Modern human society is obsessed with sex, but even a cursory glance at a natural history documentary should convince anyone that this obsession is not limited to humans. Sex is everywhere in the living world, and its consequences for almost every aspect of life, from morphology to behaviour, are profound. Given the ubiquity of sex, it is easy to forget that it is not necessary for reproduction. Indeed, there are some organisms that reproduce perfectly well without bothering with sex at all. However, the vast majority of eukaryotic species do have sex [1].
Sometimes sex is occasional, such as in the malaria parasite Plasmodium falciparum, which reproduces asexually for many generations within a host, only resorting to sex when picked up by its mosquito vector. In other species, including our own, sex and reproduction are intimately linked: the latter cannot occur without the former. However, uncovering the evolutionary forces that produced and maintain this widespread characteristic of life has proven difficult, leading one evolutionary biologist to refer to understanding sex as “the Queen of problems in evolutionary biology” [1]. In this essay, I outline why the widespread existence of sex presents a problem for evolutionary biologists and examine where the solutions to this problem might be found.
Given the ubiquity of sex, it is easy to forget that it is not necessary for reproduction
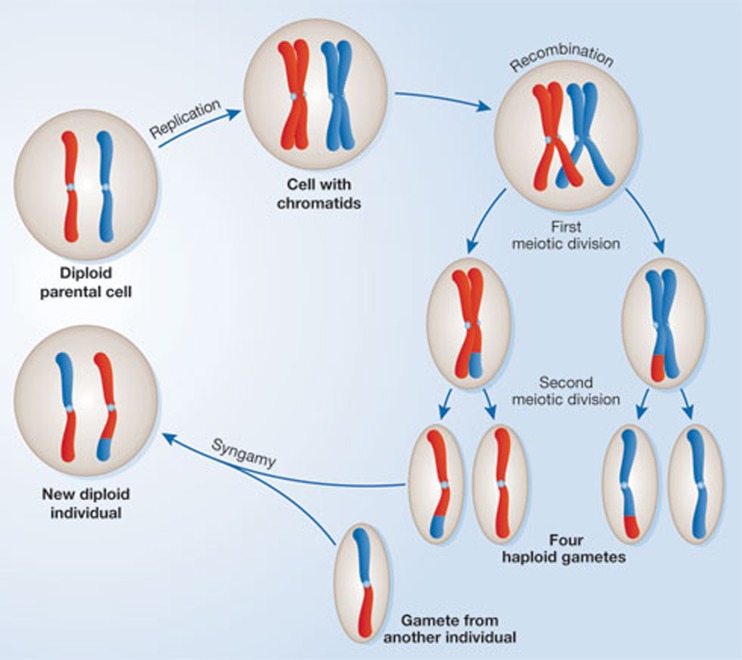
Sex means different things to different people, and so it is important to be clear about what we are trying to explain. In broad terms, sex can be viewed as any process that brings together and mixes the genetic material from different individuals into a new, single individual. By this definition, sex includes the processes of genetic exchange observed in bacteria and viruses—so-called ‘parasexual’ events—as well as the more familiar, and more elaborate, sexual cycle observed in eukaryotes [2]. However, for the purpose of this essay, when I refer to sex, I mean the eukaryotic sexual cycle (Fig 1). The reason for this is simple: whilst the outcomes of these genetic processes might be similar—and similar selective forces might even explain their early origins—the selective forces maintaining eukaryotic sex are probably fundamentally different to those that maintain the varieties of parasex in prokaryotes [3].
Figure 1.
The core aspects of sex in eukaryotes. For simplicity, the figure shows a hypothetical organism in which the whole genome is carried in a single chromosome. The sexual cycle starts with a diploid cell that contains two different copies of the genome on a pair of homologous chromosomes. Each chromosome is first replicated to produce two genetically identical chromatids. The chromosomes then line up and exchange genetic material through recombination, producing chromatids that contain a mix of genetic material from both chromosomes. A two-stage meiotic division then leads the production of haploid gametes, each containing a single chromatid—half of the genetic material of the original diploid cell. Completion of the sexual cycle requires that diploidy is restored through the fusion of two gametes, usually from two different individuals.
The key elements of the eukaryotic sexual cycle are outlined in Fig 1. Eukaryotic sex involves an alternation between haploidy and diploidy, coupled with a shuffling of genetic material. There are many variations on this general theme. Many microbial species, termed ‘isogamous’, produce gametes that are morphologically equivalent. Despite the lack of morphological distinction, the gametes usually exist in two or more mating types and fusion can only occur between gametes of different mating types. Beyond the microbial world, sexual organisms are typically anisogamous: they produce two types of morphologically distinct gamete. One type—by convention the male—is generally small and motile, whereas the other is large and stuffed with nutrients. Variation can also be seen in the ‘standard’ ploidy of certain species: some spend most of their life cycle in the diploid stage, whereas others are generally haploid, only briefly becoming diploid immediately after gamete fusion. Despite these variations on a theme, however, the core elements of the sexual cycle are remarkably conserved across eukaryotes from algae to elephants.
The ubiquity of sex, coupled with the conservation of its central elements across a diversity of organisms, suggests that the selective forces responsible must be both strong and pervasive. It is therefore surprising that finding a convincing explanation for the evolutionary success of sex has proven to be one of the most difficult challenges for modern evolutionary biologists. The question is: why?
Success in evolutionary terms is ultimately judged by an individual's success in passing on genes to future generations. The simple problem with sex, from an evolutionary perspective, is that it is an extremely inefficient way of achieving this end [4]; there are several costs associated with the sexual cycle. First, there are obvious direct costs. Unless you are a self-fertile hermaphrodite, for example, it is necessary to find a mate with whom to exchange genes. This might consume considerable energy, which could otherwise be diverted to reproducing directly; for species in which sex is an obligate part of the reproductive process, failure to find a mate leads to failure to reproduce. Similarly, for species in which one sex competes for access to the other sex, considerable efforts and energies are diverted to such competition: an asexual peacock would need no elaborate tail. Even at the cellular level, the sexual cycle requires additional time and energy, as meiotic cell division takes considerably longer than simple mitosis. This time could be devoted to other purposes if sex were avoided.
It is […] surprising that finding a convincing explanation for the evolutionary success of sex has proven to be one of the most difficult challenges for modern evolutionary biologists
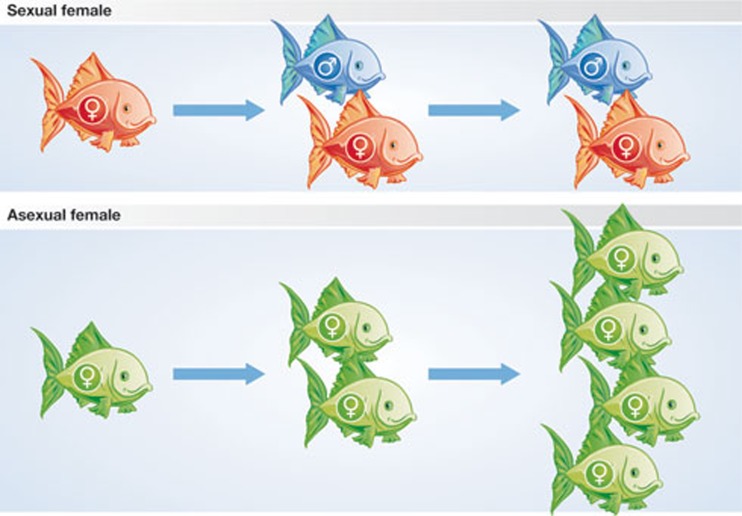
A less obvious cost to sex that occurs in anisogamous organisms has been termed ‘the cost of males’, or as evolutionary biologist John Maynard Smith put it, the two-fold cost of sex [4]. To understand this concept, consider a hypothetical species of obligate sexual fish (Fig 2). In this species, a female produces exactly two offspring in her lifetime. All things being equal, we expect her to invest equally in male and female offspring, so one of the offspring will be male and the other female. Let us assume that the brother and sister pair up to produce the next generation—the logic applies equally well if we do not assume this, but it is easier to follow if we do. Similarly to her mother, the daughter will produce two offspring during her lifetime, and so our original female has produced two grandchildren. Imagine a mutant female that is identical to the first, except that she produces offspring asexually, which are her clones. This asexual female will also produce two offspring, but they will both be female and, unlike the offspring of her sexual cousin, they will both be able to reproduce directly, providing the original asexual female with four granddaughters (Fig 2). It is easy to see how such a demographic advantage can quickly lead to the asexual lineage replacing its sexual ancestor. Put simply, a sexual female wastes up to half of her reproductive resources producing males, which do not reproduce directly themselves.
Figure 2.
The cost of males. The number of offspring of a hypothetical sexual species of fish and an asexual clone derived from it are shown. By not investing in male offspring, the asexual clone can double in frequency when rare.
There is a further potential genetic cost of recombination in that it potentially breaks up successful combinations of genes, leading to what has been termed ‘recombination load’ [4]. Genes do not generally act independently, and an individual that has survived to reproductive age has demonstrated not only that it has good genes, but that its good genes work well in combination. An asexual organism can pass on their successful genotype intact, whereas a sexual individual risks producing less successful gene combinations by mixing their genes with those of another. Unlike the cost of males, recombination load is a problem for any sexual organism.
Thus, we have a conundrum: sex is actually a costly process that ought to be lost quickly from populations. Yet, most eukaryotes are sexual. In that case, what selective benefits does sex provide that outweigh these significant costs?
The basis of the sexual cycle was present in the common ancestor of all extant eukaryotic lineages. Indeed the appearance of sex pre-dates the diversification of the eukaryotes themselves, leading to suggestions that the initial spread of sex might be an incidental consequence of the success of eukaryotes, for reasons other than their sexuality [5]. So what benefits could sex have provided to their ancient sexual ancestor?
The benefits of syngamy—the fusion of gametes to form a zygote—might be relatively easy to understand. Combining the two genomes of different parents allows for genetic complementation [4]. Essentially, syngamy compensates for the effects of deleterious genes in one genome by providing a functioning copy of the gene from the other genome. However, coming up with plausible explanations for the selective forces that led from here to the full sexual cycle complete with meiosis and recombination is less straightforward. One proposal is that meiosis provides a general way for a cell to repair DNA damage; another is that the benefits of meiosis derive from the genetic diversity that it creates among offspring. Both of these hypotheses have also been invoked to explain the maintenance of sex in extant species. It is worth remembering that the original sexual species would have been isogamous, and so without a two-fold cost of sex. Thus, in principle, the selective benefits required for the origin of sex might be far smaller than those required to explain its maintenance in species with distinct sexes.
An intriguing alternative hypothesis is that sex might have evolved as a parasitic adaptation among selfish genetic elements to allow them to spread to other genetic lineages [6]. An analogous process can be observed in bacteria such as Escherichia coli, in which genetic exchange between cells is induced and controlled by an extrachromosomal plasmid, and seems to have evolved as a mechanism for the plasmid to move through a bacterial population.
Unfortunately, the unique origin of sex, coupled with the fact that it occurred in the distant past under selective conditions about which we can only guess, and which might have changed dramatically since, makes testing theories for the evolutionary origins of sex extremely problematic. Ultimately, we might be limited to plausible stories and might never have a conclusive answer to why sex evolved in the first place.
Regardless of whether we need a selective explanation for the initial spread of sex across the tree of life, we do require one for its continued maintenance against significant evolutionary costs. Furthermore, the selective forces maintaining sex must still be operating, and operating in a diversity of species. This gives evolutionary biologists some hope of observing these forces in action, as well as independent systems in which to test directly different hypotheses. The past 50 years has seen considerable amounts of research dedicated to elucidating these selective forces.
One possibility is that sex is simply a mechanism for repairing DNA damage, in particular double-stranded DNA damage. This view has been championed by Harris Bernstein from the University of Arizona, USA, and others [7], and is in some ways a compelling idea. DNA damage is a problem for all organisms, and so selection based on repair would have the kind of universality required to explain the widespread nature of sex. Moreover, many of the enzymes involved in recombination do have roles in DNA repair and probably did evolve initially for that function, only later being co-opted for sex [4]. However, the argument is problematic on several levels [2]. Most obviously, there are organisms that never have sex, but do not apparently suffer from catastrophic DNA damage. Thus, my view—and I think it is also the view of most evolutionary biologists—is that the answer to the prevalence of sex is not repair, even if this was in part involved in its origin.
Ultimately, we might be limited to plausible stories and might never have a conclusive answer to why sex evolved in the first place
The main consequence of sex is that genetic material from two individuals is mixed together into a single individual, leading to the production of offspring that are genetically distinct from either parent. It is to this production of new genetic combinations that many evolutionary biologists have turned in search of an evolutionary advantage to sex. This has led to the conventional wisdom, even present in high-school textbook dogma, that the main function of sex is to increase genetic variability and consequently increase the rate at which a sexual species or population can evolve. This greater variability of sexual species would allow them to persist in the face of environmental change and competition with other species, and ultimately to diversify. By contrast, populations that give up sex are doomed to a short evolutionary lifespan and an early extinction. This logic gained further support from the fact that asexual groups seem to be generally found only at the tips of the tree of life, suggesting that they have a relatively short evolutionary lifespan [4].
This simple argument is faced with at least two substantial problems. The first is that, despite appearances, the genetic mixing that results from sex is not guaranteed to increase the heritable genetic variation for fitness, which is the ultimate determinant of the rate of adaptation [8]. Indeed, initial attempts to model the process showed that whilst sex could increase the efficiency of selection, the conditions required for it to do so were by no means universal, often requiring strong selection or high mutation rates and specific types of interaction between genes affecting fitness. Even under conditions where sex was beneficial, the benefits were rarely substantial enough to outweigh the two-fold costs of producing males. Perhaps most importantly, empirical work did not provide much evidence that the stringent conditions required by these models for sex to be beneficial were generally met in real organisms.
The second problem is that the process, as described above, is one of group selection; sex, it is argued, is beneficial because sexual groups are more evolutionarily successful—they have a longer evolutionary lifespan—than asexual groups [4]. Evolutionary biologists have developed a deep distrust of arguments based on group advantage, on the grounds that, despite being possible in theory, the conditions for it to operate are incredibly restrictive [9]. To understand why, consider the two forces acting on sexual reproduction. The group-selected advantage operates over a geological timescale, because changes in the frequency of sex depend on differential rates of extinction and speciation. By contrast, the benefits to an asexual mutant within a population operate much faster, as it is based on the differential birth and death rates of individuals. All things being equal, the replacement of a sexual species by a derived asexual population would be essentially instantaneous compared with the rate of group selection. Ultimately, if sex is to be maintained by this process, for every sexual population that gives up sex, a corresponding asexual population must become extinct. Unless mutations producing viable asexual mutants in sexual populations are incredibly rare—of the order of the rate of extinction of asexual populations—group selection cannot maintain sex [4]. Although I argue later that evolving asexuality might actually be difficult for some organisms, species in which sexual and asexual individuals coexist within the same populations, or in which individuals are facultatively sexual, pose extreme problems for explanations based on such long-term benefits of sex.
Essentially, sex produces higher quality genotypes, and the genes for sex hitchhike to high frequency on the back of the high-fitness genotypes that they create
Faced with these problems, evolutionary biologists were left in the awkward position of lacking a solid theoretical basis to explain one of the most widespread phenomena in nature [1, 4, 9]. Some looked for other selective explanations; for example, whether cyclical fluctuations in the environment, perhaps caused by co-evolving parasites [10], could lead to situations in which it would be selectively beneficial for offspring to be genetically different from their parents. However, these models also showed that sex is only substantially beneficial under limited and extreme conditions, which do not generally appear in nature [8].
Yet, work has shown that these problems might be more hypothetical than real. The original models made simplifying and often unrealistic assumptions about natural populations [8]. For example, most assumed that populations were infinite and well mixed, whereas most actual populations are relatively small and structured. Incorporating additional realism into the models has broadened considerably the range of conditions under which sex is predicted to be beneficial. Moreover, the original models typically examined a single evolutionary process at a time, for example the purging of deleterious mutations, or the bringing together of beneficial mutations. This was done in part for practical reasons, but also because there was a feeling that the ubiquity of sex would require a single explanation. However, new models that incorporate multiple selective processes operating simultaneously predict far more substantial benefits of sex than do those that model the effects independently [11]. These new models also show clearly that the benefits of sex can apply at the level of the gene and do not require the invocation of group or species selection [2, 8]. Essentially, sex produces higher quality genotypes, and the genes for sex hitchhike to high frequency on the back of the high-fitness genotypes that they create.
In addition to these new theoretical insights, work in experimental microbial systems has begun to examine directly the evolutionary consequences of sex. The overwhelming conclusion of this work is that sex can provide benefits in real organisms as well as in theory. For example, work from my own lab shows that populations of Chlamydomonas, a facultatively sexual single-celled alga, adapt more rapidly to new environments when they are allowed to go through occasional sexual cycles [12]. Similar patterns have been observed in other microbes.
Finally, people have begun to look again at the costs of sex, and there has been an increasing acceptance that the importance of some costs might have been overstated. The two-fold cost associated with the production of males, for example, assumes that mutations can produce ‘perfect asexuals’—organisms that produce only asexual female offspring but are otherwise identical to their sexual ancestor. In fact, once a species has been sexual for a period of time, subsequent evolutionary changes might actually make rapid reversion to cost-free asexuality extremely difficult [13]. Asexual mutants are often difficult to produce in the lab, and when they can be produced, they are often extremely sick. A process of chromosomal imprinting in mammals means that unless an individual receives chromosomes from two parents, development fails. This represents an obvious constraint to the loss of sex in this group, and similar situations might well exist in other taxa.
In a complex world in which environments are constantly changing […] the differences produced by the sexual cycle provide an important evolutionary advantage
In taxa where sex has been lost, for example, some of the vestiges of sex remain. Some whiptail lizard populations consist entirely of asexual females, but these females still have to mate, despite having lost the need for sex, as the physical act of copulation is required to stimulate egg production. Such populations achieve this end by stealing mates from the males of neighbouring sexual whiptail populations, and it means that not all of the costs associated with sex in this species have been lost [4]. Other costs might be reduced by the careful timing of sex. In many microbes, sex occurs in situations in which conditions for population growth are limited and population density is high, such as the end of the growing season. This timing might considerably reduce the opportunity costs of the time-consuming sexual cycle, as well as the costs of finding a mate [14]. Thus, smaller benefits might often be required to maintain sex in many species than has generally been assumed.
Thus, it seems that the original intuition of evolutionary biologists was correct after all: the evolutionary success of sex is down to the diversity that it creates [8, 14]. In a complex world in which environments are constantly changing, competitors, parasites and prey are constantly evolving and mutation is continually eroding adaptation, the differences produced by the sexual cycle provide an important evolutionary advantage. This advantage favours genes for sex and recombination within populations, and can also have profound implications for the evolutionary lifespan of populations and species. Still, some problems remain to be solved. In general, it seems that even occasional sex is sufficient for providing most of the associated evolutionary benefits discussed above, so the important question of why animals such as us have adopted sex as an obligate part of reproduction remains to be answered. Similarly, our understanding of how different selective forces that act on differences in species biology and ecology lead to patterns in the phylogenetic and geographical distribution of sex is still at its early stages. Finally, the favoured theories suppose that the loss of sex leads to a short evolutionary lifespan for a lineage. Thus, explaining the persistence of ‘ancient asexuals’, such as the bdelloid rotifers that apparently gave up sex more than 80 million years ago, presents a challenge [15]. Despite these outstanding issues, it seems that the ‘Queen of problems’ might be close to abdication.
Science & Society Series on Sex and Science.
Sex is the greatest invention of all time: not only has sexual reproduction facilitated the evolution of higher life forms, it has had a profound influence on human history, culture and society. This series explores our attempts to understand the influence of sex in the natural world, and the biological, medical and cultural aspects of sexual reproduction, gender and sexual pleasure.

Nick Colegrave
Footnotes
The author declares that he has no conflict of interest.
References
- Bell G (1982) The Masterpiece of Nature. The Evolution and Genetics of Sexuality. London, UK: Croom Helm Bernstein [Google Scholar]
- Barton NH, Charlesworth B (1998) Why sex and recombination? Science 281: 1986–1990 [PubMed] [Google Scholar]
- Redfield RJ (2001) Do bacteria have sex? Nat Rev Genet 2: 634–639 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J (1978) The Evolution of Sex. Cambridge, UK: Cambridge University Press [Google Scholar]
- Spiegel FW (2011) Commentary on the chastity of amoebae: re-evaluating evidence for sex in amoeboid organisms. Proc Biol Sci 278: 2096–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey DH, Rose MR (1988) The role of gene transfer in the evolution of eukaryotic sex. In The Evolution of Sex: An Examination of Current Ideas (eds Michod R, Levin BR), pp161–175. Sunderland, Massachusetts, USA: Sinauer Associates [Google Scholar]
- Bernstein H, Hopf FA, Michod RE (1988) Is meiotic recombination an adaptation for repairing DNA, producing genetic variation, or both? In The Evolution of Sex: An Examination of Current Ideas (eds Michod R, Levin BR), pp 139–169. Sunderland, Massachusetts, USA: Sinauer Associates [Google Scholar]
- Otto SP (2009) The evolutionary enigma of sex. Am Nat 174: S1–S14 [DOI] [PubMed] [Google Scholar]
- Williams GC (1975) Sex and Evolution. Princeton, New Jersey, USA: Princeton University Press [Google Scholar]
- Hamilton WD (1980) Sex versus non-sex versus parasite. Oikos 35: 282–290 [Google Scholar]
- West SA, Lively CM, Read AF (1999) A pluralist approach to sex and recombination. J Evol Biol 12: 1003–1012 [Google Scholar]
- Colegrave N (2002) Sex releases the speed limit on evolution. Nature 420: 664–666 [DOI] [PubMed] [Google Scholar]
- Engelstadter J (2008) Constraints on the evolution of asexual reproduction. Bioessays 30: 1138–1150 [DOI] [PubMed] [Google Scholar]
- Burt A (2000) Perspective: sex recombination, and the efficacy of selection—was Weismann right? Evolution 54: 337–351 [DOI] [PubMed] [Google Scholar]
- Butlin (2002) The costs and benefits of sex: new insights from old asexual lineages. Nat Rev Genet 3: 312–317 [DOI] [PubMed] [Google Scholar]