Abstract
Esophageal adenocarcinoma (EAC) is an aggressive malignancy with a poor outcome. Although targeting ERBB2 with trastuzumab is evaluated in clinical trials, the molecular mechanisms of trastuzumab resistance remain uncharacterized in EAC. The dopamine and cyclic AMP-regulated phosphoprotein of Mr 32,000 (DARPP-32), also known as PPP1R1B, is located together with ERBB2 at the 17q12-q21 amplicon. We evaluated the expression of a transcript variant of DARPP-32 (t-DARPP) and ERBB2 in 141 primary tumors and investigated the role of t-DARPP in trastuzumab resistance using OE19 and OE33 EAC cell models. Overexpression of t-DARPP mRNA was detected in two-thirds of tumors with a correlation between ERBB2 and t-DARPP overexpression levels (r=0.58, p=0.003). Cell viability and clonogenic survival assays showed that t-DARPP increased survival by 40% in response to trastuzumab (p<0.01). The Annexin-V staining and Western blot analysis indicated that t-DARPP effectively abrogated trastuzumab-induced apoptosis, inhibited cleavage of caspase-3 and blocked trastuzumab-induced dephosphorylation of ERBB2 and AKT proteins. The knockdown of endogenous t-DARPP reversed these effects and sensitized cells to trastuzumab (p<0.01). The cycloheximide-based protein degradation analysis indicated that t-DARPP extended the half-life of ERBB2 explaining the increase in the basal levels of ERBB2, p-ERBB2(Y1248), and p-AKT(S473). Coimmunoprecipitation and Western blot analysis demonstrated that t-DARPP associated with ERBB2 in a protein complex, and interfered with trastuzumab binding to the ERBB2 receptor. Using EAC-xenografted mouse model, t-DARPP enhanced tumor growth and rendered tumors unresponsive to trastuzumab. This study establishes t-DARPP as a mediator of trastuzumab resistance and underscores its potential importance in clinical trials of EAC.
Keywords: HER2, Herceptin, trastuzumab, DARPP-32
Introduction
Esophageal carcinoma is the sixth most common cause of cancer-related death worldwide (1). Histologically, esophageal cancer can be divided into adenocarcinoma and squamous cell carcinoma. The incidence of esophageal adenocarcinoma (EAC) has been rising fast in the past three decades (2, 3). There are approximately 14,000 cases of esophageal cancer per year in the United States, most of which are EAC (4, 5). The overall 5 year survival rate of EAC is less than 15% (4-7), suggesting that current treatment regimens are ineffective.
Chromosomal amplification at the 17q21 region is a frequent finding in adenocarcinomas of the stomach and esophagus (8, 9). This region is a gene-rich area that contains several candidate cancer genes (9). The ERBB2 locus, within the 17q12-q21 amplicon, has been heavily implicated in several malignancies. ERBB2 is amplified and overexpressed in approximately 15-25% of EAC tumor specimens and has been implicated in the pathogenesis of EAC (10-12). In addition to ERBB2, the amplicon region contains several other genes such as DARPP-32 (also known as protein phosphatase 1 regulatory subunit 1B (PPP1R1B), GRB7 and TOP2A. Recently, DARPP-32 and its cancer-specific truncated variant (t-DARPP) have been mapped to the ERBB2 amplicon (8). t-DARPP is overexpressed in several malignancies, such as those of the stomach, colon, breast, and prostate (13-15). We and others have shown that t-DARPP protein promotes cell growth, survival, and drug resistance through activation of AKT signaling in cancer cells (14, 16-18).
The ERBB2 gene-targeted therapy continues to be applied in several clinical trials; Trastuzumab (Herceptin), a humanized monoclonal anti-ERBB2 antibody, was first used for the treatment of ERBB2-overexpressing advanced metastatic breast cancers (19). To date, most of our understanding of ERBB2-targted therapy comes from studies in breast cancer. Although ERBB2-positive tumors initially respond to trastuzumab treatment, the majority of responders progress within 12 months of initiating therapy as a result of acquired trastuzumab resistance (20). Several studies have elucidated some of the mechanisms of trastuzumab resistance using in vitro cell models. This resistance has been attributed to disruption of interaction between ERBB2 and trastuzumab by MUC4 expression (21); compensatory signaling by other ERBB receptor members (22); compensatory signaling from other types of receptors such as IGF-IR (23); increased circulating ERBB2 ECD (24); and altered downstream signaling, including PTEN deficiency (25), increased AKT activity (26), and down-regulation of P27 (CDKN1B) (27).
Trastuzumab in combination with Cisplatin has been recently used in clinical trials to treat patients with ERBB2-positive metastatic gastric or gastroesophageal junction adenocarcinoma (28). Of note, a phase III clinical trial (RTOG 1010 protocol) is currently underway to evaluate the addition of trastuzumab to increase disease-free survival when combined with trimodality treatment (radiation plus chemotherapy followed by surgery) for EAC patients. Therefore, it is crucial to characterize novel mechanisms of trastuzumab resistance in EAC as our capabilities to identify, overcome or clinically manage this resistant phenotype in EAC are currently limited. In this study, we elucidate a novel mechanism by which t-DARPP mediates trastuzumab resistance in EAC. We demonstrate that t-DARPP binds and stabilizes the ERBB2 protein, thereby activating the AKT signaling and promoting trastuzumab resistance by interfering with trastuzumab interaction with the ERBB2 receptor.
Materials and Methods
Cell lines and reagents
The human esophageal adenocarcinoma cancer cell lines, OE19 and OE33, were obtained from the European Collection of Animal Cell Cultures (Sigma-Aldrich), and ATCC, respectively. To generate trastuzumab-resistant clones, OE19 cells were cultured with increasing concentrations of trastuzumab for over 6 months in vitro and the resistant cells were maintained with 20 μg/ml trastuzumab in culture. Cycloheximide was purchased from Sigma-Aldrich. ERBB2, AKT, p-AKT(S473), caspase-3, cleaved caspase-3, and β-actin antibodies were obtained from Cell Signaling Technology (Danvers, MA). DARPP-32 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and P-ERBB2(Y1248) antibody was obtained from Abcam (San Francisco, CA). Trastuzumab was purchased from the Vanderbilt University Hospital Pharmacy (Nashville, TN).
t-DARPP expression and small-interfering RNA
To generate stable expression cells, the flag-tagged coding sequence of t-DARPP was amplified and cloned into pcDNA3 mammalian expression vector (Invitrogen). OE19 cells stably expressing t-DARPP or pcDNA3 empty vector were generated following standard protocols as described previously (16). Flag-tagged t-DARPP coding sequence was amplified and cloned into the adenoviral shuttle vector (pACCMV), and the recombinant adenovirus was generated by cotransfecting HEK-293 cells with the shuttle and backbone adenoviral (pJM17) plasmids using the Calcium Phosphate Transfection Kit (Applied Biological Materials Inc., Richmond, BC). Control siRNA (sc-37007) and t-DARPP siRNA (sc-35173; a cocktail of 3 different oligonucleotides was obtained from Santa Cruz Biotechnology.
Cell viability assays
The CellTiter-Glo Luminescent Cell Viability Assay (Promega) was performed according to supplier instructions. Briefly, cells (5 × 103 per well) were seeded onto a 96-well plate. Approximately 18 h after seeding, cells were treated with trastuzumab (20 μg/ml) for 48 h. The luminescence was read on a Microplate Reader (FLUOstar OPTIMA). For trypan blue dye exclusion assay, viable cells for each concentration were counted on a hemocytometer after trypsinization. All experiments were performed in triplicates and repeated three times.
Clonogenic survival assay
Cells were trypsinized and harvested in single cell suspension. Cells were plated at low-density (2×103 cells per well) in 6-well plates. The following day, cells were treated with vehicle or 20 μg/ml trastuzumab. Culture media were replaced every three days with the addition of vehicle or fresh trastuzumab. After culturing for two weeks, cells were fixed with methanol:acetic acid (3:1, vol:vol) and stained with 1% crystal violet. Colonies with ≥50 cells were counted.
Apoptosis assay
OE19 cells infected with control (10 MOI) or t-DARPP (10 MOI) recombinant adenoviruses, and parental or trastuzumab resistant OE19 cells were seeded onto 60 mm culture plates. The next day, cells were treated with trastuzumab (20 μg/ml) or vehicle for 48 h. Cells were then collected and stained with Annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI) (R&D Systems, Minneapolis, MN). The samples were washed with PBS and re-suspended in binding buffer (2 ml HEPES buffered saline solution supplemented with 2.5 mmol/L CaCl2), and then subjected to fluorescence-activated cell sorting (FACS) analysis (Becton Dickinson).
Cycloheximide (CHX)-based ERBB2 protein stability assay
Cells (2×105 cells per well) were seeded into 12-well plates. The next day, cells were treated with 80 μg/ml of CHX and harvested at different time points. Proteins were extracted and analyzed by Western blotting to assess ERBB2 protein stability. Protein bands intensities were semi-quantitatively analyzed by densitometry using ImageJ software (NIH Image). ERBB2 band intensities for each treatment condition were normalized to β-actin. The protein degradation curve was generated by plotting band intensities ratios as a function of the time period of CHX treatment. Linear regression was performed and the protein half-life (t½), which is expressed as the time for degradation of 50% of the protein, was calculated from the fitted line equation (29).
Quantitative real-time RT-PCR
Frozen de-identified human tissue samples were obtained from the archives of pathology at Vanderbilt University and the National Cancer Institute Cooperative Human Tissue Network. The use of coded specimens was approved by the Institutional Review Board at Vanderbilt University. The samples included 141 adenocarcinomas of the esophagus and stomach and 51 non-tumor normal mucosae samples. Histopathological diagnosis was verified based on H&E-stained sections. The adenocarcinomas ranged from well-differentiated to poorly-differentiated, stages II-IV, with a mix of intestinal- and diffuse-type tumors. Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). Single-stranded cDNA was synthesized from 1 μg total RNA by an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Primers for ERBB2, t-DARPP, and HPRT1 were designed, and the results were normalized to HPRT1 as a stable reference gene for quantitative real-time RT-PCR. All primers were purchased from IDT (Integrated DNA Technologies, Inc.). The quantitative reverse transcription polymerase chain reaction was performed in an iCycler (Bio-Rad), with the threshold cycle number determined by iCycler software version 3.0. The relative mRNA expression levels were calculated according to the formula 2(RT-ET)/2(Rn-En), as described previously (30).
Western blot analysis
Cells were washed with phosphate buffered saline (PBS) and lysed in RIPA buffer (50 mmol/L Tris-HCl buffer, pH 7.4, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) supplemented with 1x Halt protease inhibitor cocktail and 1x Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL). Protein concentrations were determined with the Bio-Rad Protein Assay (Bio-Rad). Proteins were separated on 10-12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to Protran nitrocellulose membranes (Whatman, Boston, MA). Membranes were probed with specific primary antibodies, and horseradish peroxidase-coupled secondary antibodies (Cell Signaling). Bands were visualized using a commercial Immobilon Western Chemiluminescent horseradish peroxidase (HRP) Substrate kit (Millipore, Billerica, MA).
Immunoprecipitation
Cells were washed twice with ice-cold PBS and solubilized for 30 min at 4°C with lysis buffer (1% Triton X-100) containing 1% Halt protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL). The cell lysates were first sonicated and then spun down at 15,000 rpm for 10 min. The supernatants were collected and protein concentration was measured by the Bio-Rad Protein Assay (Bio-Rad). Total protein (200 μg) was incubated with 1 μg trastuzumab or anti-M2-flag antibodies overnight at 4°C on a rotating platform followed by incubation with 50 μl Dynabeads Protein G (Invitrogen) for 1 h at 4°C on a rotating platform. The tubes were placed in a magnetic stand and supernatants were discarded. The beads were washed three times with ice-cold PBS. The immunoprecipitated proteins were eluted by re-suspending the beads in 30 μl of 2x sample buffer and denatured by incubating at 95°C for 5 min. The eluted proteins were resolved on 10% SDS-PAGE and transferred onto nitrocellulose membranes for Western blot analysis.
To evaluate the interaction of ERBB2 receptor with trastuzumab, cells were treated with trastuzumab (20 μg/ml) for 24 h. After washing twice with ice-cold PBS, cells were solubilized for 30 min at 4°C with lysis buffer (1% Triton X-100) containing 1% Halt protease inhibitor cocktail (Thermo Fisher Scientific). Total protein (200 μg) was incubated with 50 μl Dynabeads Protein G (Invitrogen) in an Eppendorf tube for 1 h at 4°C on a rotating platform to pull down specifically trastuzumab-bound ERBB2 protein. The tubes were placed in a magnetic stand and supernatants were discarded. The beads were then processed and the bound protein was eluted as described above. Protein bands intensities were semi-quantitatively analyzed by densitometry using ImageJ software (NIH Image). Pulled-down ERBB2 protein bands intensities were depicted as ratios relative to their corresponding input ERBB2 proteins.
Nude mice xenograft experiments
Five-week-old female athymic nude - Foxn1 nu/nu mice (Harlan Laboratories Inc., IN) were purchased and maintained under specific pathogen-free conditions. The mice were randomized into two groups (10 xenografts per group). OE19 cells stably expressing t-DARPP or pcDNA3 empty vector were injected s.c. (4×106 cells, suspended in 200 μl growth factor-reduced Matrigel, per site into the flanks). Once xenografted tumors had reached a volume ≥200 mm3, mice were treated with 20 mg/kg trastuzumab diluted in sterile PBS by i.p. injection twice weekly. To determine tumor volume, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were serially measured by external caliper. Tumor volume was calculated by the formula: Tumor volume = 1/2 (length × width2). The Vanderbilt Institutional Animal Care and Use Committee approved all animal work.
Statistical analysis
Data are presented as means ± standard error of mean. All in vitro experiments were performed in triplicates. Statistical significance of the in vitro studies was evaluated by the parametric unpaired Student’s t test. The t-test, Wilcoxon Rank Sum test, and Spearman correlation test were used for analyses of primary tumors. Differences with p values ≤0.05 are considered significant.
Results
t-DARPP overexpression is associated with tumor stage and directly correlates with ERBB2 mRNA levels
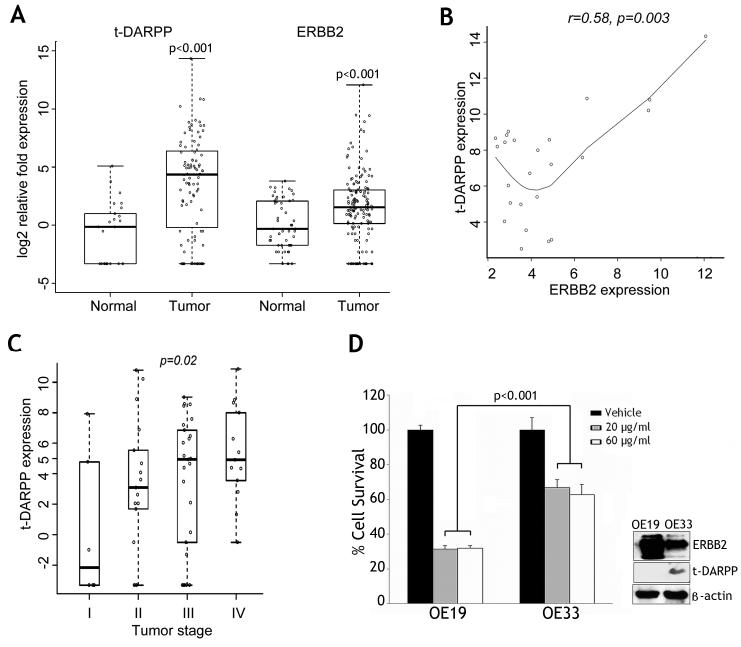
Using the t-test, t-DARPP and ERBB2 were significantly overexpressed in tumors as compared to normal samples (p<0.001) (Figure 1A). The Wilcoxon Rank Sum test showed a similar result. The expression of t-DARPP and ERBB2 showed a similar pattern in esophageal and gastric adenocarcinomas. Overexpression (log2 fold expression ≥2.0) of ERBB2 was detected in 57 tumors (40%) whereas t-DARPP was overexpressed in two-thirds of the tumors. The use of a cutoff log2 fold expression ≥4.0 demonstrated overexpression of ERBB2 and t-DARPP in 22% and 48% of the tumors, respectively. Statistical analysis using Spearman’s correlation coefficient and correlation test where the cutoff for gene expression is ≥log(5,2)=2.32, indicated that ERBB2 overexpression values in tumors were significantly correlated with those of t-DARPP (r=0.58, p=0.003) (Figure 1B). The multivariate regression model analysis indicated that tumor stage was significantly associated with t-DARPP gene expression values (p=0.02) (Figure 1C).
Figure 1. t-DARPP and ERBB2 are significantly overexpressed in adenocarcinomas.
A) Significant mRNA overexpression of t-DARPP and ERBB2 in adenocarcinomas of the esophagus and stomach (141 tumors and 51 normal tissue samples) (p<0.001). B) Spearman’s correlation coefficient and correlation test where the cutoff gene expression is ≥log(5,2)=2.32, show that t-DARPP and ERBB2 overexpression levels are significantly correlated in tumors (r=0.58, p=0.003). C) The multivariate regression model analysis indicates that tumor stage has a significant effect on t-DARPP mRNA gene expression levels (p=0.02). D) Left panel, cell viability of OE19 and OE33 cells in response to trastuzumab treatment was evaluated by Trypan blue staining. OE19 cells were two-fold more sensitive to trastuzumab than OE33 cells (p<0.001). Right panel, Western blot analysis demonstrates higher protein expression of ERBB2 in OE19 cells than OE33 cells. In contrast, t-DARPP expression was undetectable in OE19 cells but highly expressed in OE33 cells.
To establish cell models for investigating the role of t-DARPP in trastuzumab resistance in esophageal adenocarcinoma, we evaluated survival of two esophageal adenocarcinoma cell lines, OE19 and OE33, in response to trastuzumab. The results of the trypan blue dye exclusion assay indicated that OE33 cells were more resistant to trastuzumab than OE19 cells (p<0.001) as treatment with 20 μg/ml or 60 μg/ml trastuzumab decreased the survival by 70% in OE19 cells as opposed to 40% in OE33 cells (Figure 1D, left panel). In addition, the results showed that 20 μg/ml trastuzumab was a saturation concentration as a higher drug concentration (60 μg/ml) had a similar effect on survival in the two cell lines. The Western blot analysis data showed that both OE19 and OE33 cells have high protein expression levels of ERBB2, which is in line with the reported increased copy numbers of ERBB2 gene in these two cell lines (Figure 1D, right panel). While t-DARPP protein was highly expressed in OE33 cells, it was undetectable in OE19 cells (Figure 1D, right panel), suggesting that t-DARPP expression was associated with increased trastuzumab resistance in esophageal cancer cells.
t-DARPP promotes cell survival in esophageal adenocarcinoma
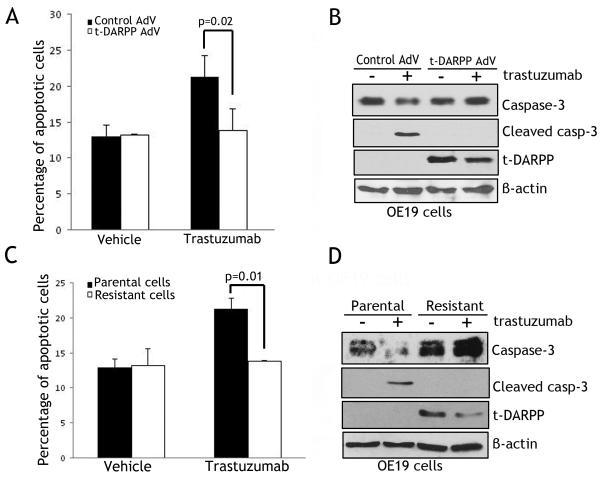
The CellTiter-Glo® viability assay results showed that transiently expressed t-DARPP increased cell survival by 40% relative to control cells in response to trastuzumab (p<0.001) (Figure 2A). Likewise, stably expressed t-DARPP in OE19 cells enhanced cell survival by approximately 40% relative to control cells after treatment with trastuzumab (p<0.01) (Figure 2B). As an additional cell model in our study, we generated trastuzumab-resistant OE19 cells through culturing sensitive parental cells in increasing concentrations of trastuzumab for 6 months. Interestingly, unlike parental cells, the resistant cells acquired expression of endogenous t-DARPP (Figure 2C, lower panel). Indeed, following treatment with trastuzumab, cell survival was significantly higher in resistant cells than parental cells (p<0.01) (Figure 2C, upper panel).
Figure 2. t-DARPP enhances survival of esophageal cancer cells.
A) Cell viability of OE19 cells infected with control adenovirus (10 MOI) or t-DARPP adenovirus (10 MOI) in response to treatment with vehicle or trastuzumab (20 μg/ml) for 48 h, was assessed by CellTiter-Glo Luminescent Cell Viability Assay. B) Cell viability of OE19 cells stably expressing pcDNA3 or t-DARPP were treated with vehicle or trastuzumab (20 μg/ml) for 48 h, and determined as in panel A. C) Cell viability of parental and trastuzumab resistant OE19 cells treated with vehicle or trastuzumab (20 μg/ml) for 48 h and evaluated as in panel A. D) OE19 cells stably expressing pcDNA3 or t-DARPP were subjected to clonogenic survival assay after treatment with vehicle or trastuzumab (20 μg/ml) for 48 h. Quantitative data are shown on the right panel. E) Parental and trastuzumab resistant OE19 cells were subjected to clonogenic survival assay after treatment with vehicle or trastuzumab (20 μg/ml) as in panel D. Quantitative data are shown on the right panel. These results show that exogenous and endogenous t-DARPP significantly promoted cell survival in response to trastuzumab in OE19 cells.
To confirm the short-term assay results, we performed long-term clonogenic survival assay using the two OE19 cell models. The results indicated that stable expression of t-DARPP doubled the cell survival relative to control (p<0.001) in response to trastuzumab (Figure 2D). Moreover, endogenous expression of t-DARPP in resistant cells tripled the cell survival relative to parental cells (p<0.01) in response to trastuzumab (Figure 2E). These results demonstrate that t-DARPP expression enhanced cell survival in response to trastuzumab in esophageal adenocarcinoma cells.
t-DARPP inhibits trastuzumab-dependent apoptosis and activation of caspase-3
The Annexin-V staining and FACS analysis results showed that adenoviral transient expression of t-DARPP suppressed early apoptosis events by approximately 25% relative to control in response to trastuzumab (P<0.02) (Figure 3A). Accordingly, Western blot analysis data revealed the cleaved caspase-3 form in control cells, but not in t-DARPP-expressing cells following treatment with trastuzumab (Figure 3B). Similarly, endogenous expression of t-DARPP in the resistant cells did not lead to an increase in apoptosis as compared to non-treated cells whereas parental cells showed an approximately doubling in apoptosis levels after treatment with trastuzumab (P<0.01) (Figure 3C). In line with this result, Western blotting showed cleavage of caspase-3 in parental cells, but not in resistant cells after treatment with trastuzumab (Figure 3D). Together, these data indicated that t-DARPP expression counteracted trastuzumab-induced apoptosis in esophageal adenocarcinoma cells.
Figure 3. t-DARPP expression blocks trastuzumab-induced apoptosis.
A) Apoptosis in OE19 cells infected with control (10 MOI) or t-DARPP (10 MOI) recombinant adenoviruses after treatment with vehicle or trastuzumab (20 μg/ml) for 48 h, was determined by Annexin-V/propidium iodide (PI) staining and FACS analysis. B) Western blot analysis of caspase-3, cleaved caspase-3, and t-DARPP proteins in OE19 cells infected with control or t-DARPP adenoviruses following treatments as described in panel A. C) Apoptosis in parental and trastuzumab resistant OE19 cells after treatment with vehicle or trastuzumab (20 μg/ml) for 48 h, was evaluated by Annexin-V/PI staining and FACS analysis. D) Western blot analysis of caspase-3, cleaved caspase-3, and t-DARPP proteins in parental and trastuzumab resistant OE19 cells after treatments as described in panel C. The data indicate that endogenous and exogenous t-DARPP expression counteracted trastuzumab-induced apoptosis in OE19 cells.
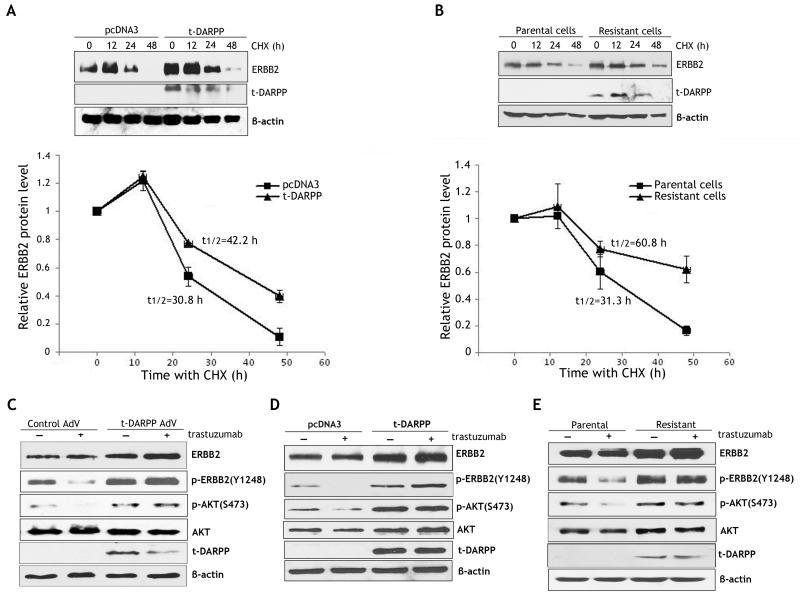
t-DARPP stabilizes ERBB2 protein and activates downstream signaling in response to trastuzumab
The cycloheximide (CHX)-based chase assay results indicated that stable expression of t-DARPP increased the half-life of ERBB2 protein to 42.2 h as opposed to 30.8 h in control cells (Figure 4A). Similarly, the protein half-life of ERBB2 was 31.3 h and 60.8 h in parental cells and resistant cells, respectively (Figure 4B). These results demonstrated that t-DARPP expression significantly enhanced ERBB2 protein stability in esophageal adenocarcinoma cells. In accordance with these data, we demonstrated by immunofluorescence analysis that ERBB2 expression on the cell surface was approximately 2 fold higher in t-DARPP-expressing cells than control cells (p<0.01) (Supplemental Figure 1).
Figure 4. t-DARPP promotes ERBB2 protein stability, inhibits trastuzumab-dependent ERBB2 dephosphorylation, and activates downstream signaling.
A) ERBB2 protein stability in OE19 cells stably expressing t-DARPP or pcDNA3 empty vector was evaluated by Western blot analysis after treatment with 80 μg/ml CHX to block new protein synthesis for the indicated times. The protein degradation data indicate that t-DARPP expression extended the protein half-life of ERBB2 from 30.8 h to 42.2 h relative to control (lower panel). B) ERBB2 protein stability in parental and trastuzumab resistant OE19 cells was assessed by Western blot analysis after treatment with CHX (80 μg/ml) for the indicated times. The protein degradation data show that endogenous t-DARPP expression in resistant cells was associated with increased ERBB2 protein half-life (60.8 h) relative to parental cells (31.3 h) (lower panel). C) Western blot analysis of p-ERBB2 (Y1248), ERBB2, p-AKT (S473), AKT, and t-DARPP proteins in OE19 cells infected with control (10 MOI) or t-DARPP (10 MOI) adenoviruses after treatment with vehicle or trastuzumab (20 μg/ml) for 24 h. The data indicate that transient expression of t-DARPP increased p-ERBB2(Y1248) and p-AKT(S473) basal protein levels, and blocked trastuzumab-dependent dephosphorylation of ERBB2 and AKT proteins. D) Western blot analysis of p-ERBB2 (Y1248), ERBB2, p-AKT (S473), AKT, and t-DARPP proteins in OE19 cells stably expressing t-DARPP or pcDNA3 vector after treatment with vehicle or trastuzumab (20 μg/ml) for 24 h. The results show that stable expression of t-DARPP increased basal levels of p-ERBB2(Y1248) and p-AKT(S473), and inhibited trastuzumab-dependent dephosphorylation of ERBB2 and AKT proteins. E) Western blot analysis of p-ERBB2(Y1248), ERBB2, p-AKT(S473), AKT, and t-DARPP proteins in parental or trastuzumab resistant OE19 cells following treatment with vehicle or trastuzumab (20 μg/ml) for 24 h. The results indicate that endogenous t-DARPP expression was associated with increased basal levels of p-ERBB2(Y1248) and p-AKT(S473), and suppression of trastuzumab-dependent dephosphorylation of ERBB2 and AKT proteins.
We further investigated the role of t-DARPP in regulating the AKT signaling pathway downstream of ERBB2 following trastuzumab treatment. Western blot analysis data showed that trastuzumab treatment induced significant dephosphorylation of ERBB2 and AKT in control OE19 cells, but this effect was suppressed in OE19 cells transiently expressing t-DARPP (Figure 4C). Likewise, stably expressed t-DARPP blocked trastuzumab-induced dephosphorylation of ERBB2 and AKT in OE19 cells (Figure 4D). Similarly, these results were confirmed in endogenous t-DARPP expression resistant cell model (Figure 4E). These data clearly demonstrated that t-DARPP expression maintained ERBB2 phosphorylation and activated the downstream AKT survival pathway in response to trastuzumab treatment. Of note, t-DARPP-mediated ERBB2 protein stability was associated with increased levels of ERBB2, p-ERBB2(Y1248), and p-AKT(S473) proteins in OE19 cells without treatment with trastuzumab (Figures 4C - 4E).
Knockdown of endogenous t-DARPP sensitizes cells to trastuzumab
The Western blot analysis data indicated that knockdown of t-DARPP and treatment with trastuzumab significantly decreased p-ERBB2(Y1248) and p-AKT(S473) protein levels relative to controls (Figure 5A). Knocking down t-DARPP alone, without treatment with trastuzumab, decreased p-AKT(S473) protein level in OE33 cells (Figure 5A). The CellTiter-Glo viability assay results revealed that knockdown of t-DARPP in combination with trastuzumab treatment decreased cell survival by 30% relative to control (p<0.01) (Figure 5B). These results clearly demonstrated that knocking down endogenous t-DARPP in OE33 cells significantly sensitized cells to trastuzumab.
Figure 5. Knockdown of endogenous t-DARPP enhances response to trastuzumab.
A) Western blot analysis of p-ERBB2(Y1248), ERBB2, p-AKT(S473), AKT, and t-DARPP proteins in OE33 cells transfected with control siRNA or t-DARPP siRNA and treated with vehicle or trastuzumab (20 μg/ml) for 48 h. The data indicate that knockdown of endogenous t-DARPP increased trastuzumab-dependent dephosphorylation of ERBB2 and AKT proteins. B) Cell viability of OE33 cells transfected with control siRNA or t-DARPP siRNA in response to treatment with vehicle or trastuzumab (20 μg/ml) for 48 h, was evaluated by CellTiter-Glo Luminescent CellViability Assay. The results revealed that knockdown of endogenous t-DARPP with treatment induced a significant decrease in cell survival (p<0.01).
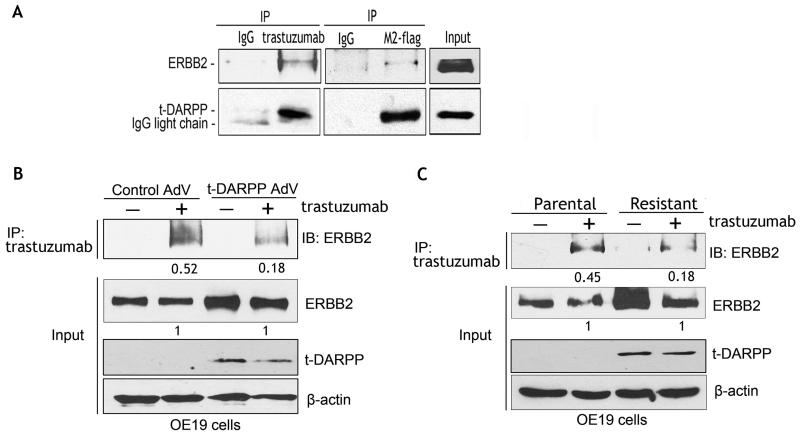
t-DARPP/ERBB2 protein interaction interferes with trastuzumab binding to ERBB2 receptor
We hypothesized that t-DARPP interacts with ERBB2, thus interfering with trastuzumab binding to ERBB2 receptor, and subsequently affecting downstream signaling. The results of two-way reciprocal immunoprecipitation assay showed that t-DARPP and ERBB2 co-immunoprecipitated, indicating their association in the same protein complex (Figure 6A). We next investigated the effect of t-DARPP expression on trastuzumab binding to ERBB2 receptor. Immunoprecipitation assay data showed that transiently expressed t-DARPP in OE19 cells decreased the trastuzumab binding to ERBB2 receptor by 2.8 fold relative to control cells (Figure 6B). Similarly, endogenous t-DARPP expression in trastuzumab resistant OE19 cells was associated with a 2.5-fold decrease in trastuzumab binding to ERBB2 relative to control cells (Figure 6C). To further confirm these data, we treated OE19 cells stably expressing t-DARPP or control vector with trastuzumab and a BS3 crosslinking reagent. The immunofluorescence staining results showed that the trastuzumab-bound ERBB2 protein level was approximately 5 fold lower in t-DARPP-expressing cells than control cells (p<0.01) (Supplemental Figure 2). In line with this finding, the immunofluorescence data demonstrated strong cytosolic expression of t-DARPP that also co-localized with the membranous ERBB2 signal. Of note, cells expressing higher levels of t-DARPP showed stronger ERBB2 signal (Supplemental Figure 2C).
Figure 6. t-DARPP associates with ERBB2 and interferes with trastuzumab/ERBB2 protein interaction.
A) Western blot analysis of co-immunoprecipitated exogenous t-DARPP and endogenous ERBB2 proteins with M2-flag or trastuzumab antibodies in OE19 cells infected with t-DARPP-flag adenovirus (10 MOI). The data demonstrate protein association of ERBB2 with t-DARPP. B) Western blot analysis of immunoprecipitated endogenous ERBB2 protein with trastuzumab antibody in OE19 cells infected with control (10 MOI) or t-DARPP (10 MOI) adenoviruses. Pulled-down ERBB2 band intensity was depicted as a ratio relative to input ERBB2 protein. The results show that exogenous t-DARPP expression blocked binding of trastuzumab to ERBB2 receptor relative to control. C) Western blot analysis of immunoprecipitated endogenous ERBB2 protein with trastuzumab antibody in parental or trastuzumab resistant OE19 cells. The band intensity of immunoprecipitated ERBB2 protein was shown as a ratio relative to input ERBB2. The data indicate that endogenous t-DARPP expression in trastuzumab-resistant cells was associated with a significant decrease in trastuzumab/ERBB2 protein interaction relative to control.
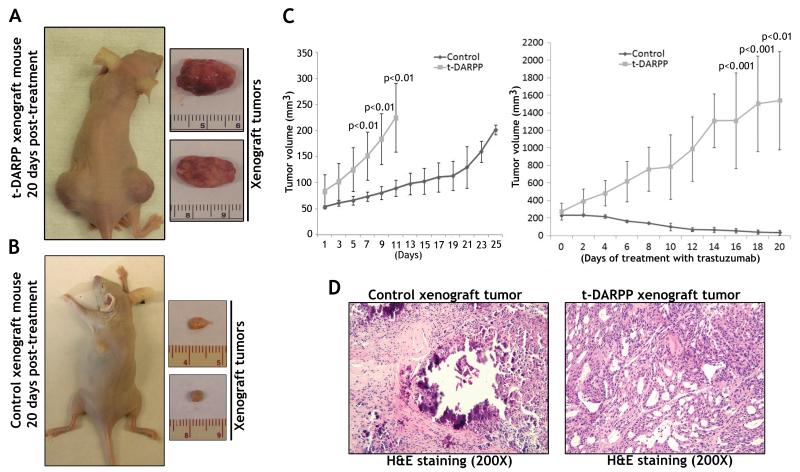
t-DARPP enhances tumor growth and inhibits response to trastuzumab in vivo
The results demonstrated that tumors derived from OE19 cells stably expressing t-DARPP failed to respond to trastuzumab treatment (Figure 7A). In contrast, trastuzumab effectively inhibited growth of control tumors (Figure 7B). Of note, t-DARPP expression significantly increased tumor growth rate (p<0.01) as compared to control (Figure 7C, left panel). The control tumors grew significantly slower than t-DARPP tumors in response to treatment with trastuzumab (p<0.001) (Figure 7C, right panel). The results from H&E staining indicated that trastuzumab effectively killed control tumors leaving fibrotic and necrotic areas with no obvious tumor cells (Figure 7D, left panel), whereas this treatment failed to affect growth of t-DARPP tumors as manifested by the presence of moderately- to poorly-differentiated tumors (Figure 7D, right panel).
Figure 7. t-DARPP overexpression promotes tumor growth and blocks response to trastuzumab treatment in vivo.
OE19 cells stably expressing t-DARPP or pcDNA3 control vector were injected subcutaneously (4 × 106 cells per site) into nude mice. When Tumor volume reached 200 mm3, the mice were treated with 20 mg/kg trastuzumab twice weekly for 20 days. A) A representative sacrificed control mouse with xenograft tumors (left panel), and resected xenograft tumors (right panel) at the end of experiment. B) A representative sacrificed t-DARPP mouse with xenograft tumors (left panel), and resected xenograft tumors (right panel) at the end of experiment. C) Tumor growth curve from OE19 cells stably expressing t-DARPP or control vector before the treatment (left panel) and after the treatment (right panel). Each data point represents the mean ± standard deviation. The data show that t-DARPP significantly enhanced tumor growth rate (p<0.01), and inhibited response to treatment with trastuzumab (p<0.001) as compared to control. D) The H&E staining at the end of trastuzumab treatment shows effectively diminished control tumors leaving necrotic and fibrotic lesions (left panel) whereas t-DARPP tumors were unaffected (right panel).
Discussion
While the incidence of EAC has increased rapidly in the last decades, especially among Caucasian men, limited progress in the treatment of EAC has been achieved (31). The prognosis of patients diagnosed with EAC remains poor with a 5-year relative survival at 10-20%. Over the past 15 years, targeted therapy approaches have made significant advances due to the rapid development of new drugs that aim treatment at specific molecular targets that are critical for cancer cell survival (32). Amplification and overexpression of ERBB2 has been identified in 15-20% of primary EAC tumor specimens and their corresponding metastases (33, 34). Therefore, in addition to breast cancer, ERBB2 has been suggested as a plausible target for treatment in esophageal cancers. Trastuzumab, a recombinant humanized monoclonal anti-ERBB2 antibody, was initially approved by the FDA for the treatment of ERBB2 metastatic breast carcinoma (19). With its successful application in breast cancer, trastuzumab antitumor activity was investigated in patients with ERBB2-positive metastatic cancer of gastroesophageal junction in combination with chemotherapy (28). Of note, a phase III clinical trial (RTOG 1010 protocol) is currently underway to evaluate the addition of trastuzumab to increase disease-free survival when combined with trimodality treatment for patients with ERBB2-positive esophageal adenocarcinoma. However, previous studies have shown that cancer patients who initially respond well to trastuzumab, develop acquired trastuzumab resistance within a year of treatment (20). Our current study provides important pre-clinical evidence suggesting that t-DARPP could mediate trastuzumab resistance in EAC.
Our results indicate that both t-DARPP and ERBB2 were significantly overexpressed in a subset of EAC tumors. Moreover, overexpression of t-DARPP was significantly associated with advanced tumor stage. Of note, both ERBB2 and t-DARPP are located inside the 17q21 chromosomal region, a commonly amplified region in adenocarcinomas of the stomach and esophagus (33, 35). Several studies have shown that ERBB2 plays an important role in activation of the pro-survival PI3K/AKT signaling pathway (36). Similarly, t-DARPP can also mediate activation of PI3K/AKT pathway (14). Based on these data, we hypothesized that t-DARPP and ERBB2 may have a functional relationship where t-DARPP-mediated activation of AKT could lead to resistance to trastuzumab. The results indicated that endogenous and exogenous t-DARPP expression can significantly enhance cell survival and block apoptosis in response to trastuzumab in esophageal adenocarcinoma cell models. The xenografted esophageal adenocarcinoma mouse model results confirmed the in vitro data and t-DARPP overexpressing tumors were unaffected by trastuzumab treatment and continued to grow. These results confirmed the role of t-DARPP in mediating resistance to trastuzumab in EAC cells. Our finding that trastuzumab-resistant OE19 cells, generated by trastuzumab selection, expressed a significantly higher endogenous level of t-DARPP than parental cells provide an additional evidence supporting the role of t-DARPP in resistance to trastuzumab. Interestingly, a breast cancer trastuzumab-resistant cell model expressed higher levels of t-DARPP (37). Taken together, these data suggest the possible presence of a small subpopulation of cells overexpressing t-DARPP among cancer cells where treatment with trastuzumab provides the appropriate selection advantage for this subpopulation to continue to grow and replace trastuzumab-sensitive cell populations.
While we and others have previously shown that t-DARPP can lead to activation of AKT and resistance to trastuzumab in breast cancer cells in vitro (14, 37) (38, 39), the mechanism by which t-DARPP activates the AKT survival pathway was not fully identified. In the current study, our data clearly indicated that t-DARPP expression, without treatment with trastuzumab, significantly increased ERBB2, p-ERBB2(Y1248), and p-AKT(S473) protein levels relative to control cells. Notably, the trastuzumab-resistant OE19 cells showed, in addition to t-DARPP overexpression, increased p-ERBB2(Y1248) and p-AKT(S473) protein levels as compared to parental cells, consistent with our findings from transient and stable expression of t-DARPP. Taken together, these results indicate that t-DARPP can significantly enhance ERBB2 protein stability thereby enhancing the AKT pathway. The possibility that t-DARPP could also enhance the protein stability of other ERBB family members remains to be investigated in EAC.
Previous studies have shown that failure to suppress the AKT pathway plays central role in resistance to trastuzumab in breast cancer. Several mechanisms have been suggested such as activating mutations in the PI3KCA, deletions of PTEN, overexpression of cMET, and overexpression of ERBB3 (40, 41). In an attempt to elucidate the mechanistic role of t-DARPP in trastuzumab resistance in EAC, we found that t-DARPP associated with ERBB2 in a protein complex. Based on this finding, we hypothesized that t-DARPP interaction with ERBB2 prevents trastuzumab binding to ERBB2 receptor, thus blocking trastuzumab-induced down-regulation of ERBB2/AKT signaling. Indeed, our results clearly demonstrated that t-DARPP significantly decreased trastuzumab binding to the ERBB2 receptor in OE19 cells. The immunofluorescence data clearly indicated that t-DARPP expression was cytosolic (Supplemental Figure 2C); strongly suggesting that t-DARPP interaction with ERBB2 cytosolic domain could alter ERBB2 protein folding and conformation, thereby interfering with trastuzumab binding to ERBB2 extracellular domain. Nagy and colleagues (42) uncovered a similar mechanism of trastuzumab resistance in JIMT-1 breast cancer cells and showed that the expression of MUC4, a trans-membrane glycoprotein, promoted resistance to trastuzumab through masking of ERBB2, which leads to diminished binding of trastuzumab. These data suggest that molecular mechanisms that lead to the impairment of binding of trastuzumab to its target, ERBB2, are important upstream determinants of therapeutic response.
In conclusion, our findings indicate that frequent overexpression of t-DARPP in ERBB2-positive EAC underlies a trastuzumab resistance phenotype. Therefore, our data suggest that t-DARPP expression status could potentially be exploited for more effective clinical management of patients with ERBB2-positive esophageal adenocarcinoma who qualify for treatment with trastuzumab.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by grants from the National Institute of Health; R01CA93999 and R01CA133738 (WER); Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Vanderbilt University.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2002;31:421–40. viii. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820–8. doi: 10.1002/(sici)1097-0142(19961015)78:8<1820::aid-cncr25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Ide H, Nakamura T, Hayashi K, Endo T, Kobayashi A, Eguchi R, et al. Esophageal squamous cell carcinoma: pathology and prognosis. World J Surg. 1994;18:321–30. doi: 10.1007/BF00316810. [DOI] [PubMed] [Google Scholar]
- 8.Varis A, Zaika A, Puolakkainen P, Nagy B, Madrigal I, Kokkola A, et al. Coamplified and overexpressed genes at ERBB2 locus in gastric cancer. International journal of cancer. 2004;109:548–53. doi: 10.1002/ijc.20001. [DOI] [PubMed] [Google Scholar]
- 9.Maqani N, Belkhiri A, Moskaluk C, Knuutila S, Dar AA, El-Rifai W. Molecular dissection of 17 q12 amplicon in upper gastrointestinal adenocarcinomas. Mol Cancer Res. 2006;4:449–55. doi: 10.1158/1541-7786.MCR-06-0058. [DOI] [PubMed] [Google Scholar]
- 10.Hardwick RH, Shepherd NA, Moorghen M, Newcomb PV, Alderson D. c-erbB-2 overexpression in the dysplasia/carcinoma sequence of Barrett’s oesophagus. J Clin Pathol. 1995;48:129–32. doi: 10.1136/jcp.48.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–68. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]
- 12.Dahlberg PS, Jacobson BA, Dahal G, Fink JM, Kratzke RA, Maddaus MA, et al. ERBB2 amplifications in esophageal adenocarcinoma. Ann Thorac Surg. 2004;78:1790–800. doi: 10.1016/j.athoracsur.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 13.El-Rifai W, Smith MF, Jr., Li G, Beckler A, Carl VS, Montgomery E, et al. Gastric Cancers Overexpress DARPP-32 and a Novel Isoform, t-DARPP. Cancer Res. 2002;62:4061–4. [PubMed] [Google Scholar]
- 14.Belkhiri A, Dar AA, Zaika A, Kelley M, El-Rifai W. t-Darpp promotes cancer cell survival by up-regulation of Bcl2 through Akt-dependent mechanism. Cancer Res. 2008;68:395–403. doi: 10.1158/0008-5472.CAN-07-1580. [DOI] [PubMed] [Google Scholar]
- 15.Beckler A, Moskaluk CA, Zaika A, Hampton GM, Powell SM, Frierson HF, Jr., et al. Overexpression of the 32-kilodalton dopamine and cyclic adenosine 3′,5′-monophosphate-regulated phosphoprotein in common adenocarcinomas. Cancer. 2003;98:1547–51. doi: 10.1002/cncr.11654. [DOI] [PubMed] [Google Scholar]
- 16.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–92. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 17.Vangamudi B, Zhu S, Soutto M, Belkhiri A, El-Rifai W. Regulation of beta-catenin by t-DARPP in upper gastrointestinal cancer cells. Mol Cancer. 10:32. doi: 10.1186/1476-4598-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vangamudi B, Peng DF, Cai Q, El-Rifai W, Zheng W, Belkhiri A. t-DARPP regulates phosphatidylinositol-3-kinase-dependent cell growth in breast cancer. Mol Cancer. 9:240. doi: 10.1186/1476-4598-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park BH, Davidson NE. PI3 kinase activation and response to Trastuzumab Therapy: what’s neu with herceptin resistance? Cancer Cell. 2007;12:297–9. doi: 10.1016/j.ccr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 21.Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–91. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 22.Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69:2191–4. doi: 10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–7. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 24.Zabrecky JR, Lam T, McKenzie SJ, Carney W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991;266:1716–20. [PubMed] [Google Scholar]
- 25.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer cell. 2004;6:117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–41. [PubMed] [Google Scholar]
- 27.Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–6. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 28.Gravalos C, Gomez-Martin C, Rivera F, Ales I, Queralt B, Marquez A, et al. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol. 13:179–84. doi: 10.1007/s12094-011-0637-6. [DOI] [PubMed] [Google Scholar]
- 29.Sun L, Trausch-Azar JS, Ciechanover A, Schwartz AL. Ubiquitin-proteasome-mediated degradation, intracellular localization, and protein synthesis of MyoD and Id1 during muscle differentiation. J Biol Chem. 2005;280:26448–56. doi: 10.1074/jbc.M500373200. [DOI] [PubMed] [Google Scholar]
- 30.El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida C, Riggins GJ, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6. [PubMed] [Google Scholar]
- 31.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–35. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 32.Tew WP, Kelsen DP, Ilson DH. Targeted therapies for esophageal cancer. Oncologist. 2005;10:590–601. doi: 10.1634/theoncologist.10-8-590. [DOI] [PubMed] [Google Scholar]
- 33.Maqani N, Belkhiri A, Moskaluk C, Knuutila S, dar A, El-Rifai W. Molecular dissection of 17q12 amplicon in upper gastrointestinal adenocarcinomas. Mol Cancer Res. 2006 doi: 10.1158/1541-7786.MCR-06-0058. in press. [DOI] [PubMed] [Google Scholar]
- 34.Reichelt U, Duesedau P, Tsourlakis M, Quaas A, Link BC, Schurr PG, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120–9. doi: 10.1038/modpathol.3800712. [DOI] [PubMed] [Google Scholar]
- 35.Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–9. [PubMed] [Google Scholar]
- 36.El-Deiry WS. Akt takes centre stage in cell-cycle deregulation. Nat Cell Biol. 2001;3:E71–3. doi: 10.1038/35060148. [DOI] [PubMed] [Google Scholar]
- 37.Belkhiri A, Dar AA, Peng DF, Razvi MH, Rinehart C, Arteaga CL, et al. Expression of t-DARPP mediates trastuzumab resistance in breast cancer cells. Clin Cancer Res. 2008;14:4564–71. doi: 10.1158/1078-0432.CCR-08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu L, Waliany S, Kane SE. Darpp-32 and its truncated variant t-Darpp have antagonistic effects on breast cancer cell growth and herceptin resistance. PLoS One. 2009;4:e6220. doi: 10.1371/journal.pone.0006220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamel S, Bouchard A, Ferrario C, Hassan S, Aguilar-Mahecha A, Buchanan M, et al. Both t-Darpp and DARPP-32 can cause resistance to trastuzumab in breast cancer cells and are frequently expressed in primary breast cancers. Breast Cancer Res Treat. 120:47–57. doi: 10.1007/s10549-009-0364-7. [DOI] [PubMed] [Google Scholar]
- 40.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nature reviews. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 41.Abramson V, Arteaga CL. New strategies in HER2-overexpressing breast cancer: many combinations of targeted drugs available. Clin Cancer Res. 2011;17:952–8. doi: 10.1158/1078-0432.CCR-09-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.