Abstract
The association of vascular reactivity between diabetes and periodontal disease has not been clarified. Gingival blood flow was measured by laser Doppler flowmetry for 31 weeks in Wistar rats, Wistar rats orally challenged with Porphyromonas gingivalis (Wistar rats + Porphyromonas gingivalis), Goto-Kakizaki rats, and Goto-Kakizaki rats orally challenged with Porphyromonas gingivalis (Goto-Kakizaki rats + Porphyromonas gingivalis). Effects of alveolar bone resorption on periodontal tissue was enhanced in Wistar rats + Porphyromonas gingivalis, and Goto-Kakizaki rats, with this effect being significantly enhanced by Goto-Kakizaki rats + Porphyromonas gingivalis. Using the L-band electron spin resonance technique, we succeeded in measuring oxidative stress as decay rate constant (K1 and K2) of 3-carbamoyl-2,2,5,5-tetramethylpyrrolidin-1-yloxy in the oral and maxillofacial region of the animal models. The decay rate constant (K1) of 3-carbamoyl-2,2,5,5-tetramethylpyrrolidin-1-yloxy was significantly greater in the oral and maxillofacial region of Goto-Kakizaki rats + Porphyromonas gingivalis compared to Wistar rats, Wistar rats + Porphyromonas gingivalis and Goto-Kakizaki rats groups. Gingival reactive hyperemia was attenuated by periodontal disease, and this effect was also remarkable in the diabetes mellitus model. Taken together, we found that vascular endothelial function was decreased in diabetes mellitus and/or periodontal disease animal models due to increasing oxidative stress in the gingival circulation.
Keywords: gingival circulation, oxidative stress, L-band ESR, diabetes mellitus, periodontitis
Introduction
The importance of oxidative stress induced by reactive oxygen species (ROS) on inflammatory pathways in the pathogenesis of type 2 diabetes and periodontitis has recently received attention.(1) Oxidative stress occurs when the production of ROS exceeds the capacity of the cell to detoxify these potentially injurious oxidants using endogenous antioxidant defense systems. Conditions associated with oxidative stress include ischemia/reperfusion, hypercholesterolemia, diabetes, and hypertension. The adhesion of circulating blood cells (leukocytes, platelets) to vascular endothelium is a key element in the pro-inflammatory and prothrombogenic phenotype assumed by the vasculature in these and other disease states that are associated with oxidative stress. There is a growing body of evidence that links the blood cell-endothelial cell interactions in these conditions to the enhanced production of ROS.(2) That an increase in insulin resistance due to diabetes mellitus causes a reduction of the nitric oxide (NO) production by endothelium dysfunction and arterial sclerosis has been demonstrated.(3,4) In research on the correlation of diabetes with periodontitis, epidemiologic studies are largely performed, but little has been reported using a basic approach to clarifying the relationship between these conditions, especially from the aspects of oxidative stress and vascular reactions. Therefore, the mechanism of why diabetes aggravates periodontal disease is still unclear.
We previously developed an electron spin resonance (ESR)-based technique for the detection of free radical reactions, including ROS, in biological systems.(5–11) Nitroxyl radicals are very useful as exogenous spin probes for measuring free radical distribution, oxygen concentration, and redox metabolism by in vivo ESR in biological systems.(5,9–12) It has been reported that nitroxyl radicals, referred to as ’nitroxyl spin probes’, lose their paramagnetism through a redox reaction when exposed to a reducing agent in biological systems.(13,14) The signal decay rate of the nitroxyl spin probe provides evidence of ROS generation and changes in redox status in biological systems.(15,16) However, no other study has specifically measured the signal decay rate as oxidative stress in experimental models of ROS-induced oxidative stress in the oral region.
This study was performed based on periodontal vascular events in a diabetic animal model, during which oxidative stress on the oral circulation occurred. We determined that gingival reactive hyperemia reflected vascular endothelial function through analysis of reactive hyperemia of the gingival tissue in animal models of diabetes mellitus and/or periodontitis with vascular disease. We succeeded through the use of an ESR in vivo technique and laser Doppler flowmetry (LDF) in evaluating gingival reactive hyperemia and found that vascular endothelial function was decreased in diabetes mellitus and/or periodontal disease animal models due to increasing oxidative stress in the gingival circulation.
Materials and Methods
Animals
In this study, Wistar rats (Ws) were used as control animals and Goto-Kakizaki rats (GKs) were used as a type 2 diabetes mellitus model. For both groups, 4-week-old male animals were purchased from CLEA Japan, Inc. (Tokyo, Japan). The procedures used in this study were in accordance with the guidelines of the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication NO. 85–23, revised 1985). Also, Institutional Animal Care Committees approved our protocols.
Animals were housed in groups of 5 per cage in a room maintained under standardized light (12:12 h light-dark cycle) and temperature (22 ± 3°C) conditions with free access to food pellets and tap water. The animals were divided into 4 groups: Group 1, Porphyromonas gingivalis (Pg)-non-infected Ws; Group 2, Ws orally challenged with Pg (Ws + Pg); Group 3, Pg-non-infected GKs, and Group 4, GKs orally challenged with Pg (GKs + Pg). Each group was comprised of 5 rats.
Bacteria and culture conditions
The bacterial strain used was Pg American Type Culture Collection (ATCC) 33277. Pg strains were grown at 37°C for 18 h in an anaerobic chamber (Anaerobox, Hirasawa, Tokyo, Japan) with an atmosphere of 85% N2, 10% H2, and 5% CO2 in brain heart infusion broth (Difco, Detroit, MI) supplemented with 5 mg/mL yeast extract, 5 µg/mL hemin, and 0.2 µg/mL vitamin K1.
Porphyromonas gingivalis infection and alveolar bone loss in rats
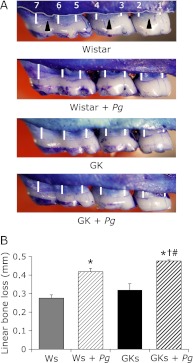
Rats were given sulfamethoxazole (1 mg/mL) and trimethoprim (200 µg/mL) in drinking water for 7 days ad libitum to reduce the original oral flora, followed by a 3-day antibiotic-free period before Pg infection. Each rat received 0.5 mL (1.5 × 109 cells per mL) Pg ATCC 33277 suspended in 5% carboxymethylcellulose (Sigma Chemical, St. Louis, MO) by oral gavage 3 times/week at the ages of 5, 6, 17, and 32 weeks. One aspect of periodontal disease is the resorption of alveolar bone. After sacrifice at the age of 33 weeks, the upper jaws were defleshed after 10 min in an autoclave at 15 pounds/inch2 and immersed in 3% hydrogen peroxide, rinsed, air dried, and stained with 1% methylene blue. Horizontal bone loss around the maxillary molars was assessed morphometrically. The distance from the cement-enamel junction (Fig. 1, white line) to the alveolar bone crest (Fig. 1, arrowhead) was measured at 7 buccal sites per rat. Measurements were made under a dissecting microscope (×40) fitted with a digital high-definition system (Digital HD microscope VH-7000, KEYENCE, Osaka, Japan) standardized to give measurements in millimeters.(17)
Fig. 1.
Measurement of bone loss in an upper jaw from 4 animals in each group. Morphometric bone levels in 33-week-old rats (A). The distance from the cement enamel junction (arrowhead in A) to the alveolar bone crest (white line in A) was measured at 7 buccal sites per rat. (B) Data are presented as mean ± SEM. (n = 4 in each group). *p<0.05 vs Ws. †p<0.05 vs GKs. #p<0.05 vs Ws + Pg.
In vivo ESR measurement
Ws and GKs were anesthetized with pentobarbital (45 mg/kg, i.p.) at the age of 11 weeks. We then analyzed the oral and maxillofacial region of Ws or GKs with in vivo L-band ESR analysis as described previously.(5,6–11,18) The ESR spectra from Ws and GKs were obtained with an L-band ESR spectrometer (JES-RE-3L; JEOL, Akishima, Japan) equipped with a 4 window loop-gap resonator under the following conditions: microwave power, 10 mW; magnetic field, 50.0 ± 10 mT; field modulation width, 0.1 mT; receiver gain, 79–320; sweep time, 0.5 min; and time constant, 0.03 s. All experiments were repeated a minimum of 4 times.
Gingival blood flow
Animals were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and were subsequently given small maintenance doses as necessary. After body weights were determined, A tail vein was tapped with 25 gauge needle, and a glucose levels was determined by a FreeStyle Freedom Glucose Meter (Nipro, Osaka, Japan). Gingival blood flow (GBF) was measured at the palatal gingiva by a laser Doppler flowmeter (TBF-LN1, Unique Medical Co. Ltd., Tokyo, Japan) with a laser Doppler probe (diameter 2.0 mm). Heart rate was monitored to determine the effect on systemic hemodynamics by agents administered or gingival reactive hyperemia. To evaluate vascular endothelial and smooth muscle functions, 100 mg/mL acethylcholine (ACh) or 5 mg/mL nitroglycerine (NTG) was topically absorbed from an area of gingival mucosa approximately 2 mm in diameter for 1 min before gingival compression for 1 min. Reactive hyperemia was elicited at the same place as ACh and NTG application by release of occlusive gingival compression by the laser Doppler probe for 1 min. The reactive hyperemia in the gingival circulation was estimated by basal blood flow (Basal), maximum response (Peak), time taken for the maximum response to fall to one half (T1/2), and increased total amount of blood flow (Mass).(19) The output signals from the flowmeter were recorded on a computer hard disc through an A/D converter and displayed simultaneously on the monitor. Recorded GBF was analyzed using data analysis software (Chart ver. 4.2 AD Instruments, Inc., Colorado Springs, CO). These GBF measurements were performed twice a month on the rats when they were from 7 to 31 weeks old. The animals were returned to the same cage after GBF measurements.
Statistical analysis
Analysis of variance and multiple comparison tests using the Tukey’s method were applied to determine differences among the 4 groups. When comparing only one pair, we used the Student’s unpaired t test. Data are expressed as the mean ± SEM.
Results
Effects of alveolar bone resorption on periodontal tissue
Plasma glucose levels of Ws and GKs were determined once every 2 weeks from 7 to 31 weeks after birth. The plasma glucose levels were significantly decreased during the experimental period in the Ws groups (Ws, 113.6 ± 6.8 mg/dL to 101.3 ± 3.5 mg/dL; Ws + Pg, 113.6 ± 4.6 mg/dL to 103.0 ± 7.5 mg/dL), while the plasma glucose levels in the GKs groups were elevated during the experimental period (GKs, 281.6 ± 14.7 mg/dL to 315.3 ± 14.8 mg/dL; GKs + Pg, 283.4 ± 14.4 mg/dL to 365.0 ± 16.5 mg/dL).
Effects of alveolar bone resorption on periodontal tissue was enhanced in Ws and GKs orally challenged with Pg, with this effect being significantly enhanced by Pg infection in GKs (Fig. 1).
Evaluation of oxidative stress in the oral and maxillofacial region in diabetes and/or periodontal disease models
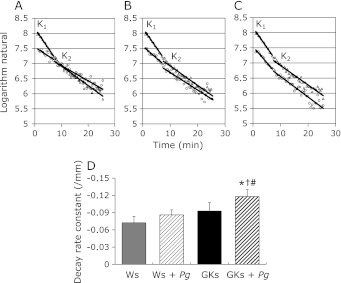
The 3-carbamoyl-2,2,5,5-tetramethylpyrrolidin-1-yloxy (C-PROXYL) is a suitable spin probe for the study of free radical reactions in the rodent skin by in vivo ESR detection.(11,18,20) These methods were also applied for the evaluation of oxidative stress of the oral and maxillofacial region in Ws, Ws + Pg, GKs and GKs + Pg. In the experiments with the oral and maxillofacial region of Ws, Ws + Pg, GKs and GKs + Pg after i.v. treatment with C-PROXYL, we initially assessed the distribution of C-PROXYL using the L-band ESR technique. With this method, we succeeded in measuring oxidative stress as decay rate constant (K1 and K2) of C-PROXYL in the oral and maxillofacial region of the animal models (Fig. 2 A–C). The decay rate constant (K1) of C-PROXYL was significantly greater in the oral and maxillofacial region of GKs + Pg compared to the Ws, Ws + Pg and GKs groups (Fig. 2D). These results suggested that increased oxidative stress occurs in the oral and maxillofacial region of the periodontal disease and/or diabetes rat models.
Fig. 2.
Assessment of oxidative stress in the oral and maxillofacial region on 10-week-old Ws, Ws + Pg, GKs and GKs + Pg. All rats were anesthetized with pentobarbital (45 mg/kg, i.p.). L-band ESR was used to determine the signal decay of C-PROXYL in the oral and maxillofacial region of all rats. Decay rate constants of (K1 and K2) C-PROXYL in the oral and maxillofacial region of 10-week-old Ws (circles in A), Ws + Pg (circles in B), GKs (circles in C) and GKs + Pg (triangles in A–C). The columns represent: K1 of C-PROXYL in the oral and maxillofacial region of 10-week-old Ws, Ws + Pg, GKs and GKs + Pg (D). Results are expressed as mean ± SD in all groups (n = 4 in each group). *p<0.05 vs Ws. †p<0.05 vs GKs. #p<0.05 vs Ws + Pg.
Evaluation of vascular responses using gingival reactive hyperemia in the diabetes and/or periodontal disease model
In experiments using non-infected Ws, compression of gingival tissue for 1 min resulted in an immediate decrease in GBF. One minute after release of gingival tissue compression, reactive hyperemia was observed in the gingiva. Peak, T1/2, and Mass of GBF were increased by gingival compression for 1 min. This effect was significantly enhanced by topical oral mucosal administration of ACh and NTG. Cardiac function was not influenced by gingival reactive hyperemia or pharmacological reagents (data not shown). These results suggest that gingival reactive hyperemia could be measured as a vascular endothelial response that did not have an effect on the systemic circulation.
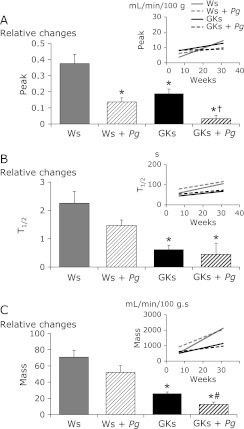
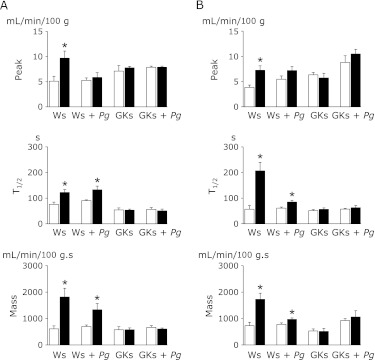
In Ws that were orally challenged with Pg there was a remarkable reduction in gingival reactive hyperemia throughout the experimental period, which was approximately 6 months. The reduction of each parameter analyzed in Pg-challenged GKs was significant, while only the reduction in Peak was significant in Ws. Pg-challenged rats, although reductions were noted in the other two parameters analyzed (Fig. 3). As shown in Fig. 4, Peak, T1/2 and Mass were all significantly increased in Ws and Ws + Pg compared to GKs and GKs + Pg after administration of ACh (A) or NTG (B) on palatal mucosa, suggesting that the effect of ACh and NTG was reduced by Pg infection of Ws. Application of ACh and NTG on the palatal mucosa did not affect reactive hyperemia in the GKs and GKs + Pg groups, indicating that the gingival circulation as shown by the gingival vascular response to ACh and NTG was reduced in GKs and GKs + Pg compared to Ws and Ws + Pg.
Fig. 3.
Alterations in gingival reactive hyperemia in Ws, Ws + Pg, GKs and GKs + Pg groups from 7 to 31 weeks old. Peak (A), T1/2 (B) and Mass (C) in gingival reactive hyperemia were plotted against time. Inserted figures represent regression lines fitted to a typical plot of laser Doppler flowmeter data obtained from Ws, Ws + Pg, GKs and GKs + Pg. The columns show slope of parameters against time. Data are presented as mean ± SEM. (n = 5 in each group). *p<0.05 vs Ws. †p<0.05 vs GKs. #p<0.05 vs Ws + Pg.
Fig. 4.
Evaluation of vascular endothelial and smooth muscle function in 10-week-old Ws, Ws + Pg, GKs and GKs + Pg. Alterations in Peak, T1/2 and Mass of gingival reactive hyperemia before (open columns) and after (solid columns) treatment with ACh (A) and NTG (B). Data are presented as mean ± SEM. (n = 5 in each group). *p<0.05 vs pretreatment with ACh or NTG.
Discussion
Periodontitis is a common, chronic inflammatory disease initiated by bacteria and has increased prevalence and severity in patients with type 2 diabetes.(1) Current evidence indicated that there is a bidirectional interrelationship between diabetes and inflammatory periodontitis. The findings that chronic periodontitis is associated with plasma TNF-α levels in subjects with type 2 diabetes support the hypothesis that periodontal infection and inflammation may contribute to insulin resistance.(21) Elevated TNF-α was observed in non-obese diabetes patients.(22) Numerous studies have long recognized that periodontitis is more prevalent in diabetic patients and worsens with the development of diabetes;(23) periodontal disease has been called as the sixth complication of diabetes.(24) A previous epidemiological survey showed that incident tooth loss caused by periodontal disease was significantly associated with peripheral arterial disease.(25) This indicates that vascular reactivity consisting of endothelial and smooth muscle functions is suppressed in the presence of circulatory failure such as in periodontal disease and diabetes. Oral challenge with Pg in Ws and GKs enhanced alveolar bone resorption, with the effect significantly enhanced in GKs (Fig. 1).
In vivo L-band ESR spectroscopy with an ESR imaging system is a highly useful technique for the non-invasive investigation of oxidative stress induced by ROS in living organisms. Several previous studies have demonstrated that quantitative ESR analysis using the spin probe C-PROXYL can be used to investigate redox status under conditions of oxidative stress in the skin of the maxillofacial region in mice.(10,11,18,20) The present study utilized in vivo L-band ESR to evaluate oxidative stress induced by Pg infection or diabetes in the oral and maxillofacial region of the rat. These results firstly showed that the ESR technique employed herein could be a powerful tool to selectively detect free radicals, including oxidative stress, and to monitor free radical reactions in vivo in the oral and maxillofacial region in the rat model (Fig. 2). We showed that the decay rate constant (K1) of C-PROXYL was significantly greater in the oral and maxillofacial region of GKs + Pg compared to Ws, Ws + Pg, and GKs (Fig. 2D). In a previous study using L-band ESR analysis, we reported that the metabolism of lipophilic nitroxyl spin probes in the brain can be divided into phase I (vascular system) and phase II (reduced excretion via the kidney), according to a two-compartment model of distribution.(5,10,11) The present study indicated that the metabolism of C-PROXYL in the oral and maxillofacial region of the rat also occurred according to a two-compartment model (K1 and K2; Fig. 2 A–C). The decay of the nitroxyl spin probe, C-PROXYL, which is reflected in ESR signal intensity in the oral and maxillofacial region, also appears to follow the usual pharmacokinetic pattern for excretion of drugs: those that tend to stay in the vascular system are excreted very rapidly (phase I; K1), while C-PROXYL show much slower excretion via the kidney (phase II; K2).(5,11,18) When Ws were challenged with Pg the level of oxidative stress in the oral and maxillofacial region was not significantly increased in comparison to that in non-treated animals. Taken together, it would be likely that the level of oxidative stress would be aggravated by Pg infection of the oral and maxillofacial region of GKs, suggesting that periodontitis may increase oxidative stress in the microcirculation in the oral and maxillofacial region of the diabetes model.
Diabetes mellitus, a major risk factor for vascular diseases, is associated with accelerated atherosclerosis and a high rate of arterial thrombotic complications such as retinopathy, nephropathy and cerebrovascular disease.(1) In diabetes, angiopathies can occur in both capillaries and large vessels. Periodontal disease is a well-known complication of diabetes, and persons with diabetes are at increased risk of developing severe periodontitis.(26–28) In addition, it has been reported that diabetes-induced insulin resistance causes endothelial dysfunction in blood vessels.(3,4) Reactive hyperemia in the gingival circulation was significantly increased in Ws after treatment with ACh and NTG. However, those effects were not observed in GKs after treatment with ACh and NTG (Fig. 4). These results suggest that the gingival microvascular function could reflect the state of the microcirculation in other vascular beds, including those in persons with diabetes mellitus. Endothelium-dependent vasodilation is abnormal in forearm resistance vessels of patients with insulin-dependent diabetes mellitus. This abnormality may be relevant to the high prevalence of vascular disease that occurs in persons with insulin-dependent diabetes mellitus.(29) In our study, we found that gingival vascular reactivity was reduced in animals challenged with Pg and that this reduction was enhanced in GKs (Fig. 3). Application of ACh and NTG to gingival mucosa did not affect GBF in 10-week-old GKs (Fig. 4). Therefore, the alteration in gingival vascular endothelial and smooth muscle functions would occur after 10 weeks in GKs and GKs + Pg.
In conclusion, results of the present study indicate that periodontal disease reduced gingival vascular reactivity and decreased reactive hyperemia, and this effect was accelerated by diabetes due to increased oxidative stress in the microcirculation of the oral and maxillofacial region of the rodent model.
Acknowlegments
Supported by grants from High-Tech Research Center Project of Kanagawa Dental College, Yokosuka, Kanagawa, Japan and Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, and Culture (19592371).
We dedicate this article to the memory of Dr. Yusuke Takahashi, our colleague and friend, who died in May, 2009.
Abbreviations
- C-PROXYL
3-carbamoyl-2,2,5,5-tetramethylpyrrolidin-1-yloxy
- ESR
electron spin resonance
- GBF
gingival blood flow
- GKs
Goto-Kakizaki rats
- LDF
laser Doppler flowmetry
- NO
nitric oxide
- Pg
Porphyromonas gingivalis
- ROS
reactive oxygen species
- Ws
Wistar rats
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Allen EM, Matthews JB, O’Connor R, O’Halloran D, Chapple IL. Periodontitis and type 2 diabetes: is oxidative stress the mechanistic link? Scott Med J. 2009;54:41–47. doi: 10.1258/rsmsmj.54.2.41. [DOI] [PubMed] [Google Scholar]
- 2.Cooper D, Stokes KY, Tailor A, Granger DN. Oxidative stress promotes blood cell-endothelial cell interactions in the microcirculation. Cardiovasc Toxicol. 2002;2:165–180. doi: 10.1007/s12012-002-0002-7. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K, Yoshino F, Takahashi SS, et al. Direct assessments of the antioxidant effects of propofol medium chain triglyceride/long chain triglyceride on the brain of stroke-prone spontaneously hypertensive rats using electron spin resonance spectroscopy. Anesthesiology. 2008;109:426–435. doi: 10.1097/ALN.0b013e318182a903. [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Miura K, Liu X, Zweier JL. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 7.Lee CI, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:9369–9376. doi: 10.1074/jbc.275.13.9369. [DOI] [PubMed] [Google Scholar]
- 8.Lee MC, Shoji H, Komatsu T, Yoshino F, Ohmori Y, Zweier JL. Inhibition of superoxide generation from fMLP-stimulated leukocytes by high concentrations of nitric oxide or peroxynitrite: characterization by electron spin resonance spectroscopy. Redox Rep. 2002;7:271–275. doi: 10.1179/135100002125000776. [DOI] [PubMed] [Google Scholar]
- 9.Lee MC, Shoji H, Miyazaki H, et al. Measurement of oxidative stress in the rodent brain using computerized electron spin resonance tomography. Magn Reson Med Sci. 2003;2:79–84. doi: 10.2463/mrms.2.79. [DOI] [PubMed] [Google Scholar]
- 10.Lee MC, Shoji H, Miyazaki H, et al. Assessment of oxidative stress in the spontaneously hypertensive rat brain using electron spin resonance (ESR) imaging and in vivo L-Band ESR. Hypertens Res. 2004;27:485–492. doi: 10.1291/hypres.27.485. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki H, Shoji H, Lee MC. Measurement of oxidative stress in stroke-prone spontaneously hypertensive rat brain using in vivo electron spin resonance spectroscopy. Redox Rep. 2002;7:260–265. doi: 10.1179/135100002125000758. [DOI] [PubMed] [Google Scholar]
- 12.Kuppusamy P, Zweier JL. Cardiac applications of EPR imaging. NMR Biomed. 2004;17:226–239. doi: 10.1002/nbm.912. [DOI] [PubMed] [Google Scholar]
- 13.Miura Y, Utsumi H, Hamada A. Effects of inspired oxygen concentration on in vivo redox reaction of nitroxide radicals in whole mice. Biochem Biophys Res Commun. 1992;182:1108–1114. doi: 10.1016/0006-291x(92)91846-i. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita K, Hamada A, Utsumi H. Mechanisms related to reduction of radical in mouse lung using an L-band ESR spectrometer. Free Radic Biol Med. 1999;26:951–960. doi: 10.1016/s0891-5849(98)00278-0. [DOI] [PubMed] [Google Scholar]
- 15.Gomi F, Utsumi H, Hamada A, Matsuo M. Aging retards spin clearance from mouse brain and food restriction prevents its age-dependent retardation. Life Sci. 1993;52:2027–2033. doi: 10.1016/0024-3205(93)90687-x. [DOI] [PubMed] [Google Scholar]
- 16.Miura Y, Ozawa T. Noninvasive study of radiation-induced oxidative damage using in vivo electron spin resonance. Free Radic Biol Med. 2000;28:854–859. doi: 10.1016/s0891-5849(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 17.Hamada N, Watanabe K, Tahara T, et al. The r40-kDa outer membrane protein human monoclonal antibody protects against Porphyromonas gingivalis-induced bone loss in rats. J Periodontol. 2007;78:933–939. doi: 10.1902/jop.2007.060245. [DOI] [PubMed] [Google Scholar]
- 18.Anzai K, Saito K, Takeshita K, et al. Assessment of ESR-CT imaging by comparison with autoradiography for the distribution of a blood-brain-barrier permeable spin probe, MC-PROXYL, to rodent brain. Magn Reson Imaging. 2003;21:765–772. doi: 10.1016/s0730-725x(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 19.Omori Y, Takahashi SS, Todoki K. Role of nitric oxide in post-ischemic gingival hyperemia in anesthetized dogs. Redox Rep. 2002;7:300–303. doi: 10.1179/135100002125000839. [DOI] [PubMed] [Google Scholar]
- 20.Takeshita K, Takajo T, Hirata H, Ono M, Utsumi H. In vivo oxygen radical generation in the skin of the protoporphyria model mouse with visible light exposure: an L-band ESR study. J Invest Dermatol. 2004;122:1463–1470. doi: 10.1111/j.0022-202X.2004.22601.x. [DOI] [PubMed] [Google Scholar]
- 21.Engebretson S, Chertog R, Nichols A, Hey-Hadavi J, Celenti R, Grbic J. Plasma levels of tumour necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J Clin Periodontol. 2007;34:18–24. doi: 10.1111/j.1600-051X.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 22.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 23.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 24.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 25.Hung HC, Willett W, Merchant A, Rosner BA, Ascherio A, Joshipura KJ. Oral health and peripheral arterial disease. Circulation. 2003;107:1152–1157. doi: 10.1161/01.cir.0000051456.68470.c8. [DOI] [PubMed] [Google Scholar]
- 26.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 27.Gibson FC, 3rd, Hong C, Chou HH, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 28.Desvarieux M, Demmer RT, Rundek T, et al. Periodontal microbiota and carotid intima-media thickness: the oral infections and vascular disease epidemiology study (INVEST) Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]