Abstract
Milk provides a well-balanced source of amino acids and other ingredients. One of the functional ingredients in milk is lactoferrin (LF). LF presents a wide variety of bioactivities and functions as a radical scavenger in models using iron-ascorbate complexes and asbestos. Human clinical trials of oral LF administration for the prevention of colon polyps have been successful and demonstrated that dietary compounds exhibit direct interactions. However, antioxidative properties of LF in distant organs require further investigation. To study the antioxidant property of LF, we employed bovine lactoferrin (bLF) using the rat model of ferric nitrilotriacetate (Fe-NTA)-induced renal tubular oxidative injury. We fed rats with bLF (0.05%, w/w) in basal chow for 4 weeks and sacrificed them after Fe-NTA treatment. After intraperitoneal administration of 9.0 mg iron/kg Fe-NTA for 4 and 24 h, bLF pretreatment suppressed elevation of serum creatinine and blood urea nitrogen levels. In addition, we observed protective effects against renal oxidative tubular damage and maintenance of antioxidant enzyme activities in the bLF-pretreated group. We thus demonstrated the antioxidative effect of bLF against Fe-NTA-induced renal oxidative injury. These results suggest that LF intake is useful for the prevention of renal tubular oxidative damage mediated by iron.
Keywords: lactoferrin, chemoprevention, ferric nitrilotriacetate, oxidative renal damage, glutathione metabolism
Introduction
Lifestyle-related pathologic conditions, such as obesity, diabetes mellitus, hypertension, cardiovascular disease, hyperlipidemia and cancer, are causatively associated with oxidative stress.(1) Therefore, daily dietary intake of antioxidative food may decrease the burden of oxidative stress and be beneficial for the promotion of health.(2) During development in mammals, the neonatal stage is sensitive to oxidative insults.(3) Dietary elements in milk might play pivotal roles in the protection of infants from harmful reactive oxygen species (ROS). Lactoferrin (LF) is one of the most functional bioavailable compounds in milk.
Previous studies have suggested that LF has beneficial effects on plasma lipids, antimicrobial, immunomodulatory, antiproliferative and antioxidant activities.(4) LF is a member of the transferrin family of non-heme iron-binding glycoproteins(5) and is expressed and secreted by a variety of glandular epithelial cells in adults of many species.(6) Despite a high degree of homology at the amino acid sequence level between transferrin and LF,(7) the function of LF differs from transferrin. Endogenous LF is induced by the exposure of iron and carcinogenic asbestos fibers in rodents, which implies a protective role for LF by forming complexes with harmful free iron in tissues in vivo.(8,9) Although, LF knockout mice do not show alterations of iron homeostasis in vivo,(10) feeding of LF-supplemented diets leads to decreased plasma triacylglycerol and free fatty acid levels and increased high-density lipoprotein levels, suggesting that LF has potential therapeutic effects in lifestyle-related diseases.(11)
The iron-binding capacity of LF might mediate antioxidant activities in studies of asbestos-induced superoxide generation in vitro.(12) Protective effects of LF have been detected in Long-Evans Cinnamon (LEC) rats,(13) neonates(3) and patients with chronic hepatitis.(14) Based on these physiological functions, LF has been evaluated in a clinical trial for metastatic renal cell carcinoma(15) and has been used successfully in clinical trials for prevention of transformation of colon polyps.(16) However, in vivo bioactivity of LF against iron-mediated oxidative stress is poorly understood.
In the present study, we employed an animal model of oxidative renal tubular damage that is induced by ferric nitrilotriacetate (Fe-NTA),(17) to examine the biological effect of bovine lactoferrin (bLF), which is a common compound in our daily life. Fe-NTA, which is an iron chelate, catalyzes the generation of ROS and accelerates lipid peroxidation in the kidney through intraperitoneal (i.p.) administration in rats and mice,(18) which revealed a high incidence of renal cell carcinoma.(19) At the acute phase, oxidatively modified molecules are increased in the kidney after Fe-NTA treatment. Increases in malondialdehydes,(20) thiobarbituric acid-reactive substances (TBARS),(18) 4-hydroxy-2-nonenal (HNE)-modified proteins,(21–23) and 8-hydroxy-2'-deoxyguanosine (8-OHdG)(24) have been demonstrated.
Regarding genetic alternations of this carcinogenetic model, homozygous deletion of the CDKN2A/2B tumor suppressor gene, which is an inhibitor for cyclin-dependent kinase has been observed(25) with monoallelic loss occurring as early as 3 weeks after the start of the protocol.(26) The other target genes in carcinogenesis and progression include annexin2,(27) ptprz1,(28) and aminoacylase1.(29)
Well-known antioxidants such as vitamin E,(30) curcumin,(31) beverage-containing fermented black soybean(32) and many other compounds,(33) have been reported to show protective effects in this carcinogenesis model. Based on these studies, this carcinogenesis model is useful for the evaluation of antioxidant properties in the presence of excess iron in vivo. In the present study, we disclosed for the first time that bLF has advantageous effects on iron-induced renal injury. Possible mechanisms and their implications will be discussed.
Materials and Methods
Chemicals
Iron nitrate enneahydrate, nitrilotriacetic acid, oxidized and reduced glutathione (GSH), and GSH reductase were purchased from Wako Pure Chem. Ind. Ltd. (Osaka, Japan). Nicotinamide adenine dinucleotide phosphate reduced, 5,5'-dithio-bis-2-nitrobenzoic acid (DTNB), bovine serum albumin, trichloroacetic acid, Tween 20 were purchased from Sigma (St. Louis, MO). Basal MF diet (powder) was from Oriental Yeast (Tokyo, Japan). The BCA assay kit was from Pierce (Rockford, IL). Normal goat serum was from Vector Laboratories (Burlingame, CA) and Histofine Simple Stain rat Max-PO (multi) was from Nichirei (Tokyo, Japan). Liquid DAB was from DAKO (Kyoto, Japan). Antibodies against HNE-modified proteins (HNE-J2)(23) were purchased from the Japan Institute for the Control of Aging (Shizuoka, Japan). All other chemicals were of the highest quality available from Wako Pure Chem. Ind. Ltd. (Osaka, Japan). bLF was a kind gift from the Morinaga Milk Co. (Tokyo, Japan). The ingredients in bLF are summarized in Table 1.
Table 1.
Ingredients of bovine lactoferrin (bLF)
| Calorie | 4 kcal/g |
| Carbohydrate | 0% |
| Protein | 99.8% |
| Fat | <0.5% |
| Purity of bLF | 96.7% |
| Ash | 190 mg/100 g |
| Sodium | 54.7 mg/100 g |
| Iron | 8.60 mg/100 g |
| Calcium | 8.54 mg/100 g |
| Phosphorus | 2.21 mg/100 g |
| Potassium | 1.94 mg/100 g |
| Magnesium | 0.58 mg/100 g |
| Zinc | 0.27 mg/100 g |
Preparation of Fe-NTA solution
The Fe-NTA solution was prepared as previously described.(34) In brief, nitrilotriacetic acid (NTA) and iron nitrate enneahydrate were dissolved in distilled water. The pH was adjusted to 7.0 with sodium bicarbonate. The molar ratio of Fe to NTA was 1:4.
Animal experiments
The Animal Care Committee of Okayama University Graduate School of Medicine and Dentistry approved these experiments. Care and handling of the animals were in accordance with the National Institutes of Health Guidelines. Male Wistar rats (4 weeks old) were purchased from Japan SLC (Hamamatsu, Japan). The rats were housed in plastic cages of temperature-controlled (25°C with alternating 12-h light/12-h dark cycles) room and allowed free access to distilled water and a standard powdered-chow diet (MF) during the experiment.
A total of 18 rats (60–80 g) were used for the following pharmacological experiments. These rats were fed with a powdered diet with different concentrations of bLF ad libitum for 4 weeks to monitor the effects on growth. In this experiment, rats were divided into six groups [1 untreated group (n = 3) and 5 bLF groups (1, 0.5, 0.1, 0.05 and 0.01%) (n = 3)].
In the investigation of antioxidant properties of bLF, 32 rats were randomly divided into 4 groups [untreated, bLF (0.05%) alone (n = 4), Fe-NTA alone (n = 6) and bLF (0.05%) + Fe-NTA (n = 6)]. These rats were fed with the regular powdered diet or powdered diet with bLF (0.05%, w/w) ad libitum for 4 weeks. Each animal received an i.p. injection of Fe-NTA (9.0 mg iron/kg body weight). After sacrificing the animals, the sera were harvested, and the kidneys were immediately excised for enzyme assays and histological examination.
Determination of creatinine, blood urea nitrogen (BUN), and serum iron-binding capacity
Blood urea nitrogen, creatinine, unsaturated iron-binding capacity (UIBC) and total iron-binding capacity (TIBC) in sera were measured using an autoanalyzer (Hitachi 7600-110S).
Determination of reduced GSH and antioxidant enzyme activities of kidney
These enzymatic activities were assayed with the post-mitochondrial supernatant of homogenized renal tissue. Reduced GSH levels were measured using DTNB as a chromogen.(35) GSH peroxidase and GSH reductase activities were measured based on NADPH oxidation in a coupled system.(35,36)
Hematoxylin and eosin (HE) staining
Kidneys were transversely cut including the renal pelvis at 5-mm thickness and immediately fixed with 10% phosphate-buffered formalin. The samples were fixed overnight and subjected to paraffin embedding. The paraffin-embedded tissues were sliced into 4-µm sections and mounted onto glass slides. These slides were used for hematoxylin and eosin staining and immunohistochemical analyses.
Immunohistochemical analysis
Immunohistochemical analyses were performed as previously described.(24,32,37)
Statistical analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA) and an unpaired t test. The difference was considered significant when p<0.05. The data were expressed as the mean ± SEM (n = 3–6) unless otherwise specified.
Results
The effect of bLF on growth and serum iron-binding capacity
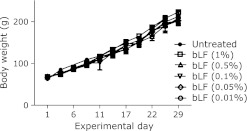
We observed no growth retardation during bLF feeding (1, 0.5, 0.1, 0.05 and 0.01%; w/w) (Fig. 1). After 4 weeks, rats received i.p. treatment of Fe-NTA (9.0 mg iron/kg body weight). We observed that 0.05% (w/w) bLF was the most efficient to protect kidney from acute tubular injury compared to other doses of bLF (data not shown). Based on these preliminary experiments, we performed the following experiments with 0.05% (w/w) bLF. Iron levels, total iron-binding capacity (TIBC) and unsaturated iron-binding capacity (UIBC) were not altered after treatment of bLF (0.05%, w/w) in rats (transferrin saturation of untreated control, 24.7 ± 2.54%; transferrin saturation of bLF alone, 22.6 ± 0.69%; n = 4; p = 0.44, untreated vs bLF alone).
Fig. 1.
The effect of bovine lactoferrin (bLF) on normal growth. Body weight curve. All rats were active and healthy following administration of different concentrations of bLF (1, 0.5, 0.1, 0.05 and 0.01% w/w) (n = 3).
Serum creatinine and BUN
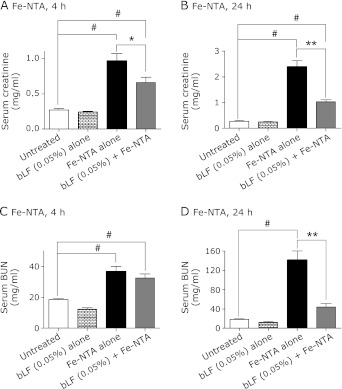
After Fe-NTA administration for 4 and 24 h, an elevation of creatinine and BUN in sera was detected. Pretreatment with bLF suppressed the elevations of these parameters at both time points (Fig. 2 A–D). A similar effect was observed in creatinine and BUN levels.
Fig. 2.
Serum markers for renal dysfunction at 4 and 24 h after Fe-NTA administration. (A) Serum creatinine: Fe-NTA, 4 h. (B) Serum creatinine: Fe-NTA, 24 h, (C) Serum BUN: Fe-NTA, 4 h. (D) Serum BUN: Fe-NTA 24 h. Fe-NTA treatment induced elevation of serum creatinine and BUN, which are markers of renal failure. Pretreatment with bLF suppressed elevation of serum creatinine and BUN. (ANOVA, p<0.0001 for A–D; #p<0.05 vs untreated; *p<0.05 vs Fe-NTA alone; **p<0.01 vs Fe-NTA alone).
Determination of reduced GSH
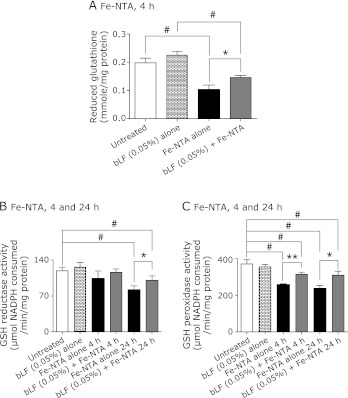
The pretreatment with bLF induced slight elevations of renal reduced GSH without statistical significance (p = 0.25). GSH was decreased 4 h after Fe-NTA administration without bLF pretreatment. These alternations were significantly suppressed by bLF pretreatment (Fig. 3A).
Fig. 3.
Determination of renal reduced glutathione (GSH) at 4 h and activities of renal antioxidative enzymes at 4 and 24 h after Fe-NTA administration. (A) GSH: Fe-NTA, 4 h. (B) GSH reductase: Fe-NTA, 4 and 24 h. (C) GSH peroxidase: Fe-NTA, 4 and 24 h. (A) Bovine lactoferrin (bLF, 0.05%, w/w) pretreatment demonstrated slight elevation of renal GSH level (p = 0.25, untreated vs bLF alone). GSH depletion was detected in Fe-NTA-administered rats. We observed that bLF pretreatment attenuated Fe-NTA-induced renal oxidative damage. (B) and (C) Protective effect of bLF (0.05%, w/w) pretreatment on Fe-NTA-induced renal oxidative damage was observed based on both parameters (ANOVA, p<0.0001, for A–C; #p<0.05 vs untreated or bLF alone; *p<0.05 and **p<0.01 vs Fe-NTA alone).
Antioxidative enzyme activity
After Fe-NTA treatment for 4 h, activities of the GSH peroxidase and GSH reductase were decreased, which was prevented by bLF pretreatment. However, no elevation of these enzymatic activities was detected following bLF pretreatment. Levels of GSH reductase and GSH peroxidase were maintained in the bLF-pretreated group during oxidative injury at 24 h after Fe-NTA treatment (Fig. 3 B and C).
Histology
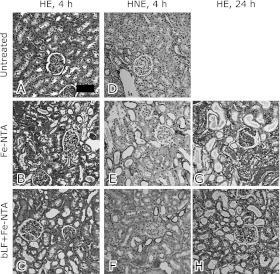
We evaluated the renal histology of Fe-NTA-treated rats 4 h after Fe-NTA treatment (Fig. 4 A–F). Acute tubular necrosis was apparent in the proximal tubules, after Fe-NTA treatment. Pretreated rats with bLF were protected from tubular injury (Fig. 4C).
Fig. 4.
Histological and immunohistochemical analyses of kidney at 4 and 24 h after Fe-NTA administration. Hematoxylin and eosin staining (HE). (A) Untreated, (B) Fe-NTA alone, 4 h, (C) or in the presence of bLF + Fe-NTA, 4 h. Immunohistochemical staining of 4-hydroxy-2-nonenal-modified proteins (HNE). (D) Untreated, (E) Fe-NTA alone, 4 h, (F) bLF + Fe-NTA, 4 h. HE staining. (G) Fe-NTA alone, 24 h, and (H) bLF + Fe-NTA, 24 h. Representative images are shown. Scattered necrotic tubules were detected (B). Only a few necrotic tubules and some degenerative tubules were observed (C). HNE immunostaining revealed accumulation of oxidatively modified proteins. No positive tubules in the HNE immunostaining were observed (D), whereas many positive tubules were detected (E). Immunopositivity was markedly decreased (F). After Fe-NTA administration for 24 h, oxidative injury destroyed massive proximal tubules (G). Pretreatment with bLF protected against oxidative injury (H). Few infiltrating inflammatory cells were observed in histological samples (bar, 50 µm).
In the cases of Fe-NTA-treated rats, degenerative proximal tubular cells with HNE-modified proteins were observed. However, the number of HNE-positive tubules was significantly decreased in the bLF-treated group. Positive tubules with apparent HNE-modified proteins were not observed in the untreated group (Fig. 4 D–F).
We evaluated the renal histology of Fe-NTA-treated rats 24 h after Fe-NTA treatment. Massive destruction of proximal tubules and cast depositions were observed in the Fe-NTA-treated group (Fig. 4G). These alternations were confined to localized areas by bLF pretreatment (Fig. 4H). Few infiltrating inflammatory cells were observed in the kidney after Fe-NTA treatment.
Discussion
Fe-NTA causes renal cell carcinoma via iron-mediated oxidative stress in rats, with homozygous deletion of CDKN2A/2B in one-third of the cases.(25,26) This is a unique experimental model, useful in search of antioxidant compounds by the pretreatment for 4 weeks and of chemopreventive compounds by the dietary administration for several months.(31–33) We observed that dietary administration of bLF (0.05%, w/w) protected renal tissue from Fe-NTA-induced damage. We demonstrated that bLF inhibited elevation of serum markers of acute renal failure and renal tubular injury. In addition, bLF pretreatment maintained the levels of reduced GSH, GSH peroxidase and GSH reductase activities. Previous studies have demonstrated that bLF pretreatment induces antioxidative enzymes in a colon cancer cell line.(38) We observed that dietary intake of bLF increased GSH and GSH reductase levels. However, this increase was not statistically significant (p = 0.25 and p = 0.55 for GSH and GSH reductase, respectively, untreated vs bLF alone).
In this study, we employed regular bovine lactoferrin (bLF), which contains apo-LF and holo-LF. Consistent with the results of previous studies,(39) no toxic effects of bLF (1%, w/w) were observed based on growth and BUN and creatine levels. In addition, we did not detect significant changes in TIBC, UIBC and transferrin saturation after bLF treatment (0.05%, w/w). In our experimental design, rats ate 20–25 g/day, which was used to estimate bLF intake as 10–12.5 mg/day. This calculation suggested that intact bLF did not enter blood stream to increase the levels of iron saturation in transferrin, which was confirmed using the conventional chemical detection method in this study and is consistent with previous reports.(10,40) Non-transferrin-bound iron (NTBI) in the sera is an emerging marker of iron toxicity.(41,42) Although we did not measure NTBI levels in the sera, quantification of NTBI might disclose evidence as a role of bLF in antioxidant modulation via chelation of Fe-NTA.
Detection of LF in the urine suggested that LF might directly interact with proximal tubules.(6) We have not determined the endogenous rat LF in the kidney. However, a possible mechanism of altered endogenous LF levels may reveal concomitant alterations in the GSH cycle in renal proximal tubules, which is directly associated with the pathologic mechanism of Fe-NTA toxicity.(18,33) Other possible mechanisms of LF-mediated attenuation of Fe-NTA-induced oxidative stress include LF-mediated effects on serum NTBI levels and Fe-NTA absorption from the peritoneum. These proposed mechanisms require further investigation.
Previous studies have revealed chemopreventive activity of bLF outside the digestive tract, such as hepatitis in LEC rats, lung tumors and chronic hepatitis.(13,14,43,44) In LEC rats, LF improves rat survival and inhibits mitochondrial 8-OHdG formation but not nuclear 8-OHdG. These results indicate that LF suppresses copper-induced oxidative stress. In female Ogg1 knockout mice, bLF suppresses lung tumor development produced via 8-OHdG accumulation. These results imply that dietary intake of bLF has antioxidative effects.
Recent clinical trials have tested the effects of dietary intake of bLF on metastatic renal cell carcinoma.(15) The mechanism of anti-tumor bioactivity was suggested that bLF may stimulate production of Interferon-gamma (IFN-γ) and IL-12, which promote a Th1 response in the intestinal mucosa.(16) This polarization of Th1-activated neutrophils, macrophages, NK cells and cytotoxic T cells enhances anti-tumor activities and may contribute to the protection of renal oxidative injury. Activation of the IL-12/IFN-γ signaling pathway is required in ischemia-reperfusion injury, where harmful ROS play a critical role in mice.(45) In our model of renal cell carcinoma, which is positive for transforming growth factor-α,(46) we observed few infiltrating inflammatory cells after a single dose of Fe-NTA (Fig. 4). We previously demonstrated that iron loading by iron saccharate enhances the production of systemic inflammatory cytokines following lipopolysaccharide stimulation.(47) However, interactions between Fe-NTA-induced renal damage and cytokines requires further investigation.
As another possible mechanism, digested peptides that are derived from bLF or secreted from the gastrointestinal mucosa following activation of immune and neuroendocrine systems might play synergistic roles to protect the kidney from iron-induced oxidative stress. A profiling analysis using microarray techniques might elucidate bLF-interacting molecules after oral administration of bLF.
In conclusion, we observed, for the first time, that bLF had protective effects against iron-induced renal tubular injury in rats. Further study is warranted to disclose human pathologic conditions or genotypes where this kind of chemoprevention is useful.
Acknowledgments
This work was supported by the Grant-in-Aid for Young Scientists (B) (23790440) from the Japan Society for the Promotion of Science and the grant from Bioactive Okayama. The authors wish to thank Morinaga Milk Co. for the kind gift and chemical analysis of bLF, Hideo Sakai and Yoko Watanabe for animal care, Emiko Okazaki for technical assistance and Masayoshi Fujisawa (Himeji Red Cross Hospital) for discussion.
Abbreviations
- ANOVA
analysis of variance
- bLF
bovine lactoferrin
- BUN
blood urea nitrogen
- DAB
3,3'-diaminobenzidine
- DTNB
5,5'-dithio-bis-2-nitrobenzoic acid
- Fe-NTA
ferric nitrilotriacetate
- GSH
glutathione
- HNE
4-hydroxy-2-nonenal
- IFN-γ
Interferon-gamma
- IL-12
interleukin-12
- i.p.
intraperitoneal
- LEC
Long-Evans Cinnamon
- LF
lactoferrin
- NTBI
non-transferrin-bound iron
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- ROS
reactive oxygen species
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Toyokuni S. Iron as a target of chemoprevention for longevity in humans. Free Radic Res. 2011;45:906–917. doi: 10.3109/10715762.2011.564170. [DOI] [PubMed] [Google Scholar]
- 2.Diplock AT, Charleux JL, Crozier-Willi G, et al. Functional food science and defence against reactive oxidative species. Br J Nutr. 1998;80 (Suppl 1):S77–S112. doi: 10.1079/bjn19980106. [DOI] [PubMed] [Google Scholar]
- 3.Raghuveer TS, McGuire EM, Martin SM, et al. Lactoferrin in the preterm infants’ diet attenuates iron-induced oxidation products. Pediatr Res. 2002;52:964–972. doi: 10.1203/00006450-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–1543S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 5.Aisen P, Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972;257:314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- 6.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 7.Metz-Boutigue MH, Jollès J, Mazurier J, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984;145:659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 8.Ghio AJ, Carter JD, Richards JH, Brighton LE, Lay JC, Devlin RB. Disruption of normal iron homeostasis after bronchial instillation of an iron-containing particle. Am J Physiol. 1998;274:L396–L403. doi: 10.1152/ajplung.1998.274.3.L396. [DOI] [PubMed] [Google Scholar]
- 9.Ghio AJ, Stonehuerner J, Richards J, Devlin RB. Iron homeostasis in the lung following asbestos exposure. Antioxid Redox Signal. 2008;10:371–377. doi: 10.1089/ars.2007.1909. [DOI] [PubMed] [Google Scholar]
- 10.Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol. 2003;23:178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi T, Shimizu H, Ando K, Harada E. Bovine lactoferrin reduces plasma triacylglycerol and NEFA accompanied by decreased hepatic cholesterol and triacylglycerol contents in rodents. Br J Nutr. 2004;91:533–538. doi: 10.1079/BJN20041090. [DOI] [PubMed] [Google Scholar]
- 12.Ghio AJ, Stonehuerner J, Steele MP, Crumbliss AL. Phagocyte-generated superoxide reduces Fe3+ to displace it from the surface of asbestos. Arch Biochem Biophys. 1994;315:219–225. doi: 10.1006/abbi.1994.1493. [DOI] [PubMed] [Google Scholar]
- 13.Tsubota A, Yoshikawa T, Nariai K, et al. Bovine lactoferrin potently inhibits liver mitochondrial 8-OHdG levels and retrieves hepatic OGG1 activities in Long-Evans Cinnamon rats. J Hepatol. 2008;48:486–493. doi: 10.1016/j.jhep.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Konishi M, Iwasa M, Yamauchi K, et al. Lactoferrin inhibits lipid peroxidation in patients with chronic hepatitis C. Hepatol Res. 2006;36:27–32. doi: 10.1016/j.hepres.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Jonasch E, Stadler WM, Bukowski RM, et al. Phase 2 trial of talactoferrin in previously treated patients with metastatic renal cell carcinoma. Cancer. 2008;113:72–77. doi: 10.1002/cncr.23519. [DOI] [PubMed] [Google Scholar]
- 16.Tsuda H, Kozu T, Iinuma G, et al. Cancer prevention by bovine lactoferrin: from animal studies to human trial. Biometals. 2010;23:399–409. doi: 10.1007/s10534-010-9331-3. [DOI] [PubMed] [Google Scholar]
- 17.Hamazaki S, Okada S, Ebina Y, Midorikawa O. Acute renal failure and glucosuria induced by ferric nitrilotriacetate in rats. Toxicol Appl Pharmacol. 1985;77:267–274. doi: 10.1016/0041-008x(85)90326-6. [DOI] [PubMed] [Google Scholar]
- 18.Hamazaki S, Okada S, Toyokuni S, Midorikawa O. Thiobarbituric acid-reactive substance formation of rat kidney brush border membrane vesicles induced by ferric nitrilotriacetate. Arch Biochem Biophys. 1989;274:348–354. doi: 10.1016/0003-9861(89)90448-7. [DOI] [PubMed] [Google Scholar]
- 19.Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int. 1999;49:91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 20.Uchida K, Fukuda A, Kawakishi S, Hiai H, Toyokuni S. A renal carcinogen ferric nitrilotriacetate mediates a temporary accumulation of aldehyde-modified proteins within cytosolic compartment of rat kidney. Arch Biochem Biophys. 1995;317:405–411. doi: 10.1006/abbi.1995.1181. [DOI] [PubMed] [Google Scholar]
- 21.Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci USA. 1994;91:2616–2620. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyokuni S, Luo XP, Tanaka T, Uchida K, Hiai H, Lehotay DC. Induction of a wide range of C2-12 aldehydes and C7-12 acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic Biol Med. 1997;22:1019–1027. doi: 10.1016/s0891-5849(96)00489-3. [DOI] [PubMed] [Google Scholar]
- 23.Toyokuni S, Miyake N, Hiai H, et al. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995;359:189–191. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 24.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 25.Tanaka T, Iwasa Y, Kondo S, Hiai H, Toyokuni S. High incidence of allelic loss on chromosome 5 and inactivation of p15INK4B and p16INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene. 1999;18:3793–3797. doi: 10.1038/sj.onc.1202707. [DOI] [PubMed] [Google Scholar]
- 26.Hiroyasu M, Ozeki M, Kohda H, et al. Specific allelic loss of p16INK4A tumor suppressor gene after weeks of iron-mediated oxidative damage during rat renal carcinogenesis. Am J Pathol. 2002;160:419–424. doi: 10.1016/S0002-9440(10)64860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T, Akatsuka S, Ozeki M, Shirase T, Hiai H, Toyokuni S. Redox regulation of annexin 2 and its implications for oxidative stress-induced renal carcinogenesis and metastasis. Oncogene. 2004;23:3980–3989. doi: 10.1038/sj.onc.1207555. [DOI] [PubMed] [Google Scholar]
- 28.Liu YT, Shang D, Akatsuka S, et al. Chronic oxidative stress causes amplification and overexpression of ptprz1 protein tyrosine phosphatase to activate beta-catenin pathway. Am J Pathol. 2007;171:1978–1988. doi: 10.2353/ajpath.2007.070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong Y, Onuki J, Yamasaki T, Ogawa O, Akatsuka S, Toyokuni S. Genome-wide analysis identifies a tumor suppressor role for aminoacylase 1 in iron-induced rat renal cell carcinoma. Carcinogenesis. 2009;30:158–164. doi: 10.1093/carcin/bgn255. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Okada S, Yu Y, Zheng P, Yamaguchi R, Kasai H. Vitamin E inhibits apoptosis, DNA modification, and cancer incidence induced by iron-mediated peroxidation in Wistar rat kidney. Cancer Res. 1997;57:2410–2414. [PubMed] [Google Scholar]
- 31.Okazaki Y, Iqbal M, Okada S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim Biophys Acta. 2005;1740:357–366. doi: 10.1016/j.bbadis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki Y, Iqbal M, Kawakami N, Yamamoto Y, Toyokuni S, Okada S. A beverage containing fermented black soybean ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J Clin Biochem Nutr. 2010;47:198–207. doi: 10.3164/jcbn.10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada S. Prevention of free-radical mediated tissue damage and carcinogenesis induced by low-molecular-weight iron. Biometals. 2003;16:99–101. doi: 10.1023/a:1020747508072. [DOI] [PubMed] [Google Scholar]
- 34.Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am J Pathol. 1979;95:663–673. [PMC free article] [PubMed] [Google Scholar]
- 35.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086–5091. [PubMed] [Google Scholar]
- 36.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- 37.Tanaka T, Nishiyama Y, Okada K, et al. Induction and nuclear translocation of thioredoxin by oxidative damage in the mouse kidney: independence of tubular necrosis and sulfhydryl depletion. Lab Invest. 1997;77:145–155. [PubMed] [Google Scholar]
- 38.Burrow H, Kanwar RK, Kanwar JR. Antioxidant enzyme activities of iron-saturated bovine lactoferrin (Fe-bLf) in human gut epithelial cells under oxidative stress. Med Chem. 2011;7:224–230. doi: 10.2174/157340611795564286. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi K, Toida T, Nishimura S, et al. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem Toxicol. 2000;38:503–512. doi: 10.1016/s0278-6915(00)00036-3. [DOI] [PubMed] [Google Scholar]
- 40.Wakabayashi H, Kuwata H, Yamauchi K, Teraguchi S, Tamura Y. No detectable transfer of dietary lactoferrin or its functional fragments to portal blood in healthy adult rats. Biosci Biotechnol Biochem. 2004;68:853–860. doi: 10.1271/bbb.68.853. [DOI] [PubMed] [Google Scholar]
- 41.Gosriwatana I, Loreal O, Lu S, Brissot P, Porter J, Hider RC. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem. 1999;273:212–220. doi: 10.1006/abio.1999.4216. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki K, Ikuta K, Tanaka H, et al. Improved quantification for non-transferrin-bound iron measurement using high-performance liquid chromatography by reducing iron contamination. Mol Med Report. 2011;4:913–918. doi: 10.3892/mmr.2011.518. [DOI] [PubMed] [Google Scholar]
- 43.Igarashi M, Watanabe M, Yoshida M, et al. Enhancement of lung carcinogenesis initiated with 4-(N-hydroxymethylnitrosamino)-1-(3-pyridyl)-1-butanone by Ogg1 gene deficiency in female, but not male, mice. J Toxicol Sci. 2009;34:163–174. doi: 10.2131/jts.34.163. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda Y, Saoo K, Hosokawa K, et al. Post-initiation chemopreventive effects of dietary bovine lactoferrin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in female A/J mice. Cancer Lett. 2007;246:41–46. doi: 10.1016/j.canlet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deguchi J, Kawabata T, Kondo A, Okada S. Transforming growth factor-alpha expression of renal proximal tubules in Wistar rats treated with ferric and aluminum nitrilotriacetate. Jpn J Cancer Res. 1993;84:649–655. doi: 10.1111/j.1349-7006.1993.tb02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hida AI, Kawabata T, Minamiyama Y, Mizote A, Okada S. Saccharated colloidal iron enhances lipopolysaccharide-induced nitric oxide production in vivo. Free Radic Biol Med. 2003;34:1426–1434. doi: 10.1016/s0891-5849(03)00143-6. [DOI] [PubMed] [Google Scholar]