Abstract
Normalization to a reference gene is the method of choice for quantitative PCR analysis. The stability of reference genes is critical for accurate gene expression analysis, as significant variations in reference gene expression can alter experimental results and conclusions. In this study, we evaluated the expression stability of five commonly used reference genes found in mouse lymphocytes. Using NormFinder and BestKeeper algorithms, we consistently show that ubiquitin C (Ubc) is the optimal reference gene for normalizing qPCR data obtained from mouse lymphocytes, whereas beta-actin (Actb) is not a suitable reference gene due to its extensive variability in expression. Our findings emphasize the importance of validating reference genes for qPCR analyses. We provide a shortlist of reference genes to use for normalization and recommend freely available software programs as a rapid approach to validate potential reference genes.
Keywords: Lymph nodes, lymphocytes, reference genes, quantitative PCR (qPCR)
1. Introduction
Quantitative PCR (qPCR) has become a standard method to compare steady-state mRNA expression between control and test samples. It is the most sensitive method for the detection and quantification of gene expression levels (VanGuilder et al., 2008), making it especially valuable for working with low RNA concentrations either due to limited tissue samples or small changes in mRNA expression levels. For example, lymphocytes obtained from mouse lymph nodes are limited in number, making it difficult to determine cytokine production via enzyme-linked immunosorbent assay or enzyme-linked immunosorbent spot assay without pooling multiple lymph nodes. Intracellular cytokine staining is a potential alternative, but these three methods all require in vitro stimulation. These methods therefore evaluate the potential of cytokine production by the isolated lymphocytes but are unsuitable for measuring actual cytokine production by the lymphocytes in vivo. Hence, qPCR is an invaluable technique that is sensitive enough to reliably evaluate cytokine production in vivo, albeit indirectly through the measurement of mRNA expression.
Major factors that dictate the quality of qPCR results include the integrity of purified RNA, primer design, quality of reverse transcribed cDNA, and normalization methods. With the development of commercially available kits, many of these factors can be controlled for consistently. Normalization, however, remains as one of the most difficult problems to resolve. Of the various normalization methods, normalizing target gene expression to that of a reference gene is the method of choice (Huggett et al., 2005; Hruz et al., 2011). Beta-actin (Actb) and glyceraldehyde-3-phophosphate dehydrogenase (Gapdh) are two of the most commonly used reference genes based on the assumption that their mRNA expression is stable throughout experimental conditions (Thellin et al., 1999; Vandesompele et al., 2002; Huggett et al., 2005; Gilsbach et al., 2006; Mamo et al., 2007; Wang et al., 2010; Hruz et al., 2011). However, recent reports demonstrate that the stability of reference gene expression can vary between tissues and be regulated under experimental conditions (Tricarico et al., 2002; Bemeur et al., 2004; Sugden et al., 2010). Furthermore, extensive variability in reference gene expression has been shown to significantly alter results and conclusions (Dheda et al., 2005; Sullivan-Gunn et al., 2011), highlighting the need to validate the selection of reference genes for every study.
In this study, the stability of gene expression of five commonly used reference genes (Table S1) was determined for mouse B cells and T cells by using publically available bioinformatics software. We identified ubiquitin C (Ubc) as the most stable reference gene. Actb expression, on the other hand, exhibited the most variability and was thus defined unsuitable as a reference gene for mouse lymphocytes.
2. Materials and methods
2.1. Mice
C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in sterile micro-isolator rooms under specific pathogen-free conditions at the Fred Hutchinson Cancer Research Center animal facility. Animals were handled in accordance with the protocols by the Fred Hutchinson Cancer Research Center Animal Care and Use Committee.
2.2. Quantitative Reverse Transcription-PCR
Inguinal lymph nodes (LNs) were harvested from 7–8 week-old C57BL/6 mice. LNs were individually minced, dispersed, and filtered to collect lymphocytes according to published methods (Kruisbeek, 2001). B cells from each LN were isolated with CD19 MACS microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The untouched fraction is termed the non-B cell population, which contains > 97% CD3+ T cells (data not shown). Total RNA was extracted from B and non-B cells using the RNAqueous kit (Ambion, Austin, TX), and 400 ng RNA per sample were reverse transcribed to cDNA using the iScript™ cDNA synthesis kit (BioRad Laboratories, Hercules, CA). The collected cDNA was analyzed via quantitative PCR using iQ™ SYBR® Green Supermix (BioRad Laboratories) according to the manufacturer’s protocol. Thermal cycling was carried out by Applied Biosystems 7900HT (Foster City, CA). All primers were designed with Primer Express 3.0 software (Applied Biosystems). Primer sequences, thermal cycling conditions, and related details are listed in Table S1.
2.3. Data Analysis
Relative quantification was calculated based on a previously developed mathematical model (Pfaffl, 2001). Applied Biosystems SDS software 2.3 and RQ Manager 1.2 were used to determine the threshold cycle (Ct) value. BestKeeper (http://www.gene-quantification.de/bestkeeper.html) and NormFinder (http://www.mdl.dk/publicationsnormfinder.htm) were used to compare gene expression stability between selected reference genes. All data are expressed as means SEM. Using Graphpad Prism 5.0 (La Jolla, CA), Student’s paired t-test was used for inter-group comparisons.
3. Results and discussion
3.1. B cells and non-B cells express significantly different levels of reference genes
Ideal reference genes are expressed at relatively high but stable levels (Huggett et al., 2005). A Ct value obtained from qPCR is defined as the number of replication cycles needed for the fluorescence signal to reach a threshold level of detection. A Ct value is therefore inversely correlated with the amount of template cDNA present in the reaction and can be directly used to compare the expression levels of the tested reference genes isolated from inguinal LN B cells and non-B cells (Fig. 1). The five reference genes tested exhibited a wide range of expression, with Ct values between 20.6 and 28.96. In general, Actb was the highest and Hprt1 was the lowest transcribed gene for both B cells and non-B cells. The expression level of each tested reference gene was significantly different between B cells and non-B cells, with the smallest difference for Ubc (ΔCt = 2.23) and greatest for Actb (ΔCt = 3.99). This finding suggests that reference gene expression can vary significantly between different cell types (B vs. T cells) even within the same tissue (inguinal LN). Studies investigating gene expression alterations between different cell types should therefore take extra care to use reference genes that are expressed at similar relative levels between these cell types.
Figure 1.
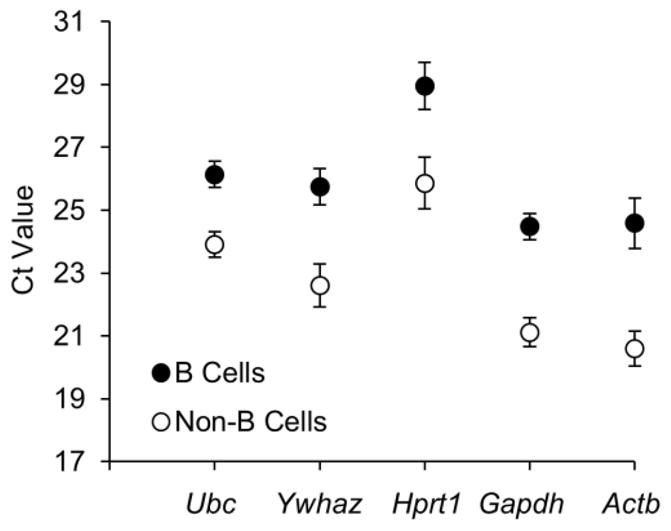
Expression levels of reference genes of B cells and non-B cells isolated from inguinal lymph nodes. Results represent two independent experiment sets, with four mice in each set. Values are given as qPCR cycle threshold numbers (Ct values) and expressed as means SEM.
3.2. Ubc is the most stably expressed reference gene in mouse lymphocytes
We next used NormFinder and BestKeeper algorithms to determine the expression stability of the tested reference genes. NormFinder identifies the optimal reference gene among a set of candidates (Andersen et al., 2004). It calculates a stability value for each candidate gene according to their expression stability by combining estimates of intra- and inter-group expression variations. NormFinder ranked Ubc and Gapdh equally as the best reference genes for both B cells and non-B cells (Fig. 2). Ubc was ranked slightly higher than Gapdh as the reference of choice for analysis of total lymphocytes. Actb was the least stably expressed reference gene for all the tested cellular populations.
Figure 2.
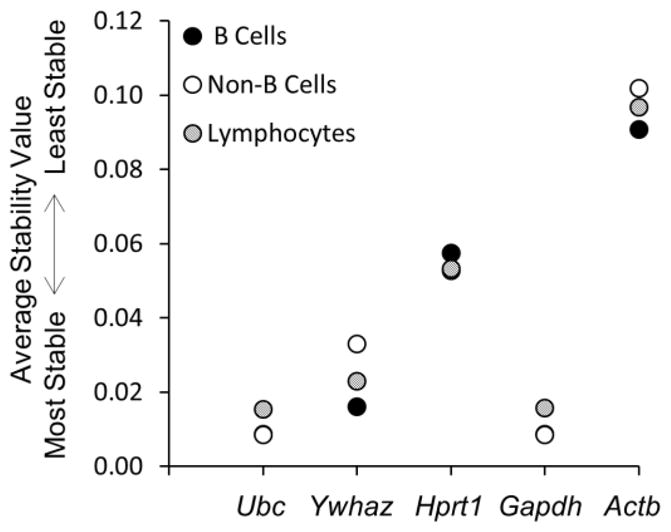
NormFinder analysis of reference genes. Results represent two independent experiment sets, with four mice in each set. See text for details.
BestKeeper evaluates reference genes based on the geometric mean and standard deviation (SD) of Ct values (Pfaffl et al., 2004). Stability of reference genes are then ranked according to the SD. Low SD represents low variation or high stability. Any Ct value mean with its SD greater than one is defined as inconsistent, and the corresponding gene should therefore not be considered as a reference gene. Of the tested reference genes for mouse lymphocytes, BestKeeper ranked Ubc as the most stably expressed gene for B cells and non-B cells, followed by Gapdh (Table 1). Actb and Hprt1 were the least stably expressed genes for B cells and non-B cells, respectively. For total lymphocytes, Ubc was the reference gene of choice, while the rest of the tested candidate genes showed high variability and should not be used as reference genes.
Table 1.
BestKeeper analysis of reference genes. Descriptive statistics of the five reference genes were calculated based on their Ct values
| Parameter | Ubc | Ywhaz | Hprt1 | Gapdh | Actb | BestKeeper Index | |
|---|---|---|---|---|---|---|---|
| B Cells | GM (Ct) | 26.12 | 25.71 | 28.90 | 24.45 | 24.50 | 25.89 |
| SD (Ct) | 0.84 | 1.10 | 1.59 | 0.90 | 1.83 | 0.96 | |
| Non-B Cells | GM (Ct) | 23.89 | 22.54 | 25.78 | 21.08 | 20.55 | 22.69 |
| SD (Ct) | 0.74 | 1.39 | 1.72 | 0.87 | 1.10 | 0.88 | |
| Total | GM (Ct) | 24.98 | 24.07 | 27.30 | 22.71 | 22.44 | 24.24 |
| Lymphocytes | SD (Ct) | 0.80 | 1.16 | 1.26 | 1.40 | 1.43 | 1.20 |
In the last column, the BestKeeper index is computed with the same descriptive parameters. Results represent two independent experiment sets, with four mice in each set.
GM (Ct), geometric mean of Ct values; SD, standard deviation of the Ct values.
For model systems in which it is not possible to identify a single reference gene due to biological or experimental processes that modulate expression of reference genes, the use of three or more highly correlated reference genes are recommended (Vandesompele et al., 2002; Pfaffl et al., 2004). We thus used BestKeeper to analyze inter-gene relationships for the tested reference genes (Table S2). Pair-wise correlation analyses were performed for all possible reference gene combinations to calculate the Pearson correlation coefficient (r) and the probability p value. Ubc and Ywhaz were best correlated for B cells and lymphocytes, whereas Ubc and Gapdh were the best combination for non-B cells. Actb correlated the least with any of the tested reference genes.
In addition to determining highly correlated reference genes, BestKeeper can also calculate a BestKeeper Index based on the weighted expression of at least three reference genes (Table 1). This index can be applied as a reference point in the same way as a single reference gene to determine whether target genes are differentially expressed. Consistent with earlier findings, the BestKeeper Index correlated best with Ubc, followed closely by Gapdh (Table S3). Interestingly, both Ubc and Gapdh outperformed the BestKeeper Index for B cells and non-B cells, and Ubc outperformed in the total lymphocyte population. This suggests that BestKeeper Index is most useful when a selection of highly correlated reference genes is used. If the correlation between the selected reference genes is not high, the use of a single reference gene may be more suitable.
Our findings conclude that Ubc is an ideal reference gene for normalizing qPCR data of mouse lymphocytes, and this holds true for various experimental conditions (data not shown). Similar conclusions were made in a study that evaluated the best overall reference genes for human lymphocytes (Vandesompele et al., 2002), suggesting that the stability of optimal reference genes may be conserved between humans and mice. Whether Ubc is an appropriate reference gene for other murine tissues should be individually evaluated, as the stability of reference genes vary between different tissues, cell types, and experimental conditions. For example, Hprt1 is more stable than Ubc in mouse oocytes and embryos (Mamo et al., 2007), and whereas Gapdh is one of the more stably expressed genes in mouse lymphocytes, it is less so in epithelial and non-epithelial cells of mouse small intestine (Wang et al., 2010).
3.3 Conclusion
To our knowledge, this is the first systematic analysis of the stability of mRNA expression of a selected number of commonly used reference genes for mouse lymphocytes. We and others (Tricarico et al., 2002; Bemeur et al., 2004; Sugden et al., 2010) show that there are significant variations in reference gene expression between different cell types within the same tissue, different tissues within the same mouse, and different experimental conditions. NormFinder and BestKeeper are freely available and easy-to-use Excel-based programs that can used to analyze the stability of reference genes. Of the five reference genes evaluated in this study, both programs identified Ubc as the most stably expressed genes for use as reference genes in qPCR analyses of mouse B cells and T cells. Despite its popular use as a reference gene for qPCR analyses, Actb was ranked the least stable gene, and its extensive variability excludes it as a suitable reference gene in mouse lymphocytes.
Normalization of qPCR data to reference genes is a simple method, but the analysis is meaningful only if the selection of the reference genes has been validated. This study provides a rapid methodology to evaluate reference genes. In situations where the expression of commonly used reference genes varies significantly, online tools are freely available to identify novel reference genes by searching from a genome-wide microarray database (Hruz et al., 2011). These potential reference genes can then be validated by the methodology presented in this study. If necessary, reference genes can be further validated by additional methods, such as Northern blot hybridization or advanced technologies that can directly detect the presence of mRNAs without amplification, thereby eliminating errors that could result from reverse transcription and amplification processes that are necessary for qPCR analysis (Geiss et al., 2008). Such technologies, however, may be prohibitively expensive for investigators who are analyzing a small number of genes but can be more cost-efficient for large-scale genetic studies.
This study highlights the importance of reference gene validation by showing that reference genes can be cell-, tissue-, and experiment-specific. We therefore provide a short list of reference genes as a starting point for investigators studying gene expression in mouse lymphocytes and propose the use of publically available bioinformatics software as a rapid approach to identify and validate reference genes for each experimental condition.
Supplementary Material
Reference genes evaluated in this study.
Repeated pair-wise correlation analysis of reference genes by BestKeeper. Genes were pair-wise correlated one with another based on coefficient of correlation (top value) and p-value (bottom value). Results represent two independent experiment sets, with four mice in each set.
Correlation analysis of reference genes against the BestKeeper Index. Reference genes were pair-wise correlated with the BestKeeper Index based on coefficient of correlation (top value) and p-value (bottom value). Results represent two independent experiment sets, with four mice in each set.
Highlights.
Validation of reference genes is critical for accurate gene expression analysis.
Ubc is the optimal reference gene for qPCR analysis of mouse B and T cells.
Actb is not a suitable reference gene due to extensive variability in expression.
Free software programs are available to rapidly validate potential reference genes.
Acknowledgments
This work was funded by the National Institute of Health (NCI R01 CA68328 to AR and T32 CA080416 to TCA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Bemeur C, Ste-Marie L, Desjardins P, Hazell AS, Vachon L, Butterworth R, Montgomery J. Decreased beta-actin mRNA expression in hyperglycemic focal cerebral ischemia in the rat. Neurosci Lett. 2004;357:211. doi: 10.1016/j.neulet.2003.12.081. [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Chang JS, Kim LU, Bustin SA, Johnson MA, Rook GA, Zumla A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem. 2005;344:141. doi: 10.1016/j.ab.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Kouta M, Bonisch H, Bruss M. Comparison of in vitro and in vivo reference genes for internal standardization of real-time PCR data. Biotechniques. 2006;40:173. doi: 10.2144/000112052. [DOI] [PubMed] [Google Scholar]
- Hruz T, Wyss M, Docquier M, Pfaffl MW, Masanetz S, Borghi L, Verbrugghe P, Kalaydjieva L, Bleuler S, Laule O, Descombes P, Gruissem W, Zimmermann P. RefGenes: identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC genomics. 2011;12:156. doi: 10.1186/1471-2164-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Kruisbeek AM. Isolation of mouse mononuclear cells. Curr Protoc Immunol. 2001;Chapter 3(Unit 3):1. doi: 10.1002/0471142735.im0301s39. [DOI] [PubMed] [Google Scholar]
- Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC developmental biology. 2007;7:14. doi: 10.1186/1471-213X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Sugden K, Pariante CM, McGuffin P, Aitchison KJ, D’Souza UM. Housekeeping gene expression is affected by antidepressant treatment in a mouse fibroblast cell line. J Psychopharmacol. 2010;24:1253. doi: 10.1177/0269881108099690. [DOI] [PubMed] [Google Scholar]
- Sullivan-Gunn M, Hinch E, Vaughan V, Lewandowski P. Choosing a stable housekeeping gene and protein is essential in generating valid gene and protein expression results. Br J Cancer. 2011;104:1055. doi: 10.1038/bjc.2011.35. author reply 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75:291. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang J, Liu D, Su Y. Normalizing genes for real-time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal Biochem. 2010;399:211. doi: 10.1016/j.ab.2009.12.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reference genes evaluated in this study.
Repeated pair-wise correlation analysis of reference genes by BestKeeper. Genes were pair-wise correlated one with another based on coefficient of correlation (top value) and p-value (bottom value). Results represent two independent experiment sets, with four mice in each set.
Correlation analysis of reference genes against the BestKeeper Index. Reference genes were pair-wise correlated with the BestKeeper Index based on coefficient of correlation (top value) and p-value (bottom value). Results represent two independent experiment sets, with four mice in each set.


