Abstract
β-Adrenergic receptors (βAR) and D2-like dopamine receptors (which include D2-, D3- and D4-dopamine receptors) activate Gs and Gi, the stimulatory and inhibitory heterotrimeric G proteins, respectively, which in turn regulate the activity of adenylyl cyclase (AC). β2-Adrenergic receptors (β2AR) and D4-dopamine receptors (D4DR) co-immunoprecipitated when co-expressed in HEK 293 cells, suggesting the existence of a signalling complex containing both receptors. In order to determine if these receptors are closely associated with each other, and with other components involved in G protein-mediated signal transduction, β2AR, D4DR, G protein subunits (Gαi1 and the Gβ1γ2 heterodimer) and AC were tagged so that bioluminescence resonance energy transfer (BRET) could be used to monitor their interactions. All of the tagged proteins retained biological function. For the first time, FlAsH-labeled proteins were used in BRET experiments as fluorescent acceptors for the energy transferred from Renilla luciferase-tagged donor proteins. Our experiments revealed that β2AR, D4DR, G proteins and AC were closely associated in a functional signalling complex in cellulo. Furthermore, BRET experiments indicated that although activation of Gi caused a conformational change within the heterotrimeric protein, it did not cause the Gβγ heterodimer to dissociate from the Gαi1 subunit. Evidence for the presence of a signalling complex in vivo was obtained by purifying βAR from detergent extracts of mouse brain with alprenolol-Sepharose and showing that the precipitate also contained both D2-like dopamine receptors and AC.
Keywords: β-adrenergic receptors, D2-like dopamine receptors, heterotrimeric G proteins, adenylyl cyclase, signalling complexes, bioluminescence resonance energy transfer, alprenolol-Sepharose
1. Introduction
A wide variety of extracellular chemical and physical signals evoke intracellular responses by activating G protein-mediated signalling pathways. The process begins when these extracellular signals, or ligands, bind to specific heptahelical G protein-coupled receptors (GPCR) resulting in activation of cognate heterotrimeric G proteins. Activated G proteins in turn regulate effector proteins such as adenylyl cyclase (AC). Cells often harbor multiple G protein-mediated signalling pathways. A substantial and growing body of evidence indicates that proteins involved in G protein-mediated signal transduction are organized into signalling complexes, an arrangement that may account for the efficacy, speed and specificity of signalling [1]. Though it has been demonstrated that a receptor, G protein and effector are all simultaneously present within the same complex [2] data support the view that these complexes are much more extensive, containing many other proteins required to organize and regulate signalling pathways [1, 3]. They are also likely to contain multiple GPCR closely associated as homo- or heteromers [4].
There are many examples of heteromeric GPCR complexes formed when receptors are exogenously expressed in cells [5] as well as a few examples of endogenous heteromeric GPCR complexes [6–9] and refs. 42, 44, 48, 51, 54 and 55 in the Data Supplement for [10]. Among the heteromeric GPCR combinations that might be expected to occur naturally are complexes containing Gs- and Gi-coupled receptors both of which regulate AC activity. This would provide a means of integrating convergent signalling events. D1-like dopamine receptors (ie. D1- and D5-dopamine receptors) are among the receptors that regulate AC through Gs. In contrast, D2-like dopamine receptors, which include D2-, D3- and D4-dopamine receptors (D2DR, D3DR and D4DR), regulate AC by activating Gi. Published data indicate that complexes of D1- and D3-dopamine receptors can be co-immunoprecipitated from rat striatum [7]. A2A-adenosine receptors, like D1-dopamine receptors, regulate AC by activating Gs and have been found to co-localize with D2DR in the same cells in rat brain [11]. Furthermore, A2A-adenosine and D2DR form heteromers when heterogenously co-expressed [12]. β-adrenergic receptors and some subtypes of somatostatin receptors stimulate AC through activation of Gs, and both receptors have been shown to co-localize with D2-like dopamine receptors in neurons from various brain regions [13, 14]. Co-localization of β-adrenergic receptors and D2-like dopamine receptors suggests that they might be associated with each other as part of a signalling complex in vivo. Data presented here showing that co-expressed D4DR and β2-adrenergic receptors (β2AR) can be co-immunoprecipitated from transfected HEK 293 cells support this hypothesis. In order to determine if this was attributable to direct protein-protein interactions between the two receptors, bioluminescence resonance energy transfer (BRET) experiments were performed. BRET was also used to probe for protein-protein interactions between these receptors and other signal transduction proteins in cellulo. In addition, alprenolol-Sepharose was used to precipitate β-adrenergic receptors from mouse brain and the purified proteins were assayed for D2-like dopamine receptors and AC to determine if complexes containing these signalling proteins are present in vivo.
2. Materials and Methods
2.1 Materials
[125I]iodosulpride (2200 Ci/mmol), [125I]iodocyanopindolol (2200 Ci/mmol), [125I]adenosine 3’,5’-cyclicphosphoric acid, 2’-O-succinyl-(iodotyrosine methyl ester) (2200 Ci/mmol) and 96-well white OptiPlates (cat. no. 6005190) were obtained from Perkin Elmer/New England Nuclear. Pertussis toxin (PTx, cat. no. 516560) and forskolin were from Calbiochem. Forskolin was dissolved in dimethyl sulfoxide at a concentration of 25 mM and stored at 4°C. 3-Isobutyl-1-methylxanthine, isoproterenol, dopamine, spiperone, 1,2-ethanedithiol (cat. no. 398020), poly(ethyleneimine) (cat. no. P3143), deoxycholic acid (cat. no. D6750), 3-([3-cholamidopropyl]dimethylammonio)-2-hydroxy-1- propanesulfonate (CHAPSO, cat. no. C4695), Disperse Blue 3 and Patent Blue V were purchased from Sigma-Aldrich. Nonidet P40 (cat. no. 5500UA) was from Bethesda Research Laboratories and 10% SDS (cat. no. 351-032-101) was from Quality Biological, Inc. SuperSignal West Pico Chemiluminescent Substrate reagents were supplied by Thermo Scientific/Pierce Protein Research Products, and the Quik-Change Multi Site-Directed Mutagenesis Kit was from Stratagene. Complete™ protease inhibitor cocktail tablets were from Roche and Protein Assay reagent (cat. no. 500-0006) was from Bio-Rad Laboratories, Inc. Lipofectamine Plus, Hanks Balanced Salt Solution (HBSS, cat. no. 14025), Ca2+- and Mg2+-free HBSS with 0.05% trypsin and 0.02% EDTA (cat. no. 25300-054), Dulbecco’s modified Eagle’s medium with 4.5 g glucose/l and 25 mM HEPES (DMEM/HEPES, cat. no. 12430), FlAsH (cat. no. T34561) and protein A-Sepharose (cat. no. 10 1042) were purchased from Invitrogen. SNAP-Surface 488 (cat. no. S9124S) and CLIP-Surface 547 (cat. no. S9233S) were from New England Biolabs. Collagen-coated glass bottom culture dishes (cat. no. P35GCol-1.5-14) were from MatTek Corp. Goat anti-cAMP was from Research Products International. Mouse monoclonal anti-β2AR (cat. no. sc-81577) was from Santa Cruz. Rabbit anti-D4DR (cat. no. ab-13318) was from Abcam. Rabbit anti-HA was supplied by Santa Cruz (cat. no. sc-805) or Abcam (cat. no. ab-9110). Alexa-488 conjugated goat anti-rabbit (cat. no. A-11008) and coelenterazine h (cat. no. C6780) came from Molecular Probes. HRP-conjugated goat antimouse (cat. no. 474-1802) was purchased from KPL. Goat serum (cat. no. 6010-25) and donkey anti-goat (cat. no. 510-10) were supplied by Linco Research, Inc. HRP-conjugated protein A (cat. no. M00089) was purchased from GenScript Corp. and reconstituted to 1 mg/ml as suggested by the manufacturer. Rabbit anti-human β2AR was obtained from rabbits immunized with the amidated peptide VPSDNIDSQGRNCSTNDSLL which corresponds to the sequence of the C-terminus of the human β2AR. The IgG fraction, referred to here as PF-12, was purified from antisera using protein A-Sepharose, and contained 5.4 mg IgG/ml. Alprenolol-Sepharose CL-4B was prepared as previously described [15]. Mouse brains (cat. no. 55005-1) were obtained from Pel-Freez and stored at −80°C until needed.
2.2 Recombinant Plasmids
There are nine isoforms of the D4DR possessing 2 (D4.2) to 10 (D4.10) hexadecapeptide tandem repeats in the third intracellular loop of the receptor [16]. All of these isoforms exhibit generally similar signalling properties [17, 18] thus we chose D4.2DR on the basis of availability. The cDNA for HA tag human D4.2DR was modified in order to create a tetracysteine motif (CCPGCC) at the C-terminus or at different locations within the third intracellular loop. The D4.2DR terminates with the dipeptide CC necessitating the addition of the tetrapeptide PGCC to produce a recombinant D4DR-PGCC protein with the potential to bind the FlAsH reagent. Two additional tetracysteine-tagged D4.2DR constructs were prepared using Stratagene’s Quik-Change Multi Site-Directed Mutagenesis Kit in conjunction with the primer CAGGACCCCTGCTGCCCGGGCTGTTGCCCCCCCGCGCCC or CCGGACCCCTGCTGCCCCGGGTGTTGTCCCCCCGACGCC to convert amino acids 258–263 or 274–279 of the human receptor to a tetracysteine motif. These are referred to as D4DR-G259C and D4DRG275C, respectively.
Recombinant human β2AR or β2AR with a C-terminal EGFP (β2AR-EGFP) or Renilla luciferase tag (β2AR-RLuc) have been described [19]. Plasmids coding for the β2AR with an N-terminal signal sequence followed by a SNAP tag (SNAP-β2AR) and the neurokinin-1 receptor with an N-terminal signal sequence followed by a CLIP tag were obtained from New England Biolabs. The sequence for the neurokinin-1 receptor was replaced with the cDNA for D4.2DR using standard molecular biology techniques so that the receptor was in frame with the signal sequence and the CLIP tag (CLIP-D4DR). Gαi1 tagged with RLuc inserted at different positions (Gαi1-RLuc 60, Gαi1-RLuc 91 and Gαi1-RLuc 122) were generous gifts from Drs. Michel Bouvier and Céline Galés [20]. The cDNA for Gαi1 and Gαs(long) both in pcDNA 3.1(+) were purchased from cDNA Resource Center (Rolla, MO). The cDNA for bovine type I adenylyl cyclase was in pcDNA 3.1(+). Information regarding recombinant plasmids coding for Gαs-RLuc, Gβ1, Gγ2, EGFP-Gγ2 can be found elsewhere [19, 21, 22]. The construction of rat type II adenylyl cyclase with a C-terminal R. reniformus luciferase tag (AC-RLuc) has been described [19]. This plasmid was mutated to introduce a stop codon between the cDNA for AC and RLuc to regenerate wild type AC. Previous studies have shown that all of the RLuc- and EGFP-tagged signalling proteins retain biological activity [19, 21–23]. pcDNA 3.1(+) containing the cDNA for Renilla luciferase was modified using standard techniques so that it coded for luciferase with a tetracysteine motif (SCCPGCC) fused to its C-terminus (RLuc-CCPGCC).
2.3 Protein Expression in HEK 293 Cells, Pertussis Toxin Treatment, and cAMP Assays
HEK 293 cells were plated at a density of 1.0–1.5 × 105 cells/cm2 of growth surface in T75 flasks (co-immunoprecipitation and ligand binding assays), in 6-well tissue culture plates (immunofluorescence and BRET assays), or polylysine-coated 24-well tissue culture plates (cAMP assays) or at 2.5 × 104 cells/cm2 in collagen-coated glass bottom culture dishes (fluorescent receptor imaging). Approximately 16 to 24 h after plating, cells were transfected with 0.005 to 0.1 µg of each recombinant plasmid per cm2 of growth surface using Lipofectamine Plus. As necessary, vector DNA was included to keep the total amount of DNA constant. Except when noted, cells used for BRET experiments were co-transfected with β2AR, D4DR, Gαs, Gαi1, Gβ1, Gγ2 and AC. The proteins bearing the tags required for a particular BRET, immunofluorescent or fluorescent receptor imaging experiment are indicated in individual figures. When D4DR-mediated regulation of endogenous AC activity was investigated, cells were transfected with β2AR-RLuc alone or in conjunction with either D4DR or tetracysteine-tagged D4DR. Regulation of exogenously expressed types I and II AC was determined in HEK 293 cells co-expressing β2AR and D4DR with and without either type I or type II AC. In some instances, cells were treated with PTx (100 ng /ml of culture media) added approximately 6 h after transfection.
For cAMP accumulation assays, the medium was replaced with DMEM/HEPES containing 1 mM 3-isobutyl-1-methylxanthine approximately 18 h after PTx addition. The cells were stimulated with 1 µM isoproterenol or dopamine, either individually or in combination for 15 min at 37°C. cAMP was extracted from the cells and measured by radioimmunoassay. For some experiments, cells were released from 6-well culture plates with a trypsin/EDTA solution, collected by centrifuging at 100 × g for 5 min, and suspended in the above medium before being assayed for cAMP accumulation.
2.4 Co-immunoprecipitation of β2AR and D4DR
HEK 293 cells were transfected so they co-expressed Gαs, Gαi1, Gβ1, Gγ2, and AC together with β2AR and/or D4DR. Approximately 24 h after transfection the media was removed and the cells washed with Dulbecco’s phosphate buffered saline (DPBS). Cells were scraped in ice-cold buffer (1 mM Tris-HCl, pH 7.4, 2 mM EDTA, Roche Complete™ protease inhibitors). Cells expressing each receptor type alone were combined to create a “mixed” population of cells. Cells transiently co-expressing Gαs, Gαi1, Gβ1, Gγ2, and AC but no exogenous receptor were 1) use as negative controls, or 2) combined with an equal amount of cells co-expressing β2AR and D4DR in order to attain the same stoichiometry between total cell protein and exogenously expressed protein expected in the “mixed” cell population. Cell suspensions were incubated on ice for 15 min before homogenization with a Polytron at maximum rpm for 30 sec. Samples were centrifuged at 4°C for 5 min at 1,000 × g to remove unbroken cells and nuclei, and the supernatants were centrifuged at 4°C for 20 min at 20,000 × g to collect the membranes. The membranes were suspended in RIPA buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 3.7 mM EDTA, 1.2 mM EGTA, 0.1% SDS, 0.1% NP-40, 0.5% sodium deoxycholate) at a protein concentration of approximately 0.5 mg/ml, and solubilization proceeded during a 1 h incubation of the samples on ice. Soluble proteins were recovered in the supernatant after centrifuging the samples at 100,000 × g for 1 h at 4°C. To immunoprecipitate receptors, 5 µg of anti-β2AR (PF-12) or 2 µg of anti-D4DR (ab-13318) was added per ml of supernatant. After incubating for 1 h at room temperature, 20 µl of a 50% slurry of protein A-Sepharose was added, and the incubation continued for an additional 30 min while shaking vigorously. Protein A-Sepharose was collected by centrifugation, and the supernatant removed. The pellet was washed twice with 0.5 ml of RIPA buffer and bound protein dissolved by adding 10 µl each of 150 mM dithiothretol and LDS-PAGE solution (100 mM Tris-HCl, pH 8.5, 6% lithium dodecylsulfate, 30% glycerol, 0.2% bromphenol blue). Samples were incubated at 60°C for 15 min followed by separation on 10% Bis-Tris NuPAGE polyacrylamide gels (Invitrogen) using MOPS-SDS running buffer (50 mM 2-(N-morpholino) propane sulfonic acid, 50 mM Tris-base, 0.1% SDS, 1 mM EDTA). Proteins were then transferred to nitrocellulose with blotting buffer (25 mM Tris-base, 192 mM glycine, 20% methanol, 0.1% (w/v) SDS, pH 8.3) using a Bio-Rad Trans-Blot SD Semi-Dry Transfer Cell. Nitrocellulose membranes were incubated for 1 h at room temperature in blocking buffer (DPBS without Ca2+ or Mg2+, 0.1% Triton X-100, 5% (w/v) non-fat dry milk). Membranes were probed for β2AR or HA-tagged D4DR overnight at room temperature with antibodies in blocking buffer (sc-81577 and ab-9110 diluted 1:1000 and 1:2000, respectively), followed by HRP-conjugates of goat anti-mouse and protein A, respectively, each diluted 1:5000 with blocking buffer. Between incubations with primary antibodies and HRP-conjugated reagents, and following incubation with the latter, membranes were washed three times for 5 min at room temperature with gentle agitation in 20 ml of Tris-buffered saline containing Tween 20 (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% Tween 20). HRP bound to the membrane was detected using chemiluminescent reagents as directed by the manufacturer.
2.5 Confocal Microscopy
Confocal microscopy was used to determine the distribution and localization of transiently expressed D4DR. HEK 293 cells were transfected so that they co-expressed β2AR-RLuc, Gαs, Gαi1, Gβ1, Gγ2, AC-RLuc and either D4DR or one of the tetracysteine-tagged D4DR constructs. Cells transiently co-expressing all of the signalling proteins except the D4DR served as a negative control. Approximately 24 h after transfection, cells were seeded on collagen-coated glass cover slips. Four hours later, the cells were washed with DPBS and fixed with 3% paraformaldehyde in DPBS for 15 min at room temperature. Paraformaldehyde was removed by washing three times with DPBS, and non-specific antibody binding was blocked by incubating cells for 1 h at room temperature in DPBS containing 5% normal goat serum. Cells were washed once with DPBS followed by overnight incubation at 4°C in a 1:500 dilution of rabbit anti-HA (sc-805). Unbound anti-HA was removed by three washes with DPBS followed by a 1 h incubation at room temperature with a 1:2500 dilution of Alexa-488 conjugated goat anti-rabbit. Antibody solutions were made in DPBS containing 1% normal goat serum. To assay for both cell surface and internal D4DR, cells were permeablized by including 0.2% Triton X-100 in the blocking, and antibody-containing solutions. Cells were examined on an inverted Zeiss LSM 510 laser-scanning microscope.
Cells expressing SNAP-β2AR and/or CLIP-D4DR were grown in collagen-coated glass bottom culture dishes or seeded on collagen-coated cover slips as described above. Approximately 24 h after transfection the media was replaced with fresh media containing 5 µM each SNAP-Surface 488 and CLIP-Surface 547 prepared as directed by the supplier. After incubating for 30 min at 37°C, the cells were washed several times with fresh culture media, and the last wash replaced with fresh media. Imaging of naive cells or cells treated with ligand was done with a Zeiss LSM 710 laser-scanning microscope. When cells were treated with ligand, a 1 mM stock solution of isoproterenol in ethanol was added so that the final ligand concentration was 10 µM, and the cells were incubated for 45 min before imaging. Imaging of cells grown in collagen-coated glass bottom culture dishes was done in a climate-controlled chamber at 37°C in an atmosphere of 5% CO2 in air. Cells grown on collagen-coated glass cover slips were fixed as described above before imaging.
2.6 BRET Measurements
For some BRET experiments, cells were treated with PTx as described above, and all BRET experiments were done approximately 24 h after transfection. When experiments involved signalling proteins tagged with RLuc and EGFP, BRET was performed as described [22]. For BRET involving D4DR tagged with a tetracysteine motif, culture medium was removed and cells gently washed three times with 1 ml of HBSS containing 10 mM glucose (HBSS+glu). The last wash was replaced with 1.5 ml of a solution containing the fluorophore FlAsH and the cells incubated for 1 h at 37°C. The FlAsH solution was prepared by mixing one volume of 2 mM FlAsH with two volumes of dimethylsulfoxide containing 25 mM 1,2-ethanedithiol and incubating for 5–10 min at room temperature before diluting the mixture with HBSS+glu to give a final concentration of 500 nM FlAsH. To remove non-specifically bound FlAsH, cells were gently washing twice with 1 ml of HBSS+glu and incubating for 20 min at 37°C in 0.5 ml of HBSS+glu containing 250 µM 1,2-ethanedithiol. After washing the cells twice with HBSS+glu they were released from the tissue culture plates with trypsin/EDTA solution, and collected by centrifuging at 100 × g for 5 min. The supernatant was removed and the cells were suspended in DPBS+ (DPBS, 1 mg glucose/ml, 1 mM ascorbate, Roche Complete™ protease inhibitors). Cell suspensions were assayed for protein using Bio-Rad Protein Assay reagent as directed by the supplier. Samples volumes of 90 µl containing 25–100 µg of cell protein were dispensed into OptiPlates, and BRET assays were initiated by adding 10 µl of 50 µM coelenterazine h in DPBS+. BRET was measured both before and after the addition of vehicle or agonist(s) (1 µM isoproterenol and/or dopamine). The ratio of light passed by a 530/25 nm filter to that passed by a 460/25 nm filter was measured with a Berthold Mithras plate reader. Cells expressing RLuc-CCPGCC were used as a positive control for BRET experiments that employed FlAsH as a fluorophore.
2.7 Purification of Signalling Complexes Containing βAR from Mouse Brain
Frozen mouse brains were thawed in cold lysis buffer (1 mM Tris, pH 7.4, 2 mM EDTA, Roche Complete™ protease inhibitors) and homogenized at maximum rpm with a Polytron tissue homogenizer for 30 sec. The homogenate was centrifuged at 1,000 × g for 5 min at 4°C, and the resulting supernatant was subsequently centrifuged at 20,000 × g for 20 min at 4°C to obtain a membrane pellet. The membranes were suspended in buffer A (50 mM Tris, pH 7.4, 120 mM NaCl, 5 mM KCl, 5 mM MgCl2, 1.5 mM CaCl2, 1 mM EDTA), assayed for protein, diluted to a concentration of 5 mg/ml, and either stored at −20 °C for later use or collected by centrifugation at 15,000 × g for 20 min at 4°C prior to being dissolved with detergent. Soluble proteins were prepared by suspending the pellet at a concentration of 2 mg membrane protein/ml in buffer A containing 0.5% (w/v) CHAPSO. After incubating for 5 min on ice the insoluble material was removed by centrifuging at 4°C for 1 h at 100,000 × g (clarifying the detergent extract by centrifuging in a microfuge at 16,000 × g produced the same results). For each experimental sample, the soluble material obtained from one mg of membrane protein was transferred to a 1.5 ml conical snap cap Microfuge tube containing 25 µl of packed Sepharose or alprenolol-Sepharose. In some cases, precipitation of βAR by alprenolol-Sepharose was blocked by adding 10 µM propranolol. The samples were incubated at room temperature with mixing for 30 min to keep the gel suspended. The gel was collected by a brief centrifugation and the supernatants removed and assayed for the presence of βAR as described below. The gel pellets were washed twice with buffer A containing 1/10th the critical micelle concentration (0.05%) of CHAPSO and assayed for either AC activity or the presence of D2-like dopamine receptors.
To assay for AC activity, gel samples were suspended in 0.05% CHAPSO in buffer A that had been diluted 10X. To 150 µl samples were added 40 µl of assay buffer (6.4 mg/ml phosphocreatine, 300 U/ml creatine phosphokinase, 125 mM Tris, pH 7.6, 5 mM theophylline, 0.5 mM ATP, 0.2 mM GTP, 0.5% bovine serum albumin, 5 mM DTT, 10 mM EDTA, 20 mM MgCl2) and 10 µl of 200 µM forskolin or vehicle. Samples were incubated at 30°C for 15 min and the reaction stopped by adding 200 µl of 0.2 M HCl. The samples were lyophilized, reconstituted in 150 µl of water and 100 µl assayed for cAMP by radioimmunoassay.
To assay for D2-like dopamine receptors the gel was suspended in buffer A containing 0.05% CHAPSO and 1 nM [125I]iodosulpride. The total volume of each sample was approximately 200 µl and non-specific radioligand binding was assessed in the presence of a 1 µM concentration of either spiperone or propranolol. The gel was kept suspended by mixing for 30 min at room temperature before adding 2 ml of cold buffer A containing 0.05% CHAPSO and rapidly filtering on a vacuum manifold through GF/F glass fiber filters that had been soaked in 0.05% poly(ethyleneimine). Filters were rapidly washed twice with 2 ml of cold buffer A containing 0.05% CHAPSO and subsequently counted using a Wallac Wizard 1470 automatic gamma counter.
[125I]Iodocyanopindolol binding was used to determine the amount of βAR remaining in the supernatant after incubation with Sepharose or alprenolol-Sepharose. Samples were incubated with 1 nM radioligand in the absence or presence of 1 µM propranolol to determine the total and non-specific binding, respectively. Sample volumes were 0.5 ml and the samples were incubated at room temperature for 30 min. Bound radioligand was precipitated by adding 1.0 ml of normal goat serum diluted 1:200 with cold buffer A and 1.0 ml of 25% PEG-8000 in cold buffer A. The samples were then filtered through GF/C glass fiber filters mounted on a vacuum manifold and the filters washed twice with 2 ml of 8% PEG-8000 in cold buffer A. The amount of βAR precipitated by the affinity matrix was determined by subtracting the amount of βAR in the supernatant from CHAPSO extracts incubated with alprenolol-Sepharose from the amount in extracts incubated with Sepharose.
3. Results
3.1 Co-immunoprecipitation of β2AR and D4DR
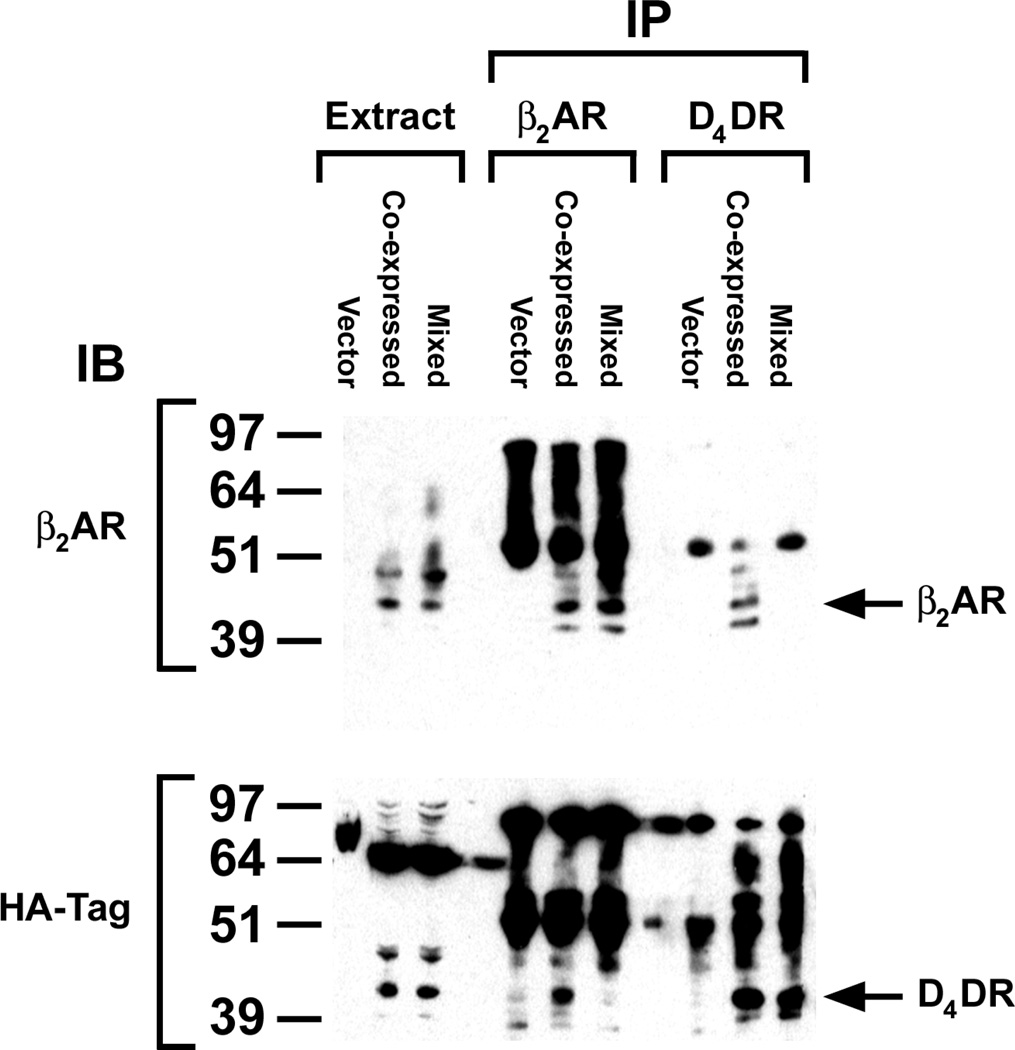
Radioligand binding revealed that HEK 293 cells used in this study expressed approximately 10 fmol of endogenous cell surface βAR/mg of cell membrane protein (data not shown), but these were not detectable by western blotting (Fig. 1). Cells exogenously expressing β2AR typically displayed about one pmol of cell surface receptors/mg of cell protein, and the major immunoprecipitable β2AR band had a molecular mass of approximately 44 kDa in agreement with the observation of others [24]. HEK 293 cells lack endogenous D2-like dopamine receptors. HEK 293 cells transfected with D4DR expressed 0.1–0.7 pmol of cell surface receptors/mg of cell protein. All of the D4DR expressed in these studies carried an N-terminal HA tag, and were identified by western blotting with either anti-HA (Fig. 1) or anti-D4 antibodies (data not shown). The major D4DR band had a molecular mass of approximately 41 kDa, a value similar to that observed by others [16]. When β2AR and D4DR were co-expressed in HEK 293 cells they were co-immunoprecipitated (Fig. 1). However, if the receptors were expressed in separate cell populations that were mixed prior to immunoprecipitation the receptors did not co-precipitate.
Figure 1. Co-immunoprecipitation of exogenously expressed β2AR and D4DR.
All cells used in these studies transiently co-expressed Gαs, Gαi1, Gβ1, Gγ2 and AC. In addition they expressed β2AR and/or the HA-tagged D4DR. Cells expressing one or the other receptor were mixed prior to preparing membranes (Mixed). So that each sample contained the same ratio of expressed receptor protein to total cell protein, cells co-expressing the receptors (Co-expressed) were mixed with cells receiving plasmid without receptor cDNA. These latter cells (Vector) were also used as a negative control. Membranes prepared from these samples were dissolved in RIPA buffer, and the soluble proteins in the supernatant following centrifugation at 100,000 × g were treated as described in Materials and Methods in order to immunoprecipitate and identify β2AR and HA-tagged D4DR on western blots. Data are representative of three independent experiments.
3.2 Cell Surface Expression and Functional Activity of Exogenously Expressed Receptors
HA-tags on D4DR are exposed to the extracellular milieu when the receptors are inserted correctly into the plasma membrane. HEK 293 cells expressing D4DR with or without the tetracysteine motif were stained by anti-HA antibodies, but cells lacking the exogenous D4DR were not (Supplementary Fig. 1). Immunofluorescent staining of D4DR in non-permeabilized cells indicated that receptors were localized to the plasma membrane, while the staining pattern in detergent-permeabilized cells revealed that there were also receptors within the cell. Membranes from HEK 293 cells expressing the various tetracysteine-tagged D4DR bound the antagonist spiperone with affinities that were indistinguishable from D4DR that lacked the tetracysteine tag (data not shown). SNAP-tagged β2AR and CLIP-tagged D4DR were co-localized in the plasma membrane prior to treatment with ligand (Supplementary Fig. 2). Exposure to isoproterenol caused both receptors to be redistributed within the cell and they remained largely co-localized.
HEK 293 cells are reported to express types I, II, III, VI and IX AC [25, 26]. The basal rate of cAMP production in cells used for these studies was 2 ± 3 pmol cAMP/mg/15 min. It increased to 80 ±10 pmol cAMP/mg/15 min (n=3) in response to isoproterenol-mediated activation of endogenous βAR. Neither dopamine stimulation nor PTx treatment of the cells had any effect on the isoproterenol-mediated stimulation of endogenous AC (Supplementary Fig. 3). cAMP accumulation in response to isoproterenol was increased in cells exogenously expressing β2AR-RLuc. When HEK 293 cells co-expressed β2AR-RLuc together with either D4DR or any of the tetracysteine-tagged D4DR constructs, a dopamine-mediated inhibition of the endogenous AC was observed. This inhibition was blocked when cells were pre-treated with PTx. Dopamine also inhibited forskolin-induced cAMP accumulation only when D4DR was co-expressed (data not shown). Taken together, these experiments demonstrated that the β2AR-RLuc and tetracysteine-tagged D4DR constructs were functional and able to regulate the endogenous AC in HEK 293 cells (Supplementary Fig. 3).
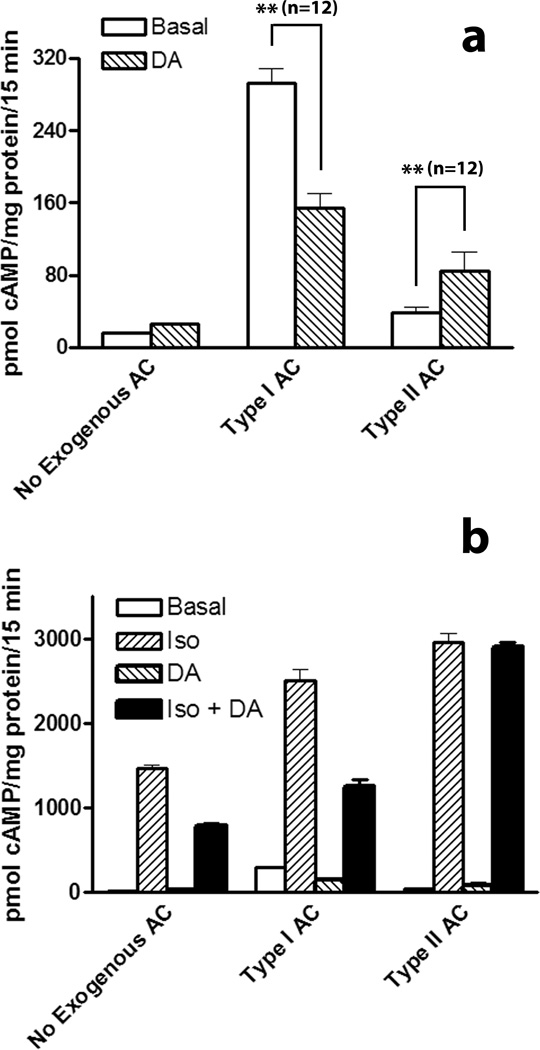
Previous studies have indicated that D4DR can regulate the activity of type II AC[27]. Type II AC differs from other types of mammalian AC in that it is stimulated by Gi rather than inhibited. Although HEK 293 are reported to naturally express type II AC, the dopamine-mediated inhibition of isoproterenol-induced cAMP accumulation observed in these studies indicated that on balance the activity of endogenous AC other than type II predominate in these cells. This simplified efforts to show that exogenously expressed RLuc-tagged type II AC (AC-RLuc) was regulated by D4DR. HEK 293 cells exogenously expressing β2AR and D4DR with or without co-expressed AC-RLuc were treated with dopamine to determine if the receptor was capable of regulating the activity of tagged type II AC. There was no significant effect of dopamine on the basal activity of endogenous AC (Fig. 2a). However, when AC-RLuc was exogenously expressed, dopamine caused a significant increase in cAMP accumulation indicating that the D4DR was capable of regulating AC-RLuc activity. In comparison, the basal activity of exogenously expressed type I AC was inhibited by dopamine. As has already been noted, isoproterenolmediated activation of endogenous AC was inhibited by dopamine in cells exogenously expressing both β2AR and D4DR (Fig 2b and Supplementary Fig. 3). This was also true of HEK 293 cells that exogenously co-expressing type I AC. In contrast HEK 293 cells co-expressing β2AR, D4DR and AC-RLuc showed no dopamine-mediated inhibition of the isoproterenol-induced cAMP accumulation indicating that the net inhibition of endogenous AC was overcome by the Gi-mediated stimulation of exogenously expressed type II AC.
Figure 2. D4DR-mediated regulation of type I and type II AC activity.
HEK 293 cells were transfected so that they expressed β2AR and D4DR with or without either type I AC or type II AC-RLuc. Panel a: Cells were then assayed for cAMP accumulation in the absence (Basal) or in the presence of dopamine (DA) as described in Materials and Methods. Panel b: Data in panel (a) are shown together with the response of cells to isoproterenol (Iso) or both Iso and DA together (Iso + DA). Data in panel (b) are shown on a different scale than the data in panel (a). Basal levels of cAMP accumulation in the absence of exogenously expressed AC (No Exogenous AC) were not significantly affected by dopamine (p>.05, n=3). Significant differences based on the indicated number (n) of samples from three independent experiments were determined with GraphPad Prism 4 software (** p<0.01).
3.3 BRET Between Signalling Proteins
In order to use BRET to determine if two proteins interact in an intact cell they must be tagged, one with the BRET donor and the other with the BRET acceptor. BRET occurs when luminescent energy from a bioluminescent donor tag is non-radiatively transferred to a fluorescent acceptor. This transfer of energy only occurs if the distance between donor and acceptor is <100 Å indicating that the proteins are closely associated in a complex. Typically the bioluminescent donor protein is R. reniformus luciferase (RLuc) and the fluorescent acceptor is a variant of green fluorescent protein (eg. EGFP). If the tagged proteins interact, then the donor and acceptor are brought into close proximity and BRET can occur. An alternative approach was used to produce a fluorescently tagged D4DR for BRET experiments. Instead of fusing the D4DR to EGFP, additional amino acids were added to the C-terminus or mutations introduced into the third intracellular loop of the receptor to generate a “tetracysteine” motif with the amino acid sequence CCPGCC. This motif binds a 480 Da biarsenical reagent known as FlAsH producing a fluorophore [28] with the potential to make the tagged D4DR an acceptor for resonance energy transfer experiments. The same strategy has been used to make other fluorescently tagged receptors for FRET experiments [29–31].
RLuc-CCPGCC was prepared as a positive control for BRET experiments involving FlAsH as the fluorescent acceptor. When cells expressing RLuc-CCPGCC were incubated first with FlAsH and then with coelenterazine h, BRET was observed, and increased in a dose-dependent manor with concentrations of FlAsH up to 500 nM (data not shown). Consequently, BRET experiments were subsequently performed after incubating transfected cells with 500 nM FlAsH. Fluorescence measurements on cell suspensions indicated that there was significantly more FlAsH fluorescence in cells expressing tetracysteine-tagged proteins other than D4DR-PGCC (see below) when compared with cells that lacked these proteins. However, fluorescence measurements as well as microscopic examination of cells that did not express any tetracysteine-tagged proteins revealed a significant amount of non-specific fluorescence. Sodium pyruvate and the non-fluorescent dyes Patent V and Disperse Blue have been reported to reduce non-specific FlAsH fluorescence [32]. These reagents had no effect on BRET in cells expressing RLuc-CCPGCC, but in our hands they were unable to significantly reduce the background fluorescence either individually or when combined.
BRET experiments were done on cells in suspension which necessitated their release from the growing surface with trypsin. To determine if this had any effect on agonist-mediated signal transduction, cells exogenously expressing β2AR and D4DR were prepared as if for use in BRET experiments, but were assayed for ligand-mediated cAMP accumulation instead. Using trypsin to release the cells from the growth surface had no effect on the response of the endogenous AC to isoproterenol and/or dopamine in HEK 293 cells exogenously expressing β2AR and D4DR (Supplementary Fig. 4).
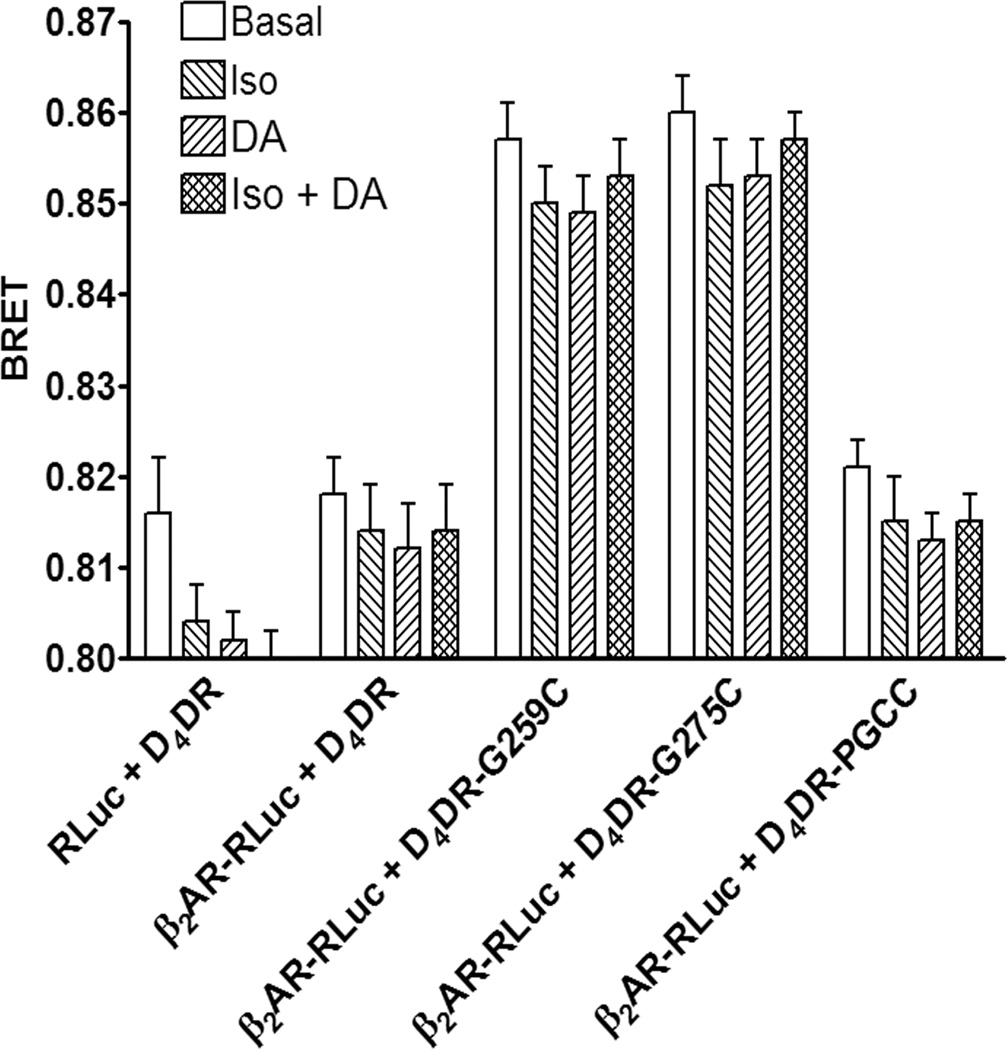
D4DR-G259C, D4DR-G275C or D4DR-PGCC was co-expressed with various RLuc-tagged signalling proteins. Although D4DR-PGCC functioned normally with respect to signal transduction, fluorescence measurements suggest that cells expressing this protein did not bind FlAsH specifically. This would explain why no BRET was observed between it and any of the RLuc-tagged signalling proteins (Fig. 3 and data not shown). Probable explanations are that binding of the FlAsH reagent was prevented because cysteines in the CCPGCC motif of this construct were oxidized to cystines or that one or more of the cysteines may have been palmitoylated [33].
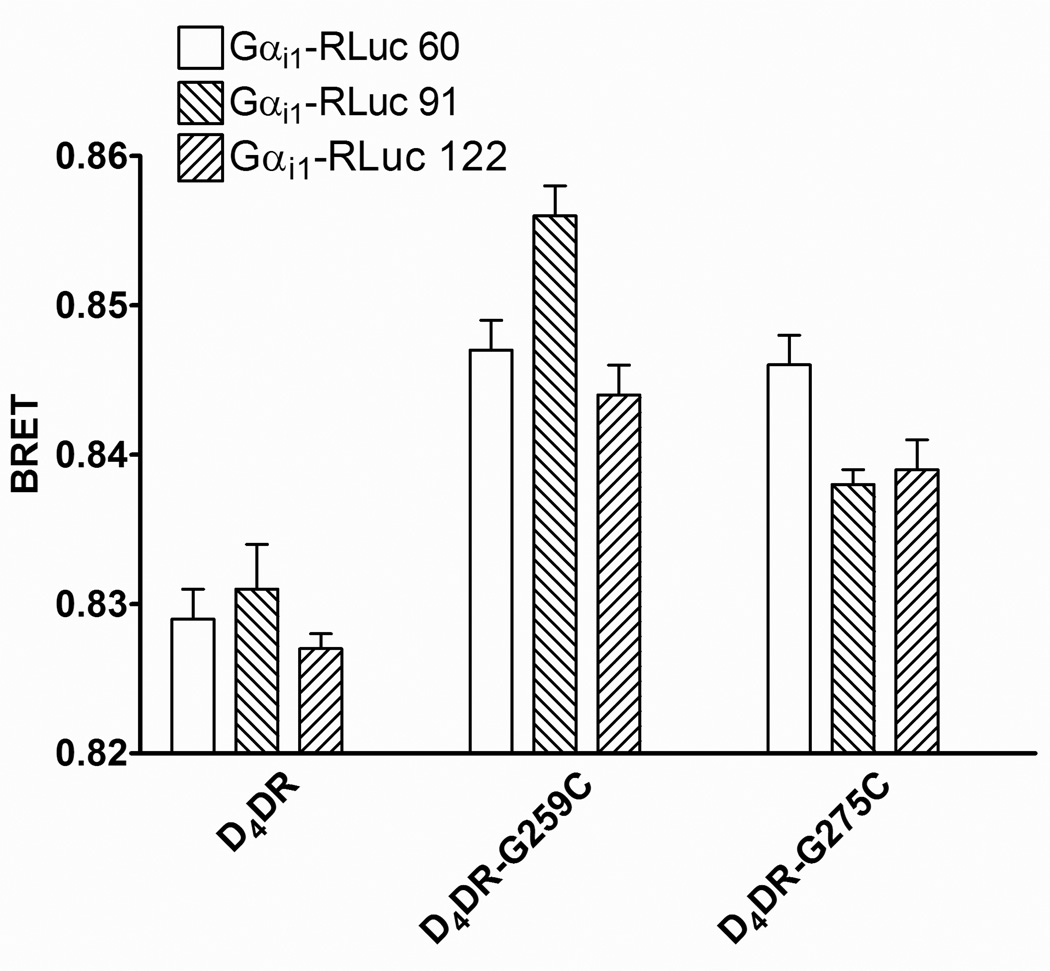
Figure 3. BRET between β2AR-RLuc and tetracysteine-tagged D4DR.
HEK 293 cells co-expressing β2AR-RLuc, Gαs, Gαi1, Gβ1, Gγ2, AC and the indicated tetracysteine-tagged D4DR were incubated with FlAsH and BRET was assayed following the addition of coelenterazine h. The effects of isoproterenol (Iso) or dopamine (DA) either individually or together on BRET were determined as described in Materials and Methods. The BRET background was determined in cells co-expressing D4DR that lacked a tetracysteine tag and either β2AR-RLuc or RLuc. There was a significant difference between the background BRET and BRET in cells expressing D4DR-G259C or D4DR-G275C (p<0.05) but not for cells expressing the D4DR-PGCC construct (p>0.05). There were no significant differences (p>0.05) between BRET in the presence and absence of agonists for a particular D4DR construct. The data are representative of five independent experiments.
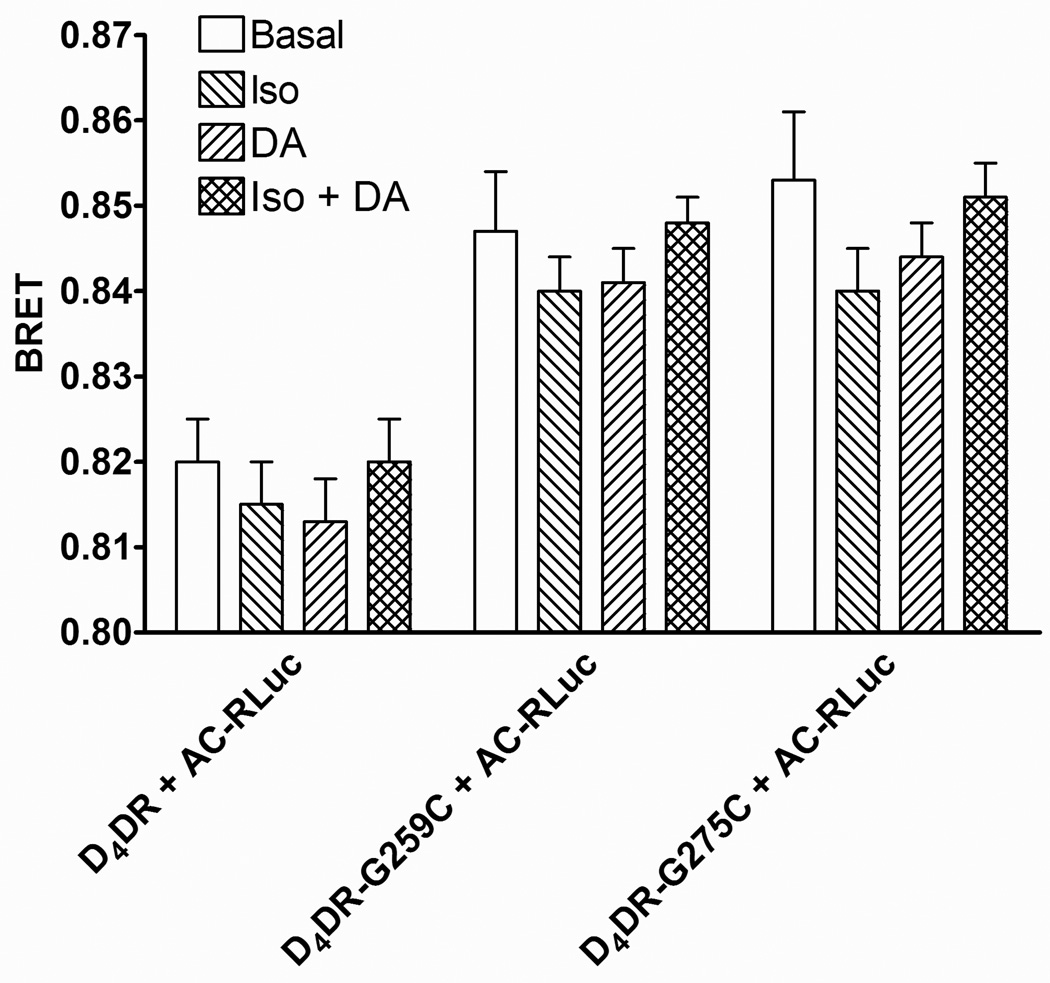
BRET was detected when β2AR-RLuc was co-expressed with either D4DR-G259C or D4DR-G275C (Fig. 3). The BRET was saturable, and no BRET was observed between D4DR-G259C and another membrane bound BRET donor, CD8-RLuc (Supplementary Fig. 5) indicating that the interaction between the receptors was specific. BRET was also observed between AC-RLuc and both tetracysteine-tagged D4DR (Fig. 4). BRET between β2AR-RLuc and tetracysteine-tagged D4DR, or between tetracysteine-tagged D4DR and AC-RLuc was not affected when cells were exposed to isoproterenol, dopamine or both agonists together, indicating that the complex persisted during signal transduction.
Figure 4. BRET between AC-RLuc and tetracysteine-tagged D4DR.
HEK 293 cells co-expressing β2AR, Gαs, Gαi1, Gβ1, Gγ2, AC-RLuc and the indicated tetracysteine-tagged D4DR were treated as described in Fig. 3. The BRET background was determined in cells co-expressing AC-RLuc and D4DR that lacked a tetracysteine tag. . There was a significant difference between the background BRET and BRET in cells expressing D4DR-G259C or D4DR-G275C (p<0.01). There were no significant differences (p>0.05) between basal BRET and BRET in the presence of isoproterenol (Iso) or dopamine (DA) for a particular D4DR construct. The data is representative of six independent experiments.
BRET also occurred between either D4DR-G259C or D4DR-G275C and Gαi1-RLuc donors (Fig. 5). The magnitude of the resonance energy transfer depended upon the position of RLuc within Gαi1 and the tetracysteine motif within the D4DR. In no case was there a significant effect of dopamine and/or isoproterenol on BRET between any combination of the tetracysteine-tagged D4DR and Gαi1-RLuc donors (data not shown).
Figure 5. BRET between Gαi1-RLuc donors and tetracysteine-tagged D4DR.
HEK 293 cells co-expressing β2AR, Gαs, Gβ1, Gγ2, AC and the indicated combination of tetracystein-tagged D4DR and Gαi1-RLuc were treated as described in Fig. 3. The BRET background was determined in cells co-expressing D4DR that lacked a tetracysteine tag. The data is representative of five independent experiments.
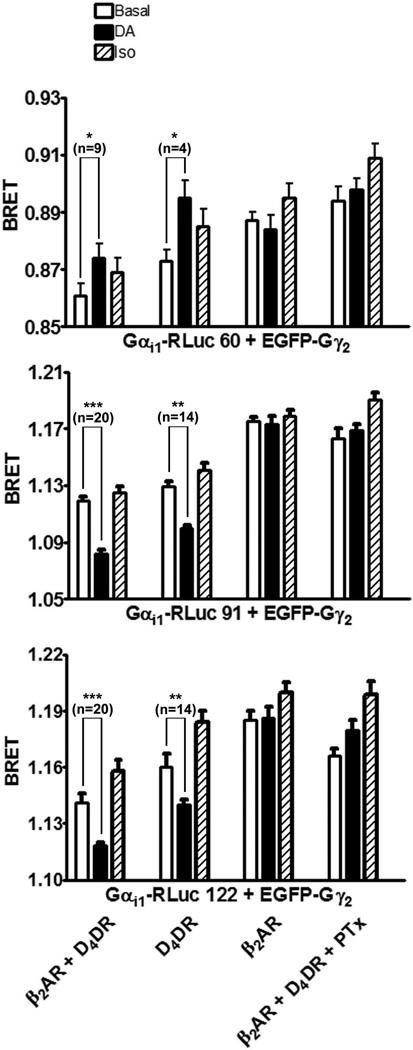
Co-expressed Gαi1-RLuc donors, EGFP-Gγ2 and Gβ1 associated to form Gi1 heterotrimers that could be detected by BRET (Fig. 6). BRET between these subunits was altered when Gi1 was activated. There was a significant increase in BRET between Gαi1-RLuc 60 and EGFP-Gγ2 when cells co-expressing the D4DR were exposed to dopamine. In contrast, dopamine caused a significant decrease in BRET when the co-expressed Gα subunit was either Gαi1-RLuc 91 or Gαi1-RLuc 122. These changes required the expression of exogenous D4DR, and they were eliminated when cells were treated with PTx before activating D4DR with dopamine. In these studies β2AR activation had no significant effect on BRET between the different Gαi1-RLuc donors and EGFP-Gγ2 even though BRET revealed that β2AR is close to Gαi1 (Fig. 7). Furthermore, BRET between β2AR-EGFP and Gαi1-RLuc donors was not affected by dopamine or isoproterenol when applied individually or together.
Figure 6. The effects of isoproterenol and dopamine on BRET between Gαi1-RLuc donors and EGFP-Gγ2.
HEK 293 cells co-expressing Gαs, Gβ1, EGFP-Gγ2, AC and the indicated combination of β2AR, D4DR and Gαi1-RLuc were assayed for BRET in the absence of ligand (Basal) or in the presence of isoproterenol (Iso) or dopamine (DA) as described in Materials and Methods. Some cells were treated with PTx for approximately 18 h before BRET was assayed. The BRET background was determined using cells expressing RLuc in place of Gαi1-RLuc and was 0.804 ± 0.009 for the experiment shown. Significant differences based on the indicated number (n) of samples from 4 to 18 independent experiments were determined with GraphPad Prism 4 software (* p<0.05, ** p<0.01, *** p<0.001).
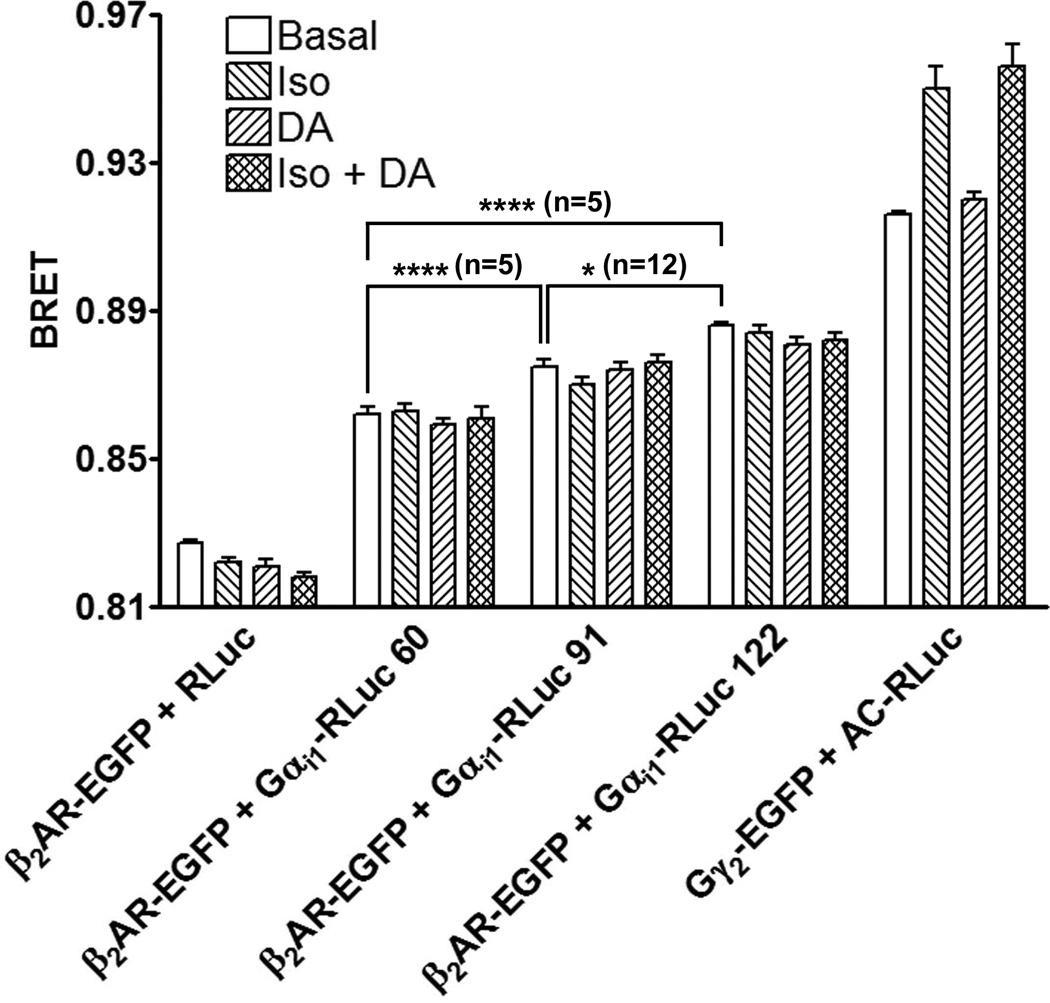
Figure 7. BRET between β2AR-EGFP and Gαi1-RLuc donors, or between heterotrimeric G proteins tagged with EGFP-Gγ2 and AC-RLuc.
HEK 293 cells co-expressing β2AR-EGFP, Gαs, Gβ1, Gγ2, AC and the indicated Gαi1-RLuc construct were assayed for BRET as described in Materials and Methods. The BRET background was determined in cells expressing RLuc in placed of Gαi1-RLuc. Cells co-expressing Gαi1, EGFP-Gγ2, and AC-RLuc in place of Gαi1-RLuc, Gγ2, and AC (experimental group on far right) were used to investigate the effects of isoproterenol (Iso) and dopamine (DA) on the interaction between heterotrimeric Gs or Gi and AC. The data are representative of five or more independent experiments depending on the RLuc-tagged construct being tested. Significant differences (* p<0.05, **** p<0.0001) are based on the indicated number (n) of experiments, and were calculated using GraphPad Prism 4 software.
Dopamine caused both regulation of AC activity (Fig. 2 and supplementary Fig. 3) and a conformational change in the Gi heterotrimer (Fig. 6) demonstrating that Gi was activated by the agonist-occupied dopamine receptor. However, Gi activation did not cause any significant change in BRET between the EGFP-tagged Gγ2 subunit of Gi1 and AC-RLuc (Fig. 7). This is in contrast to the increase in BRET between EGFP-Gγ2 and AC-RLuc following isoproterenol-mediated activation of Gs ([22] and Fig. 7) an event that could not be blocked by the dopamine-mediated activation of Gi1.
3.4 Co-purification of a Signalling Complex Containing βAR, D2-like Dopamine Receptors and AC from Mouse Brain
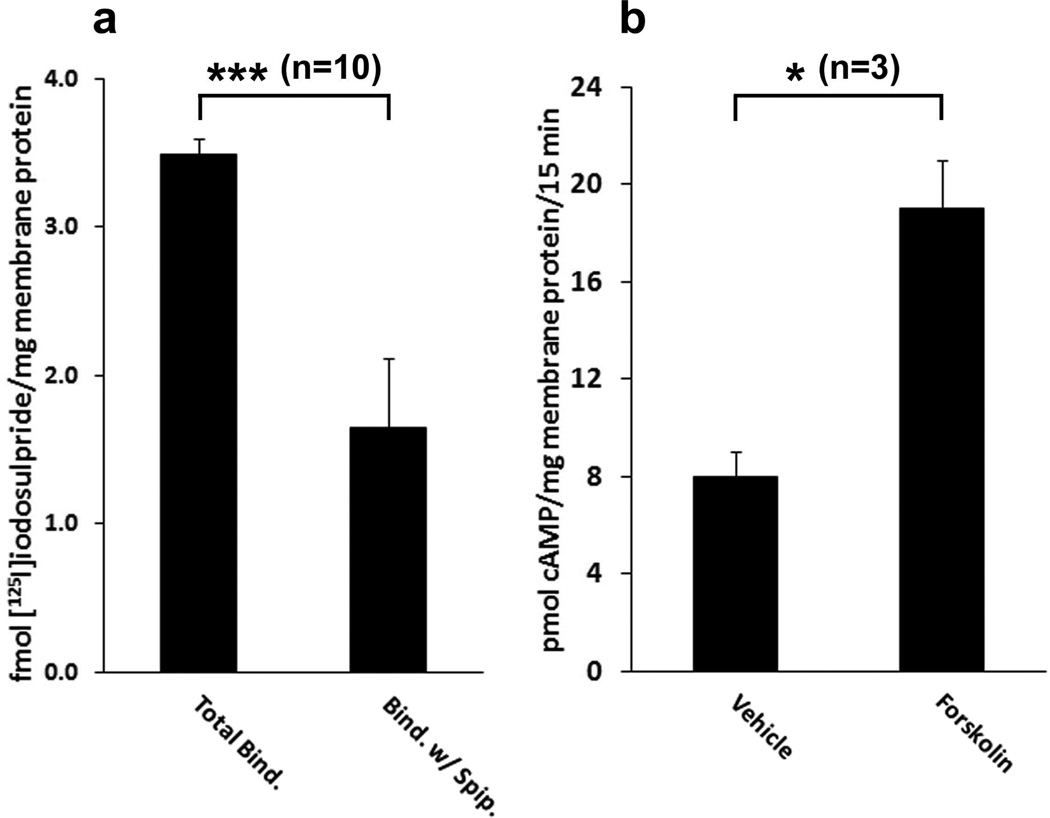
βAR and D2-like dopamine receptors present in a CHAPSO extract of mouse brain retained their ability to bind ligands. Taking advantage of this fact, these extracts were incubated with alprenolol-Sepharose in order to precipitate endogenous βAR. Alprenolol-Sepharose precipitated 19±10 fmol (n=3) of βAR from the detergent extract of one mg of mouse brain membranes which represented 55±5% (n=3) of the total βAR in the extract. The precipitate was also able to bind the dopaminergic antagonist [125I]iodosulpride (Fig. 8a). [125I]Iodosulpride binding was significantly reduced in the presence of the dopaminergic antagonist spiperone (p<0.005 n=10). Propranolol, a β-adrenergic antagonist, was unable to block binding of [125I]iodosulpride to the precipitate (P>0.05 n=9, data not shown) ruling out non-specific binding of [125I]iodosulpride to βAR. However, if propranolol was present during incubation of the CHAPSO extract with alprenolol-Sepharose, no specific [125I]iodosulpride binding could be detected in the precipitate (data not shown) indicating that the dopamine receptors were associated directly with the βAR or were part of a complex that included the βAR, and not non-specifically bound by the alprenolol-Sepharose.
Figure 8. Co-purification of D2-like dopamine receptors and AC with βAR from mouse brain.
Mouse brain membranes were dissolved in 0.5% CHAPSO and soluble material in the supernatant from a 1 h centrifugation at 100,000 × g was incubated with either Sepharose or alprenolol-Sepharose. The gel was washed to remove proteins that did not bind and assayed for the presence of dopamine receptors and AC activity. a) The presence of dopaminergic receptors bound to alprenolol-Sepharose was determined by [125I]iodosulpride binding in the presence and absence of 1 µM spiperone as described in Materials and Methods. GraphPad Prism 4 software was used to determine that spiperone significantly inhibited [125I]iodosulpride binding (*** p<0.005). b) The soluble proteins in a CHAPSO extract of mouse brain membranes were precipitated with alprenolol-Sepharose and assayed for AC activity. Forskolin (Forsk.) caused a significant (* p<0.05) increase in AC activity in precipitates. Non-specific binding of AC to the gel was determined by substituting Sepharose for alprenolol-Sepharose in the precipitation step. The data represents AC specifically bound by alprenolol-Sepharose. The statistical analyses are based on the indicated number (n) of independent experiments.
When alprenolol-Sepharose precipitates of mouse brain detergent extracts were incubated with forskolin there was an increase in the production of cAMP indicating that AC was also co-precipitated with the βAR (Fig. 8b). It is unlikely that the co-precipitated proteins were non-specifically associated with the βAR as a result of being trapped in the same detergent micelle. The reasons for this are two-fold: 1) CHAPSO has a molecular weight of 631 Da and the micelle aggregation number is 11 [34] making it unlikely that several large unassociated proteins would be present in the same micelle, and 2) the gel was washed with buffer containing 1/10th the critical micelle concentration of CHAPSO in order to disperse micelles. Furthermore, there was no forskolin-stimulated AC activity associated with the gel when the detergent extract was incubated with Sepharose instead of alprenolol-Sepharose.
4. Discussion
D4DR and βAR have a wide tissue distribution [35, 36], and the β2AR, in particular, is widely expressed [35, 37]. There is also immunohistochemical evidence that βAR and D2-like dopamine receptors are present in the same cells of the prefrontal cortex [14] raising the possibility that heteromeric complexes of these receptors exist in vivo. To determine if β2AR and D2-like dopamine receptors form complexes in cellulo, they were co-expressed in HEK 293 cells. The β2AR agonist isoproterenol increased cAMP accumulation in these cells, and dopamine attenuated this response indicating that both receptors were present on the cell surface and functionally coupled to the endogenous AC. Fluorescently tagged β2AR and D4DR co-localized in the plasma membrane and trafficked together when the cells were treated with isoproterenol suggesting that they may be associated as part of a signalling complex. A complex containing both β2AR and D4DR was immunoprecipitated from cells co-expressing them, but not when cells expressing the receptors individually were mixed prior to immunoprecipitation. The choice of detergents was critical for preserving a soluble complex containing both receptors. The complex survived when dissolved in RIPA or 0.5% CHAPSO, but not when dissolved in 0.2% Lubrol PX (data not shown). Taken together, these data suggest that the complex was not simply an artifact of dissolution with detergent.
Although co-immunoprecipitation of β2AR and D4DR indicates that they are associated with each other, the association is not necessarily by a direct protein-protein interaction. BRET experiments, on the other hand, can be used to determine if proteins interact directly. For example, BRET has been used to demonstrate that the β2AR forms a complex with Gs [21] and AC [19], and that AC forms a complex with Gs [22]. BRET has also been combined with a protein fragment complementation assay to demonstrate that all three signalling proteins, β2AR, G protein and AC, are simultaneously part of the same complex [2]. Here, BRET was used to determine if the β2AR and D4DR were in close proximity when co-expressed in HEK 293 cells. Tagging proteins for BRET usually involves fusing them to fluorescent or bioluminescent proteins with molecular masses of 27 to 36 kDa. Remarkably, the functionality of signalling proteins often remains intact after the addition of these tags, although the site of their insertion into the host protein can be critical. For example, Gβ and Gγ subunits will tolerate N-terminal EGFP or RLuc tags [38], but a C-terminal tag interferes with their function [39]. Tagging heterotrimeric G protein α subunits at either the N- or C-terminal inactivates them, but if the tags are inserted at specific locations between these termini, the subunits retain biological function [38, 40, 41]. GPCR generally tolerate a tag on their cytosolic C-termini. For example, the β2AR with a C-terminal EGFP or RLuc tag is functionally indistinguishable from the wild type receptor [23, 42]. Unfortunately, this approach of tagging the D4DR with RLuc or GFP variants rendered it nonfunctional [19]. In an attempt to circumvent this problem, a tetracysteine motif was added to the receptor that would enable it to bind the fluorophore FlAsH. This required introducing mutations that substituted three (D4DR-G259C) or four (D4DR-G275C) amino acids in the third intracellular loop, or the addition of four amino acids to the C-terminus (D4DR-PGCC). These modifications left the receptor functionally intact, and although the latter receptor was unable to bind FlAsH, both D4DR-G259C and D4DR-G275C could bind the fluorophore making them potential acceptors in BRET experiments. BRET occurred between the β2AR-RLuc and both D4DR-G259C and D4DR-G275C when FlAsH was bound to the dopamine receptor indicating that the receptors were in close proximity, and demonstrating for the first time the feasibility of using FlAsH-based fluorescence in BRET experiments. There was no effect of isoproterenol and/or dopamine on BRET suggesting that the receptor complex was stable during the time frame in which signal transduction took place.
BRET experiments also revealed that Gαi1 was closely associated with both D4DR and β2AR. The magnitude of the BRET between FlAsH bound to D4DR and the Gαi1-RLuc donors depended upon the position of the RLuc within the Gαi1 and the tetracysteine motif within the D4DR. Varying the positions of both the donor and acceptor probes within host proteins alters the distance and orientation between the probes, which in turn had a significant effect on the magnitude of BRET. For similar reasons BRET between β2AR-EGFP and the different Gαi1-RLuc donors was dependent upon the location of the RLuc within the Gαi1 subunit. The lack of any significant effect of dopamine or isoproterenol on BRET between Gαi1 and either receptor again suggests that these complexes persist during signal transduction.
Conformational changes that occur during signalling can alter the relative positions of donor and acceptor tags in BRET experiments, thereby revealing information regarding the dynamics of signalling. When Gαi1-RLuc donors were co-expressed with EGFP-Gγ2 and Gβ1, BRET occurred when the subunits associated to form heterotrimeric Gi1. Other investigators have observed conformational changes in the Gi heterotrimer triggered by the binding of isoproterenol to β2AR [20], but in these studies BRET between Gαi1-RLuc donors and EGFP-Gγ2 was not affected by isoproterenol, suggesting that the β2AR did not activate Gi1 even though they are closely associated as part of the same complex. However, BRET between Gαi1-RLuc and EGFP-Gγ2 did change in response to dopamine, but only if the cells co-expressed D4DR. PTx, which covalently modifies Gαi1 so that it can no longer be activated by the D4DR, completely blocked the ability of dopamine to regulate AC activity as well as the ability of dopamine to cause a change in BRET between Gαi1-RLuc donors and EGFP-Gγ2. Changes in the magnitude of BRET in response to dopamine were dependent upon the position of the RLuc tag within Gαi1. Similar results were obtained when Gi was activated by the α2-adrenergic receptor [20, 39]. The observation that receptor-mediated activation of Gi1 can cause either an increase or a decrease in the magnitude of the BRET between its subunits depending upon the location of the donor probe is most easily explained by the hypothesis that activation of heterotrimeric Gi1 produced a conformational change that did not cause subunit dissociation. The same conclusion has been reached regarding activated Gs [43], and cone transducin [44] using different experimental techniques. Although these data contradict the hypothesis that G protein activation is accompanied by an obligatory dissociation of the G protein α-subunit from the Gβγ heterodimer, it is consistent with evidence presented here and elsewhere [45, 46] that subunit dissociation does not normally occur when heterotrimeric G proteins are activated.
All types of mammalian AC are stimulated by Gs [47] and type II AC is also stimulated by Gi. Stimulation of type II AC by Gi is mediated by the Gβγ heterodimer [48, 49]. Furthermore, stimulation of type II AC by Gs is augmented by receptor-mediated activation of Gi [50]. In this regard activation of D4DR by dopamine has been shown to augment isoproterenol-mediated stimulation of type II AC in HEK 293 cells [27]. Data presented in this study are consistent with these findings. To determine if type II AC and D4DR interact directly, type II AC tagged with RLuc was co-expressed with either D4DR-G259C or D4DR-G275C. With FlAsH bound to the receptors, BRET was observed indicating that they formed a complex with their effector. BRET between AC and D4DR was not affected by short term exposure to dopamine or by activating the co-expressed β2AR with isoproterenol, suggesting again that this complex persisted during signal transduction.
All D2-like receptors (D2DR, D3DR and D4DR) couple to Gi, and just like D4DR, D2DR can augment Gs-mediated activation of type II AC, but curiously D3DR cannot [27]. Interpreting these results within the frame work of the G protein subunit dissociation hypothesis mentioned above led to the suggestion that Gi activation by D2DR or D4DR releases Gβγ from Gi, but that activation by D3DR does not. If this were correct and if subunit dissociation is required for regulating downstream effectors then D3DR would not be able to regulate the activity of any effectors. This is contradicted by data showing that D3DR can regulate K+ channels and mitogenesis via PTx-sensitive G proteins [51, 52]. An alternative explanation for these results is that D2DR and D4DR can regulate type II AC because they are able to form a signalling complex with the effector, while D3DR cannot. Thus, the assembly of these complexes may involve cooperative interactions between signalling proteins producing complexes that selectively include some proteins while necessarily excluding others.
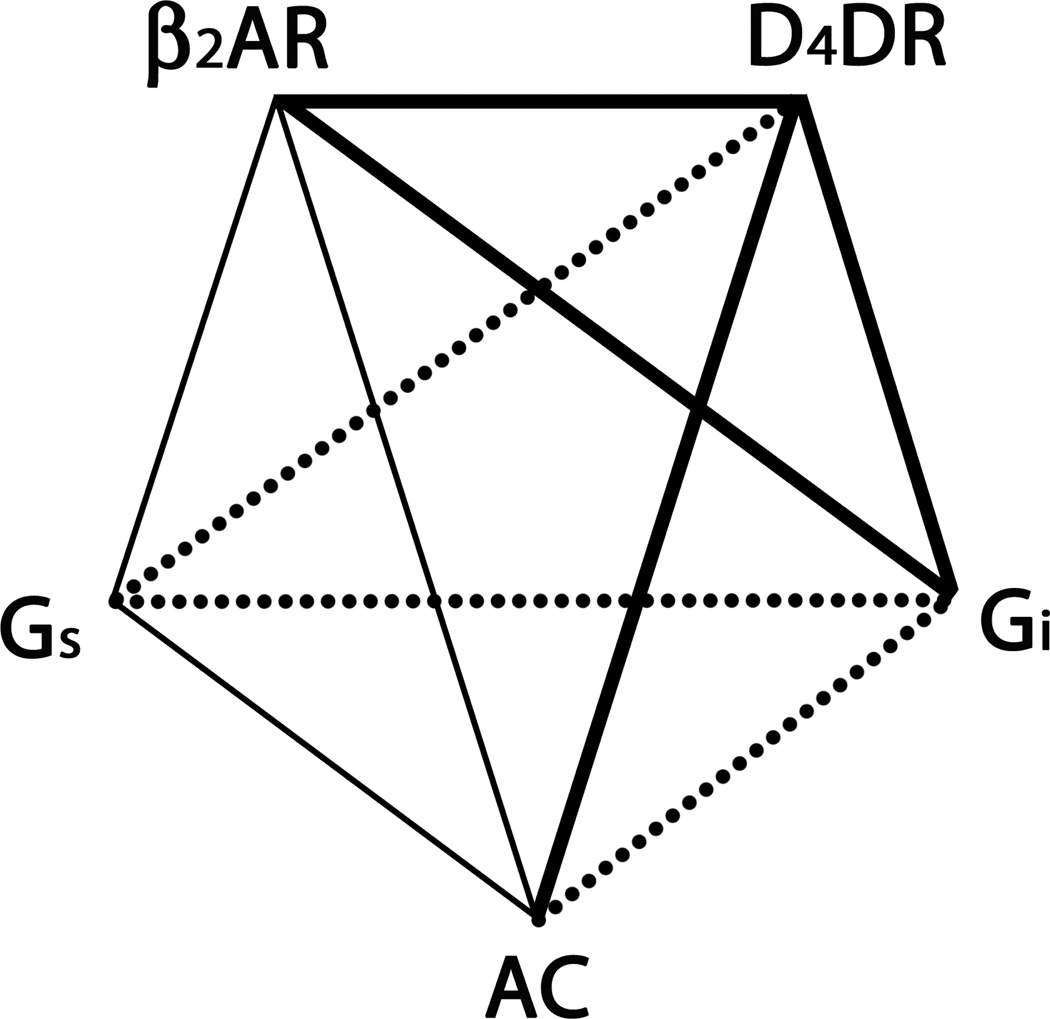
The present study provides evidence that D4DR forms a complex with β2AR, Gi and AC, and that β2AR are closely associated with Gi in cellulo. These data along with previously published data indicating that β2AR, Gs and AC associate to form a signalling complex are summarized in Fig. 9. Taken together these data support the hypothesis that a signalling complex containing all of the components needed to regulate AC activity by both Gs and Gi is present in living cells. To determine if signalling complexes containing both D2-like dopamine receptors and βAR are present in vivo, the soluble proteins from a detergent extract of mouse brain were incubated with alprenolol-Sepharose in order to precipitate the βAR. Ligand binding studies indicated that the precipitate contained D2-like dopamine receptors. In addition the precipitate contained AC that was activated by forskolin. Collectively, these data provide evidence for the existence of a signalling complex in vivo that contains at a minimum βAR, D2-like receptors and AC. Furthermore, BRET data presented here as well as evidence that Gs and Gi do not compete for binding to AC [53] are consistent with the idea that both Gs and Gi are present in the same signalling complex. Interestingly, Gs activation was accompanied by a change in BRET between its EGFP-Gγ2 subunit and AC-RLuc, but Gi activation was not. These data suggest that Gs and Gi interact differently with AC. Furthermore, activation of Gi did not prevent the change in BRET between the EGFP-Gγ2 subunit of Gs and AC-RLuc when Gs was activated, implying that Gi activation does not interfere with Gs activation. These observations are consistent with Gs and Gi regulating AC activity by an allosteric mechanism.
Figure 9. β2AR, D4DR, Gs, Gi and AC are assembled into a signalling complex responsible for integrating the regulation of AC activity by Gs and Gi.
Associations indicated by bold lines were demonstrated in this study while those designated by lighter weight solid lines were reported previously [2, 19, 21, 22]. Associations indicated by dotted lines remain to be investigated.
D4DR may play a role in attention deficit hyperactivity disorder [54] and schizophrenia [55], and aberrant signalling by dopaminergic receptors and βAR has been implicated in bipolar disorder and related diseases [56]. An understanding of how these G protein signalling systems are organized is likely to be critical for developing new and effective therapeutic treatments for these diseases. The fluorescent techniques used in these studies have been instrumental in elucidating the arrangement of signalling proteins within cells. These techniques also have the potential to reveal information about commonly used pharmaceuticals whose mechanisms of action are still poorly understood. For example, the most commonly used drugs for treating bipolar disorder (eg. carbamazepine, lithium and valproic acid) do not act as receptor binding site ligands. Although there are many hypotheses as to how these drugs work, the idea that the basis of their therapeutic effect involves modulating protein-protein interactions within these signalling complexes has not been seriously considered. This is primarily because an appreciation of the organizational complexity of these signalling systems is relatively recent. If these drugs do alter protein interactions within signalling complexes, BRET and other fluorescent techniques will be useful in ferreting out their sites of action, leading to a better understanding of their therapeutic effects, and providing a launching point for the development of new more effective drugs for the treatment of these diseases.
Highlights.
эThe fluorescent reagent FlAsH can be used as and acceptor in bioluminescence resonance energy transfer experiments. э β2-adrenergic receptors, D4-dopamine receptors, Gs, Gi and adenylyl cyclase form a functional signalling complex in cellulo. эGi activation does not cause the Giα subunit to dissociate from the Gβγ heterodimer эA signalling complex containing β-adrenergic receptors, D2-like dopamine receptors and adenylyl cyclase was isolated from mouse brain.
Acknowledgments
We thank Maya Mamarbachi for making the recombinant plasmid coding for D4DR-PGCC, and Drs. Jurgen Wess and David R. Sibley for reviewing the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Deafness and Other Communication Disorders and the National Institute of Neurological Disorders and Stroke, and by grants to TEH from the Canadian Institutes for Health Research. TEH is a Chercheur National of the Fonds de la Recherche en Santé du Québec (FRSQ).
Abbreviations
- AC
adenylyl cyclase
- βAR and β2AR
β-adrenergic and β2-adrenergic receptor(s) respectively
- BRET
bioluminescence resonance energy transfer
- D2DR, D3DR and D4DR
D2-, D3-, and D4-dopamine receptor(s) respectively
- EGFP
enhanced green fluorescent protein
- G protein
heterotrimeric guanine nucleotide binding protein
- Gi and Gs
inhibitory and stimulatory G proteins respectively
- Gαi and Gαs
α-subunits of Gi and Gs respectively
- Gβγ
heterodimeric βγ subunit complex of a G protein
- GPCR
G protein coupled-receptor
- PTx
pertussis toxin
- RLuc
R. reniformus luciferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement : None of the authors have any actual or potential conflict of interest that could inappropriately influence, or be perceived to influence, the work presented in this article.
Author Contributions:
Participated in research design: Rebois, Fishman, Hébert and Northup
Conducted experiments: Rebois, Maki and Meeks
Contributed reagents: Fishman
Performed data analysis: Rebois, Maki and Meeks
Wrote or contributed to the writing of the manuscript: Rebois, Fishman, Hébert
Other: Northup acquired funding for the research
References
- 1.Rebois RV, Hébert TE. Receptors Channels. 2003;9:169–194. [PubMed] [Google Scholar]
- 2.Rebois RV, Robitaille M, Pétrin D, Zylbergold P, Trieu P, Hébert TE. Methods. 2008;45:214–218. doi: 10.1016/j.ymeth.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Joiner MLA, Lise MF, Yuen EY, Kam AYF, Zhang MX, Hall DD, Malik ZA, Qian H, Chen YC, Ulrich JD, Burette AC, Weinberg RJ, Law PY, El-Husseini A, Yan Z, Hell JW. EMBO J. 2010;29:482–495. doi: 10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milligan G. Biochim Biophys Acta-Biomemb. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Hébert TE, Galés C, Rebois RV. Cell Biochem Biophys. 2006;45:85–110. doi: 10.1385/CBB:45:1:85. [DOI] [PubMed] [Google Scholar]
- 6.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- 8.Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fribourg M, Moreno José L, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney Scott K, Hatcher C, Eltit José M, Ruta Jeffrey D, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell Alexander D, Jr, Brezina V, Sealfon Stuart C, Filizola M, González-Maeso J, Logothetis Diomedes E. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitwieser GE. Circulation Research. 2004;94:17–27. doi: 10.1161/01.RES.0000110420.68526.19. [DOI] [PubMed] [Google Scholar]
- 11.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 12.Vidi P-A, Chemel BR, Hu C-D, Watts VJ. Mol Pharmacol. 2008;74:544–551. doi: 10.1124/mol.108.047472. [DOI] [PubMed] [Google Scholar]
- 13.Baragli A, Alturaihi H, Watt HL, Abdallah A, Kumar U. Cell Signal. 2007;19:2304–2316. doi: 10.1016/j.cellsig.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Montezinho LP, Castro MMCA, Duarte CB, Penschuck S, Geraldes CFGC, Mørk A. J Neurochem. 2006;96:1336–1348. doi: 10.1111/j.1471-4159.2005.03654.x. [DOI] [PubMed] [Google Scholar]
- 15.Caron MG, Srinivasan Y, Pitha J, Kociolek K, Lefkowitz RJ. J Biol Chem. 1979;254:2923–2927. [PubMed] [Google Scholar]
- 16.Oak JN, Oldenhof J, Van Tol HHM. Eur J Pharmacol. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- 17.Kazmi MA, Snyder LA, Cypess AM, Graber SG, Sakmar TP. Biochemistry. 2000;39:3734–3744. doi: 10.1021/bi992354c. [DOI] [PubMed] [Google Scholar]
- 18.Watts VJ, Vu MN, Wiens BL, Jovanovic V, Van Tol HHM, Neve KA. Psycopharmacology. 1999;141:83–92. doi: 10.1007/s002130050810. [DOI] [PubMed] [Google Scholar]
- 19.Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hébert TE, Van Tol HHM. J Biol Chem. 2002;277:46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 20.Galés C, Van Durm JJJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 21.Galés C, Rebois RV, Hogue M, Trieu P, Breit A, Hébert TE, Bouvier M. Nat Methods. 2005;2:177–184. doi: 10.1038/nmeth743. [DOI] [PubMed] [Google Scholar]
- 22.Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 23.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Proc Natl Acad Sci USA. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salahpour A, Bonin H, Bhalla S, Petäjä-Repo U, Bouvier M. Biol Chem. 2003;384:117–123. doi: 10.1515/BC.2003.012. [DOI] [PubMed] [Google Scholar]
- 25.Hellevuo K, Yoshimura M, Kao M, Hoffman PL, Cooper DMF, Tabakoff B. Biochem Biophys Res Commun. 1993;192:311–318. doi: 10.1006/bbrc.1993.1415. [DOI] [PubMed] [Google Scholar]
- 26.Premont RT. In: Methods Enzymol. Ravi I, editor. Vol. Volume 238. Academic Press; 1994. pp. 116–127. [Google Scholar]
- 27.Watts VJ, Neve KA. Mol Pharmacol. 1997;52:181–186. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]
- 28.Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. J Am Chem Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann C, Gaietta G, Bünemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga J-P, Tsien RY, Ellisman MH, Lohse MJ. Nat Methods. 2005;2:171–176. doi: 10.1038/nmeth742. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi J, Takarada T, Yunoki S, Kikuchi Y, Maeda M. Biochem Biophys Res Commun. 2006;343:1191–1196. doi: 10.1016/j.bbrc.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 31.Maier-Peuschel M, Frölich N, Dees C, Hommers LG, Hoffmann C, Nikolaev VO, Lohse MJ. J Biol Chem. 2010;285:8793–8800. doi: 10.1074/jbc.M109.098517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin BA, Adams SR, Jones J, Tsien RY. In: Methods Enzymol. Jeremy Thorner SDE, John NA, editors. Vol. Volume 327. Academic Press; 2000. pp. 565–578. [DOI] [PubMed] [Google Scholar]
- 33.Chini B, Parenti M. Journal of Molecular Endocrinology. 2009;42:371–379. doi: 10.1677/JME-08-0114. [DOI] [PubMed] [Google Scholar]
- 34.George M, van de Rijn I. The Journal of Immunology. 1988;140:2008–2015. [PubMed] [Google Scholar]
- 35.Sano M, Yoshimasa T, Yagura T, Yamamoto I. Life Sci. 1993;52:1063–1070. doi: 10.1016/0024-3205(93)90199-d. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Kobayashi K, Nagatsu T. Neurosci. Lett. 1995;199:69–72. doi: 10.1016/0304-3940(95)12021-u. [DOI] [PubMed] [Google Scholar]
- 37.Booze RM, Crisostomo EA, Davis JN. J Pharmaacol Exp Ther. 1989;249:911–920. [PubMed] [Google Scholar]
- 38.Janetopoulos C, Jin T, Devreotes P. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 39.Bünemann M, Frank M, Lohse MJ. Proc Natl Acad Sci USA. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes TE, Zhang H, Logothetis DE, Berlot CH. J Biol Chem. 2001;276:4227–4235. doi: 10.1074/jbc.M007608200. [DOI] [PubMed] [Google Scholar]
- 41.Yu J-Z, Rasenick MM. Mol Pharmacol. 2002;61:352–359. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- 42.Barak LS, Ferguson SSG, Zhang J, Martenson C, Meyer T, Caron MG. Mol Pharmacol. 1997;51:177–184. doi: 10.1124/mol.51.2.177. [DOI] [PubMed] [Google Scholar]
- 43.Ganpat MM, Nishimura M, Toyoshige M, Okuya S, Pointer RH, Rebois RV. Cell Signal. 2000;12:113–122. doi: 10.1016/s0898-6568(99)00078-9. [DOI] [PubMed] [Google Scholar]
- 44.Rosenzweig DH, Nair KS, Wei J, Wang Q, Garwin G, Saari JC, Chen C-K, Smrcka AV, Swaroop A, Lem J, Hurley JB, Slepak VZ. J Neurosci. 2007;27:5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levitzki A, Klein S. ChemBioChem. 2002;3:815–818. doi: 10.1002/1439-7633(20020902)3:9<815::AID-CBIC815>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 46.Rebois RV, Warner DR, Basi NS. Cell Signal. 1997;9:141–151. doi: 10.1016/s0898-6568(96)00133-7. [DOI] [PubMed] [Google Scholar]
- 47.Hanoune J, Defer N. Annual Review of Pharmacology and Toxicology. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 48.Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, Vogel Z. J Biol Chem. 1998;273:2273–2276. doi: 10.1074/jbc.273.4.2273. [DOI] [PubMed] [Google Scholar]
- 49.Taussig R, Quarmby LM, Gilman AG. J Biol Chem. 1993;268:9–12. [PubMed] [Google Scholar]
- 50.Lustig KD, Conklin BR, Herzmark P, Taussig R, Bourne HR. J Biol Chem. 1993;268:13900–13905. [PubMed] [Google Scholar]
- 51.Chio CL, Lajiness ME, Huff RM. Mol Pharmacol. 1994;45:51–60. [PubMed] [Google Scholar]
- 52.Werner P, Hussy N, Buell G, Jones KA, North RA. Mol Pharmacol. 1996;49:656–661. [PubMed] [Google Scholar]
- 53.Sprang SR, Chen Z, Du X. Advances in Protein Chemistry. Vol. Volume 74. Academic Press; 2007. pp. 1–65. [DOI] [PubMed] [Google Scholar]
- 54.Tarazi FI, Zhang K, Baldessarini RJ. J Recept Signal Transduct. 2004;24:131–147. doi: 10.1081/rrs-200032076. [DOI] [PubMed] [Google Scholar]
- 55.Sharma A, Kramer ML, Wick PF, Liu D, Chari S, Shim S, Tan W, Ouellette D, Nagata M, DuRand CJ, Kotb M, Deth RC. Mol Psychiatry. 1999;4:235–246. doi: 10.1038/sj.mp.4000522. [DOI] [PubMed] [Google Scholar]
- 56.Newberg AR, Catapano LA, Zarate CA, Manji HK. Expert Rev Neurother. 2008;8:93–110. doi: 10.1586/14737175.8.1.93. [DOI] [PubMed] [Google Scholar]