Abstract
Chronic gastric inflammation developing after Helicobacter pylori (H. pylori) infection is responsible for either dyspeptic symptom relevant to gastritis/peptic ulcer or gastric tumorigenesis, in which acid suppressants, especially proton pump inhibitors (PPIs), play role in relieving dyspepsia as well as the eradication regimen. Among several mediators engaged in propagating gastric inflammation after H. pylori infection, cyclooxygenase-2 (COX-2) might be the principal one, and several prescriptions have been made for decreasing the COX-2 levels. Multiple line of evidence are available for anti-inflammatory action of PPIs beyond acid suppression, but revaprazan, a novel acid pump antagonist launched in clinic, has also been suggested to exert significant anti-inflammatory actions as much as PPI. In the current study, we hypothesized that revaprazan could regulate H. pylori-driven COX-2 expression as one of its anti-inflammatory pharmacological actions. The changes of gastric COX-2 expression as well as responsible transcription factors were measured after H. pylori infection in the presence or absence of revaprazan. Infection of AGS cells with H. pylori induced significant up-regulation of COX-2 in time- and concentration-dependent manners, which was mediated by Akt phosphorylation. Revaprazan treatment significantly inhibited IkappaB-alpha degradation as well as Akt inactivation, resulting in attenuation of H. pylori-induced COX-2 expression. Additional rescuing action of revaprazan against H. pylori-induced cytotoxicity was noted. In conclusion, revaprazan imposed significant anti-inflammatory actions on H. pylori infection beyond acid suppression.
Keywords: Helicobactor pylori, anti-inflammatory effects, NF-κB, Akt, revaprazan
Introduction
Cyclooxygenase (COX) or prostaglandin H synthase (PGHS) is the key enzyme that catalyzes the rate-limiting step in plostagladin biosynthesis from the substrate arachidonic acid and exists in at least two isoforms designated as COX-1 and COX-2. Although COX-1 is a housekeeping enzyme, being constitutively expressed in almost all mammalian tissues, COX-2 is barely detectable under normal physiological conditions. COX-2 is induced rapidly and transiently by proinflammatory mediators or mitogenic stimuli, such as like cytokines, endotoxins, several growth factors, and oncogenes. Since COX-2 expression is markedly increased after Helicobacter pylori (H. pylori) infection in parallel with ensuing gastric inflammation and its expression is especially prominent in intestinal type gastric carcinoma as well as dysplastic premalignant lesions, COX-2 induction associated with H. pylori infection has been considered as the potential target of cancer prevention related to H. pylori-associated gastric cancer as well as H. pylori-associated chronic atrophic gastritis, a premalignant lesion of gastric cancer.(1)
NF-κB has been known to regulate COX-2 expression since the 5-promoter region of COX-2 contains two putative NF-κB binding sites, which is a member of the Rel family including p50 (NF-B1), p52 (NF-B2), Rel A (p65), c-Rel, Rel B, and Drosophila morphogen dorsal gene product.(2) Activator protein-1 (AP-1) is another redox-regulated transcription factor that plays a critical role in inflammation.(3) AP-1 consists of either homo- or heterodimers between members of the Jun and Fos families. H. pylori infection led to activation of these transcription factors immediately which can explain the pathogenic link between H. pylori infection and various gastric diseases including gastric malignancy.(4,5) Phophoinositide 3-kinase (PI3K) and its downstream target Akt/protein kinase B are also known to regulate NF-κB activation and COX-2 expression.(6,7)
In addition to efficient suppression of gastric acid secretion, proton pump inhibitors (PPIs) can further modulate inflammation either by reducing the production of cytokines and chemokines or enforcing phase II detoxifying enzyme induction through Nrf2 transcriptional regulation as well as selective cancer cell apoptosis.(8,9) The acid pump antagonist (APA) is a reversible inhibitor of gastric H+/K+-ATPase, which competes with luminal K+ ions in binding to the proton pump and dissociates from the enzyme when their blood concentration falls, after which the net outcome is similar to those of PPI.(9) Furthermore, similar to additional pharmacological actions of PPI,(8) APA can impose the direct anti-inflammatory actions against the diverse etiologic factors of gastritis including H. pylori infection, NSAID challenge, and stress.(10,11)
In this study, we examind the additional pharmacological action of revaprazan on Akt signaling and NF-κB activations related to COX-2 expression after H. pylori infection and found that the novel APA, revaprazan, can reduce COX-2 expression after H. pylori infection by inactivating Akt and NF-κB with reduced COX-2 expression in gastric mucosal cells.
Materials and Methods
Reagents
Revaprazan was supplied from Yuhan Corporation and dissolved in DMSO for treatment. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and sodium dedocylsulfate (SDS) were purchased from Sigma Chemical Co. (St. Louis, MO). Fetal Bovine Serum, penicillin/streptomycin, RPMI medium 1640 were obtained from Gibco BRL (Grand Island, NY). Rabbit polyclonal COX-2 antibody was a product of Cayman Chemical Co. (Ann Arbor, MI). Rabbit polyclonal Akt and pAkt antibodies were obtained from Cell signaling Technology Inc. (Beverly, MA). Primary antibody for IκB-α was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) Anti-rabbit, and anti-mouse horseradish peroxidased conjugated secondary antibodies were products of Zymed Laboratories (San Francisco, CA). An oligonuleotide probe containing the NF-κB consensus sequence in the human COX-2 promotor region was obtained from Bionics (Seoul, Korea). [γ-32P]ATP was purchased from Amersham Pharmacia Biotech (Buckinghamxhire, UK). The electrophoretic mobility shift assay (EMSA) kit was obtained from Gibco BRL (Grand Island, NY).
Bacterial strains and H. pylori culture conditions
The H. pylori ATCC 43504 strain, the typical S shape, gram negative rods, possessing the CagA, VacA cytotoxin, oxidase, urease, and catalase, was provided in a frozen state by American Type Culture Collection (Manassas, VA). H. pylori was cultured on tryptic soy agar (TSA) with 5% (v/v) sheep blood (Becton Dickinson) and Dent antibiotics supplement (Oxoid, Basingstoke, UK) at 37°C under microaerophilic conditions (Campy-Pak Systems; BBL, Gaithesburg, MD). The microaerophilic conditions were generated by CampyPack plus (BD) at 37°C in an atmosphere of 5% O2, 10% CO2, and 85% N2. For biphasic culture, H. pylori inoculated onto the TSA were transferred into tryptic soy broth supplemented with 10% FBS again in microaerobic conditions for the additional 5 days. Colonies with a characteristic H. pylori morphology were identified by phase contrast microscopy and stored in liquid nitrogen before use.
Cell culture
AGS cells, human gastric adenocarcinoma epithelial cell were obtained from the American Type Culture Collection. The cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Life Technologies, NY), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma) at 37°C under humidified atmosphere of 5% CO2.
Treatment of H. pylori
The number of bacterial cells in the suspension was counted by optical density measurement. AGS cells were plated appropriately for H. pylori treatment. H. pylori were treated at a bacterium/cell ratio of 100:1 [100 multiple of infection (MOI)]. For example, AGS cells (1 × 107) were treated with 100 µl of bacterial suspension (1 × 109 CFU) of H. pylori. Before treating H. pylori, each well was washed twice in 5 ml of fresh phosphate-buffered solution (PBS). AGS cells and H. pylori were co-cultured in RPMI 1640 supplemented with 10% FBS and antibiotics.
Western blot analysis
AGS cells pretreated for 2 h with vehicle or revaprazan (5, 20, 50 µM, respectively) were incubated in the presence of H. pylori for 24 h for determination of protein levels of COX-2 and Akt. To check the LPS-stimulated expression of COX2 and IκBα, AGS cells were treated H. pylori after 2 h revaprazan pretreatment. Medium was washed out twice with ice-cold PBS before harvesting the cell lysates. The nuclei and cytosolic proteins were separated from the pellets. AGS cells were lysed in RIPA lysis buffer [150 mM NaCl, 0.5% Triton × 100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 20 mM EGTA, 1 mM dithiothreitol (DTT), 1 mM Na3VO4] for 15 min at 0°C followed by centrifugation at 13,000 × g for 15 min. The protein concentration of the supernatant was measured by using the BCA reagents (Pierce, Rockfold, IL). Aliquots of supernatant containing 50 µg protein were boiled in sodium dodecylsulfate (SDS) sample loading buffer for 5 min before electrophoresis on 12% SDS-polyacrylamide gel and transferred to the PDVF membrane (Gelman Laboratory, Ann Arbor, MI). The blots were blocked with 5% non-fat dry milk-PBST buffer [PBS containing 0.1% Tween-20] for 1 h at room temperature. The membranes were incubated for 12 h at 4°C with 1:1000 dilutions of primary antibodies for COX-2, Akt or IκB-α and 2–3 days at 4°C with 1:500 dilutions of primary antibody for phospho-Akt. Blots were washed three times with PBST at 10 min interval followed by incubation with 1:5000 horseradish peroxidase-conjugated secondary antibodies (rabbit or mouse) for 1 h and washed in PBST for three times. The transferred proteins were visualized with an ECL detection kit according to the manufacturer’s instructions.
Preparation of nuclear and cytosolic fractions
AGS cells were washed with ice-cold PBS, scrapped in 5 ml PBS, and centrifuged at 3,000 rpm for 3 min at 4°C. Cell pellets were suspended in 200 µl of hypotonic buffer A [10 mM HEPES, pH 7.9; 10 mM KCl; 2 mM MgCl2; 1 mM DTT; 0.1 mM EDTA; 0.1 mM PMSF] for 15 min on ice, and 2 µl of 10% Nonidet P-40 solution was added for 5 min and centrifuged 14,000 rpm for 15 min. The nuclei were washed once with 100 µl of buffer A and centrifuged 14,000 rpm for 2 min, resuspended in 150 µl of hypertonic buffer C [50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 M PMSF and 10% glycerol], and incubated for 5 min on ice and centrifuged at 14,000 rpm for 15 min at 4°C. The supernatants containing nuclear, cytosolic proteins were stored at –70°C after determination of protein concentration.
Electrophoretic mobility shift assay (EMSA)
EMSA was performed using a DNA-protein binding detection kit according to the manufacturer’s protocol (GIBCO). Briefly, the NF-κB oligonucleotide probe (5'-AGT TGA GGG GAC TTT CCC AGG C-3') was labeled with [γ-32P] ATP by T4 polynucleotide kinase and purified on a Nick column (Amersham Phamacia Biotech). The binding reaction was carried out in a total volume of 25 µl containing 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 4% (v/v) glycerol, 0.1 mg/ml sonicated salmon sperm DNA, 10 µg of nuclear extracts, and 100,000 cpm of the labeled probe. After 50 min incubation at room temperature, 2 µl of 0.1% bromophenol blue was added, and samples were electrophoresed through a 6% non-denaturating polyacrylamide gel at 150 V in a cold room temperature for 2 h. Finally, the gel was dried and exposed to X-ray film.
MTT assay
AGS cells were plated at a density of 5 × 104 cells in 48-well plates, and cell viability was checked by the conventional MTT reduction assay. The MTT assay relies primarily on the mitochondrial metabolic capacity of viable cells and hence reflects the intracellular redox state. AGS cells were treated with DMSO or revaprazan (10, 30, 40, 50, 80, 100 µM). In another set, AGS cells were treated with revaprazan (5, 20, 50 µM) before LPS or H. pylori treatment. After incubation, cells were treated with MTT solution (final concentration, 0.25 mg/mL) for 2 h at 37°C. The dark blue formazan crystals formed in intact cells were dissolved with DMSO, and transferred to 96 well plates. The absorbance was measured at 570 nm with the ELISA reader. The results were expressed as the percentage of control cells was 100%.
Cell migration monitored with live cell image
Confluent AGS cells infected with 10 MOI H. pylori were wounded with pipette tip and observed under the live cell imager analyzer (Kongju University, Kongju, Korea), in which cell growth was monitored up to 24 h and recorded with 3 min interval. Four different groups were monitored; 10 MOI H. pylori, 10 MOI H. pylori plus pretreatment of 5 µM revaprazan, 10 MOI H. pylori plus pretreatment of 10 µM, and 10 MOI H. pylori plus pretreatment of 20 µM. With the still photo taken after 24 h, the mean velocity of cell growth was calculated according to the group and the mean levels of cell migrations were displayed.
Statistical analysis
The data are presented as means ± standard deviations (SD). The Tukey test or the Student’s t for unpaired results was used to evaluate differences between more than three groups or between two groups, respectively. Differences were considered to be significant for values of p<0.05.
Results
Revaprazan pretreatment reduced H. pylori-stimulated COX-2 expression and activity
When the AGS cells were infected with H. pylori for 24 h, expression of COX-2, one of the key proinflammatory mediators related with H. pylori infection, was significantly up-regulated (p<0.01, Fig. 1A and B). However, revaprazane pretreatment could reduce H. pylori-induced COX-2 expression, and the inhibitory effect was more pronounced at 24 h than 12 h (p<0.05, Fig. 1B). COX-2 inhibition with pretreatment of 20 µM revaprazan was significantly associated with decreased levels of PGE2 (Fig. 1C). Taken together, revaprazan could attenuate H. pylori-associated COX-2 expression and activity.
Fig. 1.
Effects of Revaprazan pretreatment on H. pylori-induced COX-2 expression in AGS gastric epithelial cells. AGS cells exposed to 100 MOI of H. pylori or revaprazan alone or in combination of revaprazan pretreatment and H. pylori for 12 and 24 h were subjected to western blot of COX-2. (A) Expression of COX-2 was determined by western blot analysis 12 h after H. pylori infection (upper) and mean levels of relative intensities as assessed with triplicate experiments. H. pylori infection was associated with significant inductions of COX-2. Though 2 h revaprazan pretreatment decreased the expressions of COX-2, it was not statistically significant. (B) Expression of COX-2 was determined by western blot analysis 24 h after H. pylori infection (upper) and mean levels of relative intensities as assessed with triplicate experiments. H. pylori infection was associated with significant inductions of COX-2. 2 h pretreatment of 20 µM revaprazan pretreatment significantly decreased the expressions of COX-2 (p<0.05). (C) Mean levels of PGE2 according to 2 h pretreatment of 20 µM revaprazan significantly decreased H. pylori-associated PGE2.
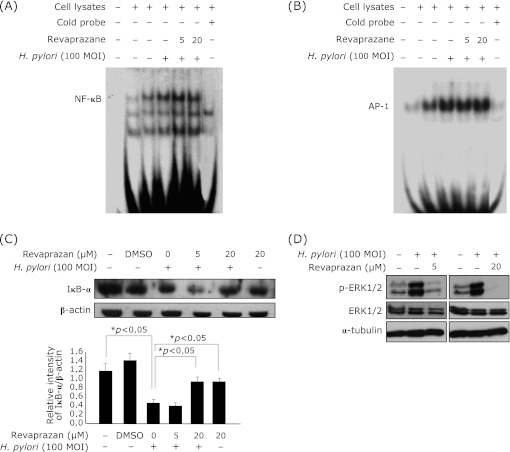
Revaprazan inactivated NF-κB-DNA binding for anti-inflammatory action
Since NF-κB and AP-1 are well-known to regulate COX-2 induction and their DNA binding activities are also associated with H. pylori infection,(12) we examined whether revaprazan pretreatment can suppress the activation of these transcription factors induced by H. pylori infection in the AGS cells. Though EMSA showed that NF-κB and AP-1 DNA-binding activities were increased by H. pylori treatment, 20 µM revaprazan pretreatment slightly inhibited transcription activity of NF-κB (Fig. 2A), but did not influence AP-1-DNA binding activities (Fig. 2B). NF-κB is normally retained in cytosolic and its nuclear import is blocked by binding with IκB protein. Therefore, the dislocation from the IκB-α protein, which is mediated by phosphorylation and subsequent proteasomal degradation, is regarded as an essential step for NF-κB activation. When we detected the IκB-α level in cytosolic extracts using Western blot analysis, expression of IκB-α was significantly decreased after H. pylori infection, signifying that significant activation of NF-κB was occurred. In this setting, revaprazan pretreatment kept IκB-α accumulation in the cytoplasmic fraction, leading to inactivation of NF-κB-DNA binding, Revaprazan effectively inactivated H. pylori-induced ERK phosphorylation (Fig. 2D).
Fig. 2.
Inhibitory effect of revaprazan pretreatment on H. pylori-induced NF-κB-DNA binding, not AP-1 binding. AGS cells were pretreated with revaprazan (5 or 20 µM) 2 h before 100 MOI H. pylori infection. Cells were scrapped 1 h after the H. pylori treatment, and nuclear protein (10 µg) was incubated with radiolabeled with either (A) NF-κB or (B) AP-1 oligonuclotide for EMSA. NF-κB-DNA bindings were increased after H. pylori infection, but its binding was decreased in 20 µM revaprazane pretreated lane. Even though the AP-1 binding was increased after H. pylori infection, there was no change in AP-1-DNA binding with revaprazane pretreatment. (C) AGS cells were treated with 5 or 20 µM revaprazan 2 h before H. pylori infection (100 MOI, 1 h) and the expression of IκB-α in cytosolic extracts was examined by western blot analysis. H. pylori infection was significantly associated with decreases in the levels of IκB-α, whereas 20 µM revaprazan effectively preserved its cytosolic levels under H. pylori infection, imposing inhibitory action of NF-κB activation with revaprazan. (D) Western blotting of ERK1/2 and phosphorylated ERK1/2.
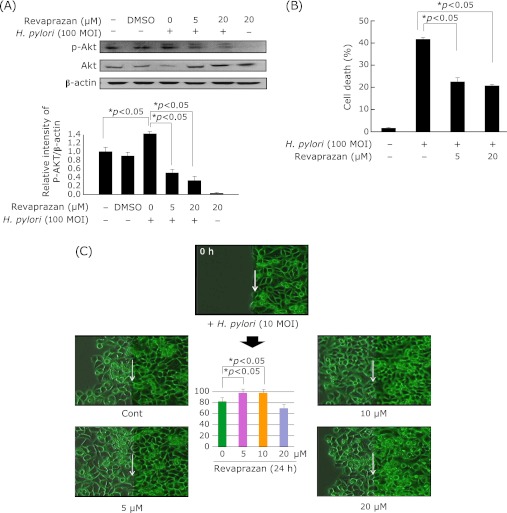
Revaprazan pretreatment reduced H. pylori-stimulated Akt activation, but preserved migration activation for regeneration
Phophoinositide 3-kinase (PI3K) and its downstream target Akt/protein kinase B are known to regulate inflammatory signaling.(13) Akt phosphorylation was elevated after H. pylori infection for 24 h. As shown in Fig. 3A, revaprazan pretreatment significantly inhibited Akt phophorylation. Since Akt activation is associated with the cell survival, we have checked whether revaprazan can rescue from H. pylori-induced cytotoxicity. Apart from Akt inactivation related to inflammation regulation with revaprazan, as shown in Fig. 3B, revaprazan significantly rescued from H. pylori-induced cytotoxicity. As shown with the live cell imaging experiment (Fig. 3C), H. pylori infection delayed wound healing, and revaprazan pretreatment significantly accelerated the wound healing, but only at concentrations lesser than 10 µM. The velocity and amounts of cell growth monitored under the live cell imager were calculated and the mean differences in the speed of cell growth were displayed in the Fig. 3C, showing that revaprazan overcome the retarded cell growth under H. pylori infection, but in concentration lesser than 10 µM. We speculated revaprazan more than 20 µM was not effective in speeding the cell growth because of simultaneous induction of apoptosis.
Fig. 3.
Effects of revaprazan on H. pylori-induced expression and activity of Akt in AGS gastric epithelial cell line. (A) AGS cells were treated with revaprazan (5, 20 µM) for 24 h under H. pylori infection. The levels of Akt and p-Akt were determined by western blot analysis. The graph presented the ratio at pAkt: β-actin. (B) H. pylori-induced cytotoxicity Revaprazan pretreatment significantly rescued from H. pylori-induced cytotoxicity. (C) Real live imaging after wound Compared to delayed wound healing in control group 24 h after H. pylori infection, revaprazan pretreatment significantly compensated the delayed reepithelialization, but in a concentration below 10 µM.
Discussion
Our study suggest that even though acid pump antagonist (APA) is a drug for suppressing gastric acids, it can also be used for ameliorating gastric inflammation caused by H. pylori infection as it efficiently attenuated COX-2 expression. In addition to current documented mechanism regarding the anti-inflammatory action of APA against H. pylori infection, we have reported other peculiar actions of revaprazan; rescuing stomach from NSAID-induced gastropathy with accentuated preservation of heat shock protein 27 (HSP27) and mitigating endoplasmic reticulum stress (ER stress) imposed by water immersion restraint stress.(10,11) Taken together with COX-2 inhibition as demonstrated in the current study, APA possesses gastroprotective pharmacological actions.
H. pylori infection widespread in humans worldwide, and its eradication can prevent the recurrence of gastroduodenal ulceration. Moreover, it has been widely accepted that there has been a strong association between H. pylori-associated gastritis and gastric cancer development. Several lines of evidence have been reported supporting that effective modulation of inflammation can confer the possibility of cancer prevention in diverse kinds of GI cancers associated with inflammation on their pathogenesis, such as chronic atrophic gastritis, chronic reflux esophagitis, cholangitis, pancreatitis, inflammatory bowel disease. Considering the link between inflammation and carcinogenesis, COX-2 seems to be one of the prime players in these connections, and NSAIDs and other anti-inflammatory agents have been documented candidates for chemoprevention. Since proinflammatory COX-2 has been considered as one of the potential targets for chemoprevention,(14) targeted inhibition of abnormally increased COX-2 can be an appropriate strategy for alleviating inflammation as well as preventing related cancer.(15,16)
H. pylori-induced COX-2 expression(17,18) was known to be regulated by NF-κB.(4,16,18) In resting cells, NF-κB is present as an inactive form in cytoplasm sequestered by its inhibitory protein IκBα. When IκBα is phosphorylated, NF-κB translocated to the nucleus to induce specific pro-inflammatory enzymes such as COX-2 and other cytokines. Our study reveals that reveprazan pretreatment suppressed H. pylori-induced COX-2 expression in the AGS cells with blocking phosphorylation of IκB-α. Even though AP-1 is an another critical transcription factor which can be activated by H. pylori and responsible for redox-sensitive inflammatory response and several reports showed that H. pylori infection of epithelial cells induced the activation of the transcription factor AP-1,(20,21) AP-1 was not engaged in anti-inflammatory action of revaprazan. Instead, we found that the inactivation of Akt signaling by APA contributed to its anti-inflammatory action.
The serine/threonine kinase Akt is basically a mitogen activated survival factor,(22,23) but PI3K and its downstream target Akt/protein kinase B are also known to regulate COX-2 expression.(24) Akt regulated COX-2 expression in endometrial cancer cells, and H. pylori strongly activated PI3K.(25,26) In this study, revaprazan exhibited an inhibitory effect on H. pylori-induced Akt phosphorylation, suggesting that revaprazan attenuated COX-2 expression partly through Akt inhibition. Several other studies have also demonstrated that PPIs like omeprazole and lansoprazole possess powerful anti-inflammatory properties against H. pylori in vitro.(27,28)
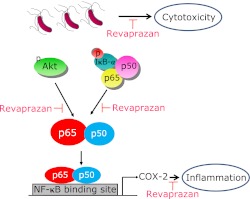
In conclusion, revaprazan could inhibit H. pylori-induced COX-2 expression by blocking phosphorylation of IκB-α and Akt signaling in AGS cells, as schematically proposed in Fig. 4. In conjunction with the our clinical data that revaprazane was very effective in the treatment of gastritis(10) and translational study that revaprazan could protect against NSAID-induced gastropathy through heat shock protein 27 accentuation(11) and ameliorated stress-related mucosal diseases through mitigating endoplasmic reticulum stress,(29) a novel APA, revaprazane possesses significant anti-inflammatory activities in H. pylori infection in addition to potent acid suppression.
Fig. 4.
A putative molecular mechanism showing the inhibition of H. pylori-induced COX-2 expression with revaprazan. Revaprazan, a novel acid pump antagonist, exerted COX-2 inhibition led by the inactivation of Akt signaling and NF-κB-DNA binding actions. Therefore, even though revaprazane is acid suppressant playing as acid pump antagonist, it can additionally impose the direct anti-inflammatory actions in H. pylori-associated gastric inflammation as well as rescuing stomach from H. pylori-induced cytotoxicity.
Acknowledgments
This work was supported by the grants from Korean Health Industry Development Institute (KHIDI) and also by National Center of Efficacy Evaluation for the Development of Health Products Targeting Digestive Disorders (NCEED).
Abbreviations
- APA
acid pump antagonist
- COX-2
cyclooxygenase-2
- H. pylori
Helicobacter pylori
- NF-κB
nuclear factor-kappaB
- PI3K
phosphoinositol-3 kinase
- PPI
proton pump inhibitor
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Saukkonen K, Rintahaka J, Sivula A, et al. Cyclooxygenase-2 and gastric carcinogenesis. APMIS. 2003;111:915–925. doi: 10.1034/j.1600-0463.2003.1111001.x. [DOI] [PubMed] [Google Scholar]
- 2.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 3.Guo YS, Hellmich MR, Wen XD, Townsend CM,, Jr Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J Biol Chem. 2001;276:22941–22947. doi: 10.1074/jbc.M101801200. [DOI] [PubMed] [Google Scholar]
- 4.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 5.Xie J, Qian J, Wang S, Freeman ME,, 3rd, Epstein J, Yi Q. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J Immunol. 2003;171:4792–4800. doi: 10.4049/jimmunol.171.9.4792. [DOI] [PubMed] [Google Scholar]
- 6.Romashkova JA, Makarov SS. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 7.Van Dross, Hong X, Pelling JC. Inhibition of TPA-induced cyclooxygenase-2 (COX-2) expression by apigenin through downregulation of Akt signal transduction in human keratinocytes. Mol Carcinog. 2005;44:83–91. doi: 10.1002/mc.20123. [DOI] [PubMed] [Google Scholar]
- 8.Takagi T, Naito Y, Okada H, et al. Lansoprazole, a proton pump inhibitor, mediates anti-inflammatory effect in gastric mucosal cells through the induction of heme oxygenase-1 via activation of NF-E2-related factor 2 and oxidation of kelch-like ECH-associating protein 1. J Pharmacol Exp Ther. 2009;331:255–264. doi: 10.1124/jpet.109.152702. [DOI] [PubMed] [Google Scholar]
- 9.Yeo M, Kim DK, Kim YB, et al. Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clin Cancer Res. 2004;10:8687–8696. doi: 10.1158/1078-0432.CCR-04-1065. [DOI] [PubMed] [Google Scholar]
- 10.Yeo M, Kwak MS, Kim DK, et al. The novel acid pump antagonists for anti-secretory actions with their peculiar application beyond acid suppression. J Clin Biochem Nutr. 2006;38:1–8. [Google Scholar]
- 11.Ock CY, Lim YJ, Kim YJ, et al. Acid pump antagonist-provoked HSP27 dephosphorylation and accentuation rescues stomach from indomethacin-induced damages. J Dig Dis. 2011;12:71–81. doi: 10.1111/j.1751-2980.2011.00482.x. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Han SU, Lee KM, Park KH, Cho SW, Hahm KB. 5-LOX inhibitor modulates the inflammatory responses provoked by Helicobacter pylori infection. Helicobacter. 2007;12:49–58. doi: 10.1111/j.1523-5378.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee KM, Hwang MK, Lee DE, Lee KW, Lee HJ. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J Agric Food Chem. 2010;58:5815–5820. doi: 10.1021/jf903698s. [DOI] [PubMed] [Google Scholar]
- 14.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 15.Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 16.D'Acquisto F, Iuvone T, Rombolà L, Sautebin L, Di Rosa, Carnuccio R. Involvement of NF-kappaB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997;418:175–178. doi: 10.1016/s0014-5793(97)01377-x. [DOI] [PubMed] [Google Scholar]
- 17.Sawaoka H, Kawano S, Tsuji S, et al. Helicobacter pylori infection induces cyclooxygenase-2 expression in human gastric mucosa. Prostaglandins Leukot Essent Fatty Acids. 1998;59:313–316. doi: 10.1016/s0952-3278(98)90079-5. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001;36:706–716. doi: 10.1080/003655201300191969. [DOI] [PubMed] [Google Scholar]
- 19.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 20.Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobacter pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003;48:257–265. doi: 10.1023/a:1021963007225. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Latif MM, Windle H, Terres A, Eidhin DN, Kelleher D, Reynolds JV. Helicobacter pylori extract induces nuclear factor-kappa B, activator protein-1, and cyclooxygenase-2 in esophageal epithelial cells. J Gastrointest Surg. 2006;10:551–562. doi: 10.1016/j.gassur.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 23.Béraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen LA, Allgood JA, Han X, Wittine LM. Phosphoinositide3-kinase regulates actin polymerization during delayed phagocytosis of Helicobacter pylori. J Leukoc Biol. 2005;78:220–230. doi: 10.1189/jlb.0205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 26.Figura N, Armellini D, Bugnoli M, Bayeli PF, Gennari C, Crabtree JE. Activity of omeprazole on Helicobacter pylori and relation to toxicity of strains. J Clin Pathol. 1994;47:440–442. doi: 10.1136/jcp.47.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauch F, Bode G, Malfertheiner P. Identification and characterization of an ATPase system of Helicobacter pylori and the effect of proton pump inhibitors. Am J Gastroenterol. 1993;88:1801–1802. [PubMed] [Google Scholar]
- 28.Lage AP, Glpczynski Y. Omeprazole exerts the same inhibitory effect on the growth of cytotoxic-positive and cytotoxic-negative Helicobacter pylori strains and does not inhibit vacuolating toxin production. Eur J Gastroenterol Hepatol. 1994;6:299–301. [Google Scholar]
- 29.Kim JH, Kim EH, Ock C, et al. Mitigating endoplasmic reticulum stress with revaprazan ameliorates stress-related mucosal disease. J Gastroenterol Hepatol. 2012;27(1):120–129. doi: 10.1111/j.1440-1746.2011.06838.x. [DOI] [PubMed] [Google Scholar]