Abstract
Objectives
To investigate one-segment strain and strain rate indices as measures of myocardial performance in asphyxiated term neonates.
Design
Quality improvement cohort study.
Setting
Newborns admitted to a neonatal intensive care unit at a Norwegian University Hospital for perinatal asphyxia and non-asphyxiated newborn recruited from the maternity ward at the same hospital.
Participants
Twenty asphyxiated and 48 non-asphyxiated term neonates.
Primary outcome measure
Strain and strain rate indices and repeatability measures. One-segment longitudinal strain and strain rate by tissue Doppler were assessed on days 1, 2 and 3 of life in nine heart walls. Repeatability was compared against measurements from two-segment analyses previously performed in the same images.
Results
The 95% limits of agreement were significantly better for the one-segment than two-segment repeatability analyses, the inter-rater peak systolic strain (PSS) was (−3.1, 3.3) vs (−11.4, 18.3)%, the inter-rater peak systolic strain rate (PSSR) was (−0.38, 0.40) vs (−0.79, 1.15)/s, the intra-rater PSS was (−2.5, 2.6) vs (−8.0, 9.8)% and the intra-rater PSSR was (−0.23, 0.25) vs (−0.75, 0.80)/s (p<0.05). The myocardial performance was lower in the asphyxiated neonates (indices closer to zero) than in the non-asphyxiated neonates, PSS was −17.8 (0.6) (mean (SEM)) vs −21.2 (0.3)%, PSSR −1.43 (0.08) vs −1.61 (0.03)/s, early diastolic strain rate 1.72 (0.11) vs 2.00 (0.11)/s and strain rate during the atrial systole 1.92 (0.17) vs 2.27 (0.10)/s (p<0.05), despite no difference in fractional shortening (29.0 (0.5) vs 29.1 (1.0)%) (p>0.05).
Conclusions
One-segment strain and strain rate assessed the reduced myocardial performance in asphyxiated neonates with significantly improved reproducibility as compared with two-segment analysis and was therefore more feasible than two-segment analyses for assessment of myocardial performance after perinatal asphyxia.
Keywords: Cardiology, Echocardiography, Neonatology, Paediatrics, Paediatric cardiology
Article summary.
Article focus
Strain and strain rate by tissue Doppler are sensitive indices for the assessment of myocardial performance in neonates.
Use of one-segment analyses might improve repeatability and make the analyses more suitable for clinical practice.
In this study, the feasibility and reliability of one-segment strain and strain rate analyses for assessment of the myocardial performance in asphyxiated and healthy term neonates are assessed.
Key messages
The variation between repeated measurements in neonates was reduced by the use of one-segment strain and strain rate analyses as compared to two-segment analyses.
One-segment strain and strain rate indices of myocardial performance in systole and diastole is reduced in perinatal asphyxia.
One-segment analysis might be a promising modality for assessing myocardial performance in the daily care of asphyxiated term neonates.
Strengths and limitations of this study
The measurements and the variability can be directly compared between the one-segment and two-segment analyses, as the same tissue Doppler images were used in both analysis procedures.
As an unselected group of asphyxiated neonates was studied, this study was not designed for quantifying the myocardial performance after perinatal asphyxia. A larger study should be conducted to study in more detail the impact from asphyxia on the myocardial performance.
Introduction
Perinatal asphyxia can lead to impaired oxygen supply to the myocardium. Affection of the heart after asphyxia is usually part of a multiorgan involvement.1–7 Myocardial injury can occur in both ventricles.8 Autopsies have shown that the necrotic areas frequently are scattered, with the papillary muscles and the subendocardial regions most prone to injury.9 10 The reduced myocardial performance following perinatal asphyxia11 12 can contribute to increase end-organ damage and mortality.13 Standard methods for cardiovascular monitoring in neonates such as blood pressure, capillary refill time, urinary output, biochemical markers and ECG are of limited value.14–16
Reduced myocardial performance after perinatal asphyxia can be assessed by cardiac ultrasound,14 17–20 traditionally based on left ventricle cavity measures such as fractional shortening and ejection fraction.21 Some studies have shown a reduction in these indices after perinatal asphyxia 14 17 19 22 while others have not.13 23–25
Newer tissue Doppler-derived indices have been introduced in neonates such as atrioventricular (AV) plane velocities26–29 and strain and strain rate in myocardial segments.29–33 Especially the AV-plane velocities25 34 and two-segment systolic longitudinal strain and strain rate33 have proven to be more sensitive than fractional shortening as markers for reduced myocardial performance after perinatal asphyxia. Strain and strain rate have the additional advantage that they are normalised for heart size, compared to velocities and AV-plane motion. Strain rate in ventricle diastole is yet to be assessed in asphyxiated neonates. Newer modalities using two-dimensional analyses of grey-scale images have been used for deformation analysis also in neonates,35 36 but in neonates its use might be limited by a low temporal resolution. At a low temporal resolution the true peak values might be missed and hence false low peak values might be assessed.
Because strain and strain rate indices have the advantages over tissue velocities that they are normalised for heart size, they may be more suitable for comparing the performance of hearts of different size and during growth. However, the signal-to-noise ratio and the reproducibility are poor in small segments37 and the analysis procedure is complex.32
We have earlier shown a low reproducibility in two-segment strain and strain rate analyses in neonates.32 33 In one-segment analyses the segments can be larger, improving both signal-to-noise ratio and reproducibility.37 38 The analysis procedure can be simplified by omitting the tracking procedure32 33 and by use of one sample area per wall instead of two32 33 or three.30 31
The main obstacles against use of strain and strain rate analyses in routine neonatal care might therefore be overcome by use of one large segment from each wall set stationary in the image sector. Accordingly, the aims of this study were to investigate one-segment strain and strain rate analyses in asphyxiated and non-asphyxiated term neonates, to compare one-segment repeatability indices against repeatability indices previously assessed for two-segment analyses in the same images33 to and assess if differences in myocardial performances between asphyxiated and non-asphyxiated neonates could be detected by one-segment analyses.
Materials and methods
Materials
The one-segment analyses were performed in all images used earlier for two-segment analyses in 20 asphyxiated and 48 non-asphyxiated neonates32 33 (table 1) obtained in March 2005–March 2007. The asphyxiated neonates were recruited among neonates born at gestational age ≥37 weeks and admitted to the Neonatal Intensive Care Unit at Oslo University Hospital, Ullevål, diagnosed with asphyxia. The decision for admittance was based on the clinical condition after birth and the department's guidelines for transferral. Hence, an unselected group of asphyxiated neonates were included. Eight neonates received isotonic saline volume expansion, three were treated with conventional mechanical ventilation, one with high-frequency ventilation and nitric oxide and one was treated with dopamine. Nineteen of them survived. The non-asphyxiated neonates were recruited from the maternity ward at Oslo University Hospital, Ullevål.
Table 1.
Demographic data and fractional shortening (long axis m-mode) in the asphyxiated and non-asphyxiated neonates
| Demographic data | Asphyxiated | Non-asphyxiated | p Value |
|---|---|---|---|
| No neonates (no. of exams) | 20 (53) | 48 (138) | |
| Gestational age (weeks), median (range) | 40 (37, 42) | 41 (37, 42) | 0.100* |
| Birth weight (kg) median (range) | 3.4 (3.0, 3.7) | 3.7 (3.5, 3.8) | 0.022† |
| Apgar score median (25 and 75 percentiles) | |||
| 1 min | 2 (1, 3) | 9 (9, 9) | <0.001* |
| 5 min | 5 (4. 6) | 9 (9, 10) | 0.001* |
| 10 min | 7 (6, 8) | ||
| Fractional shortening % (95% CI) | 29.1 (26.9 to 31.2) | 29.0 (27.9 to 30.0) | 0.922† |
*Mann-Whitney U test.
†T-test.
Measurements
Echocardiography was performed on days 1, 2 and 3 of life.32 33 Images were recorded with a 2.4 MHz probe (5S probe, Vivid 7 Dimension, V. 4.0.1 built 1644, GE Vingmed, Horten, Norway). The sector angle was 30° or less and the tissue Doppler frame rate was 170–220/s in most images. Congenital heart defects were ruled out on day 1. A grey-scale parasternal image for assessment of the fractional shortening was obtained at each examination.
Protocol
The nine walls were recorded from five apical views. The left lateral wall, the septum and the right lateral wall were recorded from the apical four-chamber view; the left inferior and left anterior wall from the apical left two-chamber view, the left inferiolateral wall and the anterior septum from the apical long-axis view; the right inferior wall from the right inferior two-chamber view; and the right superior wall from the right superior two-chamber view.32
In 19 of the asphyxiated neonates the injury of the myocardium was assessed biochemically by the cardiac troponin T (Roche Diagnostics, GmbH, Mannheim, Germany). These neonates were grouped according to the peak values. As 97 ng/l has been suggested as the upper reference limit in neonates,39 values less than 100 ng/l were regarded as normal.
Data analysis
One segment from each wall was analysed (figure 1). The end-systolic lengths of the myocardial walls parallel to the ultrasound beam were 25 mm or higher and the widths of the myocardial walls were 4 mm or higher. The sample area was set stationary in the ultrasound sector. Due to the motion of the heart during the heart cycles, a segment length of 21 mm and a width of 3 mm were therefore used, to ensure that the whole segment was within the myocardial wall throughout the cardiac cycle. The sample area was defined by a strain length of 20 mm and a length of 1 mm and width of 3 mm for the region-of-interest. The peak systolic strain (PSS), peak systolic strain rate (PSSR), early diastolic strain rate and strain rate during the atrial systole were obtained from curves averaged over three consecutive cycles.
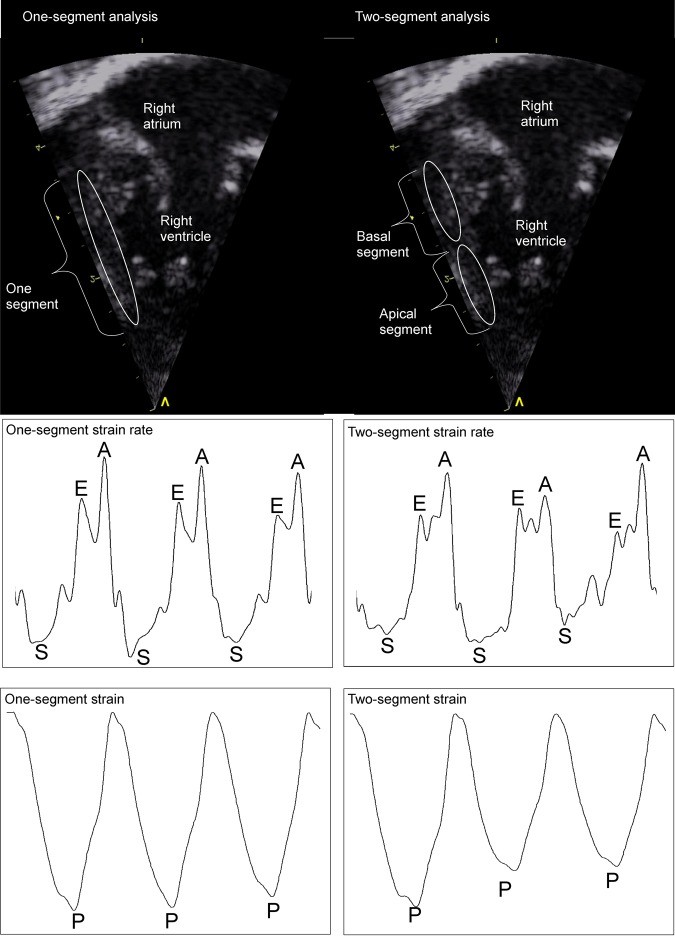
Figure 1.
One-segment and two-segment strain rate analysis. Upper row: grey-scale image showing the one-segment (left) and two-segment (right) analysis of the right lateral wall (apical four-chamber view). Mid row: strain rate curves for three heart cycles showing the lower irregularity in the one-segment analysis (left) than the two-segment analysis (right). Lower row: strain curves for three heart cycles showing the lower irregularity in the one-segment analysis (left) than the two-segment analysis (right). S, peak systolic strain rate; E, early diastolic strain rate; A, strain rate during the atrial systole; P, peak systolic strain.
For the two-segment PSS and PSSR32 analyses (figure 1) used in the repeatability analyses, a strain length of 10 mm and a length of 1 mm and a width of 3 mm for the region-of-interest had been used, leading to length of 11 mm and a width of 3 mm for the sample area. A semiautomatic tracking algorithm had been used to keep the two-segment region-of-interest centred in the segment within the ultrasound sector.
For all analyses, 40 ms Gaussian smoothing and linear drift compensation for the Lagrangian strain curves were used. The start of the strain calculation was at the start of the ventricle systole, defined by the first peak of the QRS complex. The end of the ventricle systole was defined by the notch in the tissue Doppler displacement curve caused by the closure of the aortic valve.40 There was little or no postsystolic strain in the measurements.
The fractional shortening was assessed from the parasternal long-axis view.21 41
The one-segment indices for each examination were assessed by averaging measurements from all walls eligible for analysis. Indices for the left, septum and right groups of walls were assessed by averaging measurements within the respective group. The peak systolic indices from the septum and left walls were averaged into left/septum values. Indices for each neonate were assessed by averaging the indices from the examination on day 1, 2 and 3.
All changes are described in absolute magnitudes; a PSS of −20% is regarded as a higher value than −15%, and a change to the latter is described as a decrease.
Statistics and repeatability analyses
Two-sided p values and 95% CI were used. Variables with a normal distribution were compared by one-way analysis of variance and t-tests. Least significant difference corrections were used for post hoc pair-wise comparisons. Variables with a marked non-normal distribution were compared by Mann-Whitney U-tests. Bland-Altman plots, intraclass correlation coefficients, mean errors and coefficients of variation were used in the repeatability analyses of the PSS and PSSR measurements. Seven randomly selected walls from the asphyxiated neonates and seven randomly selected walls from the non-asphyxiated neonates were analysed twice by the same researcher (EN) several months apart and once by another researcher (AS). These repeatability indices were compared against similar indices from the two-segment repeatability analyses performed earlier in the same groups.32 33 The limits of agreement between repeated measurements were compared by t-tests of the absolute differences between the paired observations. The mean errors were assessed as mean of the residuals within each paired measurements divided by the average of the paired measurements. The coefficients of variance were calculated as SD divided by the mean of the paired measurements, where SD was the standard deviation of the residuals between the paired measurements.
Ethics
Written parental consent was obtained, and the project was approved by the Norwegian South-East Regional Committee for Medical Research Ethics and by the Scientific Committee at Oslo University Hospital, Ullevål.
Results
Reproducibility measurements
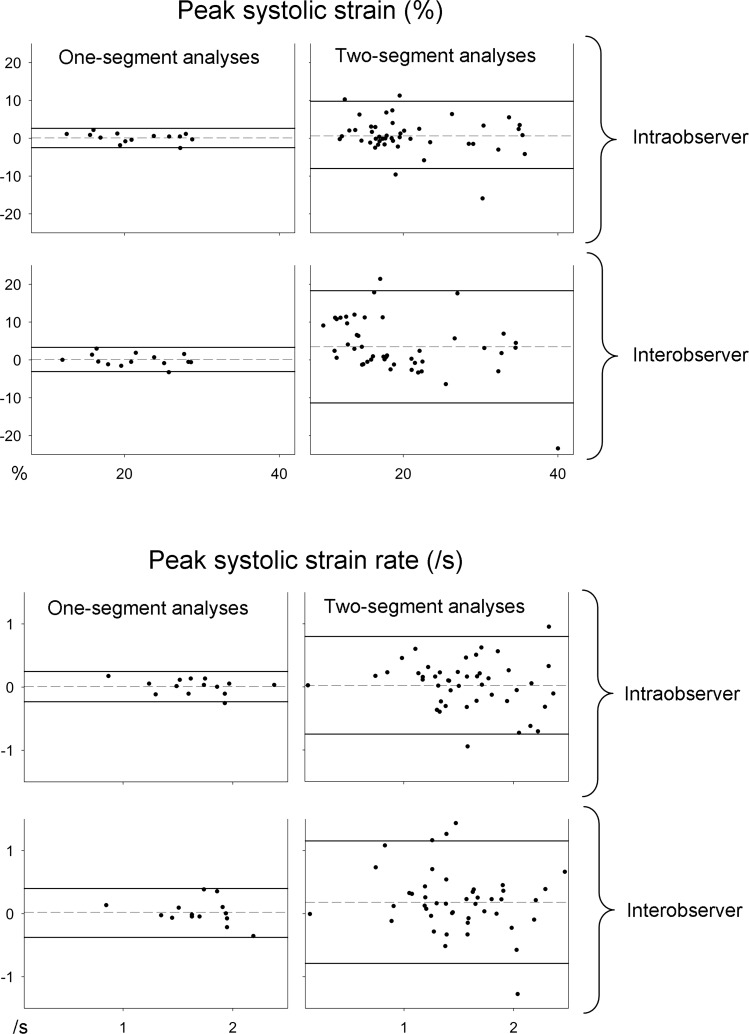
Bland-Altman plots are shown in figure 2 and repeatability indices are shown in table 2. The limits of agreement in the Bland-Altman plots were significantly different between one-segment and two-segment analyses (p<0.05). The CI for the intraclass correlations coefficients overlapped only for the inter-rater analysis of PSSR.
Figure 2.
Bland-Altman plots of the repeatability measurements for one-segment and two-segment32 33 measurements. See the text for details. X-axis: absolute mean of the repeated measurements. Y-axis: difference between the repeated measurements. Dashed line: mean difference in the repeated measurements. Solid lines: 95% limits of agreement.
Table 2.
Repeatability measurements
| One-segment intra-rater | One-segment inter-rater | Two-segment intra-rater32 33 | Two-segment inter-rater32 33 | |
|---|---|---|---|---|
| PSS ICC | 0.99 (0.95 to 1) | 0.98 (0.93 to 0.99) | 0.89 (0.80 to 0.94) | 0.76 (0.56 to 0.87) |
| PSSR ICC | 0.97 (0.92 to 0.99) | 0.93 (0.77 to 0.98) | 0.86 (0.74 to 0.92) | 0.76 (0.57 to 0.87) |
| PSS Mean error % | 5 (3 to 7) | 6 (3 to 9) | 15 (10 to 21) | 34 (23 to 44) |
| PSSR Mean error % | 6 (3 to 9) | 8 (4 to 12) | 19 (15 to 23) | 27 (18 to 36) |
| PSS COV % | 5.9 | 7.5 | 21.9 | 38.0 |
| PSSR COV % | 7.2 | 11.7 | 24.1 | 31.8 |
COV, coefficients of variance (mean); ICC, intra-class correlation coefficients (mean (95% CI)); mean error, (mean (95% CI)); PSS, peak systolic strain; PSSR, peak systolic strain rate; PSS, peak systolic strain; PSSR, peak systolic strain rate; 95% CI: 95% CI for mean.
Longitudinal strain and strain rate
The PSS, PSSR, early diastolic strain rate and strain rate during the atrial systole were all decreased in the asphyxiated neonates (table 3). The left/septum PSS and PSSR were lower in the asphyxiated than in the non-asphyxiated neonates, the PSS was −15.5 (−14.6, −16.4) (mean (95% CI)) vs −21.2 (−20.7, −21.7) % and the PSSR was −1.43 (−1.34, −1.66) vs 1.61 (−1.56, −1.66)/s (p<0.05). The one-segment PSS was 0.8 (0.4, 1.3) % lower and the PSSR 0.18 (0.15, 0.22)/s lower than the two-segment indices obtained in the same images earlier33 (p<0.05).
Table 3.
One-segment strain and strain rate measurements. See the text for details
| Asphyxiated neonates | Non-asphyxiated neonates | p Value | |
|---|---|---|---|
| All walls | |||
| Peak systolic strain (%) | −17.8 (0.6) | −21.2 (0.3) | <0.001 |
| Peak systolic strain rate (/s) | −1.43 (0.08) | −1.61 (0.03) | <0.001 |
| Early diastolic strain rate (/s) | 1.72 (0.11) | 2.00 (0.11) | 0.002 |
| Strain rate during the atrial systole (/s) | 1.92 (0.17) | 2.27 (0.10) | <0.001 |
| Left, septum and right group of walls | |||
| Peak systolic strain (%) | |||
| Left walls | −17.2 (1.2) | −19.9 (0.9) | 0.001 |
| Septum walls | −14.2 (0.9) | −15.9 (0.7) | 0.007 |
| Right walls | −21.3 (1.7) | −24.6 (0.7) | <0.001 |
| Peak systolic strain rate (/s) | |||
| Left walls | −1.40 (0.12) | −1.59 (0.06) | 0.002 |
| Septum walls | −1.34 (0.25) | −1.35 (0.06) | 0.881 |
| Right walls | −1.65 (0.15) | −1.75 (0.07) | 0.187 |
| Early diastolic strain rate (/s) | |||
| Left walls | 1.78 (0.19) | 2.24 (0.18) | 0.007 |
| Septum walls | 1.27 (0.13) | 1.43 (0.11) | 0.098 |
| Right walls | 2.01 (0.21) | 2.22 (0.14) | 0.134 |
| Strain rate during the atrial systole (/s) | |||
| Left walls | 1.86 (0.21) | 2.00 (0.14) | 0.305 |
| Septum walls | 1.39 (0.11) | 1.60 (0.11) | 0.036 |
| Right walls | 2.26 (0.28) | 2.76 (0.14) | 0.001 |
Values are mean (SE of the mean).
Left, septum and right groups of walls
Measurements are shown in table 3. The PSS was significantly decreased in the asphyxiated neonates in all wall groups. The PSSR and early diastolic strain rate were significantly lower in the left walls. The strain rate during the atrial systole was significantly lower in the septum and the right walls. The difference between left, septum and right walls was significant for all indices within both the asphyxiated and the non-asphyxiated neonates (p<0.05), but by post hoc tests the differences in PSSR between the left walls and septum in the asphyxiated neonates and in early diastolic strain rate between the left and right walls in both the asphyxiated and the non-asphyxiated neonates were not significant.
Cardiac troponin T
The highest cardiac troponin T value (1116 ng/l) was assessed in the one asphyxiated neonate that did not survive. The maximal value in the other asphyxiated neonates ranged 30–380 ng/l. The maximal value was within the normal range in 7 and elevated in 12 neonates. There were no statistically significant differences in the strain or strain rate indices between the two groups of asphyxiated neonates except for the strain rate during the atrial systole (normal 1.65 (1.45, 1.85), elevated 2.04 (1.82, 2.26)/s, p=0.022). The fractional shortening was similar in the asphyxiated neonates with normal and with elevated cardiac troponin T (normal 30.4 (27.1, 33.6), elevated 28.6 (25.2, 32.1), p>0.05).
Discussion
Reproducibility measurements
As the one-segment intra-rater and inter-rater analyses were performed by the same researchers in images selected from the same patient group as for the two-segment analyses performed earlier,32 33 we consider the one- and two-segment repeatability indices to be comparable. The indices showed a better reproducibility for the one-segment than two-segment analyses and the 95% limits of agreement in the Bland-Altman plots were of a magnitude that indicates that one-segment analyses are feasible for assessing clinically relevant differences in daily routine care. The one-segment intraclass correlation coefficients were higher than for deformation indices in adults.42 The mean errors in the one-segment analyses were similar to the mean errors both for deformation indices and for tissue velocities in adults.43 The one-segment coefficients of variance were lower than what has been found for strain analyses by use of a strain length of 10 mm in neonates33 and in tissue velocity measurements in neonates.29
The large acoustic windows in neonates give rise to good image quality. The good image quality holds the potential for high reproducibility, while a short segment length will tend to decrease the signal-to-noise ratio and hence the reproducibility. The segment length can be increased by increasing the length of the region-of-interest and by increasing the strain length. It has previously been shown that increasing the segment size by increasing the strain length improved the signal-to-noise ratio more than by increasing the length of the region-of-interest.37 In the two-segment analyses performed earlier32 33 the segment length was 11 mm and in the present analyses the segment length was 21 mm. The segment length was increased by changing the strain length from 10 to 20 mm while the size of the region-of-interest was unchanged. The regional value was calculated as the mean for all points within the region-of-interest. The one-segment and two-segment values were therefore calculated from a similar number of points. The value for each point was calculated by linear regression of all velocity measurements along the strain length, a line parallel to the ultrasound beam, centred in the point and reaching half the strain length towards the probe and half the length away from the probe. Therefore, the indices for each point within the region-of-interest were based on twice as many velocity measurements in the one-segment than the two-segment analyses. Assuming an even velocity gradient throughout the segment, the velocity differences along the strain length were twice as large in the one-segment as in the two-segment analyses. The uncertainty in each velocity measurement was similar in the one- and two-segment analyses, as the analyses were performed in the same images. Hence, the improved reproducibility in the one-segment analyses was probably caused by both an increased number of velocity measurements in the gradient estimation and by an increased difference between these velocities.
Longitudinal strain and strain rate
To our knowledge, this is the first study of one-segment longitudinal strain and strain rate in neonates and the first study of strain rate during the ventricle diastole in asphyxiated neonates.
All indices were lower in the asphyxiated neonates, despite no difference in fractional shortening.33 Fractional shortening is not always reduced in perinatal asphyxia23–25 34 and both tissue Doppler velocities of the AV-plane25 34 and two-segment strain and strain rate33 have been shown as more sensitive indices for impaired myocardial performance.
Strain and strain rate have the potential advantage over tissue velocities and atrioventricular annulus excursions44 45 that they are size-independent indices. Strain rate is annulus velocity normalised for heart size, and might therefore more readily be compared between different hearts sizes and during growth. One-segment strain is the AV-plane excursion normalised for heart size and might have the same advantage over AV-plane excursion indices such as mitral annulus excursion44 and tricuspid annular plane systolic excursion.45 As the one-segment deformation indices are based on tissue velocities from a larger sample area than the AV-plane motion indices they might be less disturbed by clutter and reverberation.
Deformation analyses by tissue Doppler have in general a low signal-to-noise ratio. The ratio is improved in large sample areas37 38 (figure 1). The one-segment peak systolic indices were lower than the two-segment indices assessed previously, possibly because the indices were assessed at false high local peaks caused by random noise. As the signal-to-noise ratio would be improved in large segments, these peaks were probably smaller and their impact on the measurements less pronounced in one-segment analyses. Strain measurements are less noisy due to time smoothing.37 38 In accordance, the differences between one- and two-segment values were relatively smaller for PSS than for PSSR.
Left, septum and right walls
Septum values were lower than left heart values and right heart values were higher than left and septum values in most cases in this study, as in the two-segment systolic analyses.33 A global impact from birth asphyxia on the myocardial performance was found, as has been shown in other studies both functionally25 32–34 and histologically.8
Cardiac troponin T
The myocardial injury can be assessed biochemically by measuring cardiac troponin T,17 24 46 47 which has shown to have a high positive predictive value in the retrospective diagnosis of asphyxia.48 Cardiac troponin T values are higher in healthy neonates than in healthy adults, 97 ng/ml has been suggested as a cut-off point between normal and elevated levels.39 As all indices except for the strain rate during the atrial systole were similar in the asphyxiated neonates with elevated cardiac troponin T as in the asphyxiated neonates with normal values, we speculate that the reduced myocardial performance following birth asphyxia might in part be caused by factors not directly related to myocardial injury.
Should one-segment or two-segment analyses be used in neonates?
The one-segment analysis procedure is fast and easy. More importantly, the reproducibility is significantly better than for two-segment analyses, and the differences between the asphyxiated and the healthy neonates were found similar by the present one-segment analyses as by the two-segment analyses previously performed in the same images.33 However by one-segment analysis, differences between the apical and basal segments within each wall, within group of walls or within the heart at gross cannot be assessed. Such differences were found in the right group of walls by two-segment analyses. In our opinion, the ease of the one-segment procedure and the benefit of improved repeatability outweigh the loss of spatialisation, especially in situations where the myocardial impairment is expected to affect each heart wall globally and in situations where the global myocardial performance is of interest. The one-segment analysis enables regional differences still to be assessed. We suggest that one-segment strain and strain rate can be used as indices of myocardial performance after perinatal asphyxia.
Assessment of deformation indices by different modalities
Deformation analysis by tissue Doppler carries a high temporal resolution due to a high frame rate, in our study typically 170–220/s. The main limitation of these measurements is that they are one dimensional and hence angle dependent, as only the deformation parallel to the ultrasound beam can be obtained. Deformation analyses based on speckle-tracking have been used in neonates.35 36 The main advantage of the speckle-tracing is that the deformation analyses are to a certain degree two dimensional, that is, the measurements are independent of the angle between the ultrasound beam and deformation within the two-dimensional ultrasound plane. However, this depends on the line density, and is poorer in the base, as well as being reduced with increased frame rate (which is obtained by reducing the number of lines). Even at the highest frame rate, however, the temporal resolution is lower as it is determined by the frame rate of the grey-scale image (typically 70–100/s), and the deformation analyses are not totally independent of angle, as deformation perpendicular to the ultrasound plane cannot be assessed. Only in three-dimensional datasets the deformation can be assessed independent of angle. Such three-dimensional datasets can be obtained by ultrasound49 and by MRI.50 The temporal resolution is low (frame rate by MRI typically 30/s and by 3D-echocardiography typically 20/s), in addition 3D ultrasound has extremely low-line density and such deformation analyses have so far to our knowledge only been used in adults. The utility of using 3D ultrasound for deformation analysis with speckle tracking in view of this low temporal resolution is still unclear.
Limitations
As an unselected group of asphyxiated neonates was studied in order to evaluate the sensitivity of the method for asphyxia in general, the study was not designed for quantifying the myocardial performance after perinatal asphyxia. The degree of asphyxia varied widely, and measurements from mechanically ventilated neonates were combined with measurements from asphyxiated neonates breathing spontaneously. To study in more detail the impact from different degree of asphyxia on the myocardial performance a larger study should be conducted.
Further studies
A larger study is needed for assessing the myocardial impairment in perinatal asphyxia. Assessment of the myocardial performance by other ultrasound indices such as AV-plane motion and excursion should be compared against deformation indices in neonates.
Conclusions
One-segment strain and strain rate analyses by tissue Doppler is feasible and reliable in term neonates and can be used for assessing the reduced myocardial performance in asphyxiated neonates with significantly improved reproducibility as compared with two-segment analyses. One-segment analyses are therefore more feasible than two-segment analyses for the assessment of myocardial performance in birth asphyxia.
Supplementary Material
Acknowledgments
Leiv Sandvik and Knut Liestøl advised on the statistics. Leif Brunvand participated in image acquisition.
Footnotes
Contributors: EN participated in the planning of the study, participated in image acquisition, analysed the images, performed the statistics and prepared the final draft. AS participated in the planning of the study, advised on technical issues, preformed the inter-rater measurements and contributed in the preparation of the final draft. DF participated in the planning of the study, recruited most patients, participated in image acquisition and contributed in the preparation of the final draft.
Funding: This work was supported by Vestfold Hospital Trust, Norwegian Extra Foundation for Health and Rehabilitation and by Renée and Bredo Grimsgaard's Foundation.
Competing interests: None.
Ethics approval: Norwegian South-East Regional Committee for Medical Research Ethics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The complete dataset is available from the corresponding author at nestaas@hotmail.com pending approval from the Vestfold Hospital Trust and Oslo University Hospital, Ullevål. Consent for data sharing was not obtained from parents or carers of neonates, but the presented data are anonymised and risk of identification is low.
References
- 1.Martin-Ancel A, Garcia-Alix A, Gaya F, et al. Multiple organ involvement in perinatal asphyxia. J Pediatr 1995;127:786–93 [DOI] [PubMed] [Google Scholar]
- 2.Ranjit MS. Cardiac abnormalities in birth asphyxia. Indian J Pediatr 2000;67:S26–9 [PubMed] [Google Scholar]
- 3.Tapia-Rombo CA, Carpio-Hernandez JC, Salazar-Acuna AH, et al. Detection of transitory myocardial ischemia secondary to perinatal asphyxia. Arch Med Res 2000;31:377–83 [DOI] [PubMed] [Google Scholar]
- 4.Bhunia BC, Basu K, Batabyal SK, et al. Myocardial changes in neonates dying of asphyxia neonatorum. Indian J Pathol Microbiol 1992;35:308–18 [PubMed] [Google Scholar]
- 5.Burnard ED, James LS. Failure of the heart after undue asphyxia at birth. Pediatrics 1961;28:545–65 [PubMed] [Google Scholar]
- 6.Rowe RD, Hoffman T. Transient myocardial ischemia of the newborn infant: a form of severe cardiorespiratory distress in full-term infants. J Pediatr 1972;81:243–50 [DOI] [PubMed] [Google Scholar]
- 7.Rowe RD, Izukawa T, Mulholland HC, et al. Nonstructural heart disease in the newborn. Observations during one year in a perinatal service. Arch Dis Child 1978;53:726–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Primhak RA, Jedeikin R, Ellis G, et al. Myocardial ischaemia in asphyxia neonatorum. Electrocardiographic, enzymatic and histological correlations. Acta Paediatr Scand 1985;74:595–600 [DOI] [PubMed] [Google Scholar]
- 9.Donnelly WH, Bucciarelli RL, Nelson RM. Ischemic papillary muscle necrosis in stressed newborn infants. J Pediatr 1980;96:295–300 [DOI] [PubMed] [Google Scholar]
- 10.Setzer E, Ermocilla R, Tonkin I, et al. Papillary muscle necrosis in a neonatal autopsy population: incidence and associated clinical manifestations. J Pediatr 1980;96:289–94 [DOI] [PubMed] [Google Scholar]
- 11.Hankins GD, Koen S, Gei AF, et al. Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol 2002;99:688–91 [DOI] [PubMed] [Google Scholar]
- 12.Shah P, Riphagen S, Beyene J, et al. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2004;89:F152–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanik E, Ozer EA, Bakiler AR, et al. Assessment of myocardial dysfunction in neonates with hypoxic-ischemic encephalopathy: is it a significant predictor of mortality? J Matern Fetal Neonatal Med 2009;22:239–42 [DOI] [PubMed] [Google Scholar]
- 14.Barberi I, Calabro MP, Cordaro S, et al. Myocardial ischaemia in neonates with perinatal asphyxia. Electrocardiographic, echocardiographic and enzymatic correlations. Eur J Pediatr 1999;158:742–7 [DOI] [PubMed] [Google Scholar]
- 15.Jedeikin R, Primhak A, Shennan AT, et al. Serial electrocardiographic changes in healthy and stressed neonates. Arch Dis Child 1983;58:605–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weindling M, Paize F. Peripheral haemodynamics in newborns: best practice guidelines. Early Hum Dev 2010;86:159–65 [DOI] [PubMed] [Google Scholar]
- 17.Costa S, Zecca E, De RG, et al. Is serum troponin T a useful marker of myocardial damage in newborn infants with perinatal asphyxia? Acta Paediatr 2007;96:181–4 [DOI] [PubMed] [Google Scholar]
- 18.Rajakumar PS, Vishnu BB, Sridhar MG, et al. Electrocardiographic and echocardiographic changes in perinatal asphyxia. Indian J Pediatr 2009;76:261–4 [DOI] [PubMed] [Google Scholar]
- 19.Gill AB, Weindling AM. Echocardiographic assessment of cardiac function in shocked very low birthweight infants. Arch Dis Child 1993;68:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dattilo G, Tulino V, Tulino D, et al. Perinatal asphyxia and cardiac abnormalities. Int J Cardiol 2011;147:e39–40 [DOI] [PubMed] [Google Scholar]
- 21.Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram. J Am Soc Echocardiogr 2006;19:1413–30 [DOI] [PubMed] [Google Scholar]
- 22.Panteghini M, Agnoletti G, Pagani F, et al. Cardiac troponin T in serum as marker for myocardial injury in newborns. Clin Chem 1997;43:1455–7 [PubMed] [Google Scholar]
- 23.Molicki J, Dekker I, de GY, et al. Cerebral blood flow velocity wave form as an indicator of neonatal left ventricular heart function. Eur J Ultrasound 2000;12:31–41 [DOI] [PubMed] [Google Scholar]
- 24.Szymankiewicz M, Matuszczak-Wleklak M, Hodgman JE, et al. Usefulness of cardiac troponin T and echocardiography in the diagnosis of hypoxic myocardial injury of full-term neonates. Biol Neonate 2005;88:19–23 [DOI] [PubMed] [Google Scholar]
- 25.Matter M, Abdel-Hady H, Attia G, et al. Myocardial performance in asphyxiated full-term infants assessed by Doppler tissue imaging. Pediatr Cardiol 2010;31:634–42 [DOI] [PubMed] [Google Scholar]
- 26.Mori K, Nakagawa R, Nii M, et al. Pulsed wave Doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart 2004;90:175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negrine RJ, Chikermane A, Wright JG, et al. Assessment of myocardial function in neonates using tissue Doppler imaging. Arch Dis Child Fetal Neonatal Ed 2012;97:F304–F306 [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Mills JF, Cheung MM. Assessment of right ventricular function using tissue Doppler imaging in infants with pulmonary hypertension. Neonatology 2009;96:193–9 [DOI] [PubMed] [Google Scholar]
- 29.Joshi S, Edwards JM, Wilson DG, et al. Reproducibility of myocardial velocity and deformation imaging in term and preterm infants. Eur J Echocardiogr 2010;11:44–50 [DOI] [PubMed] [Google Scholar]
- 30.Pena JL, da Silva MG, Faria SC, et al. Quantification of regional left and right ventricular deformation indices in healthy neonates by using strain rate and strain imaging. J Am Soc Echocardiogr 2009;22:369–75 [DOI] [PubMed] [Google Scholar]
- 31.Pena JL, da Silva MG, Alves JM, Jr, et al. Sequential changes of longitudinal and radial myocardial deformation indices in the healthy neonate heart. J Am Soc Echocardiogr 2010;23:294–300 [DOI] [PubMed] [Google Scholar]
- 32.Nestaas E, Stoylen A, Brunvand L, et al. Tissue Doppler derived longitudinal strain and strain rate during the first 3 days of life in healthy term neonates. Pediatr Res 2009;65:357–60 [DOI] [PubMed] [Google Scholar]
- 33.Nestaas E, Stoylen A, Brunvand L, et al. Longitudinal strain and strain rate by tissue Doppler are more sensitive indices than fractional shortening for assessing the reduced myocardial function in asphyxiated neonates. Cardiol Young 2011;21:1–7 [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Xu J, Xu T, et al. Left ventricular systolic function of newborns with asphyxia evaluated by tissue Doppler imaging. Pediatr Cardiol 2009;30:741–6 [DOI] [PubMed] [Google Scholar]
- 35.Petko C, Uebing A, Furck A, et al. Changes of right ventricular function and longitudinal deformation in children with hypoplastic left heart syndrome before and after the Norwood operation. J Am Soc Echocardiogr 2011;24:1226–32 [DOI] [PubMed] [Google Scholar]
- 36.Khoo NS, Smallhorn JF, Kaneko S, et al. Novel insights into RV adaptation and function in hypoplastic left heart syndrome between the first 2 stages of surgical palliation. JACC Cardiovasc Imaging 2011;4:128–37 [DOI] [PubMed] [Google Scholar]
- 37.Nestaas E, Stoylen A, Sandvik L, et al. Feasibility and reliability of strain and strain rate measurement in neonates by optimizing the analysis parameters settings. Ultrasound Med Biol 2007;33:270–8 [DOI] [PubMed] [Google Scholar]
- 38.Nestaas E, Stoylen A, Fugelseth D. Optimal types of probe, and tissue Doppler frame rates, for use during tissue Doppler recording and off-line analysis of strain and strain rate in neonates at term. Cardiol Young 2008;18:502–11 [DOI] [PubMed] [Google Scholar]
- 39.Baum H, Hinze A, Bartels P, et al. Reference values for cardiac troponins T and I in healthy neonates. Clin Biochem 2004; 37:1079–82 [DOI] [PubMed] [Google Scholar]
- 40.Aase SA, Stoylen A, Ingul CB, et al. Automatic timing of aortic valve closure in apical tissue Doppler images. Ultrasound Med Biol 2006;32:19–27 [DOI] [PubMed] [Google Scholar]
- 41.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63 [DOI] [PubMed] [Google Scholar]
- 42.Kleijn SA, Aly MF, Terwee CB, et al. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur J Echocardiogr 2012;13:159–68 [DOI] [PubMed] [Google Scholar]
- 43.Thorstensen A, Dalen H, Amundsen BH, et al. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr 2010;11:149–56 [DOI] [PubMed] [Google Scholar]
- 44.Pai RG, Bodenheimer MM, Pai SM, et al. Usefulness of systolic excursion of the mitral anulus as an index of left ventricular systolic function. Am J Cardiol 1991;67:222–4 [DOI] [PubMed] [Google Scholar]
- 45.Kaul S, Tei C, Hopkins JM, et al. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 1984;107:526–31 [DOI] [PubMed] [Google Scholar]
- 46.Awada H, Al-Tannir M, Ziade MF, et al. Cardiac troponin T: a useful early marker for cardiac and respiratory dysfunction in neonates. Neonatology 2007;92:105–10 [DOI] [PubMed] [Google Scholar]
- 47.Boo NY, Hafidz H, Nawawi HM, et al. Comparison of serum cardiac troponin T and creatine kinase MB isoenzyme mass concentrations in asphyxiated term infants during the first 48 h of life. J Paediatr Child Health 2005;41:331–7 [DOI] [PubMed] [Google Scholar]
- 48.Moller JC, Thielsen B, Schaible TF, et al. Value of myocardial hypoxia markers (creatine kinase and its MB-fraction, troponin-T, QT-intervals) and serum creatinine for the retrospective diagnosis of perinatal asphyxia. Biol Neonate 1998;73:367–74 [DOI] [PubMed] [Google Scholar]
- 49.Lilli A, Baratto MT, Del MJ, et al. Three-dimensional simultaneous strain-volume analysis describes left ventricular remodelling and its progression: a pilot study. Eur J Echocardiogr 2011;12:520–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brady BD, Knutsen AK, Ma N, et al. MRI-based multiparametric strain analysis predicts contractile recovery after aortic valve replacement for aortic insufficiency. J Card Surg 2012; 27:415–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.