Abstract
Metabolic syndrome is one of the major factors to increase the incidence of heart failure. In our study, we compared plasma fatty acid compositions among heart failure patients with and without Metabolic syndrome. Fatty acid (FA) composition of plasma phospholipids was analyzed and the activities of desaturase were estimated as the ratio of substrate and product fatty acids in 85 stable heart failure patients. Fatty acid and estimated desaturase activities were further examined for their associations with Metabolic syndrome components. Heart failure patients with Metabolic syndrome showed significant changes in fatty acid composition in comparison to those without Metabolic syndrome, which had a decreased proportion of lauric acid (C12:0) and an increased proportion of dihomo-γ-linolenic acid (C20:3n-6). Also, estimated desaturase activities (D5D and D6D) were closely related to Metabolic syndrome condition among heart failure patients. The content of dihomo-γ-linolenic acid showed positive correlations with BMI, waist circumference, and plasma triglyceride levels. D6D were positively associated with plasma triglyceride levels, whereas D5D showed a negative correlation with plasma triglyceride levels and waist circumferences. The content of dihomo-γ-linolenic acid as well as estimated D6D and D5D were altered in heart failure patients with Metabolic syndrome.
Keywords: heart failure, fatty acid, metabolic syndrome, dihomo-γ-linolenic acid, desaturase
Introduction
As one of the most common chronic diseases, heart failure (HF) has become prevalent over time in both developing and developed countries.(1,2) Even after HF diagnosis, the HF incidence barely diminished according to a population-based cohort study.(3) Along with an increasing prevalence of HF, it has become evident that the mortality and morbidity of HF is adversely affected by one of metabolic syndrome (MetS) risk factor including insulin resistance, obesity, and dyslipidemia.(4,5) Accumulating data have indicated that the importance of consideration of MetS in HF patients.(6–8) Several studies have reported that MetS was a significant predictor of HF, independent of established risk factors for HF.(9,10) These indicate that individual risk factors such as insulin resistance, dyslipidemia, obesity and hypertension reflected by MetS have involved in the underlying mechanisms explaining association between MetS and HF mortality. Besides the components of MetS, it was also reported that alterations in FAs composition in plasma and red blood cells have been related to metabolic diseases.(11) Numerous studies have studied on the roles of dietary intakes and blood compositions of FAs in various metabolic disorders such as diabetes,(12) coronary heart disease(11,13) or risk factors for MetS.(11–13) For example, elevated saturated fatty acid (SFA) intake is associated with the development of insulin resistance and thereby increased chances of progression to MetS.(14) Also, decreased plasma levels of polyunsaturated fatty acids (PUFAs) especially n-3 PUFAs were observed with individuals with MetS, compared to the healthy subjects.(15) Among newly diagnosed insulin resistance patients, differential fatty acid composition was detected, shown as a decrease in linoleic acid (18:2) and an increase in gamma-linolenic (18:3), dihomo-γ-linolenic acid (DGLA) (20:3) and arachidonic acid (20:4).(12,16) In addition, recent studies have proposed estimated desaturase activities using the ratio of a product to precursor fatty acids as an emerging risk factor for MetS.(13,17) In the case of Japanese men with MetS, FA composition from plasm cholesteryl esters indicates higher delta 9 desaturase activity and lower delta 5 desaturase activity.(17) Also, delta 5 desaturase activity has been reported to be lower in the condition of myocardial infarction.(13) These suggest that FA composition and estimated desaturase activity might have pathological role in chronic diseases either independently or as part of MetS.
Previously, it has been reported that abnormally higher or lower composition of specific FAs were associated with the risks for HF.(18–23) Also healthy subjects with MetS and without MetS were examined for their FA compositions.(17,24) However, the differences in FA composition and desaturase activities in HF patients with the MetS from those without the MetS have not been studied. MetS is a major factor accelerating HF pathological conditions in patients and their FA metabolism could be differentially affected by HF in conjunction with MetS. Therefore, in this study we aimed to compare plasma phospholipid FA compositions and estimated desaturase activities among HF patients with and without MetS to identify specific FA aberration in HF patients with MetS. We compared stearoyl-CoA desaturase (SCD), delta 6 desaturase (D6D) and delta 5 desaturase (D5D) between the groups which have been previously reported to be related to the MetS.(25–27) Correlations of MetS components with FAs or desaturase activities in HF patients were further evaluated.
Subjects and Methods
Study subjects
The total study population consisted of 177 eligible consecutive ambulatory HF patients enrolled at 2 separate sessions (91 subjects from the first and 87 subjects from the second session) from HF outpatient clinic at Yonsei Cardiovascular Center.(28) Inclusion criteria were (1) ages no greater than 80 years (2) systolic HF diagnosed with a left ventricular ejection fraction (LVEF) lower than 50% (3) stable HF on medication for at least 1 month prior to the study. We omitted patients (1) with HF with preserved ejection fraction greater than 50%, (2) who had acute myocardial infarction 3 months prior to the study, or (3) who had a severe cognitive impairment. All patients provided written informed consent. The Institutional Review Board at Yonsei University Medical Center approved the study protocol. Hospital database was accessed to obtain medical history including diagnosis, underlying disease, etiology of HF and drug use. Among the total 177 subjects, plasma FA composition data were obtained only in the 85 subjects whose samples were available for the measurement and also, those who were not taking special dietary substitutes such as omega 3 and conjugated linoleic acid supplementations. Fasting venous blood samples were collected from the forearm of the patients in EDTA-treated and plain tubes. MetS was defined when three or more of the following criteria were met, which is a modified NCEP ATP III definition (ATP III criteria and the WHO Western Pacific Region obesity criteria). (1) abdominal obesity is defined when waist circumference is greater than 90 cm in men and 80 cm in women; (2) hypertriglyceridemia is greater than 150 mg/dl; (3) hypertension is either systolic blood pressure greater than 130 mmHg or diastolic pressure greater than 85 mmHg, or on anti-hypertensive medication; (4) low HDL-cholesterol is less than 40 mg/dl in men and 50 mg/dl in women; (5) high fasting glucose is greater than 110 mg/dl or under treatment for diabetes. Waist circumference was measured twice to the nearest 0.1 cm at the level of the navel. Body weight and height were measured and BMI was calculated as the ratio of body weight (kg) to height (m2).
Blood lipid profiles and glucose concentrations
Plasma cholesterol, LDL-cholesterol, and HDL-cholesterol from fasting blood samples were measured with enzymatic methods using commercially available kits (Choongwae, Seoul, Korea). Plasma triglyceride (TG) levels were assessed by a total glycerol test kit (Roche, Basel, Switzerland). All determinants were obtained on a Hitachi 747 analyzer (Tokyo, Japan). Fasting plasma glucose levels were analyzed by the glucose oxidase method using a Beckman Glucose Analyzer (Irvine, CA).
Plasma phospholipid FA composition and estimation of desturase activities
Plasma total lipids were extracted according to the Folch method(29) and the phospholipid fraction was isolated by thin-layer chromatography using a development solvent composed of hexane, diethyl ether, and acetic acid (80:20:2). The phospholipid fractions were then methylated to FA methyl esters (FAMEs) by the Lepage and Roy method.(30) The FAMEs of individual FAs of phospholipids were separated by gas chromatography (model 6890, Agilent Technologies Inc., Palo Alto, CA), using a capillary column (Omegawaz TM 250; 30 m, Supelco, Bellefonte, PA), as previously described.(31) Peak retention times were obtained by comparison with known standards (37 component FAME mix and PUFA-2, Supelco, Bellefonte, PA; GLC37, NuCheck Prep., Elysian, MN) and analyzed with ChemStation software (Agilent Technologies). Plasma phospholipid FAs were expressed as the percentage of total FAs. The desaturase activities were calculated as the ratio of product to precursor fatty acids. SCD activities are defined by C16:1 n-7/C16:0 (SCD n-7) and C18:1 n-9/C18:0 (SCD n-9). D6D is C20:3 n-6/C18:2 n-6 and D5D is C20:4 n-6/C20:3 n-6.(25–27)
Statistical analysis
The SPSS 12.0 software package was used for statistical analysis. Data are presented as the mean ± SD. Each variable was examined for normal distribution, and abnormally distributed variables such as TG and HDL-cholesterol were log-transformed. Differences in variables between the groups were evaluated using Student’s t test and χ2 test. General Linear Model (GLM) was used to test the differences of parameters between the groups after adjusting age and BMI p value less than 0.05 was considered statistically significant.
Results
Characteristics of HF subjects with MetS or without MetS
Among the total of 85 HF subjects, 26 subjects were classified as having the MetS. Gender difference and ejection fraction were similar between subjects with MetS and without MetS. As for an average age, subjects with MetS demonstrated older average age of 66.4 ± 9.7 years than those without MetS showing 58.9 ± 13.2 years (p<0.05). Proportion of the subjects with HF-related cariomyopathy or valvular disease was not different between the two groups. Also, medical treatments for HF except ACEI and/or ARB were similar for all HF subjects. The prevalence of stroke and chronic renal failure between the two groups was similar, whereas that of type 2 diabetes mellitus was comparable between the groups (Table 1).
Table 1.
Age, gender, EF, anthropometric measurements and medical treatment between HF patients without MetS and HF patients with MetS
| HF without MetS (n = 59) | HF with MetS (n = 26) | |
|---|---|---|
| Age (yrs) | 58.9 ± 13.2 | 66.4 ± 9.7* |
| Gender (M:F)1 | 34:25 | 11:15 |
| BMI (kg/m2) | 22.9 ± 3.3 | 24.6 ± 3.7* |
| Waist circumferences (cm) | 80.7 ± 8.0 | 86.6 ± 8.7** |
| EF (%) | 32.6 ± 13.1 | 36.1 ± 11.4 |
| Etiology of HF1 | ||
| CAD (%) | 22 | 38.5 |
| Cardiomyopathy (%) | 55.9 | 42.3 |
| Valvular disease (%) | 13.6 | 11.5 |
| Hypertension (%) | 16.9 | 73.1** |
| Underlying disease1 | ||
| Type 2 diabetes mellitus (%) | 5.1 | 30.8** |
| Chronic renal failure (%) | 3.4 | 11.5 |
| Stroke (%) | 6.8 | 3.8 |
| Medical treatment1 | ||
| Diuretics (%) | 74.6 | 73.1 |
| Digitalis (%) | 28.8 | 34.6 |
| β-blocker (%) | 50.8 | 57.7 |
| ACEI and/or ARB (%) | 72.9 | 96.2* |
| Anti-platelet agents (%) | 62.7 | 69.2 |
| Hypolipidemic agents (%) | 33.9 | 42.3 |
Values are Mean ± SD. 1 χ2 test. EF: ejection fraction; CAD: coronary artery disease; ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin II receptor blocker. *p<0.05, **p<0.005.
MetS risk factors and blood lipids in HF subjects
As expected, HF subjects with MetS displayed significantly different measurements in the criteria for MetS compared to those without MetS. HF subjects with MetS showed significantly elevated plasma levels of fasting glucose (HF without MetS 100.1 ± 17.2 mg/dl vs with MetS 131.4 ± 41.0 mg/dl, p<0.001) and TG (HF without MetS 129.9 ± 80.9 mg/dL vs with MetS 199.0 ± 76.6 mg/dl, p<0.001). Also reduced levels of HDL-cholesterol (HF without MetS 49.5 ± 11.6 mg/dl vs with MetS 35.8 ± 7.7 mg/dl, p<0.001) were observed in HF subjects with MetS compared to those without MetS. On the other hand, total cholesterol (HF without MetS 169.7 ± 35.5 mg/dl vs with MetS 168.4 ± 4.09 mg/dl, ns) and LDL-cholesterol levels (HF without MetS 94.3 ± 31.1 mg/dl vs with MetS 92.7 ± 34.1 mg/dl, ns) were not significantly different between the two groups. These data confirm that HF subjects with MetS were differentiated from those without MetS and also indicate that the prevalence of metabolic syndrome among HF patients.
Plasma phospholipid fatty acid composition in HF subjects with MetS and without MetS
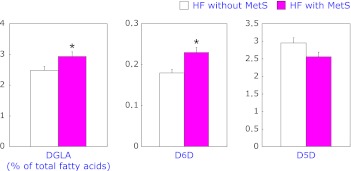
When FA compositions in plasma phospholipids were analyzed between HF patients with MetS and without MetS, significant differences in DGLA and lauric acid between the two groups were observed (Table 2). HF subjects with MetS displayed a higher proportion of C12:0 (lauric acid) (p<0.05) compared to HF without MetS. In addition, a significantly higher proportion of C20:3n-6 (DGLA) was shown in HF subjects with MetS than those without MetS (p<0.05). C20:5n-3 (EPA) showed a tendency to have a higher proportion in HF with MetS even if it was not statistically significant (p = 0.055). With respect to D5D and D6D, their activities were estimated by calculating corresponding FA product-to-precursor ratios. Estimated D5D activities were markedly lower in HF with MetS than in HF without MetS (p<0.05). However, estimated D6D activities were significantly increased in HF with MetS compared to HF without MetS (p<0.01). Overall significant elevation of DGLA (C20:5n-6) value from HF with MetS could result from the combination of decreased activity of D5D and increased activity of D6D. In GLM analysis (Fig. 1), DGLA and estimated D6D activity were proven to be higher with MetS in HF patients after adjusting for age (p<0.05 and p<0.05, respectively). In contrast, estimated D5D activity was similar between the two groups after age adjustment.
Table 2.
Proportions of FA composition in plasma phospholipids and estimated desaurase activities between HF patients with MetS and without MetS
| HF without MetS (n = 59) | HF with MetS (n = 26) | p | |
|---|---|---|---|
| 12:0 lauric acid | 0.24 ± 0.15 | 0.18 ± 0.09 | <0.05 |
| 14:0 myristic acid | 0.7 ± 0.17 | 0.66 ± 0.19 | ns |
| 16:0 palmitic acid | 27.45 ± 2.93 | 27.85 ± 2.28 | ns |
| 16:1 palmitoleic acid | 0.77 ± 0.23 | 0.83 ± 0.26 | ns |
| 18:0 stearic acid | 14.99 ± 1.94 | 14.49 ± 1.69 | ns |
| 18:1 ω9 oleic acid | 8.06 ± 1.45 | 8.04 ± 1.34 | ns |
| 18:1 t ω9 elaidic acid | 2.24 ± 0.58 | 2.15 ± 0.68 | ns |
| 18:2 ω6 linoleic acid | 14.11 ± 2.79 | 13.11 ± 2.77 | ns |
| 18:2 tt linolelaidic acid | 0.69 ± 0.3 | 0.66 ± 0.25 | ns |
| 18:3 ω6 γ-linolenic acid | 0.27 ± 0.12 | 0.26 ± 0.05 | ns |
| 18:3 ω3 α-linolenic acid | 0.59 ± 0.26 | 0.66 ± 0.25 | ns |
| 20:0 arachidic acid | 0.97 ± 0.23 | 1.0 ± 0.33 | ns |
| 20:1 ω9 gondoic acid(cis-11-eicosenoic acid) | 0.39 ± 0.15 | 0.35 ± 0.1 | ns |
| 20:2 cis-11,14-eicosadienoic acid | 1.24 ± 0.77 | 1.15 ± 0.27 | ns |
| 20:3 ω6 dihimo-γ-linolenic acid | 2.5 ± 0.62 | 2.9 ± 0.74 | <0.05 |
| 20:3 ω3 5-8-11-eicosatrienoic acid | 0.45 ± 0.25 | 0.38 ± 0.14 | ns |
| 20:4 ω6 arachidonic acid | 7.02 ± 1.61 | 7.04 ± 1.47 | ns |
| 20:5 ω3 EPA | 2.7 ± 1.2 | 3.26 ± 1.28 | ns |
| 22:0 behenic acid | 1.88 ± 0.5 | 1.91 ± 0.38 | ns |
| 22:1 erucic acid | 0.6 ± 0.21 | 0.54 ± 0.16 | ns |
| 22:2 docosadienoic acid | 0.37 ± 0.27 | 0.33 ± 0.12 | ns |
| 24:0 lignoceric acid | 1.96 ± 0.72 | 1.9 ± 0.64 | ns |
| 22:6 ω3 DHA | 8.26 ± 2.17 | 8.88 ± 1.81 | ns |
| SCD16 (16:1n-7/16:0) | 0.028 ± 0.009 | 0.03 ± 0.009 | ns |
| SCD18 (18:1n-9/18:0) | 0.54 ± 0.12 | 0.56 ± 0.11 | ns |
| D6D (20:3n-6/18:2n-6) | 0.18 ± 0.06 | 0.23 ± 0.09 | <0.01 |
| D5D (20:4n-6/20:3n-6) | 2.96 ± 0.98 | 2.53 ± 0.63 | <0.05 |
Mean ± SD. Expressed as percentage of total fatty acids.
Fig. 1.
Comparison of the proportions of DGLA, estimated D6D and D5D after age adjustment between HF patients with and without MetS (*p<0.01).
Correlations between DGLA/EPA/estimated desaturase activites and components of MetS
Among HF subjects, we evaluated correlations between DGLA and estimated desaturase indexes (D5D and D6D) and the components of MetS (BMI, waist circumference, plasma TG, HDL-cholesterol levels, and fasting glucose) (Table 3). DGLA (C20:3n-6) was positively correlated with BMI (r = 0.274, p<0.05), waist circumference (r = 0.295, p<0.01) and plasma TG levels (r = 0.390, p<0.001). There were correlations of plasma TG levels positively with estimated D6D activity (r = 0.295, p<0.01) whereas negatively with estimated D5D activity (r = –0.299, p<0.001).
Table 3.
Correlates between DGLA, estimated desaturase activities and components of MetS
| BMI | Waist | TG | HDL-cholesterol | Fasting glucose | |
|---|---|---|---|---|---|
| DGLA | 0.274* | 0.294** | 0.390*** | −0.089 | 0.078 |
| D6D | 0.146 | 0.208 | 0.295** | −0.146 | 0.043 |
| D5D | −0.163 | −0.220* | −0.299*** | 0.193 | 0.003 |
*p<0.05, **p<0.01, ***p<0.001. DGLA: dihimo-r-linolenic acid, D5D: delta-5 desaturase, D6D: delta-6 desaturase.
Discussion
In the present study, we observed significant differences in the FA compositions and estimated desaturase activities between HF patients with and without MetS, providing new information on the altered FA metabolisms in these conditions. Specifically, we found that increased proportion of DGLA and estimated D6D activity and decreased estimated D5D activity were associated with the presence of MetS among HF patients.
A few studies have reported about the associations between FA levels in plasma and the risk and severity for HF without distinguishing the MetS status.(18–23) For instance, intake of saturated fats is known to increase the risk of HF.(18,19) This was in part supported by a study showing the association of the elevated plasma levels of SFA and reduced plasma levels of DHA with the risks of HF.(20) Moreover, Oie et al.(21) and Zhao et al.(22) compared the changes in FA composition between the normal control and HF subjects and found out the roles of monounsaturated fatty acids (MUFAs) and some PUFA in HF subjects. They demonstrated that high levels of MUFA and linoleic acid and low levels of arachidonic acid were associated with those subjects.(21,22) In addition, it was reported that EPA and DHA are negatively correlated with heart rate, indicating that low proportion of either EPA or DHA increases a chance to elevate heart rate.(23)
While the evidence on the importance of FA metabolism has been accumulated, there has been limited information on the altered FA metabolism of HF patients in the presence of MetS. In this study, we have shown that MetS condition in HF patients had a positive association with elevated DGLA levels in plasma. Previously, DGLA has been implicated in the heart function by its positive association with diastolic blood pressure.(32,33) In addition, high levels of DGLA detected together with increased heart rate and insulin levels was suggested to be responsible for the development of left ventricular dysfunction.(34) These findings indicate that elevated levels of DGLA in HF patients with MetS could burden the function of the heart more aggressively than in HF patient without MetS. On the other hand, Ebbesson et al.(23) showed inconsistent results with no association of DGLA with heart rate. Therefore, further studies are needed to elucidate the exact role of DGLA in heart function. Besides the implication of DGLA in the function of heart, several previous studies have reported the associations between the proportion of DGLA and insulin resistance. The elevated level of DGLA in non-insulin diabetes mellitus (NIDDM) patients was found compared to healthy subjects.(12) Similar observation was described in old men with negative correlation of DGLA with insulin sensitivity.(35) Another report by Maruyama et al.(24) indicated higher DGLA level in subjects with MetS compared to without MetS. In line with this, our data provide additional information on MetS specific elevation of DGLA in HF patients, suggesting the relation of DGLA with MetS. It may be that MetS condition where hyperinsulinemic state could stimulate D6D activity, could further elevate proportion of DGLA in plasma phospholipids in HF patients. Alternatively, higher contents of DGLA in FA composition, in turn, could indicate the risk of insulin resistance.
Along with the changes in DGLA proportion, we observed significant differences in estimated activities of desaturases with elevated D6D activity and decreased D5D activity with MetS among HF patients, although the association with D5D disappeared after age adjustment. Similar to our results, Maruyama et al.(24) observed higher DGLA level as well as lower estimated D5D activity in subjects with MetS compared to those without MetS. Also, lower estimated D5D activity and higher estimated D6D activity were shown in Japanese men with MetS.(17) In addition, young Japanese women harboring metabolic risk factors showed similar relationship of desaturase activities.(29) Furthermore, in a population-based study, the authors suggested indices for high D6D activity and low D5D activity along with increased delta 9 desaturase activity be predictors of the development of MetS,(36) indicating the close relationship between abnormal activities of desaturase and MetS. In our study, estimated D6D activity was calculated based on the ratio of DGLA to linoleic acid and estimation of D5D activity was obtained by the ratio of arachidonic acid to DGLA. Considering that DGLA is converted to arachidonic acid by D5D, diminished D5D activity could result in increased level of DGLA among HF subjects with MetS. At the same time, DGLA is a product of D6D activity from linoleic acid (C18:2n-6). Taken together, it is likely that high contents of DGLA in plasma phospholipid in MetS subjects with HF could also be due to dysregulated activitites of D5D and D6D and act as indicators of abnormal regulation of desaturase activities. Also, this might indicate that desaturases affect metabolic processes either via their product DGLA or by their own potential capacity to influence metabolism independently.(37) Furthermore, D6D could be linked to inflammation through inflammation where PUFAs synthesized by D6D may affect the permeability and function of cell membranes.(38) Alternatively, PUFAs synthesized by D6D participate in synthesizing eicosanoids(39) which act as inflammatory mediators and also, they can be used as ligands for transcriptional factors(40) that are involved in inflammatory pathways.
In summary, we provide evidence that DGLA as well as estimated desaturase activities (D5D and D6D) were closely related to MetS condition in HF patients. Study limitations include a relatively small number of patients and no healthy control group, which discourages us to generalize these results to a population with no HF. In addition, the degrees of MetS of subjects may not be enough to detect subtle changes in FA compositions, and also the pathological condition of HF might mask the differences induced by MetS which could be easily detected only when compared with the healthy control. Despite these limitations, this is the first study to evaluate associations between various desaturase activities along with serum individual FAs and the risk for MetS in HF patients.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant No. M10642120001-06N4212-00110, 2006-2005304), and in part by a faculty research grant of Yonsei University College of Medicine for 2011 (8-2011-0015).
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- DGLA
dihomo-γ-linolenic acid
- FA
fatty acid
- HF
heart failure
- LVEF
left ventricular ejection fraction
- MetS
metabolic syndrome
- TG
triglyceride
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gomez-Soto FM, Andrey JL, Garcia-Egido AA, et al. Incidence and mortality of heart failure: a community-based study. Int J Cardiol. 2011;151:40–45. doi: 10.1016/j.ijcard.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 2.Mendez GF, Cowie MR. The epidemiological features of heart failure in developing countries: a review of the literature. Int J Cardiol. 2001;80:213–219. doi: 10.1016/s0167-5273(01)00497-1. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Gaddam KK, Ventura HO, Lavie CJ. Metabolic syndrome and heart failure—the risk, paradox, and treatment. Curr Hypertens Rep. 2011;13:142–148. doi: 10.1007/s11906-011-0179-x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto K. Metabolic syndrome and heart failure. Circ J. 2010;74:2550–2551. doi: 10.1253/circj.cj-10-0967. [DOI] [PubMed] [Google Scholar]
- 6.Miura Y, Fukumoto Y, Shiba N, et al. Prevalence and clinical implication of metabolic syndrome in chronic heart failure. Circ J. 2010;74:2612–2621. doi: 10.1253/circj.cj-10-0677. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Sarnola K, Ruotsalainen S, et al. The metabolic syndrome predicts incident congestive heart failure: a 20-year follow-up study of elderly Finns. Atherosclerosis. 2010;210:237–242. doi: 10.1016/j.atherosclerosis.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki T, Katz R, Jenny NS, et al. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2008;1:242–248. doi: 10.1161/CIRCHEARTFAILURE.108.785485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamariz L, Hassan B, Palacio A, Arcement L, Horswell R, Hebert K. Metabolic syndrome increases mortality in heart failure. Clin Cardiol. 2009;32:327–331. doi: 10.1002/clc.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Folsom AR, Eckfeldt JH. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr Metab Cardiovasc Dis. 2003;13:256–266. doi: 10.1016/s0939-4753(03)80029-7. [DOI] [PubMed] [Google Scholar]
- 12.Salomaa V, Ahola I, Tuomilehto J, et al. Fatty acid composition of serum cholesterol esters in different degrees of glucose intolerance: a population-based study. Metabolism. 1990;39:1285–1291. doi: 10.1016/0026-0495(90)90185-f. [DOI] [PubMed] [Google Scholar]
- 13.Ohrvall M, Berglund L, Salminen I, Lithell H, Aro A, Vessby B. The serum cholesterol ester fatty acid composition but not the serum concentration of alpha tocopherol predicts the development of myocardial infarction in 50-year-old men: 19 years follow-up. Atherosclerosis. 1996;127:65–71. doi: 10.1016/s0021-9150(96)05936-9. [DOI] [PubMed] [Google Scholar]
- 14.Lovejoy J, DiGirolamo M. Habitual dietary intake and insulin sensitivity in lean and obese adults. Am J Clin Nutr. 1992;55:1174–1179. doi: 10.1093/ajcn/55.6.1174. [DOI] [PubMed] [Google Scholar]
- 15.Huang T, Bhulaidok S, Cai Z, et al. Plasma phospholipids n-3 polyunsaturated fatty acid is associated with metabolic syndrome. Mol Nutr Food Res. 2010;54:1628–1635. doi: 10.1002/mnfr.201000025. [DOI] [PubMed] [Google Scholar]
- 16.Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes. 1994;43:1353–1357. doi: 10.2337/diab.43.11.1353. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima A, Sugawara S, Okita M, et al. Plasma fatty acid composition, estimated desaturase activities, and intakes of energy and nutrient in Japanese men with abdominal obesity or metabolic syndrome. J Nutr Sci Vitaminol (Tokyo) 2009;55:400–406. doi: 10.3177/jnsv.55.400. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi K, Iso H, Yatsuya H, et al. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am J Clin Nutr. 2010;92:759–765. doi: 10.3945/ajcn.2009.29146. [DOI] [PubMed] [Google Scholar]
- 19.Christopher BA, Huang HM, Berthiaume JM, et al. Myocardial insulin resistance induced by high fat feeding in heart failure is associated with preserved contractile function. Am J Physiol Heart Circ Physiol. 2010;299:H1917–H1927. doi: 10.1152/ajpheart.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi K, Nettleton JA, Folsom AR, ARIS Study Investigators Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:965–974. doi: 10.1016/j.ahj.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Øie E, Ueland T, Dahl CP, et al. Fatty acid composition in chronic heart failure: low circulating levels of eicosatetraenoic acid and high levels of vaccenic acid are associated with disease severity and mortality. J Intern Med. 2011;270:263–272. doi: 10.1111/j.1365-2796.2011.02384.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YS, Zhao SP, Tang AG. Changes in fatty acid composition of serum cholesteryl esters in patients with chronic heart failure. Hunan Yi Ke Da Xue Xue Bao. 2003;28:165–166. [PubMed] [Google Scholar]
- 23.Ebbesson SO, Devereux RB, Cole S, et al. Heart rate is associated with red blood cell fatty acid concentration: the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Am Heart J. 2010;159:1020–1025. doi: 10.1016/j.ahj.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama C, Yoneyama M, Suyama N, et al. Differences in serum phospholipid fatty acid compositions and estimated desaturase activities between Japanese men with and without metabolic syndrome. J Atheroscler Thromb. 2008;15:306–313. doi: 10.5551/jat.e564. [DOI] [PubMed] [Google Scholar]
- 25.Shin MJ, Lee KH, Chung JH, et al. Circulating IL-8 levels in heart failure patients with and without metabolic syndrome. Clin Chim Acta. 2009;405:139–142. doi: 10.1016/j.cca.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 28.Lemaitre RN, King IB, Mozaffarian D, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation. 2006;114:209–215. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]
- 29.Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 30.Steffen LM, Vessby B, Jacobs DR, Jr., et al. Serum phospholipid and cholesteryl ester fatty acids and estimated desaturase activities are related to overweight and cardiovascular risk factors in adolescents. Int J Obes (Lond) 2008;32:1297–1304. doi: 10.1038/ijo.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami K, Sasaki S, Takahashi Y, et al. Lower estimates of delta-5 desaturase and elongase activity are related to adverse profiles for several metabolic risk factors in young Japanese women. Nutr Res. 2008;28:816–824. doi: 10.1016/j.nutres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Arnlöv J, Lind L, Sundström J, Andrén B, Vessby B, Lithell H. Insulin resistance, dietary fat intake and blood pressure predict left ventricular diastolic function 20 years later. Nutr Metab Cardiovasc Dis. 2005;15:242–249. doi: 10.1016/j.numecd.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Simon JA, Fong J, Bernert JT., Jr Serum fatty acids and blood pressure. Hypertension. 1996;27:303–307. doi: 10.1161/01.hyp.27.2.303. [DOI] [PubMed] [Google Scholar]
- 34.Arnlöv J, Lind L, Zethelius B, et al. Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. Am Heart J. 2001;142:720–724. doi: 10.1067/mhj.2001.116957. [DOI] [PubMed] [Google Scholar]
- 35.Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia. 1994;37:1044–1050. doi: 10.1007/BF00400468. [DOI] [PubMed] [Google Scholar]
- 36.Warensjö E, Risérus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 37.Soriguer F, Rojo-Martinez G, de Fonseca FR, García-Escobar E, García Fuentes E, Olveira G. Obesity and the metabolic syndrome in Mediterranean countries: a hypothesis related to olive oil. Mol Nutr Food Res. 2007;51:1260–1267. doi: 10.1002/mnfr.200700021. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong VT, Brzustowicz MR, Wassall SR, Jenski LJ, Stillwell W. Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch Biochem Biophys. 2003;414:74–82. doi: 10.1016/s0003-9861(03)00159-0. [DOI] [PubMed] [Google Scholar]
- 39.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 40.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131:1129–1132. doi: 10.1093/jn/131.4.1129. [DOI] [PubMed] [Google Scholar]