Abstract
Introduction
Pancreatic adenocarcinoma is an aggressive malignancy. Oncolytic Ads are genetically modified to target tumor cells while sparing normal cells. We modified the knob domain of the adenovirus (Ad) serotype 5 with a serotype 3 knob domain and incorporated the CXCR4 promoter to regulate Ad E1A gene expression (Ad5/3-CXCR4-E1A). These modifications were made to efficiently infect and lyse pancreatic tumors.
Methods
Human pancreatic cancer lines CFPAC-1, PANC-1, AsPC-1, and BxPC-3 were obtained from ATCC. Efficiency of Ad infection in the cells was determined using an Ad construct expressing the green fluorescence protein (GFP) marker in place of the E1A gene (Ad5/3-CXCR4-GFP) and quantified by flow cytometry. Oncolytic activity in the pancreatic cancer cells was determined using the Ad5/3-CXCR4-E1A oncolytic Ad by a crystal violet staining method. To determine the oncolytic effect in vivo, pancreatic cancer cells were implanted on the flanks of 40 SCID mice (4 groups). Tumors were injected intratumorally for 3 days with Ad5/3-CXCR4-E1A, Ad5 wild-type (a positive control), or phosphate buffered saline (a no virus control). Tumor size, overall survival and body condition scale (BCS) were recorded. Statistical analyses included the Kaplan-Meier survival curve, the log-rank test, and ANOVA 1-way.
Results
The serotype 3 fiber-modified Ad with the CXCR4 promoter (Ad5/3-CXCR4-E1A) was most efficient in infecting and lysing pancreatic cancer cells compared with an Ad containing an unmodified fiber knob (Ad5-CXCR4-E1A). Treatment of pancreatic tumor xenografts in vivo with Ad5/3-CXCR4-E1A group resulted in significantly smaller tumors (p = 0.001), higher BCS (p = 0.01), and longer survival time (p = 0.04) than the other treatment groups.
Conclusion
Ad5/3-CXCR4-E1A treatment significantly prolonged survival in SCID mice pancreatic tumor xenografts. This novel construct represents a potential new therapy against pancreatic cancer.
Keywords: Ad5/3, adenovirus, chemokine receptor, CXCR4, oncolytic virus, fiber chimera, pancreatic adenocarcinoma, serotype, virotherapy
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States. Pancreatic adenocarcinoma is one of the most aggressive cancers and despite advances in the perioperative care, surgical techniques, and modern chemotherapy, significant improvement in terms of prolonging survival has been marginal [1]. Surgery remains the only treatment for potential cure; however, only a minority of patients can be considered to be truly cured [2]. Therefore, novel therapy is sorely needed.
Metastatic disease accounts for greater than 90% of cancer-related deaths [3]. The metastatic process is complex and is regulated by chemokines and their receptors [4]. Chemokines are a superfamily of small, low molecular weight proteins that induce cytoskeletal rearrangement, adhesion to endothelial cells, and directional migration once they interact with their respective chemokine receptors [4,5].
The chemokine receptor CXCR4, a seven-transmembrane G protein-coupled receptor, appears to play a pivotal role in the pathogenesis of metastatic solid tumors [6-8]. By acting as a “homing signal”, CXCR4 directs cancer cells' migration to organs that possess high concentration of the corresponding chemokine, SDF-1 [6]. CXCR4 is highly expressed in a number of cancers such as breast [6,8], colon [9], melanoma [10], and pancreas [11]. Given its importance in the pathogenesis of metastases, CXCR4 can serve as a potential molecule for the development of novel target-specific therapy.
Cancer virotherapy using oncolytic adenoviruses (Ads) is a promising therapeutic modality against a wide variety of neoplasms, including pancreatic adenocarcinoma. Oncolytic Ads are designed to replicate in tumor cells, resulting in viral-mediated oncolysis of infected tumor tissues and release of the virus progeny that are capable of further propagating to surrounding tumor cells but not in those of normal tissues. The serotype 5 (Ad5, subgroup C) is the most frequently used serotype in human clinical gene therapy trials [12]. Despite the advantages offered by the Ad5 serotype, overall efficacy of oncolytic Ads remains limited by suboptimal vector transduction in cancer tissues and nonspecific delivery to normal tissues. The process of target cell entry of Ad5 begins with its fiber knob binding to the Coxsackie Adenovirus Receptor (CAR). Unfortunately, most tumors are known to be deficient of CAR expression and consequently, tumor transduction by the unmodified Ad5 serotype is therefore limited. To increase viral transduction into tumor cells by a CAR independent pathway, we created a chimeric Ad by substituting the fiber gene of the Ad serotype 5 vector with an Ad serotype 3 fiber (Ad 5/3).
Although modifying the Ad5 can lead to an increase in transduction, it does not necessarily lead to specificity. To address this, we incorporated a characteristic that is specific to the tumor cells but not normal cells. We have found that the chemokine receptor CXCR4 is over expressed in several solid tumors, one of which is pancreatic cancers [11,13]. To exploit this, we placed the CXCR4 gene promoter before the open reading frame of the Ad E1A gene to regulate its expression. This will then restrict viral replication and oncolytic activity to cells that over express CXCR4 (i.e., pancreatic cancers) but not to those that have little or no expression of CXCR4. In this study, we present the in vitro and in vivo data to demonstrate the utility of using this novel virus to preferentially lyse and kill human pancreatic cancer cells.
Materials And Methods
Cell lines and culture conditions
The human pancreatic cancer lines CFPAC-1, PANC-1, AsPC-1, BxPC-3, and a rat breast cancer line MAT BIII (that lacks CXCR4 expression) were obtained from the American Type Culture Collection and cultured in Dubelco's Modified Eagle Medium (Invitrogen Corp, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Gemini BioProducts, Woodland, Ca) and 1% antibiotic-antimycotic solution (penicillinstreptomycin-fungizone; Sigma Chemicals Co., St. Louis, MO). The cells were maintained at 37°C in a 5% humidified CO2 incubator, and were subcultured using 1% trypsin-EDTA (Invitrogen).
Animals
Female SCID/bg mice at 4 - 6 weeks of age were obtained from a breeding colony at LSU Health Sciences Center in Shreveport using animals from Charles River Laboratories (Wilmington, MA). All animals received humane care based on guidelines set by the American Veterinary Association. The experimental protocols involving live animals were reviewed and approved by the Institutional Animal Care and Use Committee of LSU Health Sciences Center in Shreveport.
Adenoviral vectors
A conditionally replicative Ad5/3-based vector with the E1A promoter and coding regions replaced by a cassette encompassing the E1A gene placed under gene transcriptional control (Ad5/3-CXCR4-E1A) was constructed as previously described [14]. In this case an Ad shuttle vector was constructed with the human CXCR4 gene promoter inserted upstream of the Ad5 E1A gene open reading frames (ORF). Insertion into the pAd5/3-Easy-1 rescue vector to generate the Ad5/3 backbone, was accomplished in the E. coli recombination competent strain BJ-5183 by homologous recombination. The pAd5/3-Easy-1 rescue vector contains a substitution of the Ad serotype 3 fiber knob domain for the Ad serotype 5. By homologous recombination, the E1A expression cassette in the shuttle vector is flanked by Ad5 sequences located right upstream and downstream of the E1 region (right and left arm homology sequences) in the Ad5 genome and used to insert the shuttle vector cassette in place of E1A.
Western blot analysis
Treated and untreated CFPAC-1, PANC-1, AsPC-1, BxPC-3, and MAT BIII cells were harvested and solubilized in in lysis buffer (150 mM NaCl, 1.0% NP40, 20 mM Tris-HCL (pH 8.0), 5mM EDTA) containing protease inhibitors. Total protein content of each sample preparation was determined using a Bicinchoninic Acid (BCA) Total Protein assay (Pierce Biotechnology, Inc, Rockford, IL). Protein samples (20 μg) were electrophoresed on 4 - 20% Tris-HEPES-SDS precast polyacrylamide mini gel (Pierce) and subsequently transferred to PVDF membranes in a wet electrophoretic blotting system (Bio-Rad Laboratories, Hercules, CA). The PVDF membranes were incubated for 1 h in a blocking buffer of 5% non-fat dry milk in TBS buffer (136mM NaCl, 20mM Tris, 0.1% NP40), followed by overnight incubation at 4°C with buffer containing 5% non-fat dry milk. The membranes were probed either with goat polyclonal anti-CXCR4 antibody (Santa Cruz Biotechnology; Santa Cruz, CA) or with a mouse monoclonal anti-actin antibody (Sigma-Aldrich; St. Louis, MO.). The membranes were then incubated with a horseradish peroxidase (HRP) conjugated bovine anti-goat secondary antibody (Santa Cruz Biotechnology) or a HRP conjugated rabbit anti-mouse secondary antibody (Santa Cruz Biotechnology). Specific protein bands were visualized using the OPTI-4CN detection kit (Bio-Rad).
Measuring Ad infectivity
To determine the efficiency of infectivity, CFPAC-1 and PANC-1 cells were seeded at 1 × 105 cells per well in 24-well tissue culture plates one day before infection. The cells were infected with increasing titers of unmodified and fiber modified Ads expressing the green fluorescent protein (GFP) marker (Ad5-CMV-GFP, Ad5/3-CMV-GFP, or Ad5-CMV-GFP-RGD) in tissue culture medium containing 2% FBS. After infection for 2 hours, the medium was changed to 10% FBS. At 24 h and 48 h after infection, the cells were harvested by trypsinization, and GFP expression was quantified by flow cytometry using a FACSCalibur instrumend (Becton, Dickinson and Company, Franklin Lakes, NJ).
Measuring cytotoxicity in vitro
The CFPAC-1, PANC-1, AsPC-1, BxPC-3, and MAT BIII cells were seeded in 96 well plates at approximately 3 × 103 cells per well in tissue culture medium containing 10% FBS. After 16 hours the cells were infected with increasing titers of Ads (Ad5-wild-type, Ad5-CXCR4-E1A, or Ad5/3-CXCR4-E1A) at 0.1, 1, 10, 100, and 1000 ifu/cell in tissue culture medium containing 2% FBS. The cells were incubated for two hours before the media was aspirated and replaced with tissue culture medium containing 10% FBS. After five days the experiment was ended; the media was removed and the cells were rinsed with phosphate buffered saline (PBS; Invitrogen). To quantify cell killing, cells were fixed and stained with 0.1% crystal violet in 70% ethanol, followed by washing with tap water to remove excess dye. When the plates were dried, dye was dissolved with acidified ethanol (EtOH + 4% 1M HCL). Cell killing was determined by measuring absorbance at 595 nm using a SpectraMax 190 plate reader (Molecular devices, Sunnyvale, CA).
Measuring tumor response in vivo
CFPAC-1 cells were injected subcutaneously into the flanks of 40 female SCID mice (2.5 × 106 cells in 10μl PBS into each flank). The 40 SCID mice were divided into four groups of 10 mice. Once palpable tumors were established (approximately 10 mm3), then 5 × 109 viral particles of Ad5/3-CXCR4-E1A, Ad5 wild-type (a positive control virus), Ad5/3 wild-type, Ad5-CMV-GFP (a replication deficient negative control virus), or PBS alone (a no virus control) in 30 μL were injected intratumorally each day for three consecutive days. The tumors were measured with calipers every other day for 25 days. The longest dimension and corresponding perpendicular dimension (i.e. length and width) were recorded.
The physical condition and behavior of the mice were assessed and recorded daily using an established body condition scale (BCS). This scale was based on appearance, natural behavior, provoked behavior, and weight changes [15]. Specifically, scores were assigned based on appearance (bright vs. dull eyes, shiny vs. unkempt coat, hunching, piloerection), natural behavior (activity level, environmental interaction, isolation, self-mutilation), provoked behavior (moves away quickly, moves away slowly, does not respond, or reacts excessively), and body condition/weight (emaciated, thin, normal, overweight, obese). The scores from each category were then compiled, with a maximum score of 13 (perfectly healthy) and a minimum score of 1 (moribund). Mice were sacrificed if they declined to a score of 5. Scores of 6 - 10 represent early signs of clinical morbidity. The ANOVA 1-way statistical analysis was used to compare the mean tumor sizes and BCS among the groups. Survival analysis was determined by the Kaplan-Meier method and the log-rank test was used for statistical analysis.
Results
First, to determine the expression of endogenous CXCR4 in pancreatic cancer cancer cell lines, Western blot analysis was performed, and the amounts of the protein were compared to the expression of β-actin. As shown in Fig. 1, the human pancreatic cancer cell lines PANC-1, CFPAC-1, AsPC-1, and BxPC-3 showed high levels of CXCR4 expression, similar to the HeLa cervical cancer cell line. However, the rat MAT BIII breast cancer cell line demonstrated a lack of CXCR4 expression. These results suggest that using gene transcriptional control may be sufficient to target human pancreatic cancer cells for oncolytic virotherapy.
FIGURE 1. Determination of CXCR4 expression.
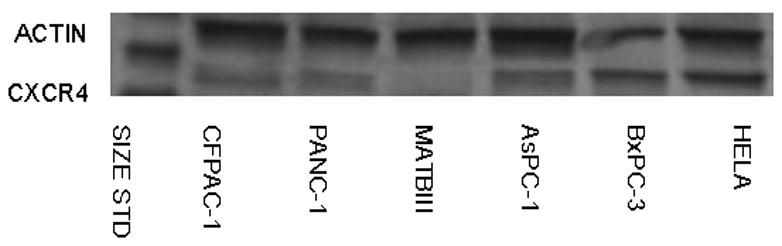
Western blot analysis was performed using lysates from pancreatic cancer cell lines PANC-1, CFPAC-1, AsPC-1, BxPC-3, and MAT BIII for expression of CXCR4 compared to the human cervical cancer cell line HeLa as a positive control.
To screen the fiber modification for viral infectivity enhancement, two capsid fiber modifications that result in Ad infection via a CAR-independent pathway, RGD [16] and F5/3 [17] were tested. Adenovirus containing the green fluorescent protein (GFP) was used to determine the efficiency of infection in the human pancreatic cell lines. All the Ad vectors had an identical backbone, the difference being the incorporation of these alternative modifications in the Ad fiber region. In order to quantify the percent of GFP positive cells, we performed flow cytometry analysis of infected cells. The cells were infected with Ad5-CMV-GFP, Ad5/3-CMV-GFP, or Ad5-CMV-GFP-RGD at ratios from 0.1:1 to 1,000:1 i.fu./cell and after 48 h post treatment they were subjected to flow cytometry analysis in order to quantify the percent of GFP positive cells. The results shown in Fig. 2, indicate the F5/3 fiber-modified Ad (Ad5/3-CMV-GFP) was most efficient in infecting PANC-1, CFPAC-1, AsPC-1, and BxPC-3 cells compared to an Ad containing an unmodified fiber (Ad5-CMV-GFP) or an Ad containing an RGD motif in the fiber knob (Ad5-CMV-GFP-RGD).
FIGURE 2. Determination of infection efficiency.
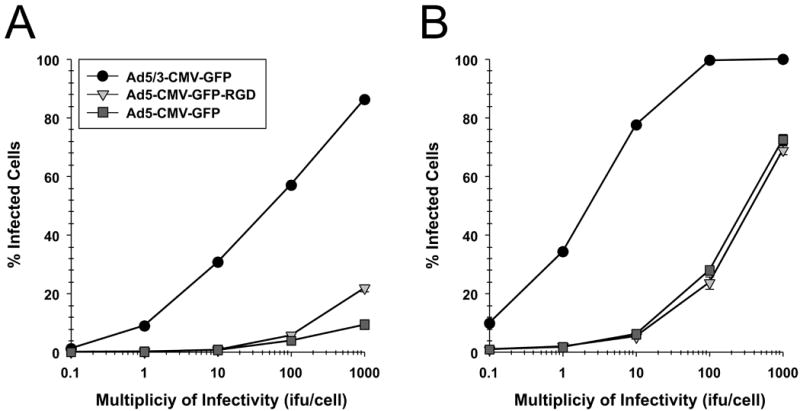
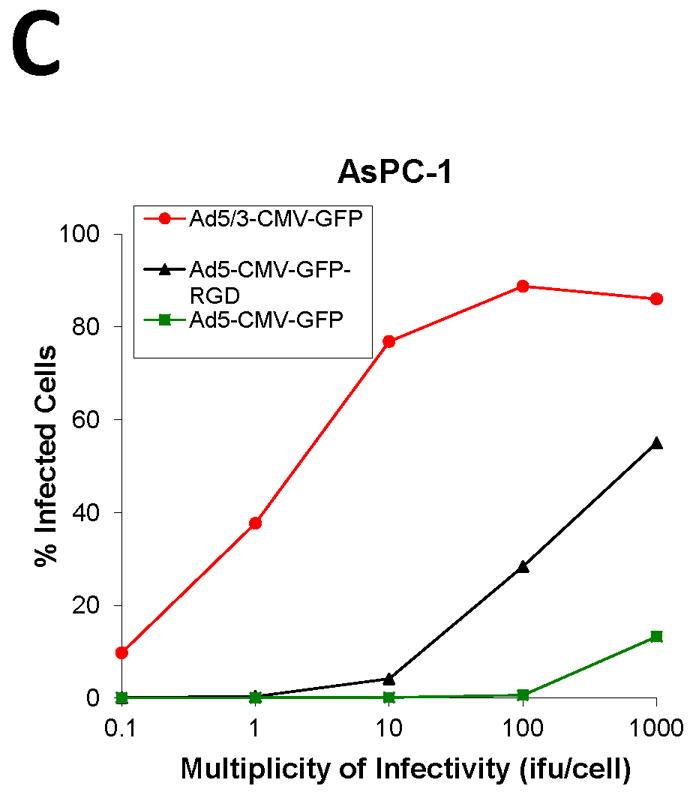
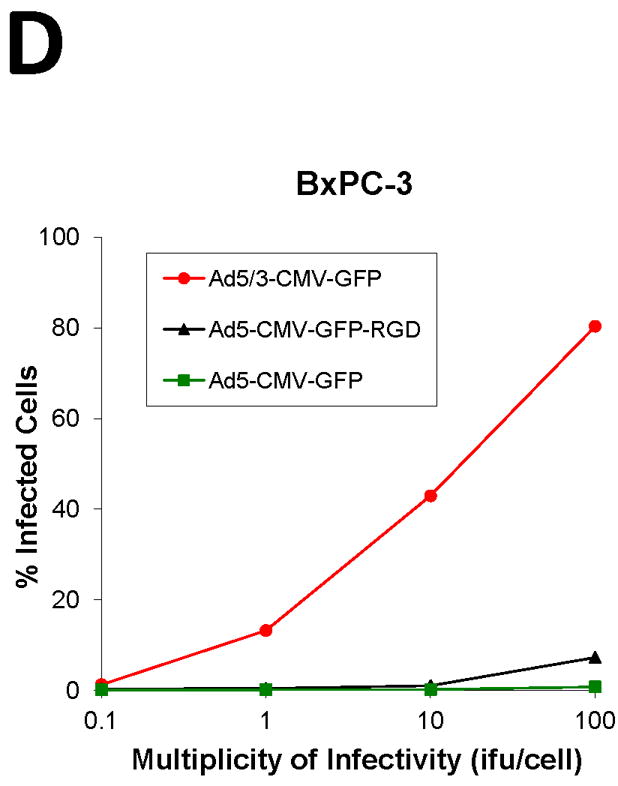
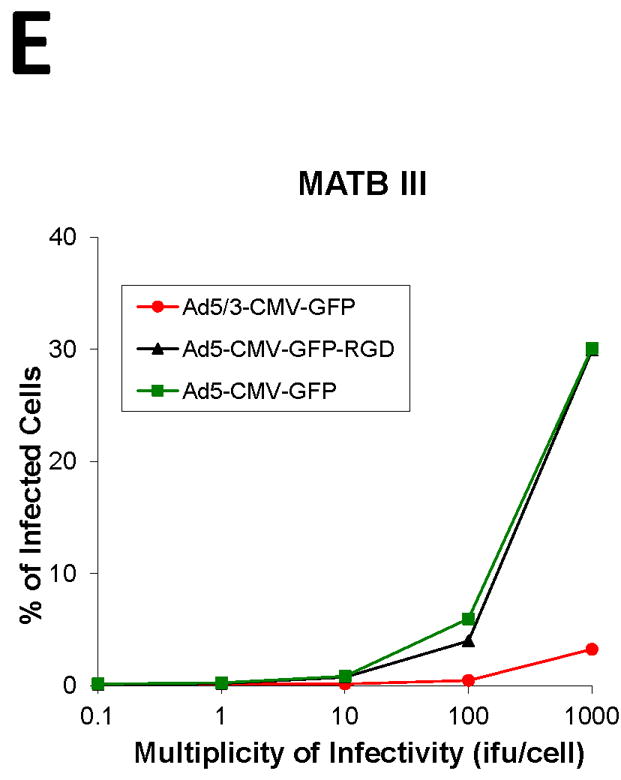
Flow cytometric analysis of (A) CFPAC-1 (B) PANC-1, (C) AsPC-1, (D) BxPC-3 human pancreatic cancer cells and (E) MAT BIII infected with Ad5/3-CMV-GFP (●), Ad5-CMV-GFP-RGD (▼) or Ad5-CMV-GFP (■) vectors expressing green fluorescent protein marker. An analysis was performed for expression of GFP at 48 h after infection with increasing doses from 0.1 i.fu./cell to 1,000 i.fu./cell. Values represent the mean ± SEM of 3 replicate measurements.
We next compared the cytotoxicity profile of oncolytic Ads in the pancreatic cancer cell lines in vitro by infection with Ad5-CXCR4-E1A, Ad5/3-CXCR4-E1A, or Ad5 wild-type (Fig. 3). Cell survival was determined by crystal violet staining and quantified by measuring absorbance at 595 nm. In the CFPAC-1, PANC-1, AsPC-1, and BxPC-3 human pancreatic cancer cell lines expressing high levels of CXCR4, the oncolytic Ad construct incorporating both the CXCR4 promoter and the serotype 3 fiber (Ad5/3-CXCR4-E1A) demonstrated highly effective oncolytic activity in pancreatic cancer cells. However, an oncolytic Ad construct incorporating the CXCR4 promoter but containing an unmodified fiber (Ad5-CXCR4-E1A) was less effective in oncolysis. This result supports our finding in Fig. 2 that a F5/3 fiber modification is more effective in infecting human pancreatic cancer cell lines. Interestingly, there was no appreciative oncolytic activity using the Ad5/3-CXCR4-E1A or Ad5-CXCR4-E1A viruses against MAT BIII cells, likely because these cells have undetectable CXCR4 expression.
FIGURE 3. Oncolytic activity of an oncolytic Ad construct in pancreatic cancer cell lines.
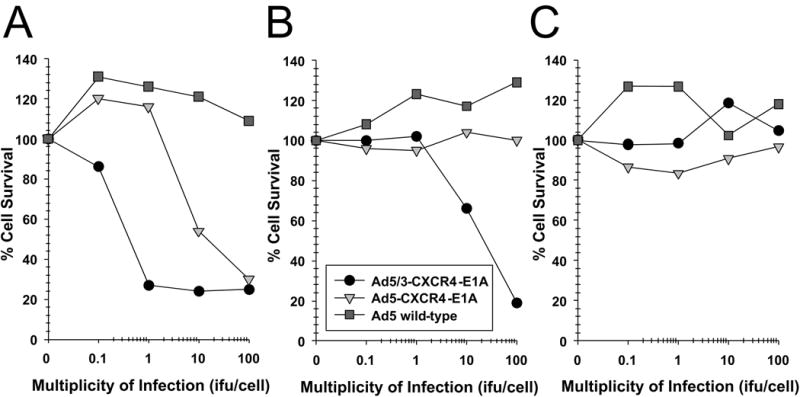
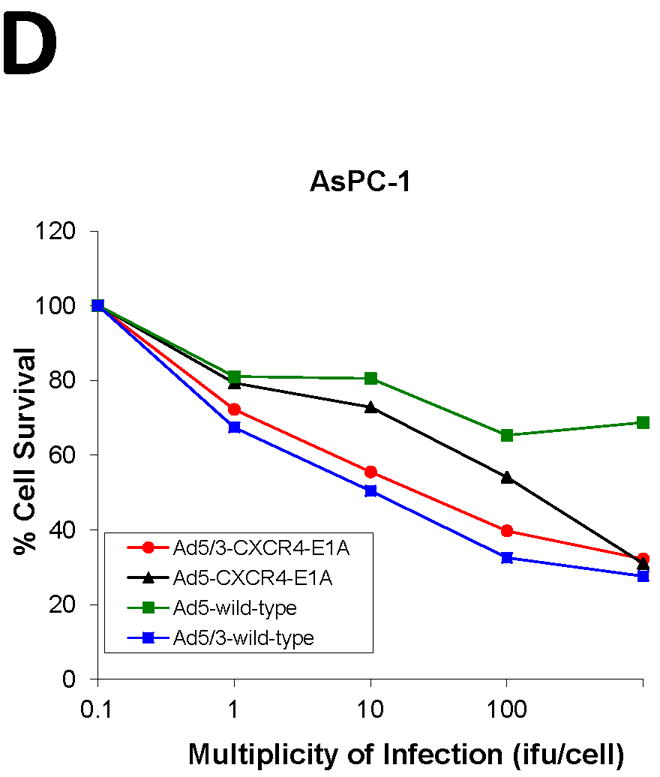
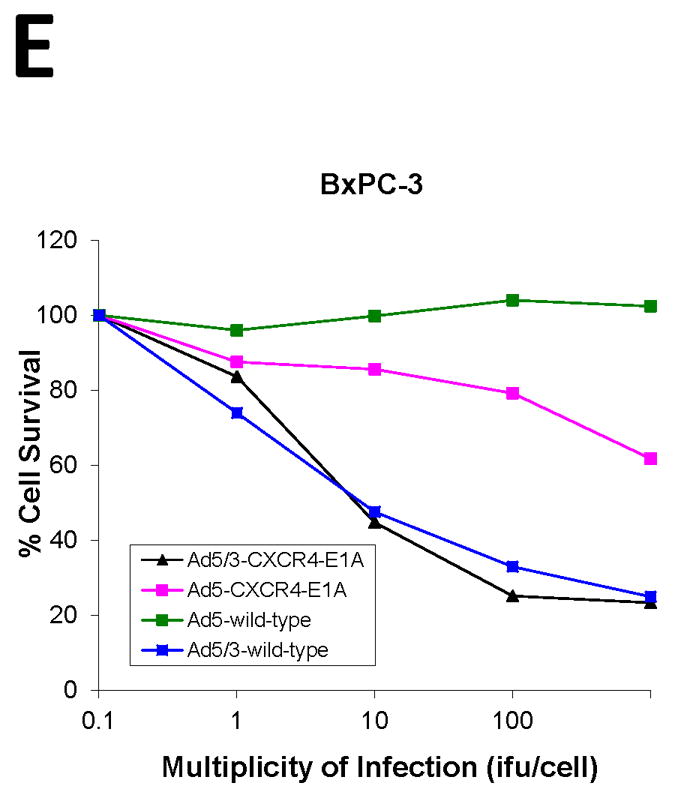
Oncolysis was evaluated by crystal violet staining in the pancreatic cancer cell lines (A) PANC-1, (B) CFPAC-1, (C) MAT BIII, (D) AsPC-1, (E) BxPC-3 after infection with increasing doses from 1 i.fu./cell to 100 i.fu./cell of Ad5/3-CXCR4-E1A (●), Ad5-CXCR4-E1A (▼), or Ad5 wild-type (■) as described in Material and Methods. Values represent the mean ± SEM of 3 replicate measurements.
Finally, the anti-tumor effect of the Ad5/3-CXCR4-E1A oncolytic Ad was analyzed in vivo using CFPAC-1 cells injected subcutaneously in an SCID mouse xenograft model. As shown in Fig. 4, treatment of Ad5/3-CXCR4-E1A resulted in an inhibition of tumor growth. In comparison, treatment with Ad5 wild-type and Ad5/3 wild-type only mediated a partial effect to inhibit tumor growth. As a control, treatment with a negative replication deficient Ad (Ad5-CMV-GFP) had no effect on tumor growth compared to mice receiving no Ad treatment (PBS).
FIGURE 4. Oncolytic activity of an oncolytic Ad construct in an orthotopic murine model of pancreatic cancer.
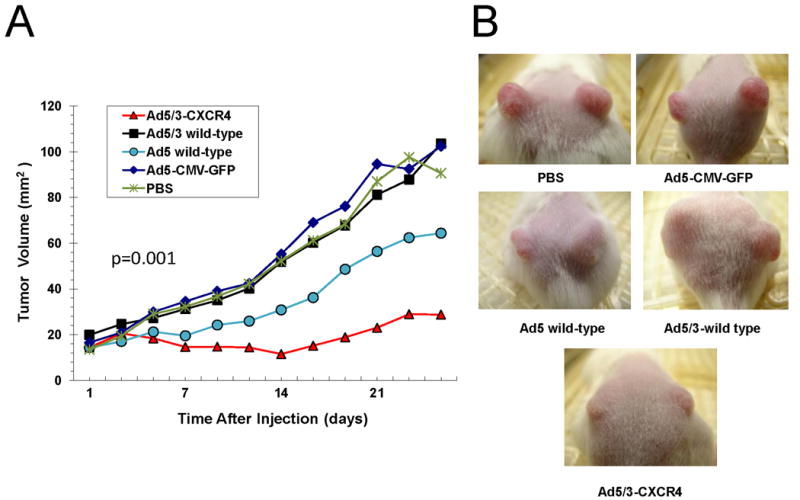
(A) Antitumor effects of oncolytic Ad agents were determined using a CFPAC-1 mouse xenograft model. When the tumor reached 10 mm3 in widest diameter, 5 × 109 i.f.u of Ad5/3-CXCR4-E1A (●), Ad5 wild-type (▼), Ad5-CMV-GFP (■) were injected intratumorally in a 30 μL volume. Control tumors were injected with an equal volume of PBS only (◆). The tumor volumes were calculated by multiplying the measured length × the measured width. Values represent the mean tumor size ± SEM. (B) Representative tumors shown in mice at 25 days after treatment.
At 25 days from the time of Ad treatment, the mean tumor sizes for the PBS, Ad-CMV-GFP, Ad5 wild type, Ad5/3 wild type, and Ad5/3-CXCR4-E1A groups were 100.73 mm2, 102.38 mm2, 64.43 mm2, 103.6 mm 2, and 28.76 mm2, respectively (p = 0.001). When the mean body condition score (BCS) of the Ad5/3-CXCR4-E1A treated group was compared with those in the Ad5 wild-type, Ad5-CMV-GFP, and PBS groups, the Ad5/3-CXCR4-E1A group had the highest mean BCS (Fig. 5). The mean BCS at 25 days from the time of oncolytic viral injection for the Ad5 wild-type, Ad5-CMV-GFP, PBS, and Ad5/3-CXCR4-E1A were 9.75, 10.75, 10.90, and 11.0, respectively (p=0.01).
FIGURE 5. The physical condition and behavior of an orthotopic murine model of pancreatic cancer.
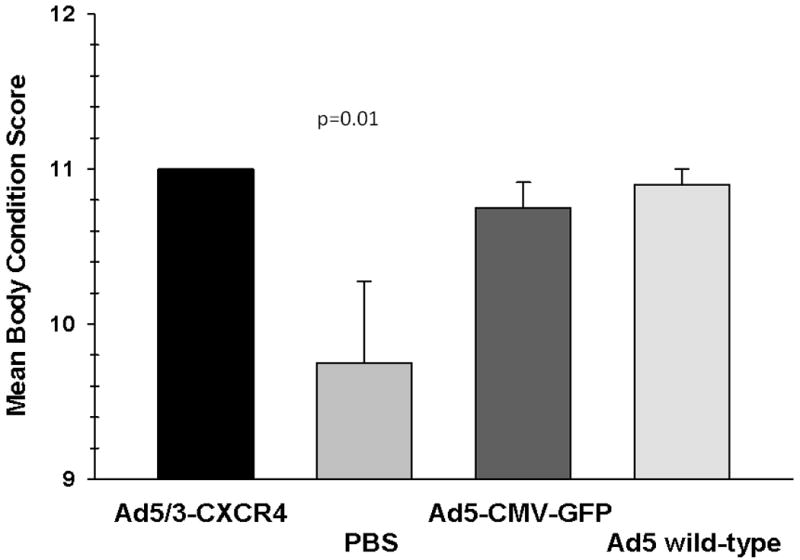
Mice treated with oncolytic Ad agents were assessed at 25 days post treatment using an established body condition scale (BCS), based on appearance, natural behavior, provoked behavior, and weight changes. Each bar represents the mean body condition score ± SEM.
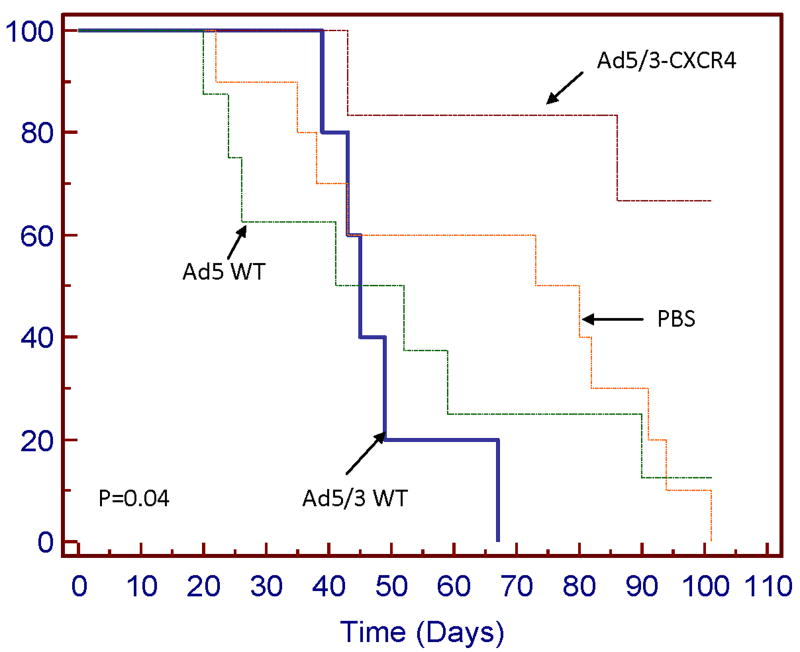
We examined whether smaller tumor burden also translates to improved overall survival for mice treated with Ad5/3-CXCR4-E1A (Fig. 6). At a follow-up time of 101 days, the median survival times at which 50% of the mice have died, for mice treated with Ad5 wild-type or PBS alone were 46.5 days and 80 days, respectively. However, the median survival time for the Ad5/3-CXCR4-E1A group had not been reached (p = 0.04). These results demonstrate when compared to the other treatment groups (Ad5 wild-type, Ad5/3 wild-type, Ad5-CMV-GFP, or PBS), the Ad5/3-CXCR4-E1A treatment group significantly inhibits tumor growth and prolongs survival in a SCID mouse pancreatic tumor model.
FIGURE 6. Kaplan-Meier overall survival curve.
Groups of SCID mice with CFPAC-1 xenograft tumors rats were compared after treatment with Ad5/3-CXCR4-E1A, Ad5 wild-type, or Ad5/3 wild-type. Control tumors were injected with an equal volume of PBS only.
Discussion
Pancreatic adenocarcinoma remains a recalcitrant disease. Today's five year survival rate (5%) for all stages of disease has not improved much compared to the survival rate seen in the early 1970's (3%) [1]. Despite recent advances, the therapeutic armamentarium against pancreatic adenocarcinoma is somewhat limited. Whether patients receive chemotherapy and/or radiation therapy before or after surgery, outcome remains dismal. It is likely that the majority of pancreatic cancers are chemo and radioresistant. Significant impact on this disease will therefore require novel therapeutics. Virotherapy represents one such option [14,18,19].
Oncolytic virotherapy is a relatively young field when compared to other traditional fields such as chemotherapy, radiation therapy, and surgery. The ability of viruses to kill cancer cells was initially observed in a patient with cervical carcinoma who experienced significant tumor response after rabies vaccination [20]. It was only recently that this field has expanded because of our increased understanding of the biology of tumorgenesis. A number of attenuated viruses have been tested in the preclinical and clinical setting; some of these include the Herpes simplex virus-1, Vaccinia virus, Influenza virus, Newcastle disease virus, Poliovirus, Reovirus, vesicular virus, and adenovirus [18,20]. The attenuated adenovirus-based vector (i.e. conditionally replicating adenoviruses or CRAds) has emerged as a promising novel antitumor agent due to its low pathogenicity, effective oncolytic capability, and enhanced transfer of therapeutic genes [21-23].
Metastatic disease accounts for greater than 90% of cancer-related deaths [3]. A better understanding of the biology underlining the process of metastasis is crucial. We found that several key molecules were involved in the metastatic process, one of which is the chemokine receptor CXCR4 [6-8]. CXCR4 is a seven-transmembrane G protein-coupled receptor that is overexpressed in a number of tumors and appears to play a pivotal role in the metastatic process of these various tumors [6-8]. Tumors cells having a high expression of CXCR4 seem to preferentially migrate to sites that produce a significant amount of the CXCR4-specific ligand, the stromal derived factor-1 (SDF-1); these distant sites are the liver, bone, and lung [6].
Given CXCR4's involvement in the metastatic process, we sought to determine whether CXCR4 is also important in the pathogenesis of pancreatic cancer. CXCR4 expression has been linked to increased proliferation, invasion, and migration of human pancreatic cancer cells [11,13,24-27]. Additionally, its expression in pancreatic cancer specimens correlated with advanced disease [11]. Using immunohistochemistry, Wehler et al compared CXCR4 expression in benign human pancreas with 103 pancreatic cancer specimens [11]. They found that, in benign tissue, there was a weak cytoplasmic and rare membranous staining of CXCR4, whereas in all 103 malignant specimens, the expression rate for CXCR4 was 100%; such expression varied from weak (10%), intermediate (61%) to strong (30%) [11]. We postulated that, by exploiting the above unique characteristic of pancreatic cancer, we can design a novel therapeutic agent that will target CXCR4 expression in pancreatic cancer.
Several obstacles will need to be overcome before adenoviral-based therapy can be considered in the clinical setting. Efficient viral transduction is necessary, especially considering that Ads require CAR expressions, yet most tumors are low in CAR expressions. To address this, we substituted the fiber knob of the wild type Ad5 with the Ad3 (Ad5/3), thereby increasing viral tropism to pancreatic tumor cells via the CAR independent pathway. Similar to our previous study that investigated viral tropism in breast cancer cells [14], we also demonstrated increased viral tropism in PANC-1 and CFPAC-1 human pancreatic cells with the modified Ad5/3 viruses.
Another obstacle to overcome in virotherapy is replication specificity. Non-specific viral transduction and propagation in normal cells can lead to unintended toxicity. To circumvent this, we placed a tumor selective promoter (TSP), the chemokine receptor CXCR4 which has been found to be overexpressed in pancreatic cancers but not in normal cells, into the open reading frame of the viral genome to provide transcriptional control. Indeed, we demonstrated that such chimeric viruses (Ad5/3-CXCR4-E1A) preferentially replicated and lysed cells that demonstrated high CXCR4 expression (i.e., PANC-1 and CFPAC-1) while having no oncolytic activity against cells that demonstrated low in CXCR4 expression (i.e., MAT BIII).
Our in vivo studies with the pancreatic cancer SCID mice further support the efficacy of our oncolytic Ad. We demonstrated that not only did intratumoral injection of the Ad5/3CXCR4-E1A had resulted in a significantly lower tumor growth, it also led to a significantly higher overall survival compared to the groups that received intratumoral injections from the unmodified wild type Ad5 or control viruses.
It is noteworthy to recognize that the oncolytic viruses seemed to halt tumor growth rather than reduce it. We postulated that one of the reasons might be because of the suboptimal concentration of the oncolytic virus that was used to inject the tumors. Another possibility is the lack of adequate number of days of injection; we only injected the mice for three consecutive days. Perhaps by increasing both the dosage and the number of days of injection, we might be able to demonstrate cessation of tumor growth. These issues will be addressed in the next set of experiments.
Our results are very similar to those reported by Stoff-Khalili et al [14]. Using the same modified oncolytic virus, these investigators demonstrated therapeutic efficacy against a number of breast cancer cell lines, primary breast cancer tissue slices, and xenograft breast cancer mouse model.
An interesting observation in our study is that the mean body condition score (BCS) of the group that had the Ad5/3-CXCR4 was significantly higher than the other groups. This score was used to assess the physical condition and behavior of the mice; the higher the score, the healthier the mice. This observation is in line with our in vitro results mainly that our oncolytic Ad preferential kill targeted tumor cells while sparing normal tissues.
Hepatotoxicity from these viruses is also a major concern with adenoviral gene therapy. Using a tissue slice model system, we demonstrated that the Ad-CXCR4 exhibits a “tumor on/liver off” phenotype [23]. In other words, the Ad-CXCR4 preferentially infects tumor cells but not normal liver tissue.
It is also known that CXCR4 is expressed in bone marrow hematopoietic stem cells and what impact Ad-CXCR4 has on these cells remains unknown. To address this concern, our group intends to test the Ad-CXCR4 on the Syrian hamster model. The Syrian hamster model is ideal to study the systemic effects of Ad5 derived oncolytic vectors because the virus replicates well in Syrian hamster tissues [28].
In summary, we demonstrated through a step-wise manner that a Ad5/3-CXCR4 oncolytic Ad is not only able to efficiently transduce pancreatic cancer cells via a CAR independent pathway, but that it can also specifically lyse pancreatic tumor cells in vitro and increase survival in vivo in a xenograft model. Further study into this novel oncolytic Ad may lead to its potential testing in the clinical arena.
Acknowledgments
This work was supported, in part, by NIH grants R42-CA114921 and 5P50CA101955 and funding from the Louisiana Gene Therapy Research Consortium and the Feist-Weiller Cancer Center.
Footnotes
Presented at the 6th Annual Academic Surgical Congress, February 1-3, 2011, Huntington Beach, CA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J for Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chu QD, Khushalani N, Javle M, Douglass H, Gibbs J. Should adjuvant therapy remain standard of care for patients with resected adenocarcinoma of the pancreas? Ann Surg Oncol. 2003;10:539–45. doi: 10.1245/aso.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Wong D, Korz W. Translating an antagonist of chemokine receptor CXCR4 from bench to bedside. Clin Canc Res. 2008;14:7975–80. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Baruch A. The Multifaceted Roles of Chemokines in Malignancy. Cancer Metast Rev. 2006;25:357–371. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 6.Mueller A, Homey B, Soto H. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 7.Salvucci O, Bouchard A, Baccarelli A, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–83. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 8.Chu QD, Panu L, Holm N, Li B, Johnson L, Zhang S. High chemokine receptor CXCR4 level in triple negative breast cancer specimens predicts poor clinical outcome. J Surg Res. 2010;159:689–95. doi: 10.1016/j.jss.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Ottaniano A, Franco R, Aiello Talamanca A, et al. Overexpression of Both CXC Chemokine Receptor 4 and Vascular Endothelial Growth Factor Proteins Predicts Early Distant Relapse in Stages II-III Colorectal Cancer Patients. Clin Cancer Res. 2006;12:2795–2803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 10.Murakami T, Maki W, Cardones AR, et al. Expression of CXC Chemokine Receptor-4 Enhances the Pulmonary Metastatic Potential of Murine B16 Melanoma Cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 11.Wehler T, Wolfert F, Schimanski C, Gockel I, Herr W, Biesterfeld S, et al. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncology Reports. 2006;16:1159–64. [PubMed] [Google Scholar]
- 12.Short J, Rivera A, Wu H, Walter M, Yamamoto M, Mathis J, Curiel D. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficiency. Mol Cancer Ther. 2010;9(9):OF1–9. doi: 10.1158/1535-7163.MCT-10-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas R, Kim J, Revelo-Penafiel M, Angel R, Dawson D, Lowy A. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut. 2008;57:1555–60. doi: 10.1136/gut.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoff-Khalili M, Rivera A, Stoff A, Mathis J, Rocconi R, Matthews Q, et al. Combining high selectivity of replication via CXCR4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer. Int J Cancer. 2006;120:935–41. doi: 10.1002/ijc.22338. [DOI] [PubMed] [Google Scholar]
- 15.Paster EV, Villines KA, Hickman DL. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med. 2009;59:234–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Krasnykh V, Dmitriev I, Navarro J, Belousova N, Kashentseva E, Xiang J, et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Research. 2000;60:6784–7. [PubMed] [Google Scholar]
- 17.Uil T, Seki T, Dmitriev I, Kashentseva E, Douglas J, Rots M, et al. Generation of an adenoviral vector containing an addition of a heterologous ligand to the serotype 3 fiber knob. Cancer Gene Therapy. 2003;10:121–4. doi: 10.1038/sj.cgt.7700543. [DOI] [PubMed] [Google Scholar]
- 18.Nishimoto T, Yoshida K, Miura Y, Kobayashi A, Hara H, Ohnami S, et al. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Therapy. 2009;16:669–80. doi: 10.1038/gt.2009.1. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Cody J, Rivera A, Waehler R, Wang M, Kimball K, et al. Conditionally replicating adenovirus expression TIMP2 for ovarian cancer therapy. Clin Cancer Res. 2011;17:538–49. doi: 10.1158/1078-0432.CCR-10-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everts B, van der Poel H. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Therapy. 2005;12:141–61. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]
- 21.Curiel D. The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395–99. [PubMed] [Google Scholar]
- 22.Alemany R, Balague C, Curiel D. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–7. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 23.Stoff-Khalili M, Stoff A, Rivera A, Banerjee N, Everts M, Young S. Preclinical evaluation of transcriptional targeting strategies for carcinoma of the breast in a tissue slice model system. Breast Cancer Research. 2005;7:R1141–52. doi: 10.1186/bcr1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchesi F, Monti P, Leone B, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–7. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 25.Saur D, Seidler B, Schneider G, et al. CXCR4 expression increases liver and lung metastatsis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237–50. doi: 10.1053/j.gastro.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Mori T, Doi R, Koizumi M, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 27.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer:a possible role for tumor progression. Clin Can Res. 2000;6:3530–5. [PubMed] [Google Scholar]
- 28.Spencer J, Sagartz J, Wold W, Toth K. New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster. Cancer Gene Therapy. 2009;16:912–22. doi: 10.1038/cgt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]