Abstract
We examined whether non-enzymatic antioxidant defense systems are disrupted in the brain of rats with water-immersion restraint stress. When rats were exposed to water-immersion restraint stress for 1.5, 3 or 6 h, the brain had decreased ascorbic acid and reduced glutathione contents and increased lipid peroxide and nitric oxide metabolites contents at 3 h and showed further changes in these components with a reduction of vitamin E content at 6 h. Increased serum levels of stress markers were found at 1.5, 3 or 6 h of WIRS. Oral pre-administration of L-ascorbic acid (1.5 mmol/kg) or vitamin E (0.5 mmol/kg) to rats with 6 h of water-immersion restraint stress attenuated the increases in lipid peroxide and nitric oxide metabolites contents and the decrease in vitamin E content in the brain. Pre-administered L-ascorbic acid attenuated the decreases in brain ascorbic acid and reduced glutathione contents at 6 h of water-immersion restraint stress, while pre-administered vitamin E enhanced the decreases in those contents. Pre-administered L-ascorbic acid or vitamin E did not affect the increased serum levels of stress markers in rats with 6 h of water-immersion restraint stress. These results indicate that water-immersion restraint stress causes disruption of non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and nitric oxide generation in the brain of rats with water-immersion restraint stress.
Keywords: water-immersion restraint stress, rat brain, non-enzymatic antioxidant defense systems, lipid peroxide, nitric oxide
Introduction
The brain is especially vulnerable to oxidative damage because the tissue has high oxygen consumption, abundant lipid content, and a relative paucity of antioxidant defense systems to prevent ongoing oxidative damage compared with other tissues.(1) It is known that lipid peroxide (LPO) level in the brain of rats is the highest among main nine tissues, i.e., brain, adrenal, liver, kidney, heart, lung, skeletal muscle, spleen, and testis, while the levels of reduced glutathione (GSH) and vitamin E determined as α-tocopherol (α-Toc) in the brain are relatively low among the nine tissues, although the level of ascorbic acid (AsA) (vitamin C) is relatively high among the nine tissues.(2)
Acute immobilization/restraint stress induces oxidative stress in the brain of rats, which might be involved in stress-induced neurobehavioural alterations.(3–9) Liu et al.(3) have shown that exposure of rats to 8 h of immobilization stress enhances lipid peroxidation in the cerebral, cortex, cerebellum, hippocampus, and midbrain of the brain, although LPO level is simultaneously increased in the serum and liver. Zaidi and Banu(4) have reported that exposure of rats to 6 h of immobilization causes a decrease in GSH level and an increase in LPO level in the brain and that pre- or post-administration of vitamin A, vitamin E or vitamin C attenuates GSH depletion and enhanced lipid peroxidation in the brain of rats with immobilization stress. Pal et al.(5) have reported that exposure of rats to 1 h of restraint stress increases LPO levels in the serum and brain and that pre-administration of L-AsA or α-Toc used as vitamin E for five consecutive days attenuates increased LPO levels in the serum and brain. Gulati et al.(6) have shown in rats with restraint stress that 1 h of stress causes an increase in LPO level and decreases in the levels of GSH and nitric oxide (NO) metabolites (nitrite/nitrrate, NOx) in the brain, while 6 h of stress causes an increase in NOx level with similar levels of increased LPO and decreased GSH in the brain. However, it has been shown that exposure of rats to 6 h of cold (2–4°C)-restraint stress has no effect on LPO level in the serum and brain and on GSH level in the brain.(10) Thus, there is a clear difference in the changes in the levels of GSH, a major member of non-enzymatic antioxidant defense systems, and LPO in the brain between rats with acute immobilization/restraint stress and cold-restraint stress.
Water-immersion restraint stress (WIRS) consists of immobilization/restraint stress and a kind of cold stress in which the exposed temperature is usually around 23°C. WIRS is widely used to evaluate stress-induced acute gastric mucosal lesions in experimental animals. We have reported that exposure of rats to WIRS for 3 to 6 h causes oxidative damage associated with disruption of non-enzymatic antioxidant defense systems and enhanced lipid peroxidation and NO• generation in the liver as well as the gastric mucosa.(11–16) Furthermore, it has been shown that exposure of rats to 6 h of WIRS causes oxidative damage in the liver, kidney, heart, and skeletal muscle and that vitamin E pre-administered to the stressed rats reduces oxidative damage in the liver, kidney, heart, and skeletal muscle.(17) It has also been shown that vitamin E and L-AsA pre-administered to rats with WIRS reduce oxidative damage by attenuating disrupted non-enzymatic antioxidant defense systems and enhanced lipid peroxidation and NO• generation in the gastric mucosa and liver.(15,16) However, there is no information on whether WIRS disrupts non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and NO• generation in the brain of rats.
In the present study, therefore, we attempted to clarify whether WIRS disrupts non-enzymatic antioxidant defense systems in the brain of rats by examining the time courses of the changes in AsA, GSH, vitamin E, LPO, and NOx levels in the brain of rats exposed to WIRS for a period of 6 h and the effects of pre-administered L-AsA and vitamin E on these changes in the brain of stressed rats. In addition, we examined WIRS-induced changes in the serum levels of adrenocorticotropic hormone (ACTH), glucocorticoide, and glucose, which are stress markers, in rats pre-administered with and without either L-AsA or vitamin E, because it is known that serum levels of these stress markers are increased in rats with WIRS.(16,17)
Materials and Methods
Materials
RRR-α-Toc used for vitamin E administration was purchased from Sigma-Aldrich (St. Louis, OM). L-AsA, corticosterone, α,α'-dipyridyl, 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB), ethylenediaminetetraacetic acid (EDTA), GSH, 2-thiobarbituric acid, α-Toc and δ-Toc used for vitamin E determination, and other chemicals were obtained from Wako Pure Chemical Ind. Ltd. (Osaka, Japan). All chemicals used were of reagent grade and were not further purified.
Animals
Male Wistar rats aged six weeks were purchased from Nippon SLC Co. (Hamamatsu, Japan). The animals were housed in cages in a ventilated animal room with controlled temperature (23 ± 2°C) and relative humidity (55 ± 5%) with 12 h of light (7:00 to 19:00). The animals were maintained with free access to rat chow, Oriental MF (Oriental Yeast Co., Tokyo, Japan) and tap water for one week. All animals received humane care in compliance with the Guidelines of the Management of Laboratory Animals in Fujita Health University. The animal experiment was approved by Institutional Animal Care and Use Committee.
Induction of WIRS and AsA and vitamin E administrations
Seven-week-old rats were starved for 24 h prior to experiments, but were allowed free access to water. Rats were restrained in wire cages and immersed up to the depth of the xiphoid process in at 23°C water bath to induce WIRS, as described in our previous reports.(11–17) In the time course experiment, rats were subjected to WIRS for 1.5, 3 or 6 h. In the experiment of L-AsA and vitamin E administrations, L-AsA and vitamin E (RRR-α-Toc) were dissolved in 5% Tween 80 and the prepared L-AsA or vitamin E solution was orally administered to rats with and without 6 h of WIRS at a volume of 1 ml/100 g body weight with a stomach tube at 0.5 h before the onset of WIRS. The doses of administered L-AsA and vitamin E were 1.5 and 0.5 mmole/kg body weight, respectively. The doses of L-AsA and vitamin E used were determined based on our previous report.(15) Stressed and unstressed rats not administered with L-AsA or vitamin E received the same volume of 5% Tween 80 in the same manner.
Determinations of serum and brain components
All rats used for the determinations of serum and brain components were sacrificed under ether anesthesia at which time blood was collected from the inferior vena cava. Serum was obtained from the collected blood by centrifugation. Immediately after sacrifice, whole brain was removed from each rat. The collected serum and brains were stored at –80°C until use. Serum LPO was assayed by the fluorometric thiobarbituric acid method of Yagi(18) using tetramethoxypropane as a standard. The concentration of serum LPO is expressed as that of malondialdehyde (MDA) equivalents. Serum ACTH was assayed using a commercial kit, ACTH EIA kit (Phonix Pharmaceutical Inc., Burlingame, CA). Serum corticosterone was measured by the fluorometric method of Guillemin et al.(19) using authentic corticosterone as a standard. Serum glucose was assayed using a commercial kit, Glucose-CII Test Wako (Wako Pure Chemical Ind. Ltd., Osaka, Japan). The isolated brain was homogenized in 9 volumes of ice-cold 50 mM Tris-HCl buffer (pH 7.4) containing 1 mM EDTA to prepare 10% homogenate. The brain homogenate was used for the determinations of AsA, GSH, vitamin E, LPO, and NOx. AsA in the brain homogenate was determined by the α,α'-dipyridyl method(20) using L-AsA as a standard. GSH in the brain homogenate was assayed by the DTNB method of Sedlak and Lindsay(21) using GSH as a standard. Vitamin E in the brain homogenate was assayed by the high-performance liquid chromatographic method with electrochemical detection using δ-Toc as an internal standard as described in our previous report.(22) The amount of brain vitamin E is expressed as that of α-Toc. LPO in the brain homogenate was assayed by the colorimetric thiobarbituric acid method of Ohkawa et al.(23) using tetramethoxypropane as a standard except that 1 mM EDTA was added to the reaction mixture. The amount of brain LPO is expressed as that of MDA equivalents. NOx (nitrite/nitrate), an index of NO• generation, in the brain tissue was assayed by the Griess reaction-dependent method of Green et al.(24) For this assay, the prepared brain homogenate was sonicated two times on ice for 30 s using a Handy Sonic model UR-20P (Tomy Seiko Co., Tokyo, Japan). The sonicated homogenate was centrifuged at 10,000 × g for 20 min at 4°C and the resultant supernatant was filtrated at 4°C under centrifugation using a membrane filter Ultrafree-MC (Millipore Co., Bedford, MA). NOx in the filtrate was determined using a nitric oxide assay kit (Roche-Diagnostics Co., Tokyo, Japan).
Statistical analysis
All results obtained are expressed as means ± SD. The statistical analyses of the results were performed using a computerized statistical package (StatView). Each mean value was compared by one-way analysis of variance (ANOVA) and Fisher’s protected least significance (PLSD) for multiple comparisons as the post-hoc test. The significance level was set at p<0.05.
Results
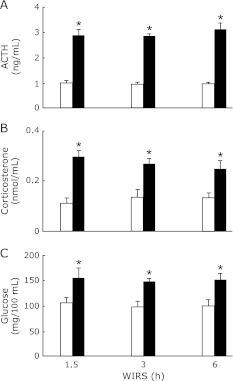
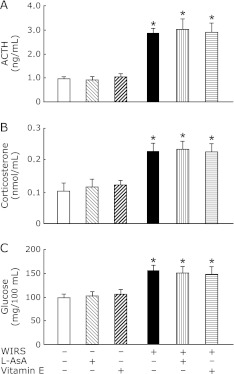
Serum ACTH, corticosterone, and glucose concentrations in rats exposed to WIRS for 1.5, 3 or 6 h were significantly higher than those in the corresponding rats without stress and there were no differences in the increased serum ACTH, corticosterone, and glucose concentrations in stressed rats between each time point of WIRS (Fig. 1).
Fig. 1.
Time courses of changes in serum ACTH (A), corticosteron (B), and glucose (C) concentrations in rats with WIRS. Fasted rats were exposed to WIRS for 1.5, 3 or 6 h. Serum ACTH, corticosterone, and glucose were assayed at each time point after the onset of WIRS as described in Materials and Methods. Open bar, control rats without WIRS; closed bar, rats with WIRS. Each value is a mean ± SD (n = 5 for unstressed rats; n = 8 for stressed rats). *Significantly different from unstressed control rats, p<0.05.
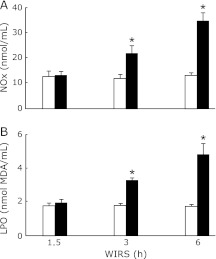
Serum NOx and LPO concentrations in rats exposed to WIRS for 3 or 6 h were significantly higher than those in the corresponding unstressed rats and the increases in serum NOx and LPO concentrations in stressed rats occurred time-dependently (Fig. 2).
Fig. 2.
Time courses of changes in serum NOx (A) and LPO (B) concentrations in rats with WIRS. Fasted rats were exposed to WIRS for 1.5, 3 or 6 h. Serum NOx and LPO were assayed at each time point after the onset of WIRS as described in Materials and Methods. Open bar, control rats without WIRS; closed bar, rats with WIRS. Each value is a mean ± SD (n = 5 for unstressed rats; n = 8 for stressed rats). *Significantly different from unstressed control rats, p<0.05.
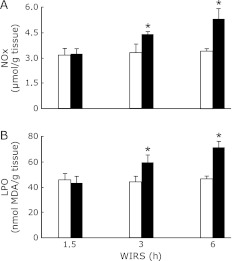
Brain NOx and LPO contents in rats exposed to WIRS for 3 or 6 h were significantly higher than those in the corresponding unstressed rats and the increases in brain NOx and LPO contents in stressed rats occurred time-dependently (Fig. 3).
Fig. 3.
Time courses of changes in brain NOx (A) and LPO (B) contents in rats with WIRS. Fasted rats were exposed to WIRS for 1.5, 3 or 6 h. Brain NOx and LPO were assayed at each time point after the onset of WIRS as described in Materials and Merhods. Open bar, control rats without WIRS; closed bar, rats with WIRS. Each value is a mean ± SD (n = 5 for unstressed rats; n = 8 for stressed rats). *Significantly different from unstressed control rats, p<0.05.
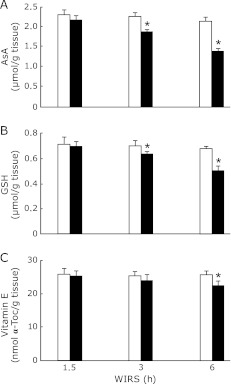
Brain AsA and GSH contents in rats exposed to WIRS for 3 or 6 h were significantly higher than those in the corresponding unstressed rats, although brain AsA content in rats with 1.5 h of WIRS tended to be lower than that in the corresponding unstressed rats (Fig. 4 A and B). The decreases in brain AsA and GSH contents in stressed rats occurred time-dependently, although the extent of decrease in brain AsA content was larger than that of decrease in brain GSH content at 3 or 6 h of WIRS (Fig. 4 A and B). Brain vitamin E content in stressed rats was significantly lower than that in unstressed rats at 6 h of WIRS (Fig. 4C).
Fig. 4.
Time courses of changes in brain AsA (A), GSH (B), and vitamin E (C) contents in rats with WIRS. Fasted rats were exposed to WIRS for 1.5, 3 or 6 h. Brain AsA, GSH, and vitamin E were assayed at each time point after the onset of WIRS as described in Materials and Methods. Open bar, control rats without WIRS; closed bar, rats with WIRS. Each value is a mean ± SD (n = 5 for unstressed rats; n = 8 for stressed rats). *Significantly different from unstressed control rats, p<0.05.
When L-AsA (1.5 mmole/kg) or vitamin E (0.5 mmole/kg) was orally administered to rats with 6 h of WIRS at 0.5 h before the onset of stress, the pre-administered L-AsA and vitamin E had no effect on the increases in serum ACTH, coeticosterone, and glucose concentrations at 6 h of WIRS (Fig. 5). Administration of the same dose of L-AsA or vitamin E to unstressed rats in the same manner did not affect the serum ACTH, corticosterone, and glucose concentrations at all (Fig. 5).
Fig. 5.
Effects of pre-administered L-AsA and vitamin E on changes in serum ACTH (A), corticosterone (B), and glucose (C) concentrations in rats with 6 h of WIRS. Fasted rats with and without 6 h of WIRS were orally administered with L-AsA (1.5 mmol/kg) or vitamin E (0.5 mmol/kg) at 0.5 h after the onset of stress. Serum ATCH, corticosterone, and glucose were assayed as described in Materials and Methods. Each value is a mean ± SD (n = 5 for unstressed groups; n = 8 for stressed groups). *Significantly different from control rats without WIRS and any administration, p<0.05; #Significantly different from rats with WIRS alone, p<0.05.
The pre-administration of L-AsA or vitamin E significantly attenuated increased NOx and LPO concentrations in the serum of rats with 6 h of WIRS, although the attenuating effect of L-AsA was smaller than that of vitamin E (Fig. 6). Administration of the same dose of L-AsA or vitamin E to unstressed rats in the same manner had no effect on the serum NOx and LPO concentrations (Fig. 6).
Fig. 6.
Effects of pre-administered L-AsA and vitamin E on changes in serum NOx (A) and LPO (B) concentrations in rats with 6 h of WIRS. Fasted rats with and without 6 h of WIRS were orally administered with L-AsA (1.5 mmol/kg) or vitamin E (0.5 mmol/kg) at 0.5 h after the onset of stress. Serum NOx and LPO were assayed as described in Materials and Methods. Each value is a mean ± SD (n = 5 for unstressed groups; n = 8 for stressed groups). *Significantly different from control rats without WIRS and any administration, p<0.05; #Significantly different from rats with WIRS alone, p<0.05.
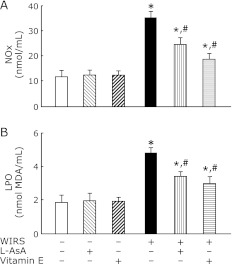
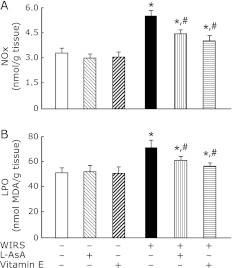
The pre-administration of L-AsA or vitamin E significantly attenuated increased NOx and LPO contents in the brain of rats with WIRS, although the attenuating effect of L-AsA was smaller than that of vitamin E (Fig. 7). The pre-administration of vitamin E returned the increased brain LPO content to near the level of unstressed rats (Fig. 7B). Administration of the same dose of L-AsA or vitamin E to unstressed rats in the same manner had no effect on the brain NOx and LPO contents (Fig. 7).
Fig. 7.
Effects of pre-administered L-AsA and vitamin E on changes in brain NOx (A) and LPO (B) contents in rats with 6 h of WIRS. Fasted rats with and without 6 h of WIRS were orally administered with L-AsA (1.5 mmol/kg) or vitamin E (0.5 mmol/kg) at 0.5 h after the onset of stress. brain NOx and LPO were assayed as described in Materials and Methods. Each value is a mean ± SD (n = 5 for unstressed groups; n = 8 for stressed groups). *Significantly different from control rats without WIRS and any administration, p<0.05; #Significantly different from rats with WIRS alone, p<0.05.
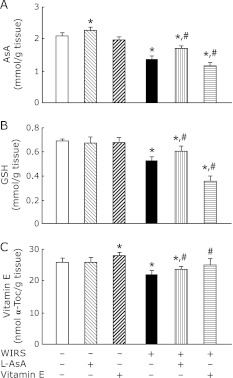
The pre-administration of L-AsA to rats with 6 h of WIRS significantly attenuated decreased brain AsA, GSH, and vitamin E contents at 6 h of WIRS (Fig. 8). The pre-administration of vitamin E to rats with 6 h of WIRS significantly caused further reductions of decreased brain AsA and GSH contents at 6 h of WIRS, while the vitamin E pre-administration significantly attenuated decreased brain vitamin E content at 6 h of WIRS and returned the decreased brain vitamin E content to near the level of unstressed rats (Fig. 8). Administration of the same dose of L-AsA to unstressed rats in the same manner increased the brain AsA content significantly, while administration of the same dose of vitamin E to unstressed rats in the same manner increased the brain vitamin E content significantly (Fig. 8).
Fig. 8.
Effects of pre-administered L-AsA and vitamin E on changes in brain AsA (A), GSH (B), and vitamin E (C) contents in rats with 6 h of WIRS. Fasted rats with and without 6 h of WIRS were orally administered with L-AsA (1.5 mmol/kg) or vitamin E (0.5 mmol/kg) at 0.5 h after the onset of stress. Brain AsA, GSH, and vitamin E were assayed as described in Materials and Methods. Each value is a mean ± SD (n = 5 for unstressed groups; n = 8 for stressed groups). *Significantly different from control rats without WIRS and any administration, p<0.05; #Significantly different from rats with WIRS alone, p<0.05.
Discussion
The present study has clearly shown that WIRS causes disruption of non-enzymatic antioxidant defense systems composed of AsA, GSH, and vitamin E thorough enhanced lipid peroxidation and NO• generation in the brain of rats. Rats exposed to WIRS for 1.5, 3 or 6 h showed a similar extent of stress response at each time point of the stress, judging from the serum levels of stress markers such as ACTH, corticosterone, and glucose. Disruption of brain non-enzymatic antioxidant defense system in rats exposed to WIRS for a period of 6 h occurred 3 and 6 h after the onset of stress, i.e., considerably later than the appearance of stress response. Such a timing of the disruption of non-enzymatic antioxidant defense systems in the brain of rats with WIRS was similar to that in the gastric mucosa and liver.(13,14)
It is known that exposure of rats to 1 h of immobilization/restraint stress enhances lipid peroxidation in the brain.(4–9) It is also known that brain NO• generation is lower in rats with restraint stress than in unstressed rats at 1 h after the onset of stress, while that generation is higher in stressed rats than in unstressed rats at 6 h.(5) In the present study, time-dependent increases in NOx and LPO contents in the brain of rats with WIRS occurred 3 and 6 h after the onset of stress, i.e., considerably later than the appearance of stress response. These results suggest that WIRS enhances NO• generation and lipid peroxidation in the brain of rats under lasting stress conditions. In addition, time-dependent increases in serum NOx and LPO concentrations occurred in rats exposed to WIRS and were well consistent with those in brain NOx and LPO contents. It has been suggested that excessive glucocorticoids induce oxidative stress in cultured rat hippocampal slices by downregulating the gene expression of glutathione peroxidase, an enzyme to metabolize hydrogen peroxide and lipid hydroperoxides in the presence of GSH, and by upregluating the gene expression of NADPH oxidase, an enzyme to generate superoxide radical, one of reactive oxygen species (ROS).(25) It is known that lipid peroxidation is caused via ROS in the brain, which is involved in the pathogenesis of neurodegenerative diseases.(26) Therefore, excessive corticosterone being secreted continuously from the adrenal gland through the hypothalamus-pituitary-adrenal axis may enhance lipid peroxidation in the brain of rats with WIRS by stimulating the generation of ROS in the hippocampus. It has been reported that exposure of rats to 6 h of immobilization stress activates the expression of inducible nitric oxide synthase (iNOS) through the mechanism involved in excitatory amino acids and subsequent activation of nuclear factor κB in the brain cortex, resulting in excessive iNOS-mediated NO• generation in the brain.(27) It has been shown that NO• induces oxidative stress through the generation of ROS in neuronal cells.(28) Therefore, there seems to be a possibility that exposure of rats to WIRS causes excessive NO• generation through activation of iNOS in the brain cortex.
It has been shown that GSH, a major member of non-enzymatic defense systems, is depleted in the brain of rats with immobilization/restraint stress.(4–9) When compared with unstressed rats, brain AsA and GSH contents were decreased in rats with 3 or 6 h of WIRS of which decreases were reversely related to the increases in brain NOx and LPO contents. The brain AsA content tended to decrease in rats with 1.5 h of WIRS. The extent of decrease in AsA content was larger than that in GSH content in the brain of rats with WIRS. Furthermore, rats with 6 h of WIRS had lower brain vitamin E content than unstressed rats. It is known that AsA (reduced form) is oxidized to dehydroasacorbic acid (oxidized form) to regenerate vitamin E from vitamin E radical in the liquid/aqueous interface, resulting in support of the chain-breaking antioxidant action of vitamin E for lipid peroxidation.(29) It is also known that GSH recycles AsA from dehydroascorbic acid through a non-enzymatic reaction and/or an enzymatic reaction.(30) It has been shown that AsA is oxidized first with subsequent vitamin E oxidation for protection of brain against oxidative damage.(31) These findings suggest that, in the brain of rats with WIRS, AsA is consumed first followed by GSH consumption with subsequent vitamin E consumption. Thus, brain non-enzymatic antioxidant defense system was disrupted in rats with WIRS. These findings also suggest that WIRS could disrupt non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and NO• generation in the brain of rats with WIRS.
It has been reported that pre- or post-administrations of vitamins C and E prevents oxidative stress in the brain of rats exposed to immobilization stress for 6 h by attenuating decreased GSH level and increased LPO level in the tissue, although pre- or post-administration of vitamins C and E in combination dose not cause additive prevention of immobilization stress-induced oxidative stress in the brain.(4) We examined the effects of L-AsA and vitamin E pre-administered alone on the changes in AsA, GSH, vitamin E, LPO, and NOx contents in the brain of rats with 6 h of WIR in order to confirm the disruption of non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and NO• generation in the brain of stressed rats. When L-AsA (1.5 mmole/kg) or vitamin E (0.5 mmole/kg) was orally administered to rats with 6 h of WIRS at 0.5 h the onset of stress, both pre-administered vitamins did not affect the increases in serum ACTH, corticosterone, and glucose concentrations at 6 h of WIRS at all. These results indicate that L-AsA and vitamin E pre-administered to rats with WIRS have no effect on stress response. Vitamin E functions not only as a chain-braking antioxidant for lipid peroxidation but also as a scavenger of ROS and NO•.(32,33) It is known that vitamin E inhibits NO•-induced lipid peroxidation in rat brain homogenates.(34) It is also known that AsA not only supports the chain-braking antioxidant action of vitamin E for lipid peroxidation but also functions as a scavenger of ROS.(29,35) The pre-administered L-AsA and vitamin E attenuated the increases in serum LPO and NOx concentrations and brain LPO and NOx contents in rats with 6 h of WIRS. These results support the above-described suggestion that WIRS disrupts non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and NO• generation in the brain of rats with WIRS. The pre-administration of L-AsA attenuated decreased AsA, GSH, and vitamin E contents in the brain of rats with 6 h of WIRS. In addition, the same dose of L-AsA administered to unstressed rats in the same manner increased the brain AsA content. These results indicate that pre-administered L-AsA transported to the brain of rats with 6 h of WIRS restores AsA, GSH, and vitamin E by exerting antioxidant action in a direct manner and/or an indirect manner in the tissue. In contrast, the pre-administration of vitamin E enhanced the decreases in AsA and GSH contents in the brain of rats with 6 h of WIRS, although the vitamin E pre-administration returned the decreased brain vitamin E content to near the level of unstressed rats. The same dose of vitamin E administered to unstressed rats in the same manner increased the brain vitamin E content. As described above, vitamin E pre-administered to rats with 6 h of WIRS attenuated increased LPO and NOx contents in the brain. These results indicate that pre-administered vitamin E transported to the brain of rats with WIRS exerts antioxidant action in the tissue. It is known that not only AsA but also GSH can recycle vitamin E from vitamin E radical.(27,36) Therefore, the further reductions of decreased brain AsA and GSH contents by vitamin E pre-administration in rats with 6 h of WIRS seems to be explained as follows: reduced AsA and GSH in the brain of rats with 6 h of WIRS are further consumed to maintain pre-administered vitamin E transported to the brain, resulting in prevention of enhanced lipid peroxidation and NO• generation by the transported vitamin E in the tissue. These findings allow us to confirm that WIRS disrupts non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and NO• generation in the brain of rats.
In conclusion, the results of the present study indicate that WIRS causes disruption of non-enzymatic antioxidant defense systems through enhanced lipid peroxidation and NO• generation in the brain of rats. It has been reported that immobilization/restraint stress induces oxidative stress through disruption of enzymatic antioxidant defense systems as well as non-enzymatic antioxidant defense systems in the brain of rats.(4,7,9) It has also been reported that inflammation associated with expression of iNOS and cyclooxygense-2, nuclear factor κB activation, and release of cytokines such as tumor necrosis factor-α is involved in immobilization/restraint stress-induced oxidative stress in the brain of rats.(26,37,38) Therefore, further investigation is needed to verify whether and how disruption of non-enzymatic defense systems contributes to oxidative stress in the brain of rats with WIRS.
Abbreviations
- ACTH
adorenocorticotropic hormone
- AsA
ascorbic acid
- DTNB
5,5'-dithiobis(2-nitrobenzoic acid)
- EDTA
ethylenediaminetetraacetic acid
- GSH
reduced glutathione
- iNOS
inducible nitric oxide synthase
- LPO
lipid peroxide
- MDA
malondialdehyde
- NO•
nitric oxide
- ROS
reactive oxygen species
- Toc
tocopherol
- WIRS
Water-immersion restraint stress
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 2.Azhar S, Cao L, Reaven E. Aleration of the adrenal antioxidant defense system during aging in rats. J Clin Invest. 1995;96:1414–1424. doi: 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Wang X, Shigenaga MK, Yeo HC, Mori A, Ames BN. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996;10:1532–1538. [PubMed] [Google Scholar]
- 4.Zaidi SM, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. 2004;340:229–233. doi: 10.1016/j.cccn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Pal R, Gultai K, Chakraborti A, Banerjee B, Ray A. Role of free radicals in stress-induced neurobehavioural changes in rats. Indian J Exp Biol. 2006;44:816–820. [PubMed] [Google Scholar]
- 6.Gulati K, Chakrabotri A, Ray A. Differential role of nitric oxide (NO) in acute and chronic stress induced neurobehavioral modulation and oxidative injury in rats. Pharamcol Biochem Behav. 2009;92:272–276. doi: 10.1016/j.pbb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabotri A, Gulatri K, Banerjee BD, Ray A. Possible involvement of free radicals in the differential neurobehavioral responses to stress in male and female rats. Behav Brain Res. 2007;179:321–325. doi: 10.1016/j.bbr.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Gulatri K, Chakrabotri A, Ray A. Modulation of stress-induced neurobehavioral changes and brain oxidative injury by nitric oxide (NO) mimetics in rats. Behav Brain Res. 2007;183:226–230. doi: 10.1016/j.bbr.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Méndez-Cuesta LA, Márquez-Valadez B, Pérez-De la Cruz V, et al. Early changes in oxidative stress markers in a rat model of acute stress: effect of L-carnitine on the striatum. Basic Clin Pharmacol Toxicol. 2011;109:123–129. doi: 10.1111/j.1742-7843.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 10.Capel ID, Dorrell HM, Smallwood AE. The influence of cold-restraint stress on some components of the antioxidant defence system in the tissues of rats of various ages. J Toxicol Enviror Health. 1983;11:425–436. doi: 10.1080/15287398309530356. [DOI] [PubMed] [Google Scholar]
- 11.Nishida K, Ohta Y, Kobayashi T, Ishiguro I. Involvement of the xanthine-xanthine oxidase system and neurotrophils in the development of acute gastric mucosal lesions in rats with water immersion restraint stress. Digestion. 1997;58:340–351. doi: 10.1159/000201464. [DOI] [PubMed] [Google Scholar]
- 12.Nishida K, Ohta Y, Ishiguro I. Relation of inducible nitric oxide synthase activity to lipid peroxidation and nonprotein sulfhydryl oxidation in the development of stress-induced gastric mucosal lesions in rats. Nitric Oxide. 1998;2:215–223. doi: 10.1006/niox.1998.0178. [DOI] [PubMed] [Google Scholar]
- 13.Ohta Y, Kamiya Y, Imai Y, Arisawa T, Nakano H. Role of gastric mucosal ascorbic acid in gastric mucosal lesion development in rats with water immersion restraint stress. Inflammopharmacology. 2005;13:249–259. doi: 10.1163/156856005774423881. [DOI] [PubMed] [Google Scholar]
- 14.Ohta Y, Chiba S, Tada M, Imai Y, Kitagawa A. Development of oxidative stress and cell damage in the liver of rats with water-immersion restraint stress. Redox Rep. 2007;12:139–147. doi: 10.1179/135100007X200218. [DOI] [PubMed] [Google Scholar]
- 15.Ohta Y, Imai Y, Kaida S, Kamiya Y, Kawanishi M, Hirata I. Vitamin E protects against stress-induced gastric mucosal lesions in rats more effectively than vitamin C. Biofactors. 2010;36:60–69. doi: 10.1002/biof.73. [DOI] [PubMed] [Google Scholar]
- 16.Kaida S, Ohta Y, Imai Y, Kawanishi M. Protective effect of L-ascorbic acid against oxidative damage in the liver of rats with water-immersion restraint stress. Redox Rep. 2010;15:11–19. doi: 10.1179/174329210X12650506622925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohta Y, Kaida S, Chiba S, et al. Involvement of oxidative stress in increases in the serum levels of various enzymes and components in rats with water-immersion restraint stress. J Clin Biochem Nutr. 2009;45:347–354. doi: 10.3164/jcbn.09-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 19.Guillemin R, Clayton GW, Lipscomb HS, Smith JD. Fluorometric measurement of rat plasma and adrenal corticosterone concentration; a note on technical details. J Lab Clin Med. 1959;53:830–832. [PubMed] [Google Scholar]
- 20.Zannoni V, Lynch M, Goldstein S, Sato P. A rapid micromethod for the determination of ascorbic acid in plasma and tissues. Biochem Med. 1974;11:41–48. doi: 10.1016/0006-2944(74)90093-3. [DOI] [PubMed] [Google Scholar]
- 21.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 22.Kamiya Y, Ohta Y, Imai Y, Arisawa T, Nakano H. A critical role of gastric ascorbic acid in the progression of acute gastric mucosal lesions induced by compound 48/80 in rats. World J Gastroenterol. 2005;11:1324–1332. doi: 10.3748/wjg.v11.i9.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 25.You JM, Yun SJ, Nam KN, Kang C, Won R, Lee EH. Mechanism of glucocorticoid-induced oxidative stress in rat hippocamapal slice cultures. Can J Physiol Pharmacol. 2009;87:440–447. doi: 10.1139/y09-027. [DOI] [PubMed] [Google Scholar]
- 26.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 27.Madrigal JL, Moro MA, Lizasoain I, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor κB-mediated mechanisms. J Neurochem. 2001;76:532–538. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 28.Wei T, Chen C, Hou J, Xin W, Mori A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim Biophys Acta. 2000;1498:72–79. doi: 10.1016/s0167-4889(00)00078-1. [DOI] [PubMed] [Google Scholar]
- 29.Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and corenzyme Q. J Bioenerg Biomenbr. 1994;26:349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- 30.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 31.Vatassery GT. In vitro oxidation of vitamins C and E, cholesterol, and thiols in rat brain synaptosomes. Lipids. 1995;30:1007–1013. doi: 10.1007/BF02536285. [DOI] [PubMed] [Google Scholar]
- 32.Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23:147–169. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- 33.d’Ischia M, Novellino L. Nitirc oxide-induced oxidation of α-tocopherol. Bioorg Med Chem. 1996;4:1747–1753. doi: 10.1016/0968-0896(96)00191-5. [DOI] [PubMed] [Google Scholar]
- 34.Escames G, Guerrrero JM, Reiter RJ, et al. Melatonin and vitamin E limit nitric oxide-induced lipid peroxidation in rat brain homogenates. Neurosci Lett. 1997;230:147–150. doi: 10.1016/s0304-3940(97)00498-9. [DOI] [PubMed] [Google Scholar]
- 35.Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr. 1991;54 (Suppl 6):1119S–1124S. doi: 10.1093/ajcn/54.6.1119s. [DOI] [PubMed] [Google Scholar]
- 36.Niki E, Tsuchiya J, Tanimura R, Kamiya Y. Regeneration of vitamin E from α-chromanoxy radical by glutathione and vitamin C. Chem Lett. 1982:789–792. [Google Scholar]
- 37.Madrigal JL, Moro MA, Lizasoain I, et al. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- 38.Carcía-Burno B, Mardrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC. The anti-inflamatory prostaglandin 15d-PGJ2 decreases oxidative/nitrosative mediators in brain after acute stress in rats. Psychopharmacology (Berl) 2005;180:513–522. doi: 10.1007/s00213-005-2195-5. [DOI] [PubMed] [Google Scholar]