Abstract
Parkinson’s disease is a major neurodegenerative disease involving the selective degeneration of dopaminergic neurons and α-synuclein containing Lewy bodies formation in the substantia nigra. Although α-synuclein is a key molecule for both dopaminergic neuron death and the formation of inclusion bodies, the mechanism of α-synuclein induction of Parkinson’s disease-related pathogenesis is not understood. In the present study, we found that the interaction between dopamine and α-synuclein requires the oxidation of dopamine. Furthermore, we examined the protective effect of chlorogenic acid, a major polyphenol contained in coffee, against α-syn and dopamine-related toxicity. Chlorogenic acid inhibits several DA/α-synuclein-related phenomenon, including the oxidation of dopamine, the interaction of oxidized dopamine with α-synuclein, and the oligomerization of α-synuclein under dopamine existing conditions in vitro. Finally, we showed that the cytoprotective effect against α-synuclein-related toxicity in PC12 cells that can be controlled by the Tet-Off system. Although the induction of α-synuclein in catecholaminergic PC12 cells causes a decrease in cell viability, chlorogenic acid rescued this cytotoxicity significantly in a dose dependent manner. These results suggest that the interaction of oxidized DA with α-synuclein may be a novel therapeutic target for Parkinson’s disease, and polyphenols, including chlorogenic acid, are candidates as protective and preventive agents for Parkinson’s disease onset.
Keywords: chlorogenic acid, polyphenol, α-synuclein, Parkinson’s disease, dopamine
Introduction
Parkinson’s disease (PD) is a neuropathological disorder involving the selective degeneration of dopaminergic neurons and α-synuclein (α-syn) containing Lewy body (LB) formation in the substantia nigra, and the subsequent loss of their terminals in the striatum.(1,2) The ensuing loss of dopamine (DA) causes most of the debilitating motor disturbances associated with PD.
Abundant risk factors, including genetic and environmental factors, that can induce and/or enhance PD onset have been reported.(3,4) The molecule, α-syn, is an important molecule associated with the pathogenesis of familial and sporadic PD. α-syn is a major component of LBs, and a pathogenic molecule that can induce familial PD, PARK1 and PARK4.(3) In the PARK4 family, multiplication of the α-syn gene, which results in the overexpression of α-syn, induces the loss of dopaminergic neurons and typical parkinsonism.(5–7) Furthermore, the overexpression of α-syn, including pathogenic mutations found in the PARK1 family, have been reported to undergo DA-related cell death.(8) Previously, we reported that the overexpression of α-syn in catecholaminergic PC12 cells shows DA-related vulnerability to several toxicities including ER stress.(9) However, it is not understood why dopaminergic neurons specifically are disturbed by the overexpression of α-syn.
The regions of α-syn are classified to three major parts: an N-terminal region (residue 1–60) that encodes repetitive sequence including KTKEGV motif, a middle part (residue 61–95) that encodes an NAC domain forming the amyloidogenic core of α-syn, and a C-terminal sequence (residue 94–140) with a negatively charged region.(10–12) Oligomerization of α-syn is believed to be an important step for pathogenic toxicity, and several post-translational modifications such as phosphorylation,(13,14) nitration or oxidation at tyrosine residue,(15) glycosylation,(16) and lipid-modified form(17) have been reported as initiators for oligomerization and/or aggregation. However, these modifications are not phenomena specific to DA neurons.
It has been reported that DA can be converted to dopaminequinone (DAQ) following oxidation, and its oxidized form is believed to be toxic to cells.(18,19) In general, quinone attacks cysteine residues in proteins, and its oxidative modification of proteins may cause dysfunction. However, there is no cysteine residue in human α-syn. Therefore, it is unclear how α-syn induces cytotoxicity in the dopaminergic cells. A recent paper suggests that oxidized catechol interacts with the C-terminal sequence of the α-syn, and its interaction determines the oligomerization of α-syn.(20)
Antioxidants and/or quinone scavengers can inhibit the toxic effects of oxidized α-syn,(19) and numerous compounds including polyphenols have been examined as to their protective effects against PD-related cytotoxicity.(21,22) The results of a large scale cohort study suggest that coffee intake is inversely associated with the onset of PD, and one of the major components of coffee, caffeine, may play an important role on this prevention.(23,24) Although the effects of chlorogenic acid (CGA), a major component of coffee, have not been well investigated, an inhibitory effect of CGA on the oligomerization of α-syn in vitro has been reported, as well as other polyphenols.(25,26) In addition, since free radical scavenging effects of CGA have been also reported,(27,28) it seems to be useful to prevent PD onset. Therefore, we investigated the protective effects of CGA on DA and α-syn-related cytotoxicity in catecholaminergic PC12 cells.
Materials and Methods
Chemicals and antibodies
Nerve growth factor (NGF) was purchased from Invitrogen (Carlsbad, CA). Tet System Approved fetal bovine serum (FBS) and doxycycline (Dox) were obtained from Clontech (Palo Alto, CA). Mouse monoclonal antibody against human α-syn and rabbit polyclonal antibody against β-actin were purchased from BD Transduction laboratory (Lexington, KY) and Cell Signaling Technology (Beverly, MA), respectively. HRP-linked anti-mouse IgG and anti-rabbit IgG were from Amersham Biosciences (Buckinghamshire, UK). Cysteine, L-DOPA, DA, and 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrasodium bromide (MTT) were obtained from Wako (Osaka, Japan). α-Methyltyrosine (aMT) was purchased from Pfaltz & Bauer (Waterbury, CT). Chlorogenic acid was obtained from MP Biomedicals, LLC (Irvine, CA). PBA was purchased from Sigma. 3H-DA was prepared from Perkin Elmer (Norwalk, CT). Recombinant α-syn was obtained from Bio Mol (Plymouth Meeting, PA)
Preparation and culture of α-syn expressing PC12 cells
α-syn expressing PC12 cells controlled Tet-Off system (Clontech) (PC12-α-syn-Tet Off) were prepared as described previously.(9) During the amplification of PC12-α-syn-Tet Off cells, cells were maintained at 37.0°C in 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM)/F12 medium, supplemented with 5% FBS, 10,000 unit/ml penicillin (PS) and 100 mg/ml streptomycin (SM) and 2 ng/ml Dox. In order to induce α-syn expression, the culture medium was changed to Dox-free medium (DMEM/F12, 2.5% Tet System Approved FBS, and PS/SM). For induction of neural differentiation, 50 ng/ml NGF was added during culture. The culture medium was exchanged for fresh medium in order to delete Dox completely. For the present experiments, the cells were cultured for 3–7 days.
Cell viability assay (MTT assay)
Cell viability was measured using the MTT assay following the protocol described previously with some modifications.(24) For the MTT assay, 1 × 103 cells/well were seeded in 96-well plates, cultured for 3 or 7 days, and then incubated with MTT for 2 h at 37°C. After adding 100 µl of 0.05 N HCl in 2-propanol and mixing thoroughly to dissolve the dark blue crystal, the MTT reduction was measured with a microplate reader (Bio-Rad; wavelength of 570 nm). The data were presented as percent post-treatment recovery (percent live cells) where the absorbance from the control, non-treated cells was defined as 100% live cells.
For analyzing the role of catecholamine on α-syn cytotoxicity in PC12 cells, PC12 cells were cultured with or without aMT, a specific inhibitor of tyrosine hydroxylase. Similarly, in order to investigate the protective effect of cysteine and CGA on α-syn toxicity in PC12 cells, each compound was added to the culture medium, and cell viability was measured by MTT assay.
SDS-PAGE and western blot analyses
For SDS-PAGE and immunoblot analyses, cells were harvested from 6-well culture plates, and lysed in SDS-sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1 mM PMSF, 2 mM EDTA). Aliquots (20 µg) were separated by size on 12% polyacrylamide gels. SDS-PAGE was performed following the usual Laemmli’s protocol and western blotting was performed as described previously.(29)
α-syn oligomerization analysis
For in vitro aggregation analyses, a mixture of 20 µM α-syn, 10 mM Tris-HCl, pH 7.8, and 1 mM DA or L-DOPA was incubated at 30°C for 12 h. To investigate whether CGA can inhibit the oligomerization of α-syn, a comparison study with or without CGA was performed. Reaction mixture was lysed in SDS-sample buffer, and subjected to SDS-PAGE. Soluble oligomer of α-syn was detected using a silver staining kit (Wako, Osaka, Japan) according to the manufacturer’s protocol.
Measurement of DA/DOPA oxidative products by spectrophotometer
Catecholamine is known to be oxidized into catecholamine-quinone in alkaline pH. DA and DOPA are oxidized into DAQ and DOPA-quinone (DOPAQ). These are auto-oxidized into dopaminochrome (DAch)/dopachrome (DOPAch) and melanins, which are detectable by measuring the absorbance at 490 nm.DA (100 µM) or DOPA (100 µM) was incubated in 10 mM Tris-HCl, pH 7.8 buffer with or without 1 mM of CGA for 0–20 h. Equal amounts of the reactive products were plated into 96 well micro plates and the absorbance was measured by a microplate reader (Perkin Elmer; 490 nm) to quantify the amount of the DA/DOPA oxidative products. To examine the inhibitory effect of cysteine and CGA on the formation of DA/DOPA oxidation, each compound was added to the reaction mixture.
Direct binding of 3H-DA and α-syn
α-syn (2 nM) and 3H-DA (10 nM ) were incubated in 200 µl of 10 mM Tris-HCl buffer of various pH (range, 6.6–8.2) at 30°C for 30 min. After incubation, the reaction was terminated by adding 200 µl of activated charcoal suspension. After 30 min of absorption, α-syn bound 3H-DA and unbound 3H-DA (absorbed by the charcoal) were separated by centrifugation for 3 min at 1,000 × g. Aliquots (350 µl) of the supernatant were transferred to another tube and 3 ml of scintillation cocktail (Perkin Elmer) was added. α-syn bound 3H-DA in the supernatant was analyzed by liquid scintillation counter. α-syn (2 nM) and 3H-DA (10 nM) were incubated in 10 mM Tris-HCl, pH 7.8 along with an increasing concentration of CGA (0–10 µM) for 30 min at 37°C, and the α-syn bound 3H-DA was measured by the same method described above.
Aminophenylboronate agarose (PBA) isolation of DAQ-modified α-syn
Analyses for the binding between α-syn and DAQ using PBA were carried out following the protocol described by Bisaglia(30) with some modifications. Briefly, α-syn was dissolved at a final concentration of 0.5 µg/ml in 500 µl of 10 mM Tris-HCl, pH 8.2 in the presence of 1 mM DA. PBA suspension (100 µl) was added and mixtures were incubated at 4°C for 12 h. After 3 × 30 min washes with 14 ml each of 1 mM Tris buffer (pH 8.6), α-syn bound to PBA was eluted with 2 × 500 µl of 50 mM glycine. Finally, samples were analyzed by SDS-PAGE and western blotting for α-syn. As a control, we performed identical experiments in the absence of DA.
Results
Overexpression of human α-syn causes a decrease of cell viability in PC12 cells
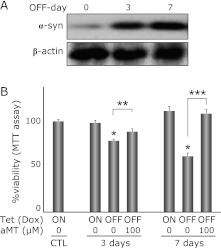
Withdrawal of Dox induced a marked increase of α-syn expression (Fig. 1A) and gradually decreased cell viability (72.5% on day 3, 56.6% on day 7 vs CTL, respectively) in PC12 cells (Fig. 1B). aMT, a specific inhibitor of tyrosine hydroxylase, was used to evaluate the association between α-syn toxicity in PC12 cells and endogenous catecholamine metabolism. Simultaneous administration of aMT (100 µM) abolished the α-syn-related cytotoxicity significantly (81.8% on day 3, 99.2% on day 7, vs CTL, respectively).
Fig. 1.
α-syn expression induces catecholamine dependent cell death in PC12 cells. (A) Withdrawal of Dox for 3 or 7 days induces α-syn expression. Day 0 is the α-syn suppressed condition (2 µg/ml Dox). Top panel shows inducible expression of α-syn, and the bottom panel shows β-actin as a loading standard. (B) Withdrawal of Dox for 3 or 7 days decreases cell viability (MTT assay) to 72.5 and 56.6%, respectively. Simultaneous treatment with aMT (100 µM) abolishes the toxicity by α-syn and upregulates those viabilities to 81.8 and 99.2%, respectively, suggesting that α-syn-related toxicity is associated with catecholamine metabolism. Each column is an average of 6 independent experiments, and the bar means SE. Asterisk means statistically significance *p<0.01 vs CTL, **p<0.05, ***p<0.01, respectively (ANOVA).
DA and DOPA enhance the oligomerization of α-syn in vitro
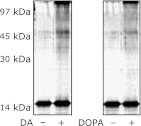
In order to investigate the effects of DA and DOPA on α-syn oligomerization, a simple experiment using recombinant human α-syn protein and DA or DOPA was performed in vitro. Under alkaline conditions, DA and DOPA induced a soluble oligomer of α-syn (Fig. 2).
Fig. 2.
DA and DOPA mediate formation of α-syn soluble oligomers. α-syn was incubated alone or in the presence of DA (100 µM) or DOPA (100 µM) in Tris-HCl, pH 7.8 at 37°C for 24 h. Co-incubation of α-syn with DA or DOPA enhances α-syn oligomerization in vitro. The reaction mixture was separated by SDS-PAGE, and α-syn monomer and oligomer are detected by silver staining.
DA is converted to the oxidized forms DAQ and/or DAch and binds to α-syn under alkaline condition
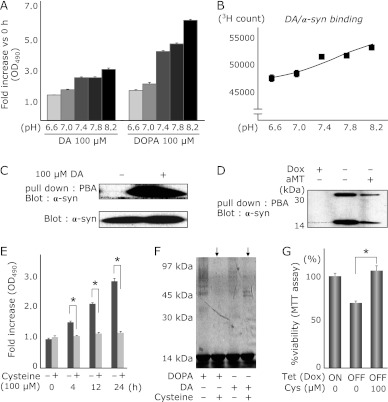
Since DA and DOPA can be converted to the oxidized forms, DAQ/DOPAQ or DAch/DOPAch, under alkaline condition, we quantified the oxidized forms of DA and DOPA under several pH conditions. Both DA and DOPA were converted to oxidized forms, especially under alkaline conditions (Fig. 3A).
Fig. 3.
Oxidized DA or DOPA is required for the oligomerization of α-syn. (A) pH dependent oxidation of DA and DOPA. DA (100 µM) or DOPA (100 µM) was incubated in increasing pH Tris-HCl buffer (range, 6.6–8.2) at 37°C for 0–20 h, and 100 µl of the reaction mixtures were analyzed by a spectrometer at 490 nm. Alkaline condition dependent DA/DOPA oxidation is shown. (B) α-syn/DA binding assay using 3H-labeled DA. pH dependent interaction between α-syn and DA (its oxidative DA; DAQ) is demonstrated. DA (or its metabolites) and α-syn interact with each other in the alkaline condition rather than in the acidic condition. (n = 3, mean ± SE). (C) Aminophenylboronate agarose (PBA) isolation of DAQ-modified α-syn in vitro. α-syn is co-precipitated with PBA only in the DA existing condition. (D) PBA isolation of DAQ-modified α-syn from the cell extracts of PC12-α-syn-Tet Off. In a Dox withdrawal condition (α-syn inducing), α-syn is co-precipitated with PBA (2nd lane). From a cell extract treated with aMT in order to inhibit endogenous catecholamine metabolism, little α-syn is co-precipitated with PBA (3rd lane). (E) Co-incubation with cysteine inhibits oxidation of DA and DOPA in vitro. DA or DOPA was incubated with or without cysteine in pH 7.8 buffer. (F) Cysteine suppresses DA or DOPA-related oligomerization of α-syn. (G) Continuous treatment of cysteine significantly decreases DA/α-syn-related cytotoxicity in PC12 cells (*p<0.01, ANOVA).
We also performed DA/α-syn binding assays under several pH conditions using 3H-labeled DA. Higher binding activity between DA (or its metabolites) and α-syn was observed under alkaline conditions where DA can be converted to the oxidized form (Fig. 3B).
We hypothesized that the oxidized form of DA may bind to α-syn directly, therefore, we examined the binding between DAQ and α-syn using PBA following the protocol described previously.(30) In vitro experiments demonstrated the binding activity between DAQ and α-syn (Fig. 3C). Moreover, we investigated whether intracellular α-syn binds to endogenous DA using cell lysate prepared from PC12-α-syn-Tet Off conditions. Under Tet Off conditions, induced α-syn was co-precipitated with PBA/DAQ (Fig. 3D). On the other hand, in the experiment using extract from cells that endogenous metabolism of catecholamine was inhibited by aMT administration, little α-syn was precipitated compared with results using cells capable of catecholamine metabolism (Fig. 3D).
The quinone scavenger cysteine suppresses DAQ/DAch formation, α-syn oligomerization, and α-syn-related cytotoxicity in PC12 cells
In the present study, results suggest that the oxidized form of DA, DAQ, may play an important role on α-syn-related cytotoxicity. In order to confirm this hypothesis, we examined the protective effects of the quinone scavenger cysteine on α-syn-related cytotoxicity in PC12 cells. Co-incubation of cysteine decreased DAQ/DAch formation and α-syn oligomerization under DA existing condition in alkaline pH (Fig. 3 E and F). Furthermore, we confirmed the cytoprotective effects of cysteine against DA/α-syn-related toxicity in PC12 cells. Simultaneous treatment with cysteine rescued the cells from DA/α-syn induced toxicity significantly (Fig. 3G).
CGA inhibits DA-related oligomerization of α-syn and rescues the cells from α-syn cytotoxicity
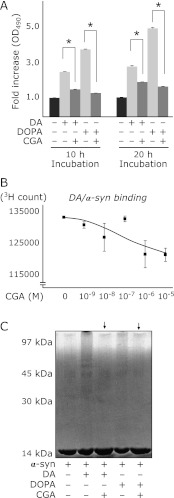
We investigated whether the presence of some antioxidative substance could prevent DA oxidization and subsequent α-syn oligomerization. Since CGA has been reported to have an inhibitory effect against α-syn oligomerization,(25) we examined the effects of CGA on α-syn oligomerization and DA/α-syn-related toxicity.
We investigated the effects of CGA on DA and/or DOPA oxidation under alkaline condition. CGA dramatically inhibited DAQ/DAchrome formation in alkaline pH buffer (Fig. 4A). We examined the inhibitory effects of CGA on α-syn oligomerization under DA existing and alkaline conditions. CGA also inhibited direct binding of 3H-DA (or its oxidized metabolites) and α-syn in a dose dependent manner (Fig. 4B). Co-incubation of CGA with DA /DOPA and α-syn showed suppressive effects of CGA against both DA- and DOPA-related soluble oligomer formation of α-syn (Fig. 4C).
Fig. 4.
CGA inhibits the interaction of DAQ with α-syn, the oxidation of DA and DOPA, and the oligomerization of α-syn. (A) Co-incubation of CGA dramatically inhibits oxidation of DA and DOPA in vitro. DA or DOPA was incubated with or without CGA in pH 7.8 buffer. (B) α-syn/DA binding assay using 3H-labeled DA. CGA inhibits the interaction between α-syn and oxidized DA at pH 7.8 in a dose dependent manner. (C) CGA suppresses DA or DOPA-related oligomerization of α-syn.
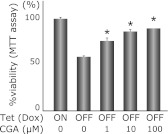
Finally, we examined the protective effects of CGA on PC12-α-syn-Tet Off cells. Simultaneous and continuous treatment of CGA rescued the PC12 cells against α-syn-related cytotoxicity in a dose dependent manner (Fig. 5).
Fig. 5.
The protective effect of CGA on α-syn/DA-related toxicity in PC12 cells. PC12-α-syn-Tet Off cells were cultured under Dox-free condition (α-syn inducing) with several concentrations of CGA. The result shows the protective effect of CGA against α-syn/DA-related cytotoxicity in a concentration dependent manner (63.9% in 0 µM CGA, 75.6% in 1 µM CGA, 85.8% in 10 µM CGA, 89.1% in 100 µM CGA vs ON condition, respectively). Columns are the average of 6 independent experiments, and SE is indicated by the bar (*p<0.01, ANOVA).
Discussion
α-syn is a key molecule in PD pathogenesis that relates to not only familial PD, but also LB formation in the brain from sporadic PD.(1–4) Overexpression of α-syn in the PARK4 family resulted in a typical parkinsonian phenotype and cell loss of dopaminergic neurons, as well as usual sporadic PD.(5–7) However, it has not been well understood why high expression levels of α-syn induces selective cell death of the dopaminergic neurons. Some reports suggest that α-syn shows cytotoxic character only in the dopaminegic cells, but not in the non-dopaminergic cells.(8,9,31) Conway reported that some compounds with the catechol structure, including DA and DOPA, form adducts with α-syn,(32) and its unstable protofibril may one of the causes for α-syn-related toxicity. Indeed, several reports suggest that DA and/or catecholamine-associated materials interact with α-syn, especially with C-terminal sequence in α-syn. Moreover, the association between DA/DAQ and PD-related molecules, including parkin,(33) tyrosine hydoxylase,(34) dopamine transporter,(35) α-syn,(36,37) ubiquitin,(28) has been reported. These results suggest that the functional interaction between DA (and/or related materials) and α-syn should be considered as important in the pathogenesis in PD.
We previously established PC12-α-syn-Tet Off cells that can be controlled in the expression level of α-syn by Dox withdrawal from the culture medium.(9) In this cell line, catecholamine metabolism can be also inhibited by aMT. Therefore, it may be a useful model for the investigation of the pathogenic interaction between DA and α-syn. Using this cell line, we confirmed catecholamine metabolism-related cytotoxicity of α-syn.
In the present study, we demonstrated that DA/DOPA are required to be oxidized to form α-syn oligomers. Indeed, recent studies revealed that the oxidization of DA and DOPA are responsible for oligomerization of α-syn. DA and DOPA are known to be oxidized to DAQ and DOPAQ in alkaline condition, and these are subsequently auto-oxidized to DAch and melanin. We investigated the association between DA/DOPA oxidation and α-syn oligomerization. The results showed an increased oxidation of DA and DOPA in pH dependent manner, especially in alkaline pH, and α-syn easily forms a soluble oligomer under alkaline conditions, suggesting that oxidized DA may enhance the oligomerization of α-syn. We also showed a direct interaction of oxidized DA with α-syn, and this interaction may be an initial step of α-syn oligomerization under DA existing condition.
Cysteine, a scavenger for quinone and an antioxidant, decreased DA oxidation and α-syn oligomerization (Fig. 3 E and F). Furthermore, cysteine rescued the cells from DA/α-syn-related toxicity in PC12 cells (Fig. 3G). This result suggests that antioxidative compounds may be candidates as protective materials against DA/α-syn-related toxicity in PD pathogenesis.
Polyphenols are a structural class of natural and/or synthetic organic chemicals characterized by the presence of phenol structure. Most polyphenols have antioxidative activity,(38,39) and a number of polyphenols have been reported as anti-parkinsonian compounds.(40–43) CGA is a polyphenol that is abundant in coffee. We investigated the protective effect of CGA on DA/α-syn-related toxicity. CGA showed not only inhibitory effects against DA oxidation (Fig. 4A) and direct interaction of oxidized DA to α-syn (Fig. 4B) but also α-syn oligomerization (Fig. 4C). As a result, CGA showed cytoprotective effects against α-syn toxicity in PC 12 cells (Fig. 5).
Taken together, the interaction of oxidized DA (or related compounds) with α-syn may be a novel therapeutic target for PD, and polyphenols including CGA are candidates as therapeutic or preventive materials for PD onset.
Acknowledgments
This study was supported by the grant from All Japan Coffee Association, and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
Abbreviations
- DMEM
Dulbecco’s modified eagle medium
- EDTA
ethylenediaminetetraacetic acid
- HCl
hydrogen chloride
- PMSF
phenylmethylsulfonyl fluoride
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Spillantini MG, Crowther RA, Jakes R, Masegawa M, Goedert M. α-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuno Y, Hattori N, Kubo S, et al. Progress in the pathogenesis and genetics of Parkinson’s disease. Philos Trans R Soc Lond B Biol Sci. 2008;363:2215–2227. doi: 10.1098/rstb.2008.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 5.Singleton AB, Farrer M, Johnson J, et al. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 6.Chatier-Harlin MC, Kachergus J, Roumier C, et al. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 7.Ibanez P, Bonnet AM, Debarges B, et al. Causal relation between α-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson’s disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 9.Ito S, Nakaso K, Imamura K, Takeshima T, Nakashima K. Endogenous catecholamine enhances the dysfunction of unfolded protein response and α-synuclein oligomerization in PC12 cells overexpressing human α-synuclein. Neurosci Res. 2010;66:124–130. doi: 10.1016/j.neures.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Uéda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer’s disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwai A, Yoshimoto M, Masliah E, Saitoh T. Non-A beta component of Alzheimer’s disease amyloid (NAC) is amyloidogenic. Biochemistry. 1995;34:10139–10145. doi: 10.1021/bi00032a006. [DOI] [PubMed] [Google Scholar]
- 12.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neuosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara H, Hasegawa M, Dohmae N, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Periquet M, Wang X, et al. Tyrosine and serine phosphorylation of α-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119:3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giasson BI, Duda JE, Murray IV, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 16.Shimura H, Schlossmacher MG, Hattori N, et al. Ubiquitination of a new form of α-synuclein by parkin from human brain: implication for Parkinson’s disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 17.De Franceschi G, Frare E, Pivato M, et al. Structural and morphological characterization of aggregated species of α-synuclein induced by docosahexaenoic acid. J Biol Chem. 2011;286:22262–22274. doi: 10.1074/jbc.M110.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res. 2003;5:165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki I, Asanuma M. Approaches to prevent dopamine quinone-induced neurotoxicity. Neurochem Res. 2009;34:698–706. doi: 10.1007/s11064-008-9843-1. [DOI] [PubMed] [Google Scholar]
- 20.Mazzulli JR, Armakola M, Dumoulin M, et al. Cellular oligomerization of α-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J Biol Chem. 2007;282:31621–31630. doi: 10.1074/jbc.M704737200. [DOI] [PubMed] [Google Scholar]
- 21.Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s disease. J Nutr Biochem. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008;33:2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 23.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson’s disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 24.Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Masuda M, Suzuki N, Taniguchi S, et al. Small molecule inhibitors of α-synuclein filament assembly. Biochemistry. 2006;45:6085–6094. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- 26.Masuda M, Hasegawa M, Nonaka T, et al. Inhibition of α-synuclein fibril assembly by small molecules: analysis using epitope-specific antibodies. FEBS Lett. 2009;583:787–791. doi: 10.1016/j.febslet.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda H, Kimura Y, Masaki M, Iwahashi H. Caffeic acid inhibits the formation of 1-hydroxyethyl radical in the reaction mixture of rat liver microsomes with ethanol partly through its metal chelating activity. J Clin Biochem Nutr. 2011;48:187–193. doi: 10.3164/jcbn.10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minakata K, Fukushima K, Nakamura M, Iwahashi H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe3+ and NADPH. J Clin Biochem Nutr. 2011;49:207–215. doi: 10.3164/jcbn.11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimoto Y, Nakaso K, Nakashima K. L-dopa and dopamine enhance the formation of aggregates under proteasome inhibition in PC12 cells. FEBS Lett. 2005;579:1197–1202. doi: 10.1016/j.febslet.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 30.Bisaglia M, Tosatto L, Munari F, et al. Dopamine quinones interact with α-synuclein to form unstructured adducts. Biochem Biophys Res Commun. 2010;394:424–428. doi: 10.1016/j.bbrc.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Bisaglia M, Greggio E, Maric D, Miller DW, Cookson MR, Bubacco L. α-synuclein overexpression increases dopamine toxicity in BE2-M17 cells. BMC Neurosci. 2010;11:41. doi: 10.1186/1471-2202-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the α-synuclein protofibril by dopamine-α-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 33.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Stokes AH, Roskoski R, Jr., Vrana KE. Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J Neurosci Res. 1998;54:691–697. doi: 10.1002/(SICI)1097-4547(19981201)54:5<691::AID-JNR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead RE, Ferrer JV, Javitch JA, Justice JB. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J Neurochem. 2001;76:1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- 36.Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010;33:559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with α-synuclein. J Biol Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- 38.Vinson JA. Flavonoids in foods as in vitro and in vivo antioxidants. Adv Exp Med Biol. 1998;439:151–164. doi: 10.1007/978-1-4615-5335-9_11. [DOI] [PubMed] [Google Scholar]
- 39.Readerstorff D. Antioxidant activity of olive polyphenols in humans: a review. Int J Vitam Nutr Res. 2009;79:152–165. doi: 10.1024/0300-9831.79.3.152. [DOI] [PubMed] [Google Scholar]
- 40.Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008;33:2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 41.Mandel SA, Amit T, Weinreb O, Reznichenko L, Youdim MB. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson’s diseases. CNS Neurosci Ther. 2008;14:352–365. doi: 10.1111/j.1755-5949.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Shi JS, Zhou H, Wilson B, Hong JS, Gao HM. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78:466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem. 2011;347:135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]