Introduction
Ablation of atrial fibrillation (AF) by using strategies that eliminate AF triggers via pulmonary vein (PV) isolation1 produces a single procedure success of 45%-65% at up to 1 year.2 Importantly, while it is known that PV ectopy may trigger AF, the mechanisms that actually sustain human AF after it has been triggered are unclear.
There are 2 prevailing hypotheses. The multiwavelet hypothesis is based on evidence that complex activation in AF represents continuously meandering electrical waves,3 while the localized source hypothesis is based on experimental models in which rapidly activating reentrant circuits (rotors)4 or focal impulses5 cause disorganized AF. There has until now been indirect6 but little7,8 or no3 direct evidence to support sustaining rotors in human AF.
We present a gentleman referred because of persistent AF despite prior left atrial radiofrequency Maze and endocardial ablation procedures. In the electrophysiological study, novel computational mapping developed in our laboratory revealed one AF rotor in the right atrium and another in the left atrium. Brief 3-5-minute ablation applications at each rotor (focal impulse and rotor modulation [FIRM]) abruptly terminated AF to sinus rhythm, with non-inducibility on aggressive testing and no AF recurrence by continuous ECG monitoring over 6 months. A video recording of the entire case is provided as an online supplement.
Case report
We present a 63-year-old Caucasian gentleman with persistent AF for 6 years causing palpitations, chest pain, dyspnea, and fatigue. The patient has coronary disease for which, at an outside center 6 years ago, he underwent bypass grafting of the left internal thoracic artery to the left anterior descending artery, and vein grafts to the first diagonal and first obtuse marginal arteries. Concomitant radiofrequency Maze ablation was also performed. Unfortunately, persistent AF continued postoperatively. He underwent further left atrial endocardial ablation at an outside center 2 years ago that also failed to improve his symptoms. He has had trials of sotalol, dofetilide, dronedarone, and amiodarone. At this time, all 7 electrocar-diograms (ECGs) taken for over 10 months show AF with a cycle length (CL) of 160-180 ms.
The patient has treated hyperlipidemia and benign prostatic hyperplasia. Recurrent angina was treated by the placement of drug-eluting stents to the right coronary artery 10 months ago. The patient is euthyroid and has a remote history of excessive alcohol use and smoking. He currently takes aspirin, clopidogrel, warfarin, metoprolol, rosuvastatin, and tamsulosin. Transthoracic echocardiography revealed left atrial diameter of 5.6 cm and left ventricular ejection fraction of 58%. After a discussion of risks and benefits, the patient provided written consent for FIRM-guided AF ablation as recently reported9 and for video recording of his case.
The patient was intubated and mechanically ventilated. He presented at the commencement of the procedure in sinus rhythm, but AF initiated (CL ≈ 174 ms) as catheters were advanced via the femoral veins to the coronary sinus and via transseptal cannulation to the left atrium. Intravenous heparin was infused to a target activated clotting time of >350 seconds, meeting or exceeding the recommendations for all equipment used in the case. A basket catheter (Constellation, Boston Scientific, MA) was advanced through an 8.5-F SL1 sheath to the right atrium (Figure 1A). We recorded simultaneously from 64 simultaneous unipoles (filtered at 0.05-500 Hz with notch filter “On”) and exported digitized electrograms from our electrophysiological recorder (Bard Labsystem Pro, Natick, MA).
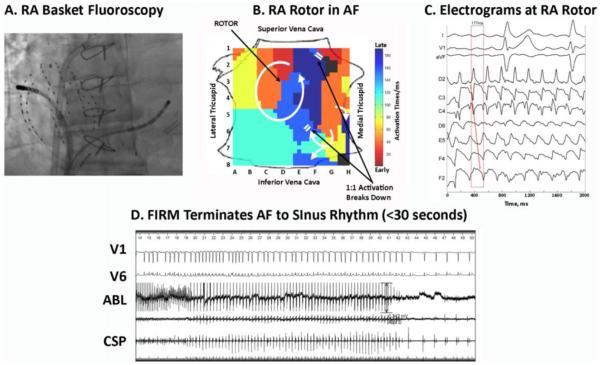
Figure 1.
Right atrial FIRM mapping and ablation during AF. A: Basket and ablation catheters in the right atrium during AF (anteroposterior fluoroscopy). B: Counterclockwise rotor in posterolateral right atrium centered over C3, with collision beyond the spiral arms (indicated by white double lines; supplementary movie). C: Electrograms around the RA rotor site, indicating counterclockwise rotation with variability at CL ≈ 177 ms. D: FIRM ablation at RA rotor terminates AF to sinus rhythm within 30 seconds. ABL = ablation electrogram; AF = atrial fibrillation; CL = cycle length; CSP = proximal coronary sinus; FIRM = focal impulse and rotor modulation; RA = right atrial.
FIRM mapping was achieved by using a novel mapping system (RhythmView; Topera Medical, San Diego, CA) in which the right atrium is projected with the lateral tricuspid annulus folded laterally (Figure 1B). Right atrial FIRM movies of AF revealed a counterclockwise rotor, indicated by an early-to-late activating spiral in the posterolateral right atrium, whose center of rotation migrated over a small region (red-to-blue region in Figure 1B; time stamp 02:09–03:32 in online movie). These maps was generated by computational analysis of AF electrograms (Figure 1C) to plot activation sequences (Figure 1B) or animate the raw unipolar voltages over time to create movies (supplemental movie). After QRS removal,10 AF CL was computed in the context of our studies of human atrial repolarization,11 which determine the maximum AF rate, and rate-related conduction slowing.12 For instance, in Figure 1B, direct propagation between 2 adjacent sites with widely separate activation times (for instance, the juxtaposed red and blue) would require unphysiologically slow conduction. To increase targeting accuracy, maps were generated by using linear interpolation of the phase state between each electrode and its nearest neighbors. The resolution provided by this approach is likely sufficient to map AF sources for ablation, despite suboptimal basket electrode spacing, since the diameter of each ablation lesion (≈7 mm) requires few lesions to span electrodes near the basket poles (≈5-mm separation) or even at the equator where separation is greater.
FIRM-guided ablation in the right atrium was achieved by advancing an ablation catheter (Blazer; Boston Scientific, Natick, MA) to electrodes BC23. High-output pacing was performed to exclude phrenic nerve capture. The center of rotation (guided by movies) was then ablated at 45-50 W with a target temperature of 52°C. Remarkably, AF terminated abruptly to sinus rhythm within 30 seconds (Figure 1C). Ablation continued for 2.5 minutes to cover the center of rotation on FIRM maps. Burst pacing to CL = 140 ms now induced cavotricuspid isthmus-dependent flutter, confirmed by entrainment with concealed fusion, that self-terminated. Cavotricuspid isthmus ablation was performed, and bidirectional cavotricuspid isthmus block was verified. Aggressive AF reinduction was now attempted, using burst pacing to CL = 140 ms and isoproterenol infusion (2-10 μg/min). Sustained AF was reinduced with CL = 180 ms. Repeat FIRM mapping in the right atrium showed elimination of the previous rotor and no additional sources.
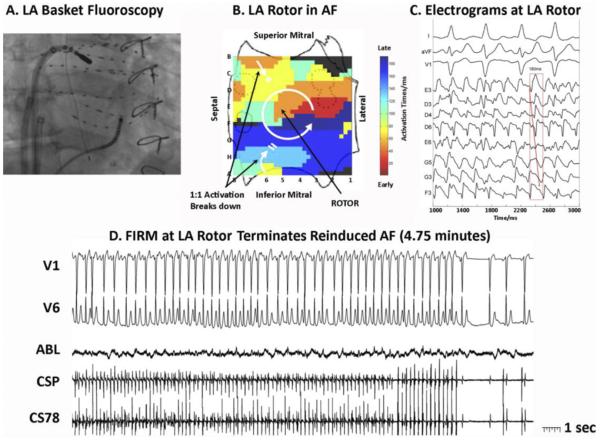
Left atrial FIRM mapping was performed by placing the basket catheter through an SL1 sheath into the left atrium (Figure 2A), oriented with the left atrium cut across its equator and projected with superior mitral annulus folded upward (Figure 2B). Left atrial FIRM movies in AF revealed a counterclockwise rotor centered at electrodes DE5-6 with precession (Figure 2B; time stamp 06:53-07:36 in online movie). FIRM-guided ablation at the rotor center organized then terminated AF to sinus rhythm after 4.75 minutes (Figure 2C). AF was now noninducible, with no triggers on isoproterenol (after 8 minutes total FIRM ablation).
Figure 2.
Left trial FIRM mapping and ablation of reinduced AF. This AF required vigorous reinduction to be sustained after right atrial FIRM. A: Basket and ablation catheters in the left atrium during AF (right anterior oblique 30o fluoroscopy). B: Counterclockwise rotor in midposterior left atrium, with collision beyond spiral arms (indicated by double lines). Pulmonary vein ostia indicated by dashed lines. C: Electrograms around the LA rotor showing counterclockwise rotation with variability. D: FIRM ablation at the LA rotor terminates AF to sinus rhythm (4.75 minutes). ABL = ablation electrogram; AF = atrial fibrillation; CS = coronary sinus; FIRM = focal impulse and rotor modulation; LA = left atrial.
Finally, the PVs were checked by using a circular catheter (Lasso; Biosense-Webster, Diamond Bar, CA) and showed isolation of the left and right superior veins, with small potentials at the inferior veins that were reisolated. There were no complications, and in our experience atrial mapping with the compliant basket has been extremely safe.
The patient underwent implantation of a loop recorder (Reveal XT; Medtronic, Minneapolis, MN), as in the Conventional Ablation for AF with or without Focal Impulse and Rotor Modulation trial.9 Anti-arrhythmic medications were discontinued after a 3 month “blanking period.” In the first 6 months since ablation (including the blanking period), the patient’s symptoms were eliminated and continuous ECGs from the implanted loop recorder showed no sustained AF (total AF/AT burden 0.0%) with only a single nonsustained 26-second episode during blanking.
Discussion
This is the first demonstration of clinical AF therapy based on the detection and targeted ablation of sustaining rotors. In this 63-year-old gentleman with persistent AF for at least 10 months despite prior Maze and then endocardial ablation procedures, novel computational mapping revealed sustained rotors in the right and left atria. Targeted (FIRM) ablation at each rotor abruptly terminated AF to sinus rhythm. This was achieved rapidly prior to any PV mapping or ablation. The patient is AF-free at 6 months using continuous implanted ECG monitoring since his ablation.
This case exemplifies the approach that we recently reported in the Conventional Ablation for AF with or without Focal Impulse and Rotor Modulation trial,9 and provides compelling evidence that human AF can be sustained by localized rotors. First, the isochrones and movies showed reentrant spiral waves that continued for tens of minutes during mapping (thousands of cycles; 15-20 shown for each atrium). Second, activation emanated from the rotor to the surrounding atrium with fibrillatory conduction. Third, brief localized ablation at rotor centers alone acutely terminated AF, with extreme difficulty in reinduction thereafter. We believe that these lines of evidence strongly support the conclusion that rotors were the primary drivers of AF in this gentleman. Absence of AF after FIRM ablation, using continuous ECG monitoring as in the Conventional Ablation for AF with or without Focal Impulse and Rotor Modulation trial,9 further supports this conclusion.
Although it is possible that prior procedures contributed to these AF mechanisms, it is also possible that these rotors were present at the outset and missed by prior ablations. This is particularly true since those procedures did not substantially alter the AF presentation in this gentleman and did not ablate the lateral right atrium (that harbored a rotor). Notably, the modes of AF termination in this FIRM-guided case differ from AF termination using conventional ablation, which often requires prolonged ablation and terminates AF to atrial tachycardia rather than sinus rhythm.13 Detailed mapping studies are necessary to define differences in the mechanisms of AF termination by each strategy. Interestingly, reconnection of the inferior PVs in this case, which we detected after FIRM ablation had terminated AF and rendered AF noninducible, was likely unrelated to the perpetuation of AF in this gentleman. While studies have shown that the PVs may reconnect in patients with postablation AF recurrence,14 studies also show that the PVs may reconnect in patients without recurrent AF.15 Future studies are thus required to examine the functional interplay between PV triggers and localized AF-sustaining rotors or focal impulses.
Conclusions
We present a gentleman with persistent AF sustained by localized rotors, where brief targeted ablation (FIRM) abruptly terminated AF to sinus rhythm and rendered AF non-reinducible before PV isolation. On initial follow-up using continuous implanted ECG monitors, FIRM-guided ablation has eliminated what was previously intractable persistent AF. FIRM ablation may provide a mechanistically guided, patient-tailored ablation approach for patients with AF.
Supplementary Material
Acknowledgments
This work was supported by grants to Dr Narayan from the National Institutes of Health (grant numbers HL70529, HL83359, and HL83359-S1) and the Doris Duke Charitable Foundation and from the American Heart Association to Dr Krummen. Dr Narayan is a coauthor of intellectual property owned by the University of California Regents and licensed to Topera, Inc. Dr Narayan holds equity in Topera. Topera does not sponsor any research, including that presented here. Dr Narayan reports having received honoraria from Medtronic, St Jude Medical, and Biotronik.
ABBREVIATIONS
- AF
atrial fibrillation
- CL
cycle length
- ECG
electrocardiogram
- FIRM
focal impulse and rotor modulation
- PV
pulmonary vein (Heart Rhythm 2012;xx:xxx)
Footnotes
Appendix
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/doi:10.1016/j.hrthm.2012.03.055.
References
- 1.Calkins H, Kuck KH, Cappato R, et al. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;2012;9:632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 3.Allessie MA, de Groot NM, Houben RP, et al. The electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 4.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu K, Shroff SC, Sahadevan J, Martovitz NL, Khrestian CM, Stambler BS. Mapping of atrial activation during sustained atrial fibrillation in dogs with rapid ventricular pacing induced heart failure: evidence for a role of driver regions. J Cardiovasc Electrophysiol. 2005;16:1348–1358. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 6.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 7.Cuculich PS, Wang Y, Lindsay BD, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atienza F, Calvo D, Almendral J, et al. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan SM, Shivkumar K, Mittal S, et al. Conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation: the CONFIRM trial. Heart Rhythm. 2011;8:LB–04. Late Breaking Clinical Trial Abstract. [Google Scholar]
- 10.Ng J, Kadish AH, Goldberger JJ. Technical considerations for dominant frequency analysis. J Cardiovasc Electrophysiol. 2007;18:757–764. doi: 10.1111/j.1540-8167.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 11.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalani G, Gibson M, Schricker A, Rostamanian A, Krummen DE, Narayan SM. Dynamic conduction slowing precedes human atrial fibrillation initiation: insights from bi-atrial basket mapping during burst pacing. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 14.Verma A, Kilicaslan F, Pisano E, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 15.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–143. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.