EMBO Reports (2012) 13: 9, 855–860. doi:; DOI: 10.1038/embor.2012.100
The endoplasmic reticulum (ER) is essential for the synthesis and folding of proteins that transport through the secretory pathway. ER stress is implicated in several diseases, including cancer, neurodegeneration, diabetes and inflammatory conditions. To cope with stress, cells activate a complex signal transduction pathway known as the unfolded protein response (UPR), which integrates information about the intensity and duration of the stress, to adapt and recover ER homeostasis or trigger apoptosis of irreversibly damaged cells [1]. However, beyond individual cells, the UPR is important in maintaining the homeostasis of full organs, including the liver, pancreas and salivary glands. Recent studies from the Aballay group—including one published in this issue of EMBO reports [2]—show that the UPR is under cell-nonautonomous control by the nervous system to facilitate the organismal response to stress.
The most conserved UPR signalling branch is initiated by the ER stress sensor inositol-requiring enzyme 1 (IRE1), a type I ER protein with kinase and RNase activities. Upon activation, IRE1 processes the mRNA encoding the transcription factor X-box binding protein 1 (XBP1), splicing out a 26-nucleotide intron. This shifts the reading frame of the mRNA, leading to the expression of a stable and active transcription factor. XBP1 upregulates a subset of UPR target genes involved in protein folding and quality control, thereby attenuating stress levels [1].
IRE1 also has an intrinsic ability to detect and bind to misfolded proteins directly through its ER luminal domain, indicating a cell-autonomous mechanism to detect the stress. Although low (sublethal) levels of ER stress are observed in many physiological processes related to high secretory activity, uncontrolled ER stress is detrimental to cell function and survival [1]. Therefore, a coordinated UPR response between individual cells at the organ level is necessary to maintain homeostasis.
Many diseases—such as bowel disease, atherosclerosis and diabetes—are associated with the co-activation of ER stress and inflammatory responses. Studies indicate that ER stress could amplify inflammatory reactions [3]. For example, in macrophages, specific UPR signalling modules—such as IRE1/XBP1—are downstream targets of Toll-like receptors, enhancing the production of proinflammatory cytokines. An ancient association between ER stress and inflammation might exist, as phylogenetic studies suggest that UPR stress sensors are evolutionarily related to regulators of innate immunity [3]. Studies in Caenorhabditis elegans also highlight the conserved role of the IRE1/XBP1 pathway in innate immunity [4]. Infection with Pseudomonas aeruginosa results in an innate immune response mediated by the mitogen-activated kinase PMK-1/p38 and the downstream activation of the IRE1 pathway. However, the biological source of ER stress in this infection model is unknown.
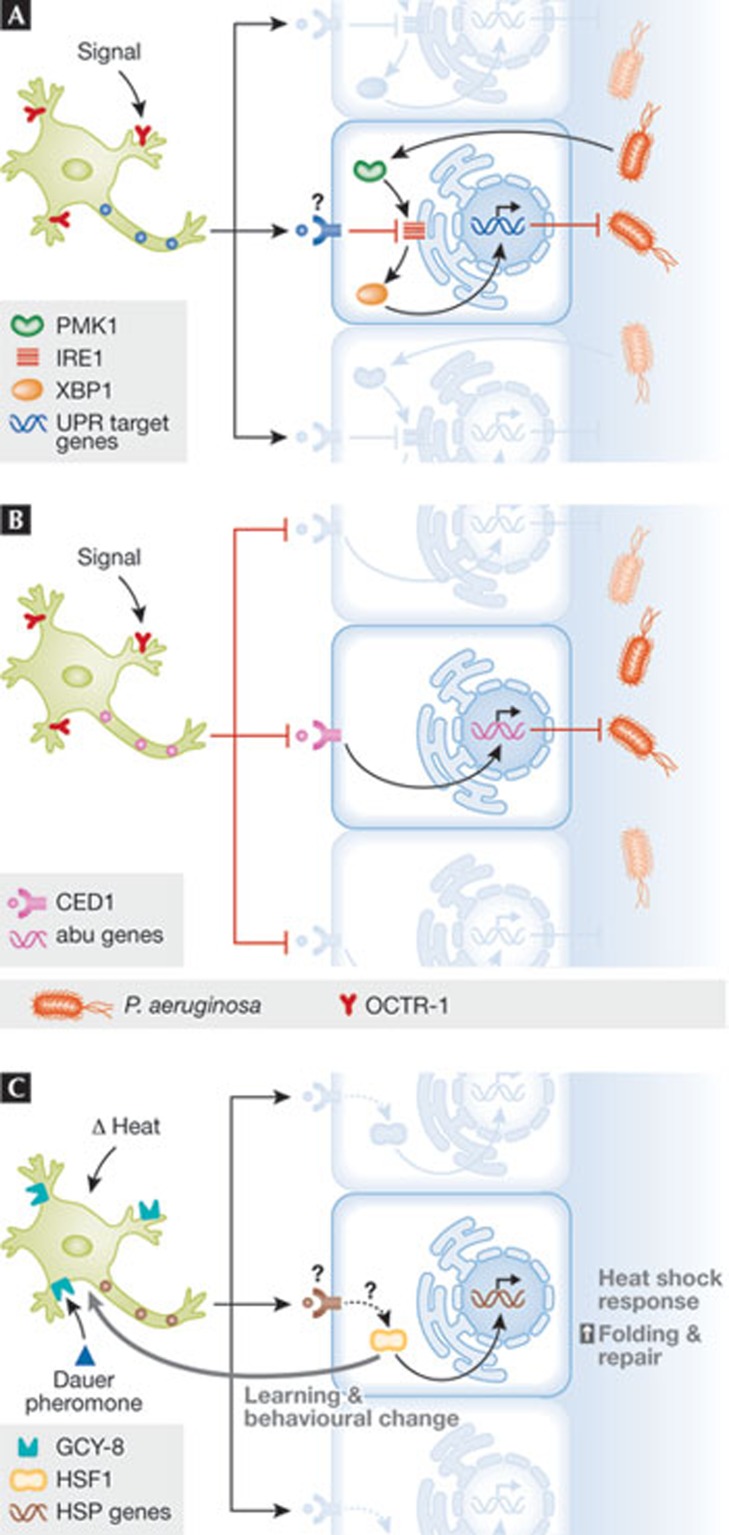
Insight into the involvement of the UPR in innate immunity was provided by the Aballay group through studies in C. elegans [2,5,6]. They initially identified a neuronal circuit that governs innate immunity against bacterial infections through the expression of NPR-1 in neurons, a G-protein-coupled receptor (GPCR; [5]). They then showed that another GPCR expressed in sensory neurons—termed OCTR-1—actively suppresses innate immunity by downregulating the activity of a non-canonical UPR pathway in non-neuronal cells during development [6]. OCTR-1-deficient worms were more resistant to death caused by P. aeruginosa, involving the downstream activation of PMK-1. This pathway selectively affected innate immunity and not pathogen avoidance behaviour, and did not engage the XBP1–UPR pathway [6]. Neuronal signalling suppressed a family of genes classified as abu (activated in blocked UPR; Fig 1B), which were initially identified as genes activated in xbp-1 mutant worms exposed to pharmacological ER stress. Most abu genes are expressed in the intestine and pharynx, which are primary sites for exposure to bacterial pathogens.
Figure 1.
Cell-nonautonomous control of the UPR and HSR. (A) Organismal control of UPR responses in peripheral tissue by the nervous system in adult Caenorhabditis elegans. The activity of OCTR-1, expressed in sensory neurons, represses the IRE1/XBP1 pathway, modulating innate immunity. PMK-1 might also modulate this pathway. (B) A related pathway also operates to control innate immunity during C. elegans development, involving the direct regulation of a non-canonical UPR response (abu genes). This pathway might be regulated by the phagocytic receptor CED-1, and does not engage a classical UPR response. (C) The HSR is negatively regulated by thermosensory neurons though GCY-8 and is modulated by the dauer pheromone. A feedback loop from peripheral tissue to sensory neurons modulates behavioural responses against fluctuations in body temperature. CED-1, cell death abnormal 1; GCY-8, guanylyl cyclase 8; HSR, heat shock response; IRE1, inositol-requiring enzyme 1; OCTR-1, octopamine receptor 1; PMK-1, mitogen-activated kinase 1; UPR, unfolded protein response; XBP1, X-box binding protein 1.
In contrast to the situation during C. elegans development, the Aballay group now shows that OCTR-1-expressing sensory neurons also regulate the classical IRE1/XBP1 pathway at the young adult stage, and this is an important response mechanism to bacterial infections [2]. OCTR-1 deficiency protects animals against P. aeruginosa, possibly due to the higher protein folding capacity provided by increased XBP1 expression [2]. Whether the production of cytokine-like molecules is modulated by XBP1 in this setting remains to be determined. This study provides the first evidence for a cell-nonautonomous control of the UPR by the nervous system, in which OCTR-1 signalling modulates disturbances in ER-protein homeostasis in the intestine. The authors speculate that the ability of the nervous system to respond rapidly to environmental changes, or pathogens, might help integrate information to maintain immune homeostasis at the organismal level through the regulation of the UPR and antimicrobial mechanisms. Whether this pathway operates in mammals is unknown. Interestingly, the propagation of ER stress responses from cell to cell through inflammatory signals has been proposed in cancer models [7].
The idea that the fine-tuning of proteostasis can be controlled by cell-nonautonomous mechanisms has also been proposed in the context of the heat shock response (HSR). The HSR is an ancient adaptive stress pathway governing the transcription of several chaperones of the heat shock protein family. Rick Morimoto's group showed that C. elegans' thermosensory neurons, which underlie temperature-dependent behaviour, control both the magnitude and the time-course of HSR gene expression in non-neuronal cells mediated by the GPCR protein GCY-8, thereby orchestrating full organism thermotolerance [8]. These neurons integrate temperature and environmental signals to control thermotaxis behaviour, modulating growth and metabolism through the dauer pheromone. Remarkably, systemic control of the HSR by thermosensory neurons is also dependent on the dauer pheromone. Because thermosensory neurons do not directly innervate tissues where the HSR is affected, the authors proposed that a neuroendocrine signal might facilitate this regulatory circuit of organismal-stress control. Morimoto's group then compared the cell-nonautonomous control of the HSR under mild and transient heat shock to chronic exposure, and to protein folding stress generated by the expression of mutant proteins related to neurodegeneration [9]. Unexpectedly, acute exposure to heat shock—which triggers a transient shift in protein homeostasis—is not recognized and decoded by sensory neurons in the same manner as prolonged exposures to stress [9]. Furthermore, a feedback loop also exists in the opposite direction, whereby the HSR in peripheral tissue can modulate the activity of the thermosensory neurons, influencing learning and behavioural changes (Fig 1C; [10]). Together, these exciting studies suggest the existence of a highly orchestrated, global stress response that combines and integrates the perception of environmental changes through the nervous system, and fine-tunes protein homeostasis networks in the affected tissue.
These advances in the field add a new layer of complexity to our understanding of how protein homeostasis is regulated in multicellular organisms. The integration of local cell-autonomous responses—intrinsic alterations in cell function—with a high-ordered control by the nervous system suggests an evolutionary mechanism by which homeostatic shifts in complex tissues on stress are coordinated. Due to the rapid and propagative nature of neuronal responses, the cell-nonautonomous mechanisms that control the UPR and HSR could transmit a ‘danger signal’ to pathogen-exposed tissues and pre-adapt them to a coming injury or environmental challenge. Sensory neurons can detect some pathogen antigens and measure subtle fluctuations in temperature, pressure and other external stimuli. Thus, neuronal signals might serve to predict further changes and avoid subsequent irreversible damage to organ homeostasis in non-neuronal tissues. In agreement with this, the function of XBP1 in B-cell differentiation has been shown to be independent of the protein folding stress triggered by immunoglobulin synthesis, pre-adapting the differentiating cell to future demands in protein synthesis [1]. Cell-nonautonomous control of proteostasis therefore emerges as an important concept to understand the adaptability of organisms to homeostatic fluctuations, in which the nervous system is fundamental to cope with cellular proteotoxicity.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hetz C (2012) Nat Rev Mol Cell Biol 13: 89–102 [DOI] [PubMed] [Google Scholar]
- Sun J, Liu Y, Aballay A (2012) EMBO Rep (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Glimcher LH (2010) Curr Opin Immunol 23: 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CE, Kooistra T, Kim DH (2010) Nature 463: 1092–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer KL et al. (2008) Science 322: 460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J et al. (2011) Science 332: 729–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan NR et al. (2011) Proc Natl Acad Sci USA 108: 6561–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Cornelius T, Morimoto RI (2008) Science 320: 811–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Morimoto RI (2011) Proc Natl Acad Sci USA 108: 14204–14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T, Nishida Y, Mori I (2011) Nat Neurosci 14: 984–992 [DOI] [PubMed] [Google Scholar]