Abstract
An impaired generation of nitric oxide has been associated with diabetic renal disease. In order to elucidate the underlying molecular mechanisms into how nitric oxide synthesis is impaired in diabetic renal disease, we examined changes in activities and expressions of some renal enzymes involved in nitric oxide production during the development of diabetic nephropathy in type II diabetic Otsuka Long-Evans Tokushima Fatty rats. Ten-week old Otsuka Long-Evans Tokushima Fatty (n = 40) and control Long-Evans Tokushima Otsuka rats (n = 20) were given drinking water containing 20% sucrose to accelerate the development of diabetic nephropathy. Otsuka Long-Evans Tokushima Fatty rats developed diabetic nephropathy in an age-dependent manner. Renal nitric oxide synthase activities in Otsuka Long-Evans Tokushima Fatty rats gradually declined with the progression of diabetic mellitus and were significantly lower than those of age-matched Long-Evans Tokushima Otsuka rats after 22 weeks of age. The lower activities of renal nitric oxide synthase in Otsuka Long-Evans Tokushima Fatty rats were correlated with relatively higher levels of renal free asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, and were also correlated with decreased activities of dimethylargininedimethylaminohydrolase which metabolizes asymmetric dimethylarginine to citrulline. These results imply that dimethylargininedimethylaminohydrolase dysregulation may play an important role in the development of diabetic nephropathy by increasing asymmetric dimethylarginine levels, which leads to inhibition of renal nitric oxide synthesis.
Keywords: diabetic nephropathy, nitric oxide, nitric oxide synthase, dimethylarginine dimethylaminohydrolase, asymmetric dimethylarginine
Introduction
Endothelial vasodilator dysfunction is the central pathophysiologic denominator for cardiovascular complication of diabetes including nephropathy.(1) About 30–40% of diabetic patients develop nephropathy which has emerged as the leading cause of end-stage renal disease (ESRD) and also contributes to cardiovascular mortality.(2–4) Although about 90% of diabetic patients are affected with type II diabetes, most of the studies on the pathogenesis of diabetic nephropathy have been conducted with streptozotocin (STZ)-induced type I diabetic models. In addition, the renal lesions in type II diabetes are heterogeneous and more complex than those found in type I diabetes.(5) For these reasons, pathophysiological studies are needed to elucidate the molecular mechanisms of nephropathy in type II diabetic models.
Nitric oxide (NO), identified as an endothelium-derived relaxing factor (EDRF), plays an important role in vascular function and homeostasis, and therefore is a critical modulator of both renal function and morphology.(6) NO is synthesized through the oxidation of L-arginine by three NO synthase isoforms (NOS). Within the kidney, neuronal NOS (nNOS; type I) is abundantly distributed in the cytoplasm of the macula densa (MD) region (distal tubule and renal nerves) and has been involved in the regulation of tubulogomerular feedback and glomerular capillary pressure.(7) Endothelial NOS (eNOS; type III) is typically expressed in endothelial cells along the renal vascular tree and has been identified in the renal vasculature.(8) Constitutive NOSs (nNOS and eNOS), both Ca2+/calmodulin-dependent enzymes, release small amounts of NO (pmol). In contrast, Ca2+/calmodulin-independent inducible NOS (iNOS; type II) generates large amounts of NO (nmol) upon stimulation with inflammatory cytokines, such as IL-1β, IFN-γ and TNF-α, in various cell types, including glomerular mesangial cells and tubular cells along the nephron and cortex.(9) NO generation depends on the bioavailability of the NOS substrate L-arginine which is shared by arginase, which metabolizes L-arginine to form urea and ornithine in the urea cycle.(10) Although the NO-NOS system in the diabetic kidney has been extensively studied using different experimental settings, contradictory findings have been reported.(8)
NG, NG-Dimethyl-L-arginine (asymmetric dimethylarginine; ADMA) is an endogenous potent NOS inhibitor and may play a regulatory role in the L-arginine-NO pathway. ADMA and NG-monomethyl-L-arginine (NMMA), but not NG, NG'-dimethyl-L-arginine (symmetric dimethylarginine, SDMA), competitively inhibit NOS activity. Evidence from animal experiments and clinical studies suggest that elevated levels of ADMA contribute to reduce NO generation and pathogenesis of various diseases, including hypertension, chronic renal failure and diabetes mellitus.(11–13) Free methylarginines are released by the proteolysis of methylated L-arginine residues in various proteins which are formed by arginine N-methyltransferase (PRMT)-1 as a posttranslational modification of proteins. The metabolic pathways for the removal of these products are, in part, mediated by the renal excretion system. In addition, ADMA and NMMA are mainly hydrolytically degraded to L-citrulline in the cytoplasm by dimethylargininedimethylaminohydrolases (DDAH) which are expressed as the type 1 and 2 isoforms.(14) DDAH1, which colocalizes with nNOS and is predominantly expressed in the proximal tubules of the kidney, may be important for the metabolism of ADMA. DDAH2, which is colocalized with eNOS and expressed in the vasculature, may be involved in the regulation of local ADMA levels and renal hemodynamics.(15)
Previous studies have reported that levels of serum ADMA are increased in insulinopenic diabetes, and regulation and localization of DDAH isoforms are altered in STZ-induced type I diabetic rat kidney, suggesting that site-specific regulation of the ADMA metabolism is impaired.(16) However, the effects of spontaneously developed type II diabetes on the activity and expression of various renal enzymes involved in the regulation of L-arginine-NO metabolism (including NOS isoforms, DDAH isoforms and arginase) have not been explored. For these reasons, we have investigated whether the renal free levels of ADMA and L-arginine-NO-metabolizing enzymes are altered in type II diabetic model rats (OLETF, Otsuka Long-Evans Tokushima Fatty rats), which spontaneously develop type II diabetes mellitus, and show glomerular sclerosis similar to those seen in human diabetic nephropathy.(17) It has been reported that the cause of diabetes in OLETF rats seems to be a combination of insulin resistance and impaired insulin secretion.(18,19) Using these type II diabetic model rats, we demonstrate here that decreased activities of DDAH observed in OLETF rat kidney leads to increased levels of ADMA and eNOS inhibition, which may be associated with the development and progression of diabetic nephropathy.
Materials and Methods
Materials
Nicotinamide adenine dinucleotide phosphate reduced form (NADPH), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), (6R)-5,6,7,8-tetrahydrobiopterin (BH4), Dowex® AG50W-X8 cation-exchange resin (mesh size, 100–200), and ammonium formate were purchased from Sigma Chemicals (St. Louis, MO). NG-Hydroxy-nor-arginine (nor-NOHA) was obtained from Cayman Chemicals (Ann Arbor, MI). 2,3,3,4,4,5,5,-D7-ADMA and U-13C6,U-15N4-L-arginine were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). ECL Western Blotting Detection Reagent and Hybond-ECLTM and secondary antibodies were purchased from GE Healthcare, (Buckinghamshire, UK). All other chemicals were obtained from Wako Pure Chemical Ind., Osaka, Japan.
Animals
The protocols for the present animal experiments were approved by the Committee of the Institutional Animal Experiments (#2008-02036). All handling and procedures were carried out in accordance with the Institutional Animal Care Guidelines. Four-week-old male OLETF rats (type II diabetic model, n = 40) and age-matched Long-Evans Tokushima Otsuka rats (LETO, non-diabetic control, n = 20) were supplied by the animal center of Tokushima Research Institute (Otsuka Pharmaceutical Co., Tokushima, Japan). They were housed at a temperature of 22–24°C and a humidity of 50–60%, with a 12-h light cycle and had free access to tap water and a diet of standard chow (CE-2, CLEA, Tokyo, Japan). At the age of 10 weeks, OLETF and LETO rats were given drinking water containing 20% sucrose in the subsequent experimental period. Groups of eight OLETF and four LETO rats were sacrificed at the ages of 10, 14, 18, 22, and 27 weeks with pentobarbital anesthesia (60 mg/kg) after fasting for 6 h. Blood samples collected via the abdominal aorta were centrifuged at 3000 rpm for 15 min at 4°C to obtain the plasma fraction, which were kept at –80°C. Various organs were removed from the rats and were immediately frozen in liquid nitrogen and then kept at –80°C until analysis.
Oral glucose tolerance test
An oral glucose tolerance test (OGTT) was performed for each rat one week before the sacrifice. Rats were fasted overnight (from 19:00 to 9:00) before the test and then a 20% glucose solution was orally administered to the rats (2 g glucose/kg body weight). Blood samples were obtained from the tail vein by capillary with lithium heparinat 0, 15, 30, 60, 120, and 180 min after the administration of glucose. Glucose concentrations in plasma were determined by a Glucose-CII Test (Wako Pure Chemical). Insulin concentrations in plasma obtained at 0, 30, 60 and 120 min were determined by an ELISA Rat insulin kit (Shibayagi Co. Ltd., Gunma, Japan). Plasma adiponectin concentrations were measured by an Adiponectin ELISA kit (Otsuka Pharmaceutical Co.). Other plasma biochemical parameters including plasma HDL cholesterol, total cholesterol, LDL cholesterol, triglyceride and glycoalbumin were measured by BML, Inc. (Tokyo, Japan).
Histological study
Parts of each kidney were fixed in 70% ethyl alcohol, dehydrated in a series of graded alcohol solutions, and then embedded in paraffin. Kidney sections of 7 µm thickness were stained with hematoxylin and eosin (H&E) or periodic acid Schiff (PAS).
Assays for renal NOS and DDAH activities
For assays of NOS activities, kidney tissues (0.2 g) were homogenized in 0.5 ml of ice-cold buffer consisting of 50 mM Tris-HCl (pH 7.4), 1 mM phenylmethylsulfonyl fluoride, 0.5 mM sodium orthovanadate and a cocktail of protease inhibitors (Roche, Basel, Switzerland). The homogenates were centrifuged at 5000 rpm for 20 min at 4°C. Protein concentrations in the supernatant were determined by a BCA protein assay kit (Thermo FisherScientific, Waltham, MA) using bovine serum albumin as a standard. The NOS activities were determined using aliquots (0.4 mg of protein) by monitoring the conversion of L-[14C(U)]-arginine (Moravek, Brea, CA) to L-[14C(U)]-citrulline as previously described.(20) For assays of DDAH activities, the tissues were homogenized using a buffer consisting of 0.1 M sodium phosphate buffer (SPB, pH 6.5). The DDAH activity was determined using aliquots (1 mg of protein) by monitoring the conversion of L-[4,5-3H]-NMMA (American Radiolabeled Chemicals, Inc., St. Louis, MO) to L-[3H]-citrulline as previously described.(20)
Western blot analyses
Aliquots (80 µg of protein) of kidney homogenates were separated by 7.5% SDS-polyacrylamide gel. Proteins transferred onto a nitrocellulose membrane were blocked with 5% milk solution. The membranes were probed with primary antibodies against eNOS, DDAH1 and DDAH2 (Santa Cruz Biotechnology, CA), or iNOS (Abcam, Cambridge, MA) and were incubated overnight at 4°C. After incubation with horseradish peroxidase-conjugated secondary antibody, the bands were visualized by ECL reagents using a LAS3000 mini (Fujifilm, Tokyo, Japan). Membranes were reprobed and incubated again with a primary antibody against β-actin (Sigma, St. Louis, MO) and the obtained signals were used as an internal standard.
Analysis of free dimethylarginines and L-arginine
Plasma and tissue levels of ADMA, SDMA and L-arginine were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Briefly, 10 µl of an internal standard solution containing 2,3,3,4,4,5,5,-D7-ADMA and U-13C6,U-15N4-L-arginine at 100 nM was added to 200 µl of plasma (10 µg of protein) or kidney homogenates (100 µg of protein). Proteins were removed from samples by mixing with 500 µl of acetonitrile. Samples were left on ice for 10 min and then centrifuged at 5000 rpm for 5 min at 4°C. Supernatants were dried down in a vacuum centrifuge and the residues were dissolved with 1 ml of milliQ water. Samples were applied to preconditioned columns (Oasis MCX SPE 50 mg/1 cc, Waters, Milford, MA), which were washed with 1 ml of 100 mM HCl followed by 1 ml of MeOH and then the amino acids were eluted with 1 ml of elution buffer (ammonia/water/MeOH = 1:4:5). Eluents were dried and dissolved with 200 µl of 30% acetonitrile water for LC-MS/MS analysis. LC-MS/MS was performed on an Agilent 1200 series HPLC system using an Atlantis® HILIC Silica column (3 µm 2.1 × 150 mm, Waters, MA) and an Agilent G6410B triple quadrupole tandem mass spectrometer with an electrospray ionization device running in positive ionization mode. The mobile phase (isocratic) consisted of 0.1% formic acid in acetonitrile: 10 mM ammonium formate: isopropyl alcohol = 60:35:5 at a flow rate of 0.2 ml/min. The collision activated dissociation gas was set at medium using nitrogen as the collision gas. The detection of ADMA, SDMA and L-arginine was performed using the multiple reaction monitoring (MRM) mode. The ion transitions monitored for ADMA, SDMA and L-arginine were m/z 203.1/46.1, 203.1/172.0, and 175.1/70.1, respectively. Under these conditions, the recoveries of ADMA, SDMA and L-arginine were 98.3 ± 5.4, 90.0 ± 3.0, and 87.3 ± 6.5% (n = 5), respectively.
Statistical analysis
Representative results are expressed as the mean ± SD. Differences between experimental groups were investigated by analyses of variance, and the significance of differences between groups was compared statistically using an unpaired t test.
Results
Body weight, plasma glucose and insulin levels during an oral glucose tolerance test
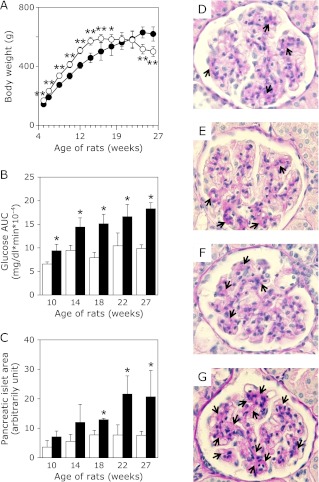
OLETF rats were significantly heavier than LETO rats between 8 and 14 weeks of age (Fig. 1A). However, further increases in the body weights of OLETF rats were not observed after 18 weeks of age, but rather their body weights were markedly decreased between 24 and 27 weeks and were lower than those of LETO rats at the same age. This reduction of body weights was partly due to a severe decrease in the weights of abdominal fats in OLETF rats (data not shown). Fig. 1B shows the results of total areas under the curve (AUC) for the plasma glucose levels during OGTT in both rats. AUC values of plasma glucose in OLETF were increased age-dependently. Although glucose AUCs in LETO rats were slightly increased by the sucrose administration, they were significantly less than those of OLETF. Moreover we observed impaired insulin secretion in OLETF rats (Supplemental Fig. 1*). By histological analyses, we also observed enlarged pancreatic islet areas (Fig. 1C and Supplemental Fig. 2*) and varying extents of connective tissue proliferation (data not shown) in OLETF, but not in LETO. PAS staining showed that the mesangial areas were significantly dilated in OLETF kidney glomerulus at 27 weeks compared with those in OLETF rats at 10 weeks of age or LETO rats at 10 and 27 weeks of age, suggesting that their mesangial cells proliferated (Fig. 1 D–G). Moreover, OLETF rats developed various metabolic abnormalities including increased visceral fat weight, hyperlipidemia as well as hypoadiponectimia and increased glycoalbumin levels (data not shown). These parameter changes suggest that OLETF rats developed severe diabetes mellitus age-dependently and also had increased risk of developing diabetic vascular complications. On the other hand, LETO rats appeared to develop low-grade diabetic symptoms, which might be caused by administration of 20% sucrose during the experimental period.
Fig. 1.
Alteration of pathophysiological parameters in LETO and OLETF rats. A, Body weight changes of LETO rats (○, n = 4 for each time point) and OLETF rats (I, n = 8 for each point) during the course of the experiment. B, Plasma glucose AUC of LETO rats (open bars, n = 4 in each time points) and OLETF rats (closed bars, n = 8 in each points) in OGTT. C, Alteration of the pancreatic islet area of LETO (open bars, n = 3 in each time point) and OLETF (closed bars, n = 3 in each time point) in OGTT. Areas of islet (3–46 in each rats) on pancreatic section after HE staining were measured by NIH image software. D–G, Histological features of the glomeruli in LETO rats and OLETF rats. PAS staining demonstrates expansion of mesangial area (arrows) in LETO rat at 10 (D), 27 (E) weeks and in OLETF rats at 19 (F), 27 (G) weeks. Values are mean ± SD. Statistically significant: *p<0.05, **p<0.01 when compared with age-matched LETO rats.
Renal activity and protein expression of NOS
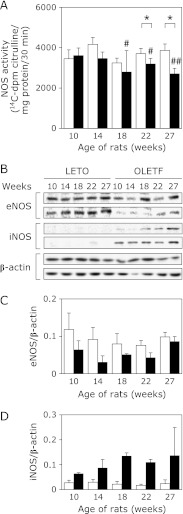
It has been reported that diabetic subjects with renal disease often have an impaired generation of NO, which is a key vasodilator involved in keeping the endothelium functioning. Here we examined the pathophysiological alterations involved in the regulation of NO production. Fig. 2A shows NOS activities measured in homogenates of the whole kidney from both OLETF and LETO rats at different ages. Although renal NOS activities in LETO rats were not significantly changed during the entire experimental period, they were decreased age-dependently in OLETF rats. The renal NOS activities of 27-week old OLETF rats were about 30% lower than those of LETO rats at the same age (Fig. 2A). By contrast, protein expression of eNOS in the whole kidney was detected in all LETO rats at different ages and the levels were not obviously altered with increasing age (Fig. 2 B and C). However, the eNOS expression levels in the kidney of OLETF rats were markedly reduced with increasing age (Fig. 2 B and C). Immunohistochemical analyses showed that higher levels of eNOS, but not iNOS, located at kidney glomerulus were detected in LETO compared to those in OLETF (Supplemental Fig. 3*). Immunoblot analyses also revealed that although iNOS protein was undetectable in the kidney of LETO rats, they were remarkably increased in that of OLETF rats of different ages, with a peak at 18 weeks of age (Fig. 2 B and D). nNOS expression in the kidney was undetectable in both rat groups (data not shown). Taken together, although iNOS was markedly induced in OLETF but not in LETO, total renal NOS activities in OLETF were significantly lower than those of LETO, indicating that eNOS was dominant in renal NOS activity and the localization of eNOS and iNOS in kidney tissues may be important in the pathology of diabetic nephropathy.
Fig. 2.
Renal NOS activities and protein expression levels in LETO and OLETF rats. A, NOS activities measured in whole kidney homogenates of LETO rats (open bars, n = 4) and OLETF rats (closed bars, n = 7–8). B, Expression of eNOS, iNOS and β-actin proteins in the kidney of LETO rats (n = 2) and OLETF rats (n = 2) determined by western blotting analyses. C and D, summarize eNOS/β-actin iNOS/β-actin expressions. Values are mean ± SD. Statistically significant: *p<0.05, **p<0.01 when compared to age-matched LETO rats, and #p<0.05, ##p<0.01 when compared with 10 week old rats.
Renal activity and protein expression of DDAH
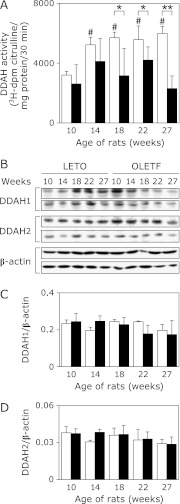
DDAH activities were also measured in the kidney of both LETO and OLETF rats. DDAH activities were markedly elevated in LETO rats with increasing age after sucrose administration (Fig. 3A). In contrast, OLETF rats did not show such an age-dependent increase in DDAH activities. Although the DDAH activities were not statistically significant among the different age groups of the OLETF rats, they tended to increase transiently after the start of sucrose-feeding and then declined at 27 weeks (Fig. 3A). When the kidney DDAH activities were compared between OLETF and LETO rats at the same age, the activities in OLETF rats were gradually and significantly decreased after 18 weeks and had reduced to about 40% of those of LETO rats at 27 weeks (Fig. 3A). Although protein expression levels of DDAH1 and 2 tended to decrease age-dependently (Fig. 3 B–D), these were not correlated to their age-dependent alteration of DDAH activities. Therefore these results suggest that alteration of DDAH activities observed in LETO and OLETF kidney may be mediated by posttranslational modifications rather than protein expression levels.
Fig. 3.
Renal DDAH activities and protein expression levels in LETO and OLETF rats. A, DDAH activities in whole kidney homogenates of LETO rats (open bars, n = 4) and OLETF rats (closed bars, n = 7–8). B, Expression of DDAH1, DDAH2, and β-actin proteins in the kidney in LETO rats (n = 2) and OLETF rats (n = 2). C and D, summarize DDAH1/β-actin and DDAH2/β-actin expressions. Values are mean ± SD. Statistically significant: *p<0.05, **p<0.01 when compared to age-matched LETO rats, and #p<0.01 when compared with 10 week old rats.
Quantitative analyses of renal free ADMA
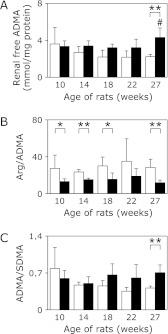
To investigate the association between lower DDAH and NOS activities in OLETF rats, we analyzed the levels of arginine, endogenous NOS inhibitor ADMA, and its symmetric isomer dimethylarginine (SDMA) using LC-ESI-MS/MS. Fig. 4A shows that levels of renal free ADMA in LETO rats gradually declined in an age-dependent manner. This decrease in ADMA levels was positively correlated with the increased DDAH activities. The levels of renal free ADMA in OLETF rats were not reduced during this experimental period, and therefore were higher than those of age-matched LETO rats (Fig. 4A). Moreover, the ratios of arginine/ADMA in OLETF rats were significantly lower than that of LETO rats (Fig. 4B). Also, the alteration of ADMA/SDMA ratios was clearly correlated with the levels of renal free ADMA (Fig. 4C), indicating that elevated levels of ADMA in OLETF rats were probably due to the low DDAH activities. Additionally, levels of plasma ADMA were not significantly different between LETO rats and OLETF rats, and may be due to systemic circulation (data not shown). Taken together, our results suggest that increased ADMA levels in the OLETF rat kidney were correlated with decreased DDAH activities and also possibly caused a decline in NOS activities during the development of type II diabetes in these rat kidneys.
Fig. 4.
Levels of renal free ADMA, SDMA, and arginine in LETO and OLETF rats. Renal free ADMA, SDMA, and arginine (Arg) in LETO rats (open bars, n = 3–4) and OLETF rats (closed bars, n = 5–6) were analyzed by LC-ESI-MS/MS as described in Materials and Methods section. Levels of ADMA (A), ratio of Arg/ADMA (B) and ADMA/SDMA (C) were summarized. Values are mean ± SD. Statistically significant: *p<0.05, **p<0.01 when compared to age-matched LETO rats, and #p<0.05 when compared with 10 week old rats.
Discussion
Diabetic nephropathy is currently one of the most serious complications of longstanding diabetes and has emerged as the most common cause of end-stage renal disease worldwide. Recently it has been shown that diabetic subjects with renal disease often have an impaired generation of NO, which is a key vasodilator involved in maintaining endothelium function.(8) It has been reported that eNOS knockout mice develop classic features of advanced diabetic nephropathy.(21) Moreover numerous studies have suggested that the accumulation of the NOS inhibitor, ADMA, may contribute to vasodilator dysfunction, and is closely correlated with the pathogenesis of a variety of chronic illnesses, including chronic renal failure and diabetes.(11,12) In this study, we investigated the pathophysiological roles of enzymes involved in the regulation of NO production in the development of diabetic nephropathy in type II diabetic mellitus. Here we employed OLETF rats, which spontaneously develop type II diabetes mellitus with induced glomerular sclerosis similar to what is observed in human diabetic nephropathy.(17) In our experimental protocol, it was confirmed that OLETF rats developed diabetic mellitus and nephropathy and control LETO rats also developed low-grade insulin resistance, which may be due to the administration of 20% sucrose in drinking water during the experimental period (Fig. 1). Using these diabetic model rats, we found the dysregulation of homeostasis involved in the renal L-arginine-NO pathway, which led to the pathogenesis of impaired NO generation in rat kidney during the development and progression of diabetic nephropathy.
Renal NOS activities and protein expression levels of eNOS and iNOS were not obviously altered in control LETO rats during the entire experimental period, although these rats developed low-grade insulin resistance. These results were in good agreement with previous reports showing that eNOS protein levels were unchanged or increased, and iNOS protein levels were undetectable in the glomeruli and renal cortex of rats with early diabetic nephropathy induced by STZ.(22,23) In contrast, the NOS activities in OLETF rats were significantly decreased age-dependently, with the activities being reduced to about 70% of those of LETO rats at 27 weeks of age. Activities of renal arginase, which competes with NOS for their common substrate L-arginine, were not different between LETO and OLETF rats, and also among rats with different ages (data not shown). Therefore the reduction in the NOS activities in OLETF rats may be mainly due to the decreased expression of eNOS protein levels, which were only about half of those detected in LETO rats. On the other hand, iNOS expression in the kidney of OLETF rats was significantly elevated in an age-dependent manner, suggesting that inflammation caused by diabetic nephropathy triggered the induction of iNOS expression. These results are in accordance with those reported for human glomerulonephritis, which showed that a loss of eNOS and an increase of iNOS immunoreactivities were correlated with the severity of glomerular injury.(24)
We also demonstrated here that the renal DDAH activities in LETO rats increased age-dependently although the protein expressions of two DDAH isoforms were not markedly altered. In contrast, although the activities of renal DDAH in OLETF rats were not significantly altered during the course of the development of diabetes, they were significantly lower than those of LETO rats after 18 weeks of age. It has been reported that the DDAH activity is affected by a posttranslational modification mediated by reactive oxygen species generated via NADPH oxidase.(25) In addition, Leiper et al.(26) have reported that DDAH activity is directly modulated by NO produced by cytokine induced-iNOS through nitrosylation of the reactive cysteine residue (Cys-249) present in the active site of DDAH. On the basis of these previous reports and our findings in the present study, we can speculate that the DDAH activity may be up-regulated to reduce levels of the endogenous NOS inhibitor ADMA, which leads to an increased production of a vasodilator (NO) in the early stage of diabetes. However iNOS is induced to produce high concentrations of NO which would nitrosylate the cysteine residues of the renal DDAH protein to reduce its activity during the course of diabetic nephropathy development.
In the present study, we found that renal DDAH activity was elevated in the kidney of low-grade diabetic LETO, which enabled them to metabolize ADMA to citrulline and thus potentially prevented the inhibition of eNOS by ADMA. Although the ratios of renal free arginine to ADMA in OLETF rats were lower than in LETO rats, they did not show this age-dependent alteration. These data suggest that ADMA excretion is impaired or arginine methyltransferase activities are elevated constitutively in OLETF rats. On the other hand, during the development of diabetes in OLETF rats, excessive oxidative/nitrative stress could be induced by prolonged exposure to high blood glucose and might suppress the DDAH activity, leading to increased ADMA concentration and subsequent disruption of normal eNOS function. Further studies are required to elucidate the molecular mechanism for dysregulation of the renal NO-producing system. Our results from this study support the notion that impaired generation of endothelium-dependent vasodilator NO, which is caused by ADMA-mediated inhibition of eNOS due to decreased DDAH activities, should play an important role in the pathogenesis of diabetic vascular dysfunction. Our results indicate that dysregulations of ADMA metabolism and/or DDAH would be the most important clinical pathophysiological findings associated with the diabetic nephropathy. It is hoped that further elucidation of the pathogenic mechanisms for the dysregulations of ADMA metabolism and/or DDAH enzyme functions will help to develop new strategies for the prevention of diabetic nephropathy and other serious complications in diabetic patients.
Acknowledgments
The authors thank Dr. Toshinao Goda and Dr. Kazuki Mochizuki for valuable discussions. This work was supportedby the Japanese Society for the Promotion of Science (2011572 to YLL); and by a Grant-in-Aid (21300280 to HO and 21680052 to NM) from the Ministry of Education, Culture, Sport, Science, and Technology; a Grant-in-Aid for Cancer Research (21-1-2) from the Ministry of Health, Labour, and Welfare, Japan; and the Global COE program at the University of Shizuoka.
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Alteration of plasma insulin levels in glucose-loaded OLETF (A, n = 7–8) and LETO (B, n = 4) rats during OGTT. An OGTT was performed for each rat one week before the sacrifice (at 10, 14, 18, 22, and 27 weeks of age). Rats were fasted overnight and then a 20% glucose solution was orally administered to the rats (2 g/kg body weight). Blood samples were collected from the tail vein by capillary with heparin. Insulin concentration in plasma obtained at 0, 30, 60 and 120 min after glucose loading were determined by an ELISA rat insulin kit.
Histological analysis of the islets in LETO (A–C) and OLETF (D–F) rats at 10 (A, D), 14 (B, E), 22 (C, F) weeks of age. Rat pancreas were fixed with formalin, dehydrated with ethanol, and embedded in paraffin. Standard hematoxylin and eosin staining were performed using the paraffin sections to examine the size of islets indicated by arrows.
Immunohistochemical analysis of eNOS (A) and iNOS (B). Rat kidneys were prepared for immunohistochemical analysis to determine the localization of eNOS (frozen section) and iNOS (formalin-fixed paraffin section). After blocking the endogenous peroxidase activity by incubating with 0.3% H2O2 in methanol for 15 min, the rat renal tissues were incubated overnight at 4°C with anti-eNOS or anti-iNOS antibody, washed, and reacted with secondary antibody conjugated with peroxidase-labeled polymer amino acid for 60 min. The peroxidase activity was visualized using 3,3'-diaminobenzidine. Counter staining was performed with Meyer’s hematoxylin. Arrows are indicating positive staining.
References
- 1.Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;24:333–344. doi: 10.1016/j.semnephrol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- 5.Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3:1458–1466. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 7.Ichihara A, Inscho EW, Imig JD, Navar LG. Neuronal nitric oxide synthase modulates rat renal microvascular function. Am J Physiol. 1998;274:F516–F524. doi: 10.1152/ajprenal.1998.274.3.F516. [DOI] [PubMed] [Google Scholar]
- 8.Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol. 2003;284:F1121–F1137. doi: 10.1152/ajprenal.00265.2002. [DOI] [PubMed] [Google Scholar]
- 9.Narita I, Border WA, Ketteler M, Noble NA. Nitric oxide mediates immunologic injury to kidney mesangium in experimental glomerulonephritis. Lab Invest. 1995;72:17–24. [PubMed] [Google Scholar]
- 10.Wu G, Morris SM,, Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surdacki A, Nowicki M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33:652–658. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 13.Fard A, Tuck CH, Donis JA, et al. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2000;20:2039–2044. doi: 10.1161/01.atv.20.9.2039. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989;264:10205–10209. [PubMed] [Google Scholar]
- 15.Tojo A, Kimoto M, Wilcox CS. Renal expression of constitutive NOS and DDAH: separate effects of salt intake and angiotensin. Kidney Int. 2000;58:2075–2083. doi: 10.1111/j.1523-1755.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes. 2008;57:172–180. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- 17.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, Asahi Y, Toide K, Nakayama N. Insulin resistance in skeletal muscle of the male Otsuka Long-Evans Tokushima Fatty rat, a new model of NIDDM. Diabetologia. 1995;38:1033–1041. doi: 10.1007/BF00402172. [DOI] [PubMed] [Google Scholar]
- 19.Ishida K, Mizuno A, Min Z, Sano T, Shima K. Which is the primary etiologic event in Otsuka Long-Evans Tokushima Fatty rats, a model of spontaneous non-insulin-dependent diabetes mellitus, insulin resistance, or impaired insulin secretion? Metabolism. 1995;44:940–945. doi: 10.1016/0026-0495(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 20.Lai YL, Aoyama S, Nagai R, Miyoshi N, Ohshima H. Inhibition of L-arginine metabolizing enzymes by L-arginine-derived advanced glycation end products. J Clin Biochem Nutr. 2010;46:177–185. doi: 10.3164/jcbn.09-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 22.Ishii N, Patel KP, Lane PH, et al. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol. 2001;12:1630–1639. doi: 10.1681/ASN.V1281630. [DOI] [PubMed] [Google Scholar]
- 23.Veelken R, Hilgers KF, Hartner A, Haas A, Böhmer KP, Sterzel RB. Nitric oxide synthase isoforms and glomerular hyperfiltration in early diabetic nephropathy. J Am Soc Nephrol. 2000;11:71–79. doi: 10.1681/ASN.V11171. [DOI] [PubMed] [Google Scholar]
- 24.Furusu A, Miyazaki M, Abe K, et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney Int. 1998;53:1760–1768. doi: 10.1046/j.1523-1755.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 25.Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW, Li YJ. Lysophosphatidylcholine-induced elevation of asymmetric dimethylarginine level by the NADPH oxidase pathway in endothelial cells. Vascul Pharmacol. 2006;44:143–148. doi: 10.1016/j.vph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA. 2002;99:13527–13532. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alteration of plasma insulin levels in glucose-loaded OLETF (A, n = 7–8) and LETO (B, n = 4) rats during OGTT. An OGTT was performed for each rat one week before the sacrifice (at 10, 14, 18, 22, and 27 weeks of age). Rats were fasted overnight and then a 20% glucose solution was orally administered to the rats (2 g/kg body weight). Blood samples were collected from the tail vein by capillary with heparin. Insulin concentration in plasma obtained at 0, 30, 60 and 120 min after glucose loading were determined by an ELISA rat insulin kit.
Histological analysis of the islets in LETO (A–C) and OLETF (D–F) rats at 10 (A, D), 14 (B, E), 22 (C, F) weeks of age. Rat pancreas were fixed with formalin, dehydrated with ethanol, and embedded in paraffin. Standard hematoxylin and eosin staining were performed using the paraffin sections to examine the size of islets indicated by arrows.
Immunohistochemical analysis of eNOS (A) and iNOS (B). Rat kidneys were prepared for immunohistochemical analysis to determine the localization of eNOS (frozen section) and iNOS (formalin-fixed paraffin section). After blocking the endogenous peroxidase activity by incubating with 0.3% H2O2 in methanol for 15 min, the rat renal tissues were incubated overnight at 4°C with anti-eNOS or anti-iNOS antibody, washed, and reacted with secondary antibody conjugated with peroxidase-labeled polymer amino acid for 60 min. The peroxidase activity was visualized using 3,3'-diaminobenzidine. Counter staining was performed with Meyer’s hematoxylin. Arrows are indicating positive staining.