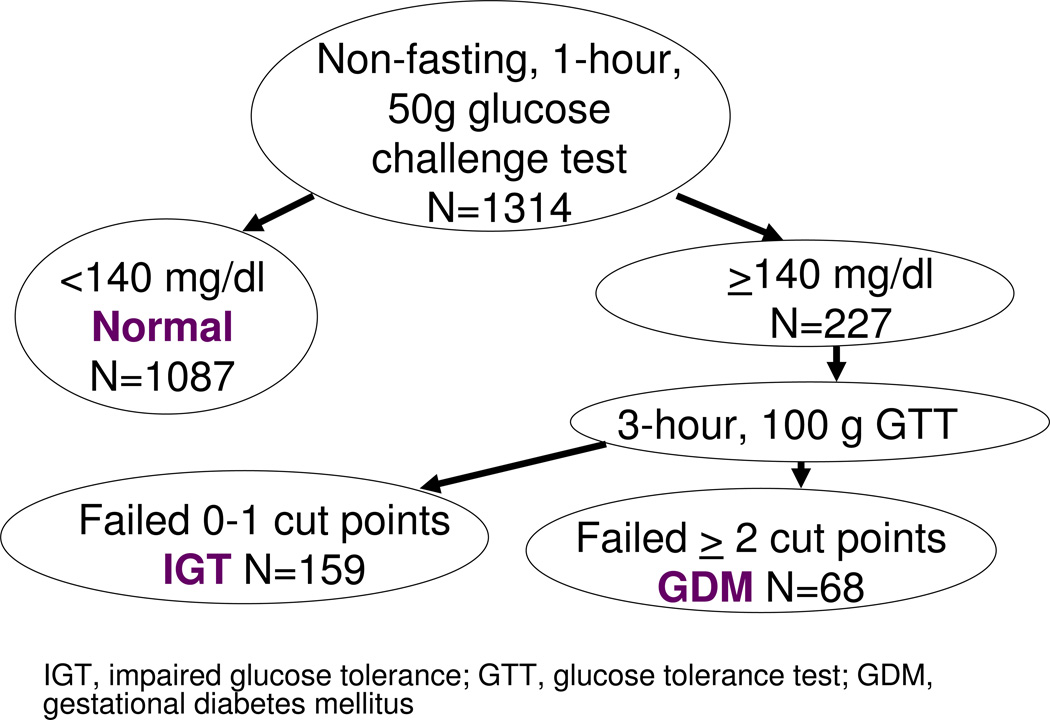
We obtained data on GDM from the clinical record. Women underwent routine screening for gestational diabetes at 26–28 weeks of gestation with a non-fasting oral glucose challenge test. If one hour after a non-fasting 50-g oral glucose load, the glucose was ≥140 mg/dL, the participant was referred for a 100-g fasting glucose 3-hour tolerance tests. Normal results were a fasting blood glucose <95 mg/DL at baseline, <180 mg/dL at 1 hours and <155 mg/dL at 2 hours, and below 140 mg/dL at 3 hours.25 We categorized participants with a normal glucose screening as having normal glucose tolerance and participants who failed the screening as having impaired glucose tolerance (Figure 1). We classified women with at least two abnormal results on the fasting glucose tolerance test as having GDM.
Figure 1.
Categorization of glucose tolerance based on 50 g, 1-hour, non-fasting glucose challenge test, and subsequent, fasting 100 g, 3-hour glucose tolerance test, Project Viva.
Assessment of covariates
Through interviews, study questionnaires and medical record reviews, we collected information on self-designated maternal age, race/ethnicity, parity, smoking habits, education, marital status and household income. We calculated gestational age in weeks at the time of the blood sample by subtracting the date of the last menstrual period from the date of the blood draw. Eighty-six percent of the participants had ultrasound data available at 16–20 weeks. For approximately 12% of the ultrasounds gestational age estimates differed by more than 10 days from the LMP pregnancy dating and for these we used the dating obtained from the ultrasound. We calculated prepregnancy body mass index (BMI) based on self-reported prepregnancy height and weight. We calculated gestational weight gain at 20 weeks’ gestation by subtracting the self-reported prepregnancy weight from the weight measured at 20 weeks’ gestation. We defined physical activity as hours spent walking or time spent performing light-to-moderate or vigorous activities in the 3 months prior to the 26–28 week blood draw.19 We obtained dietary intake data (fish and micronutrient intake including calcium and vitamin D) with a previously validated, semi-quantitative food frequency questionnaire administered in the first and second trimesters of pregnancy.26, 27 We adjusted micronutrient intake for energy intake using a residuals model.28
Statistical analysis
We first performed bivariate analyses to determine maternal and infant characteristics associated with previously described clinical categories of vitamin D status:29–32 severe deficiency (<25 nmol/L), deficiency (25 to <50 nmol/L), insufficiency (50 to <75 nmol/L), and sufficiency (≥75 nmol/L). We further dichotomized 25(OH)D levels at 25 nmol/L because preliminary analyses of the odds of GDM demonstrated a threshold; compared to women with 25(OH)D levels <25 nmol/L, women with levels 25–<50, 50–<75 and ≥75 had unadjusted odds ratios for GDM of 0.25, 0.32, and 0.20, respectively. We used multivariable-adjusted multinomial logistic regression models to examine associations between severe vitamin D deficiency and the odds of developing GDM or IGT compared to women with normal glucose tolerance. We excluded covariates that did not confound the relationship between 25(OH)D level and the odds of GDM.
To analyze the relationship between vitamin D status and blood glucose measurement after the 1-hour glucose challenge screening test, we conducted analyses predicting glucose as a continuous variable using multivariate linear regression models. In these models, we used 25(OH)D as a continuous independent variable and adjusted for the same covariates as the GDM analyses.
As is common in large epidemiologic studies, many covariates were missing on some subjects. We used chained equations to multiply impute values for these covariates.33–35 We generated 10 imputed data sets; all model results are generated by appropriately combining these results.36 To avoid incorrect imputations, all 2128 Viva subjects were used in the imputation process;34 however, only those with observed 25OHD are included in the analysis. All analyses were performed in SAS version 9.3.
Results
Second Trimester 25-hydroxyvitamin D levels
Mean [SE] 25(OH)D level was 59 [0.6] nmol/L. 25(OH)D levels were lower among women with higher BMI, lower pregnancy weight gain, lower vitamin D intake, and lower calcium intake (Table 1). Women with lower levels were more likely to have less than a college education, be single, be from households with <$70,000 annual income, and be non-white. Counter to our expectations, physical activity appeared to be inversely associated with 25(OH)D level, with women in the lowest category (<25 nmol/) reporting 9.3 hours/week of physical activity versus 7.2 hours/week among women with 25(OH)D levels ≥75 nmol/L. Younger women in our cohort reported more physical activity (Pearson correlation coefficient −0.15, P<0.001) and had lower 25(OH)D levels than older women (Pearson correlation coefficient 0.15, P<0.001). We do not have data on whether physical activity was indoor or outdoor.
Table 1.
Characteristics of 1314 participants in Project Viva, overall and by 2nd trimester 25(OH)D category
| No imputation | Complete data | Category of 2nd trimester 25(OH)D (nmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n=1314) | <25 (n=62) |
25–<50 (n=385) |
50–<75 (n=620) |
>=75 (n=247) |
|||||
| N | Mean | SE | Mean | SE | Mean | ||||
| 2nd trimester 25(OH)D (nmol/l) | 1314 | 59 | 0.6 | 59 | 0.6 | 20 | 39 | 62 | 90 |
| Maternal age at enrollment (years) | 1314 | 32 | 0.1 | 32 | 0.1 | 30 | 31 | 33 | 33 |
| Prepregnancy BMI (kg/m2) | 1308 | 24.7 | 0.1 | 24.7 | 0.1 | 28.6 | 25.8 | 24.1 | 23.7 |
| Gestational age at blood draw (weeks) | 1314 | 28 | 0.04 | 28 | 0.04 | 28 | 28 | 28 | 28 |
| Pregnancy weight gain to 20 weeks (kg) | 1302 | 5.9 | 0.1 | 5.9 | 0.1 | 4.8 | 5.7 | 6.2 | 5.5 |
| Blood glucose (mg/dL) 1 hour after 50 gram glucose load | 1307 | 114 | 0.7 | 114 | 0.7 | 122 | 116 | 114 | 111 |
| Physical activity during pregnancy (hours/week) | 1096 | 6.9 | 0.2 | 7.1 | 0.2 | 9.3 | 7.5 | 6.6 | 7.2 |
| Total vitamin D intake (IU/day) | 1217 | 547 | 4.8 | 541 | 4.9 | 373 | 489 | 571 | 588 |
| Total calcium intake (mg/d) | 1217 | 1381 | 10.1 | 1371 | 10.1 | 1142 | 1250 | 1436 | 1455 |
| Fish intake (servings/week) | 1217 | 1.6 | 0.04 | 1.6 | 0.04 | 1.8 | 1.5 | 1.5 | 1.6 |
| N | % | % | % | ||||||
| Education | |||||||||
| Less than college graduate | 420 | 32 | 32 | 66 | 39 | 28 | 22 | ||
| College graduate | 888 | 68 | 68 | 34 | 61 | 72 | 78 | ||
| Marital status | |||||||||
| Single | 91 | 7 | 7 | 18 | 10 | 5 | 4 | ||
| Married or cohabitating | 1216 | 93 | 93 | 82 | 90 | 95 | 96 | ||
| Smoking status | |||||||||
| Never | 863 | 68 | 68 | 76 | 66 | 68 | 68 | ||
| Former | 253 | 20 | 20 | 10 | 19 | 20 | 23 | ||
| During pregnancy | 158 | 12 | 12 | 14 | 15 | 12 | 9 | ||
| Household income | |||||||||
| ≤$70,000/year | 457 | 38 | 39 | 78 | 45 | 35 | 31 | ||
| >$70,000/year | 760 | 62 | 61 | 22 | 55 | 65 | 69 | ||
| Parity | |||||||||
| 0 | 622 | 47 | 47 | 29 | 46 | 50 | 48 | ||
| 1+ | 692 | 53 | 53 | 71 | 54 | 50 | 52 | ||
| Race/ethnicity | |||||||||
| White | 943 | 72 | 72 | 22 | 61 | 79 | 85 | ||
| Black | 186 | 14 | 14 | 55 | 20 | 9 | 8 | ||
| Hispanic | 76 | 6 | 6 | 13 | 8 | 5 | 4 | ||
| Other | 103 | 8 | 8 | 10 | 11 | 8 | 4 | ||
| Glucose tolerance | |||||||||
| GDM | 68 | 5 | 5 | 15 | 4 | 5 | 4 | ||
| IGT | 159 | 12 | 12 | 15 | 15 | 11 | 9 | ||
| Normal | 1087 | 83 | 83 | 71 | 81 | 83 | 87 | ||
BMI, body mass index; GDM, gestational diabetes; IGT, impaired glucose tolerance
Gestational Diabetes Mellitus and Impaired Glucose Tolerance
Sixty-eight (5.2%) women met criteria for GDM. Unadjusted analysis revealed that women with 25(OH)D levels <25 vs. ≥25 nmol/L had significantly increased odds of GDM (OR 3.6, 95% CI 1.7, 7.8) (Table 2). Adjustment for race/ethnicity, age, education, marital status, smoking, parity and season of blood draw made little difference to this estimate (OR 3.1, 95% CI 1.3, 7.4). Additional adjustment for maternal BMI attenuated the association and the confidence interval included the null value (OR 2.2, 95% CI 0.9, 5.6). Further adjustment for pregnancy weight gain made little difference (OR 2.3, 95% CI 0.9, 5.7). Addition of physical activity and dietary intakes of fish and calcium also made little difference (OR 2.2, 95% CI 0.8, 5.5).
Table 2.
Odds of gestational diabetes and impaired glucose tolerance (vs. normal glucose tolerance) by 25-hydroxyvitamin D level <25 nmol/L vs. >=25 nmol/L among 1314 participants from Project Viva.
| 25(OH)D exposure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Odds Ratio (95 % CI) | ||||
| GDM vs. Normal Glucose Tolerance | 3.6 (1.7, 7.8) | 3.1 (1.3, 7.4) | 2.3 (0.9, 5.7) | 2.2 (0.8, 5.5) |
| IGT vs. Normal Glucose Tolerance | 1.4 (0.7, 3.0) | 1.6 (0.7, 3.5) | 1.4 (0.6, 3.2) | 1.4 (0.6, 3.3) |
Model 1. Unadjusted
Model 2. Adjusted for gestational age and season at blood draw, maternal age, race/ethnicity, education, marital status, smoking, and parity
Model 3. Model 2 + prepregnancy BMI and pregnancy weight gain to 20 weeks’ gestation
Model 4. Model 3 + physical activity during pregnancy and dietary intakes of fish and calcium
CI, confidence interval; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; 25(OH)D, 25-hyroxyvitamin D;BMI, body mass index
For the 159 (12.1%) women with IGT, we did not detect an association between IGT and 25(OH)D level <25 vs. ≥25 nmol/L (unadjusted OR 1.4, 95% CI 0.7, 3.0; adjusted OR 1.4, 95% CI 0.6, 3.3).
Blood glucose measurements
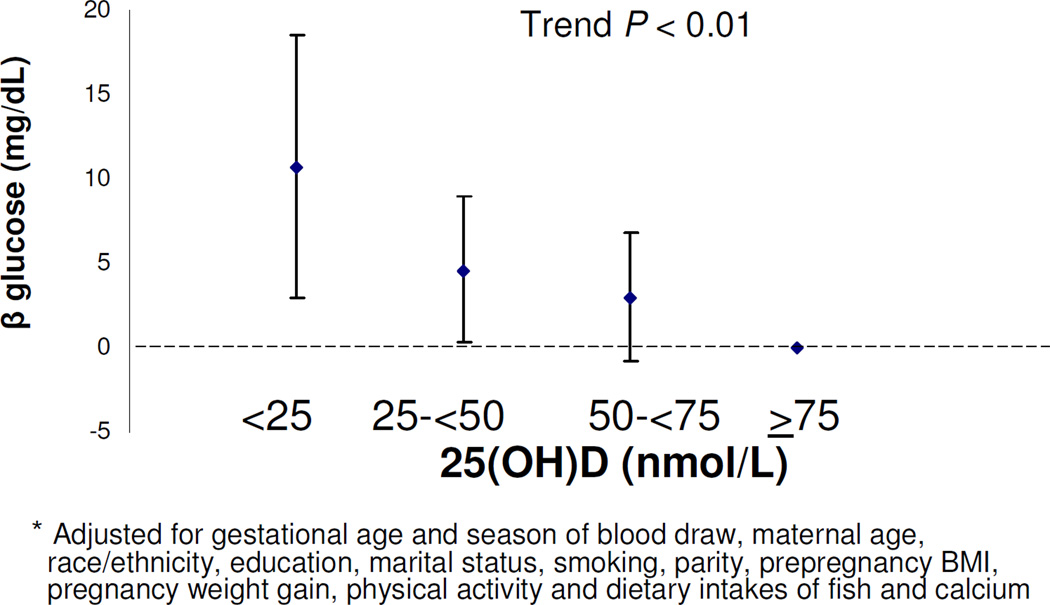
Mean (SE) blood glucose after 50 gram, 1-hour oral glucose load was 114 (0.7) mg/dl. Women with 25(OH)D levels <25 vs. ≥25 nmol/L had higher glucose levels (8.1 mg/dl [95% CI 1.3, 14.9) (Table 3). Adjustment for the same covariates as the GDM analysis did not materially change this estimate (7.2 [95% CI 0.2, 14.2]). For each 25 nmol/L decrease in continuous 25(OH)D level, blood glucose levels were 2.7 mg/dl higher (95% CI 1.0, 4.4). Adjustment for the same covariates as the models for GDM did not materially change this estimate (2.4 (95% CI 0.6, 4.2). With each increase in 25(OH)D category, blood glucose levels decreased (P Trend <0.01) (Figure 2).
Table 3.
Differences (beta coefficients) of blood glucose (mg/dl) after 50 gram 1-hour glucose challenge test for GDM by 25-hydroxyvitamin D level among 1314 participants from Project Viva.
| 25(OH)D exposure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Blood glucose β (mg/dl) | ||||
| Continuous 25(OH)D (per 25 nmol/L Decrease) | 2.7 (1.0, 4.4) | 3.2 (1.4, 5.0) | 2.3 (0.6, 4.1) | 2.4 (0.6, 4.2) |
| 4-categories of 25(OH)D level (nmol/L) | ||||
| <25 | 11.4 (4.0, 18.8) | 13.5 (5.8, 21.3) | 10.6 (2.8, 18.3) | 10.7 (2.9, 18.5) |
| 25–<50 | 5.4 (1.2, 9.6) | 6.3 (2.0, 10.7) | 4.6 (0.4, 8.9) | 4.6 (0.3, 9.0) |
| 50–<75 | 3.3 (−0.6, 7.2) | 3.7 (−0.2, 7.6) | 3.1 (−0.7, 7.0) | 3.0 (−0.8, 6.8) |
| ≥75 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| <25 v. ≥25 nmol/L | 8.1 (1.3, 14.9) | 9.1 (2.0, 16.1) | 7.1 (0.1, 14.0) | 7.2 (0.2, 14.2) |
Model 1. Unadjusted
Model 2. Adjusted for gestational age and season at blood draw, maternal age, race/ethnicity, education, marital status, smoking, and parity
Model 3. Model 2 + prepregnancy BMI and pregnancy weight gain to 20 weeks’ gestation
Model 4. Model 3 + physical activity during pregnancy and dietary intakes of fish and calcium
CI, confidence interval; GDM, gestational diabetes mellitus; 25(OH)D, 25-hyroxyvitamin D; BMI, body mass index
Figure 2.
Adjusteda glucose β (95% CI) after 50g, 1-hour, GCT by 25(OH)D) category
Comment
The association between 25(OH)D levels and GDM in the literature is not entirely clear. Farrant et al studied 559 pregnant women in India and found no association between second trimester 25(OH)D levels and GDM.18 However, among a subset of mothers with levels <50 nmol/L, they found an inverse association between 25(OH)D levels and 30-minute glucose concentrations after a glucose load, a finding consistent with our results. This study did not adjust for dietary factors or physical activity. A recent study by Makgoba et al of 90 cases of GDM and 158 controls reported no association between first trimester blood samples and subsequent development of GDM, however, consistent with our findings, they did find an inverse correlation between 25(OH)D levels and glucose measurements after a 2-hour fasting glucose tolerance test.17 This case-control study also did not adjust for dietary factors or physical activity which may confound the association between vitamin D status and GDM.
A few studies have found a link between low 25(OH)D level and GDM however, none adjusted for dietary factors or physical activity. Soheilykhah et al found in a matched case-control study of 54 women with GDM and 39 women with impaired glucose tolerance (IGT) compared to 111 non-GDM control women in Iran, that maternal 25(OH)D concentrations at 24–28 weeks of gestation were significantly lower than non-GDM controls.16 They found that 83% of GDM women had 25(OH)D levels <50 nmol/L vs. 71% of controls. Maghbooli et al found in a study of 741 women in Iran that among the 29 % of participants with 25(OH)D levels <15 nmol/L, the prevalence of GDM was significantly higher compared to women with 25(OH)D levels ≥ 35 nmol/L.13 Clifton-Bligh et al found in a study of 264 women that among the 32% of women with GDM, 25(OH)D levels were significantly lower than among women without gestational diabetes.14 Zhang et al. found in a nested case-control study of 57 cases of GDM, that maternal 25(OH)D levels at 16 weeks’ gestation were 20% lower among women who later developed GDM.15 Our finding of an inverse association between glucose and 25(OH)D concentration are consistent with these studies.
We noted a threshold phenomenon between 25(OH)D <25 nmol/L vs. ≥ 25 and the odds of GDM but an inverse linear relationship with glucose levels obtained during the 50-gram, 1-hour glucose load screening test. Compared to women with 25(OH)D levels <25 nmol/L, women at each level (25–<50, 50–<75 and ≥75 nmol/L) had similarly decreased odds of GDM (unadjusted odds ratios: 0.25, 0.32 and 0.20 respectively), but they had successively lower glucose measurements with rising category of 25(OH)D (Figure 2). We recognize that there is continued uncertainty regarding the clinical significance of various glucose levels after the 1-hour glucose load screening test in the absence of a GDM diagnosis and we speculate that the difference in the pattern of associations between 25(OH)D levels and these two outcomes may be a function of the different test characteristics between the screening and diagnostic tests. The 3-hour GTT may be more of a blunt instrument that that does not detect subtle differences in glucose tolerance. As a post-hoc analysis, we examined the glucose measurements from the 3-hour fasting glucose tolerance test for the 194 women who failed the 1-hour, 50 gram glucose load screening test. At each of the time points, we did not observe a threshold effect nor a linear relationship with 25(OH)D and glucose values. For example, compared to women with 25(OH)D levels ≥ 75 nmol/L, fasting glucose levels were 6.5, −0.5 and 2.2 mg/dL higher among women with 25(OH)D levels <25, 25–<50, and 50–<75 nmol/L respectively. We speculate that the absence of a relationship between blood glucose and 25(OH)D observed during the 3-hour testing may be secondary to chance given the small numbers of women who underwent this confirmatory testing.
Our study has several strengths including a large sample size, a healthy population, and the ability to account for dietary factors and physical activity. Adjustment for self-reported dietary intakes of calcium and fish and physical activity did not materially change the higher odds of GDM among women with 25(OH)D levels <25 vs. ≥25 nmol/L suggesting vitamin D has an independent association with glucose tolerance from foods and nutrients with which it tracks closely. We were initially surprised by our finding that women with the lowest 25(OH)D levels reported the most physical activity during pregnancy since this differs from population data in predominantly non-pregnant adults.37 This relationship was likely confounded by age as younger women in our cohort reported more physical activity. Additionally, we did not have data on whether activity was indoor or outdoor and it was entirely based on self-report. The significant attenuation by incorporating self-reported BMI into models estimating the odds ratio for GDM (OR of 3.1, 95% CI 1.3, 7.4 to an OR of 2.2, 95% CI 0.9, 5.6) among women with 25(OH)D levels <25 nmol/L vs ≥ 25 is likely due to confounding. Obesity is closely associated with both GDM8, 9 and low 25(OH)D levels.10–12 However, while our adjusted confidence interval crossed 1, our data do not eliminate the likelihood of a persistent association between low 25(OH)D and GDM among obese women. Additionally, we postulate that low 25(OH)D levels may be along the causal pathway between obesity and GDM. Subjects with low 25(OH)D levels can have secondary hyperparathyroidism12 which can increase insulin resistance.38, 39 Our work raises the possibility that obese women may benefit from vitamin D supplementation and that such an intervention might decease their risk of GDM. Randomized controlled trials are needed to confirm this assertion.
Our study is limited by its cross-sectional design. We measured 25(OH)D levels from the same blood draw as the screening 1-hour glucose load test. While we sampled the blood before the formal diagnosis of GDM, the onset of glucose intolerance likely predates the blood draw. Thus, we cannot rule out reverse causation such as physiologic changes resulting from hyperglycemia that might lower 25(OH)D levels. We think this is unlikely because our results are consistent with Zhang et al. who sampled blood much earlier in pregnancy presumably prior to the onset of glucose intolerance.15 Furthermore, we think it is unlikely that patient behaviors such as dietary changes are responsible our findings because we obtained 25(OH)D level prior to the patients and caregivers learning the results of the screening test and prior to the diagnosis of GDM. However, if reverse causation is responsible for our findings, this could be clinically useful, as women with GDM are more likely to have low 25(OH)D levels and may benefit from initiation of vitamin D supplementation for general health benefits. With regard to the potential benefits of supplementation on GDM, randomized trials are needed to support or refute this possibility. Lastly, we acknowledge the uncertainty among researchers about the optimal method of measuring 25(OH)D levels.40, 41 We measured 25(OH)D using two established assays (CLIA 23and RIA24) and used the average of the two values to estimate the 25(OH)D levels of each participant for analysis.
In conclusion, our results suggest that second trimester 25(OH)D levels <25 vs. ≥25nmol/L may be associated with increased odds of GDM but not IGT, and that 25(OH)D levels were inversely correlated with blood glucose measurement after a 1-hour, 50 gram glucose load. As maternal obesity rates increase,42 and the incidence of GDM also rises,2 it is becoming increasingly important to understand modifiable risk factors such as vitamin D status. Ultimately, randomized controlled trials will be needed to test if vitamin D supplement affects GDM risk and thereby leads to improved maternal and perinatal outcomes.
Acknowledgements
This work was funded by the NIH (R01 HD034568, R01 HD064925, K24 HL 06804) the Harvard Pilgrim Health Care Foundation, and the Klarman Scholars Program at Beth Israel Deaconess Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST/PERSONAL FINANCIAL DISCLOSURE:
“Disclosure: None of the authors have a conflict of interest.”
Presentation information: This research was presented as a poster at the Obesity Society's 29th Annual Scientific Meeting, Orlando, FL, October 1–5, 2011.
References
- 1.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286:2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103:526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 3.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 4.Harlev A, Wiznitzer A. New insights on glucose pathophysiology in gestational diabetes and insulin resistance. Curr Diab Rep. 2010;10:242–247. doi: 10.1007/s11892-010-0113-7. [DOI] [PubMed] [Google Scholar]
- 5.Dror DK. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Curr Opin Obstet Gynecol. 2011;23:422–426. doi: 10.1097/GCO.0b013e32834cb791. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozfirat Z, Chowdhury TA. Vitamin D deficiency and type 2 diabetes. Postgrad Med J. 2010;86:18–25. doi: 10.1136/pgmj.2009.078626. quiz 24. [DOI] [PubMed] [Google Scholar]
- 8.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 9.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 10.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 11.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Maghbooli Z, Hossein-Nezhad A, Mirzaei K, et al. Association between retinol-binding protein 4 concentrations and gestational diabetes mellitus and risk of developing metabolic syndrome after pregnancy. Reprod Sci. 2009;17:196–201. doi: 10.1177/1933719109351097. [DOI] [PubMed] [Google Scholar]
- 14.Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet Med. 2008;25:678–684. doi: 10.1111/j.1464-5491.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Qiu C, Hu FB, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soheilykhah S, Mojibian M, Rashidi M, Rahimi-Saghand S, Jafari F. Maternal vitamin D status in gestational diabetes mellitus. Nutr Clin Pract. 2010;25:524–527. doi: 10.1177/0884533610379851. [DOI] [PubMed] [Google Scholar]
- 17.Makgoba M, Nelson SM, Savvidou M, Messow CM, Nicolaides K, Sattar N. First-trimester circulating 25-hydroxyvitamin d levels and development of gestational diabetes mellitus. Diabetes Care. 2011;34:1091–1093. doi: 10.2337/dc10-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrant HJ, Krishnaveni GV, Hill JC, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108:1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Looker AC. Do body fat and exercise modulate vitamin D status? Nutr Rev. 2007;65:S124–S126. doi: 10.1301/nr.2007.aug.s124-s126. [DOI] [PubMed] [Google Scholar]
- 21.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 22.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201:58, e1–e8. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 25.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 27.Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20:35–42. doi: 10.1111/j.1365-3016.2006.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett W. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 29.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 30.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202:436, e1–e8. doi: 10.1016/j.ajog.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes VA, Barnes MS, Alexander HD, McFaul P, Wallace JM. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr. 2009;102:876–881. doi: 10.1017/S0007114509297236. [DOI] [PubMed] [Google Scholar]
- 32.van den Ouweland JM, Beijers AM, Demacker PN, van Daal H. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1163–1168. doi: 10.1016/j.jchromb.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 33.Little RJA, Rubin DB. Statistical analysis with missing dataWiley series in probability and statistics. Hoboken, N.J.: Wiley; 2002. [Google Scholar]
- 34.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 35.van Buuren S, Oudshoom CGM. Flexible multivariate imputation by MICE. Leidon: TNO Preventie en Gezonheid, TNO/PG 99.054. http://web.inter.nl.net/users/S.van.Buuren/mi/docs/rapport99054.pdfhttp://web.inter.nl.net/users/S.van.Buuren/mi/docs/rapport99054.pdf 1999.
- 36.Rubin DB. Multiple imputation for nonresponse in surveysWiley classics library. Hoboken, N.J.: Wiley-Interscience; 2004. [Google Scholar]
- 37.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168:577–586. doi: 10.1093/aje/kwn163. discussion 587-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty MF, Thomas CA. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med Hypotheses. 2003;61:535–542. doi: 10.1016/s0306-9877(03)00227-5. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am J Clin Nutr. 2010;92:1344–1349. doi: 10.3945/ajcn.110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross AC Institute of Medicine (U.S.) Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. [PubMed] [Google Scholar]
- 41.de la Hunty A, Wallace AM, Gibson S, Viljakainen H, Lamberg-Allardt C, Ashwell M. UK Food Standards Agency Workshop Consensus Report: the choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br J Nutr. 2010;104:612–619. doi: 10.1017/S000711451000214X. [DOI] [PubMed] [Google Scholar]
- 42.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet. 2011;115(Suppl 1):S6–S10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]