Abstract
The periodontal ligament (PDL) is a fibrous connective tissue that attaches the tooth to the alveolar bone. We previously demonstrated the ability of PDL fibroblast-like cells to construct an endothelial cell (EC) marker-positive blood vessel-like structure, indicating the potential of fibroblastic lineage cells in PDL tissue as precursors of endothelial progenitor cells (EPCs) to facilitate the construction of a vascular system around damaged PDL tissue. A vascular regeneration around PDL tissue needs proliferation of vascular progenitor cells and the subsequent differentiation of the cells. Transforming growth factor-β (TGF-β) is known as an inducer of endothelial-mesenchymal transition (EndMT), however, it remains to be clarified what kinds of TGF-β signals affect growth and mesenchymal differentiation of PDL-derived EPC-like fibroblastic cells. Here, we demonstrated that TGF-β1 not only suppressed the proliferation of the PDL-derived EPC-like fibroblastic cells, but also induced smooth muscle cell (SMC) markers expression in the cells. On the other hand, TGF-β1 stimulation suppressed EC marker expression. Intriguingly, overexpression of Smad7, an inhibitor for TGF-β-induced Smad-dependent signaling, suppressed the TGF-β1-induced growth inhibition and SMC markers expression, but did not the TGF-β1-induced downregulation of EC marker expression. In contrast, p38 mitogen-activated protein kinase (MAPK) inhibitor SB 203580 suppressed the TGF-β1-induced downregulation of EC marker expression. In addition, the TGF-β1-induced SMC markers expression of the PDL-derived cells was reversed upon stimulation with fibroblast growth factor (FGF), suggesting that the TGF-β1 might not induce terminal SMC differentiation of the EPC-like fibroblastic cells. Thus, TGF-β1 not only negatively controls the growth of PDL-derived EPC-like fibroblastic cells via a Smad-dependent manner but also positively controls the SMC-differentiation of the cells possibly at the early stage of the translineage commitment via Smad- and p38 MAPK-dependent manners.
Keywords: Ligament, TGF-β, Differentiation, Endothelial Cell, Smooth Muscle Cell
Introduction
The periodontal ligament (PDL) is a fibrous connective tissue located between the tooth root and alveolar bone. PDL contains a heterogeneous mixture of cell types, including PDL fibroblasts, cementoblasts, osteoblasts, epithelial cells (rests of Malassez), vascular endothelial cells (ECs), smooth muscle cells (SMCs), and certain types of nerve cells 1. Some PDL cells have the capacity to reconstruct the periodontal structure in response to oral pathological and physiological environmental alterations such as periodontitis, wounding, and tooth movement due to orthodontic treatment. For tissue reconstruction, multipotent progenitor cells or putative stem cells must be present in the PDL. The paravascular zones in the adult PDL contain the progenitors of fibroblastic and mineralized tissue-forming cells such as the osteoblast- and cementoblast-lineage cells 2. Recently, several studies have indicated that PDL fibroblast-like cells share biological characteristics with bone marrow mesenchymal cells, suggesting that the mineralized tissue-forming cells may have originated from a common progenitor cell 3-6. PDL fibroblast-like cells have also been shown to undergo cementoblastic/osteoblastic and adipogenic differentiation in vitro and to potentially generate cementum/PDL-like tissue in vivo, suggesting that multipotent stem cells are present in the PDL 7.
We previously demonstrated the ability of a swine PDL fibroblast-like cell line, TesPDL3, to express EC and osteoblast (OB) markers in addition to PDL markers 8, 9. We investigated whether fibroblast-like cells can differentiate into putative ECs to construct blood vessels with mature lumina by evaluating the ability of the cells to vascularize in a 3-dimensional type I collagen scaffold. We established several single cell-derived cultures (SCDCs) from a primary culture of rat fibroblast-like cells derived from PDL 10. Intriguingly, each SCDC expressed EC-specific markers in addition to mesenchymal stem cell- and ligament cell-specific markers. SCDC2 cells, which abundantly expressed the EC marker Tie-2, vigorously constructed blood vessel structures in a phosphoinositide 3-kinase activation-dependent manner, suggesting that SCDC2 cells possess endothelial progenitor cell (EPC)-like characteristics. However, whether SCDC2 cells transdifferentiate into cell lineages other than ECs still needs to be investigated. We previously demonstrated that human umbilical vein endothelium-derived cells (HUVE-DCs) retain their ability to transdifferentiate into SMC after stimulation with activin A, a member of the transforming growth factor-β (TGF-β) superfamily 11, 12. However, it remains to be clarified that how the TGF-β superfamily affect the status of growth and differentiation in the PDL-derived EPC-like fibroblastic cells which were expected to regenerate damaged periodontal tissue by a construction of vascular networks.
The TGF-β superfamily comprises 2 families: the TGF-β/activin/Nodal family, and the bone morphogenetic protein (BMP)/growth and differentiation factor (GDF)/Mullerian inhibiting substance (MIS) family (reviewed in 13). Upon ligand binding to type II receptors on the cell surface, 2 type I and 2 type II receptors form a tetrameric complex. In the ligand-bound complex of type I and type II receptors, the type II receptor kinase activates the type I receptor kinase. The type I receptor then propagates the signal through phosphorylation of receptor-activated Smads (R-Smads) (reviewed in 14-17). Smad proteins are the central mediators for TGF-β superfamily signaling and are classified into 3 groups. The first group is the R-Smads, of which Smad1, Smad5, and Smad8 are primarily activated by the BMP-specific type I receptors, whereas Smad2 and Smad3 are activated by the TGF-β-specific type I receptors. The second group is the common mediator Smad (Co-Smad; e.g., Smad4). The third Smad group includes inhibitory Smads (I-Smads; e.g., Smad6 and Smad7). Activated R-Smads form complexes with the Co-Smad, which translocate into the nucleus, where they can regulate the transcription of specific target genes together with other partner proteins. I-Smads can inhibit the activation of R-Smads by competing with R-Smads for type I receptor interaction and by recruiting specific ubiquitin ligases or phosphatases to the activated receptor complex, thereby targeting it for proteosomal degradation or dephosphorylation, respectively (reviewed in 18). In addition to the signaling via the canonical Smad pathway described above, TGF-β can also signal by activating the other signaling molecules such as mitogen-activated protein kinases (MAPKs) in a cell-type dependent manner (reviewed in 19). Intriguingly, TGF-β-induced Smad2/3- and p38 MAPK-dependent signals cooperated to induce the cellular event as an expression of tumor suppressor gene Arf in mouse embryo fibroblasts 20.
Recently, Fujii et al. demonstrated that TGF-β1, which was demonstrated to be expressed and secreted by PDL fibroblasts 21, 22, reduced the proliferation of some PDL fibroblasts and upregulated the expression of α-smooth muscle actin (α-SMA), an early-phase SMC differentiation marker, suggesting that TGF-β1 may promote the SMC-like differentiation of PDL fibroblasts 23. However, they did not evaluate abilities of the cells to express EC-markers and construct vessel structure as vascular regenerative cells. Moreover, it remains unknown what kinds of TGF-β-induced intra-cellular signals influence the status of growth and vascular differentiation of the PDL-derived fibroblast-like cells, respectively. In the present study, we investigated how the TGF-β1-induced Smad and p38 signals affected the status of proliferation and expression of EC-markers (Tie-2 and VE-cadherin) and SMC-markers (α-SMA, h1-calponin and SM22α) in the PDL-derived EPC-like fibroblastic cells. In addition, in order to clarify whether the TGF-β1-induced SMC-transdifferentiation of the cells was an early or a late event in the differentiation process, we examined whether fibroblast growth factor (FGF), which was reported to negatively modulate TGF-β superfamily-induced cellular differentiation 11, reversed the TGF-β-induced translineage commitment in the cells.
Materials and Methods
Reagents
The TGF-β receptor (TGF-βR) inhibitor SB-431542, MAPK/extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor U0126, p38 MAPK inhibitor SB 203580 and FGF receptor inhibitor SU-5402 were purchased from Calbiochem (La Jolla, CA).
Cell culture
Isolation of rat PDL fibroblast-like cells and establishment of SCDCs were as previously described 10. SCDC2 cells were routinely cultured on type I collagen-coated plastic plates (Sumilon Celltight Plate, Sumitomo Bakelite Co., Tokyo, Japan) in Ham's F-12 (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; PAA Laboratories GmbH, Pasching, Austria), 10 ng/mL FGF-1 (R&D Systems Inc., Minneapolis, MN), 15 μg/mL heparin (Sigma), 100 μg/mL kanamycin (Sigma), 20 units/mL penicillin, and 20 μg/mL streptomycin (Pen Strep, Gibco, New York) in a humidified atmosphere of 5% CO2 at 37°C. Heparin was included to achieve optimal FGF-1 activity 24.
For cell differentiation assays, the cells were seeded on culture plates with Ham's F-12 supplemented with 5% FBS, with or without TGF-β1 (0.3, 1 or 3 ng/mL; PeproTech, Rocky Hill, NJ), TGF-β2 (1 or 3 ng/mL; PeproTech, Rocky Hill, NJ), or TGF-β3 (1 or 3 ng/mL; PeproTech, Rocky Hill, NJ), for 48-72 h. Then, in some of the plates, the culture medium was replaced with 5% FBS-supplemented Ham's F-12, with or without 30 ng/mL FGF-1, and the cells were cultured for an additional 96 h. In addition, some of the SCDC2 cultures were treated with inhibitors of various intracellular signals such as TGF-β receptor inhibitor SB-431542 (10 μM) and SB 203580 (30 μM), which were added 30 min before the TGF-β administration, and SU-5402 (10 μM) and U0126 (10 μM), which were added 30 min before the FGF-1 administration, respectively.
Cell proliferation assay
The status of SCDC2 cell proliferation was evaluated using an alamarBlue assay (AbD Serotec, Oxon, UK) according to the manufacturer's instruction. This assay reagent includes an indicator that fluoresces and undergoes colorimetric change when reduced by mitochondrial respiration, which is proportional to the amount of living cells. Briefly, cells were first subcultured in 96-well plates at a density of 1 × 103-2.5 × 103 cells/well in Ham's F-12 supplemented with 5% FBS and maintained for 24 h. Then, the culture medium was replaced with Ham's F-12 supplemented with 5% FBS, with or without TGF-β1 (0.03-3 ng/mL), TGF-β2 (0.01-10 ng/mL), or TGF-β3 (0.01-10 ng/mL), for 6 days. The culture medium was changed every 2 days. Some of the SCDC2 cultures were treated with the SB-431542 (10 μM) or SB203580 (30 μM), which were added 30 min before to the TGF-β administration. Each well was washed once with PBS. The alamarBlue working solution (100 μL; 10% alamarBlue in Ham's F-12) was then added to each well, and the culture was incubated at 37°C in 5% CO2 for 1.5 h. Absorbance was measured using a microplate reader, with 570 nm and 600 nm wavelengths for reduced and oxidized forms of the reagent, respectively. The assay (n = 7) was performed independently at least 3 times.
Immunofluorescence analysis of cultured cells
For immunofluorescence analysis of cultured cells, cells were subcultured in individual wells on type I collagen-coated 8-chamber slides at a density of 1 × 104 cells/well (BD Biosciences, NJ) and maintained in Ham's F-12 supplemented with 5% FBS, with or without TGF-β1 (3 ng/mL), for 3 days. Cells were then fixed in 4% paraformaldehyde for 30 min and permeabilized with 0.2% Triton X-100 in PBS. After background inhibition with 2% (w/v) bovine serum albumin in PBS, cells were labeled with anti-α-SMA rabbit polyclonal antibody (1:100; Abcam, Cambridge, UK), anti-h1-calponin rabbit monoclonal antibody (1:100; Abcam), or anti-Tie-2 rabbit polyclonal antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 h, and then at 4°C overnight. After being washed with 0.2% Triton X-100 in PBS to remove the excess primary antibody, the cells were incubated with Alexa Fluor® 568-conjugated rabbit anti-mouse IgG (1:1000; Molecular Probes, Leiden, The Netherlands) for 30 min at room temperature. After being washed with 0.2% Triton X-100 in PBS to remove the excess secondary antibody, the cells were labeled with Alexa Fluor® 488 phalloidin (1:500; Invitrogen, Paisley, UK) and DAPI (1:500; KPL, Gaithersburg, MD). The fluorescent signal was detected using a fluorescence microscope.
RNA isolation and qRT-PCR
Total RNA from SCDC2 cells was isolated with ISOGEN reagent (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. First-strand cDNA was synthesized from total RNA using the PrimeScript RT reagent Kit (Takara-Bio, Shiga, Japan). qRT-PCR was performed on a Thermal Cycler Dice Real Time System (Takara-Bio) using SYBR Premix Ex Taq II (Takara-Bio) with specific oligonucleotide primers (Table 1). The mRNA expression levels of Tie-2, VE-cadherin, α-SMA, h1-calponin, SM22α, Smad6 and Smad7 were normalized to that of β-actin, and the relative expression levels were shown as fold increase or decrease relative to the control.
Table 1.
Primers used for qRT-PCR
| Specificity | Ologonucleotide sequence ( 5'-3' ) | Predicted size ( bp ) |
|---|---|---|
| Tie-2 | TGCCCAGATATTGGTGTCCTTAAAC | 106 |
| AGCAGAACAGTCAATTCCTGCGTA | ||
| VE-cadherin | CCAGAATTTGCCCAGCCCTA | 103 |
| TTACTGGCACCACGTCCTTGTC | ||
| α-smooth muscle actin (α-SMA) | AGCCAGTCGCCATCAGGAAC | 90 |
| CCGGAGCCATTGTCACACAC | ||
| h1-calponin | ACACTTTAACCGAGGTCCTGCCTA | 80 |
| CACGCTGGTGGTCGTATTTCTG | ||
| SM22α | GAGTCACGAAGACTGACATGTTCCA | 90 |
| TGCCCAAAGCCATTACAGTCC | ||
| Smad6 | CATCACTGCTCCGGGTGAA | 83 |
| AGTATGCCACGCTGCACCA | ||
| Smad7 | TGCTGTGCAAAGTGTTCAGGTG | 177 |
| CCATCGGGTATCTGGAGTAAGGA | ||
| β-actin | GGAGATTACTGCCCTGGCTCCTA | 150 |
| GACTCATCGTACTCCTGCTTGCTG |
Western blot analysis
Cells were lysed in RIPA buffer [50 mM Tris-HCl (pH 7.2), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS] containing a protease and phosphatase inhibitor cocktail (Sigma, St. Louis, MO). The protein content of the samples was measured using BCA reagent (Pierce, Rockford, IL). Samples containing equal amounts of protein were separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). After being blocked with 5% nonfat dry milk in T-TBS (50 mM Tris-HCl, pH 7.2, 150 mM NaCl, and 0.1% Tween 20), the membrane was incubated with primary antibodies including anti-Smad2/3 purified mouse antibody (1:1000; BD Transduction LaboratoriesTM, Franklin Lakes, NJ), anti-phosphoSmad2 rabbit polyclonal antibody (1:1000; Millipore), anti-FLAG mouse monoclonal antibody (anti-FLAG M2) (1:1000; Sigma), anti-p38MAPK rabbit polyclonal antibody (1: 1000; Cell Signaling), anti-phospho-p38MAPK rabbit polyclonal antibody (1: 1000; Cell Signaling), and anti-β-actin mouse monoclonal antibody (1:2000; ACTB, clone C4, Santa Cruz Biotechnology) as a loading control for normalization. The blots were then incubated with alkaline phosphatase-conjugated secondary antibody and developed using the BCIP/NBT membrane phosphatase substrate system (KPL). For the evaluation of Smad2 phosphorylation status in the cells transfected with adenoviral expression vectors, we firstly probed the membrane with anti-phospho-Smad2 antibody, then, stripped the first antibody from the membrane, and then reprobed the stripped membrane with anti-total-Smad2/3 antibody. The proteins of interest were then detected by using appropriate horseradish peroxidase-conjugated antibodies and an Amersham ECLTM Prime Western Blotting Detection Reagent (GE Healthcare).
Adenovirus vectors
A recombinant E1-deleted adenoviral vector carrying Smad7 cDNA ligated with a FLAG-epitope 25 under the control of cytomegalovirus promoters was generated (AdCMV-Smad7) and purified as described previously 26, 27. The expression level of exogenous Smad7 in SCDC2 cells was evaluated by western blotting with anti-FLAG antibody as previously described.
Statistical analysis
The data were presented as the mean ± SD (n = 3 or 7). The data were statistically analyzed by Student's t-test, and *P <0.05, **P <0.02, and ***P <0.01 were considered significant. The results shown in all experiments were representative of at least 3 separate experiments.
Results
TGF-β1 inhibited the proliferation of ligament-derived EPC-like fibroblastic cells in a Smad signal-dependent manner
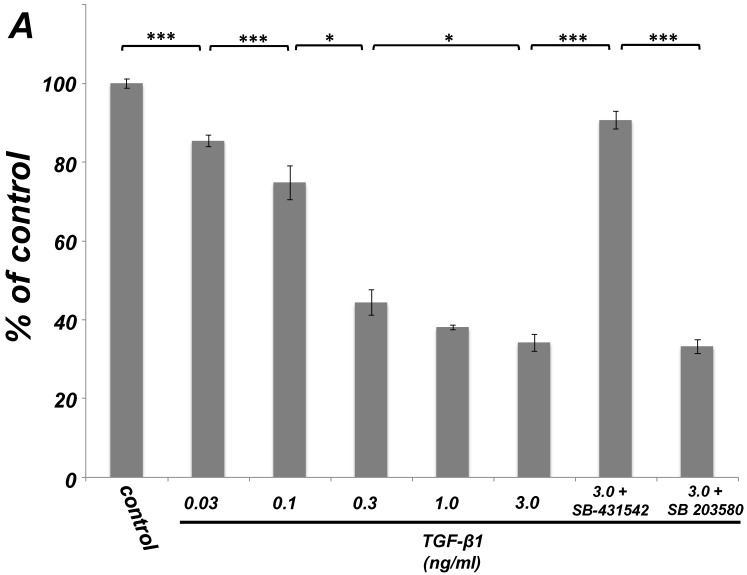
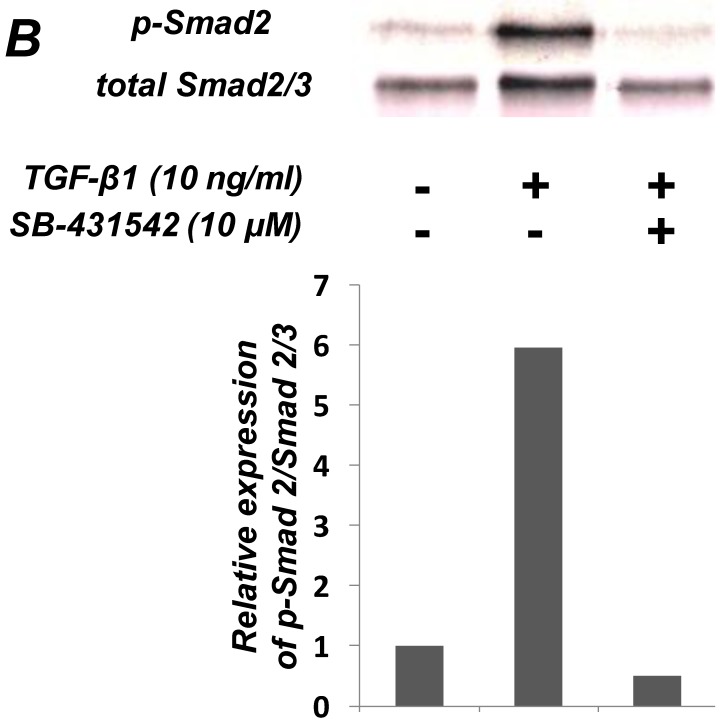
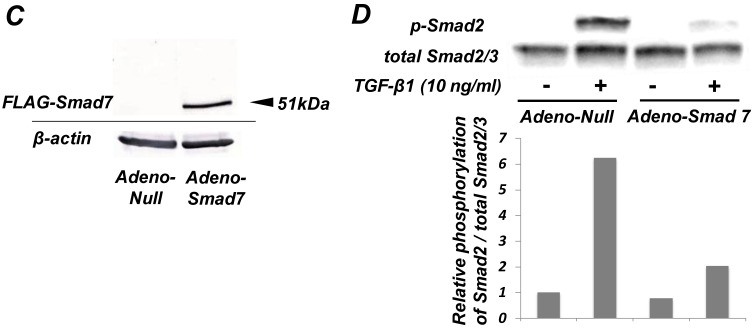
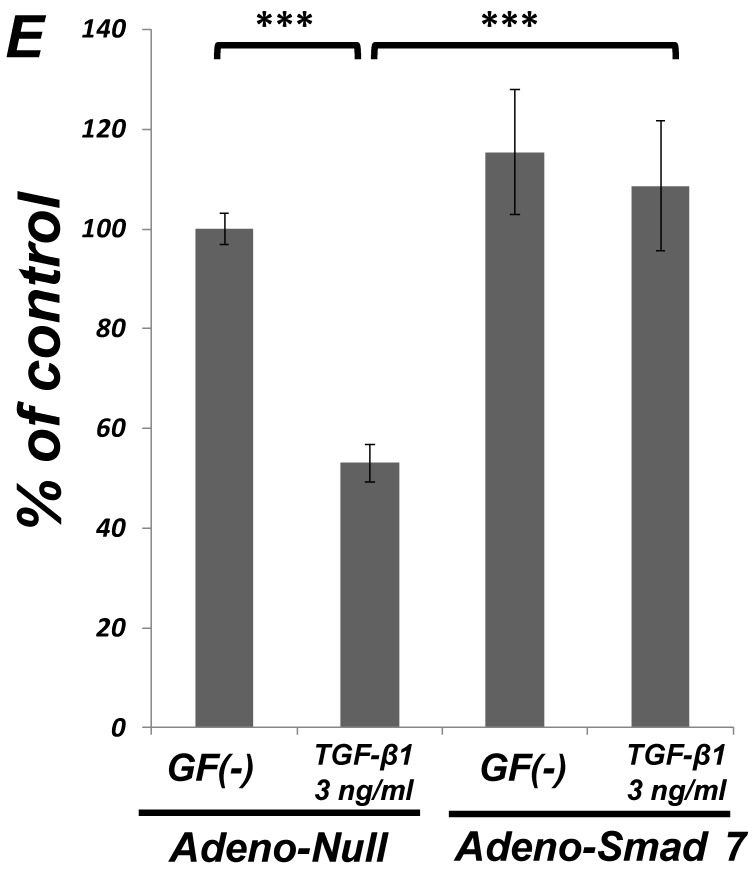
As shown in Figure 1A, TGF-β1 dose-dependently inhibited the growth of SCDC2 cells. The maximum growth inhibitory effects of TGF-β1 were observed at concentrations of 0.3-3 ng/mL. The TGF-β receptor inhibitor SB-431542 (10 μM), which inhibits TGF-β- and activin-induced phosphorylation of Smad2 but not BMP-induced phosphorylation of Smad1 28, suppressed TGF-β1-induced growth inhibition. In addition, western blotting showed that Smad2 phosphorylation was upregulated at 1 h after treatment with 10 ng/mL TGF-β1 (Figure 1B). Moreover, TGF-β1-induced Smad2 phosphorylation was suppressed by treatment with the SB-431542 (10 μM). In order to examine how Smad7, which is an inhibitor for the TGF-β1-induced Smad-dependent intracellular signaling, affects TGF-β1-induced growth inhibitory activity in SCDC2 cells, the cells were transfected with adenoviral expression vector for Smad7. Figure 1C depicts the expression of FLAG-tagged Smad7 protein in the Adeno-Smad7 transfectants. In addition, overexpression of Smad7 significantly but not completely suppressed TGF-β1-induced phosphorylation of Smad2 in SCDC2 cells (Figure 1D). Intriguingly, Smad7 clearly suppressed the TGF-β1-induced growth inhibition (Figure 1E). In contrast, the p38 MAPK-specific inhibitor SB 203580 (30 μM) did not suppress the TGF-β1-induced growth inhibition (Figure 1A), whereas it was confirmed that TGF-β induced the phosphorylation of p38 MAPK as shown in Figure 4A. These results strongly suggested that the TGF-β1-induced growth inhibition of ligament-derived EPC-like fibroblastic cells was mainly mediated by the Smad-dependent signal, but not mediated by p38 MAPK-dependent signal.
Figure 1.
TGF-β1 inhibited the growth of PDL-derived EPC-like fibroblastic cells in a Smad-dependent manner. In A, SCDC2 cells were treated with TGF-β1 at the indicated doses for 3 days, and cell growth was evaluated using the alamarBlue assay as described in Materials and Methods. alamarBlue working solution [10% alamarBlue (AbD Serotec, Oxon, UK) in Ham's F-12] was added to each well, and the culture was incubated at 37°C in 5% CO2 for 1.5 h. Some of the wells were treated with the TGF-β receptor inhibitor SB-431542 (10 μM) and p38 MAPK inhibitor SB 203580 (30 μM), which were added to the culture 30 min before the TGF-β administration. Absorbance was measured using a microplate reader, with 570 nm and 600 nm wavelengths for reduced and oxidized forms of the reagent, respectively. The data are presented as mean ± SD (n = 7). *P <0.05 and ***P <0.01 were considered significant. In B, SCDC2 cells were treated with 10 ng/mL TGF-β1 for 1 h, and then the phosphorylation status of Smad2 in TGF-β1-treated cells was evaluated by western blotting with an anti-phosphoSmad2 and anti-Smad2/3 antibodies as described in Materials and Methods. Some of the cells were treated with the TGF-β receptor inhibitor SB-431542 (10 μM). In C, D and E, cells were firstly transfected with AdCMV-Smad7 (or AdCMV-null as a control vector) then the statuses of Smad2 phosphorylation (D) and cell growth (E) with or without the TGF-β treatment were evaluated as described above. In C, the ectopic expression of FLAG-tagged Smad7 in the AdCMV-Smad7-transfected cells was detected by western blot analysis as described in Materials and Methods. In D, the phosphorylation status of Smad2 in the AdCMV-Smad7- or AdCMV-null-transfected cells was evaluated by western blot analysis as described in Materials and Methods. In E, the data are presented as mean ± SD (n = 7). ***P <0.01 was considered significant.
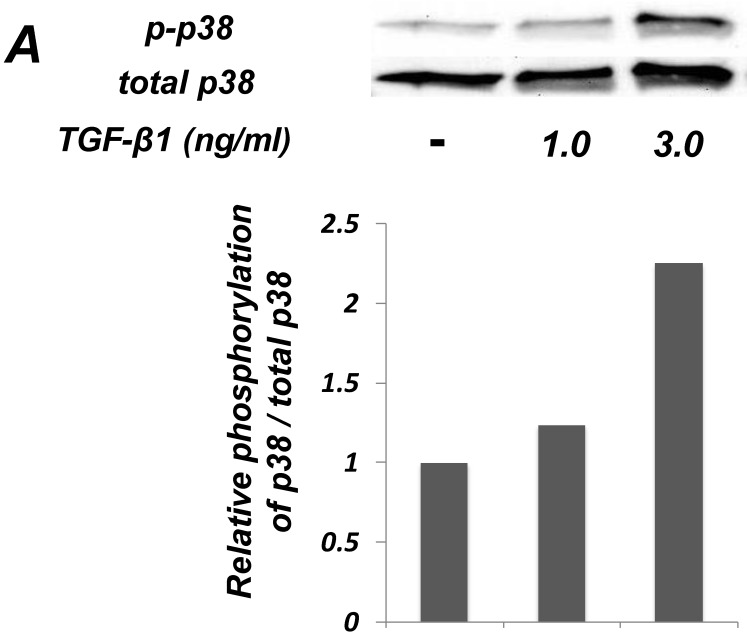
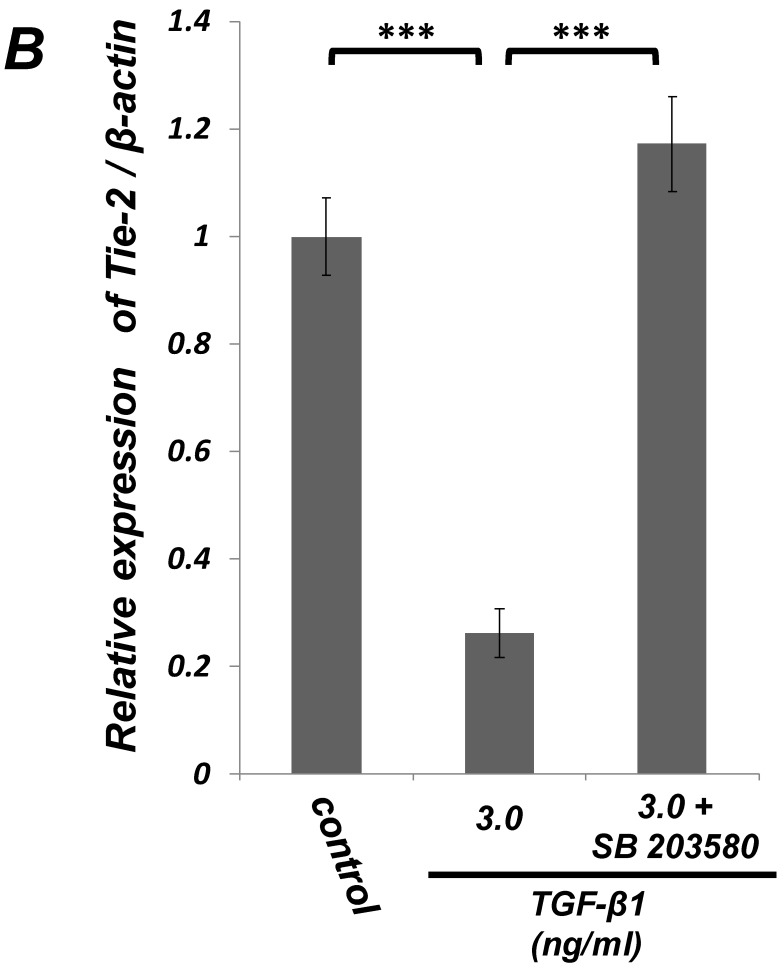
Figure 4.
Inhibition of p38 MAPK activity clearly suppressed the TGF-β-induced downregulation of EC marker (Tie-2) expression in PDL-derived EPC-like fibroblastic cells. In A, the status of p38 MAPK phosphorylation at 1 h after 1-3 ng/mL TGF-β1 treatment in SCDC2 cells was evaluated by western blot with anti-phospho-p38 MAPK- and anti-p38 MAPK-antibodies as described in Materials and Methods. In B, it was evaluated whether the inhibition of p38 MAPK activity by SB 203580 affected the status of TGF-β1-induced downregulation of Tie-2 expression in SCDC2 cells. The cells were stimulated with TGF-β1 (3 ng/mL) for 72 h with or without SB 203580 (30 μM). The relative mRNA expression level of Τie-2 in the cells was analyzed by qRT-PCR as described in Materials and Methods. Data represent the mean ± SD (n = 3). ***P <0.01 was considered significant.
TGF-β1 induced expression of SMC markers (α-SMA, h1-calponin and SM22α) but suppressed expression of the EC marker (Tie-2) in ligament-derived EPC-like fibroblastic cells
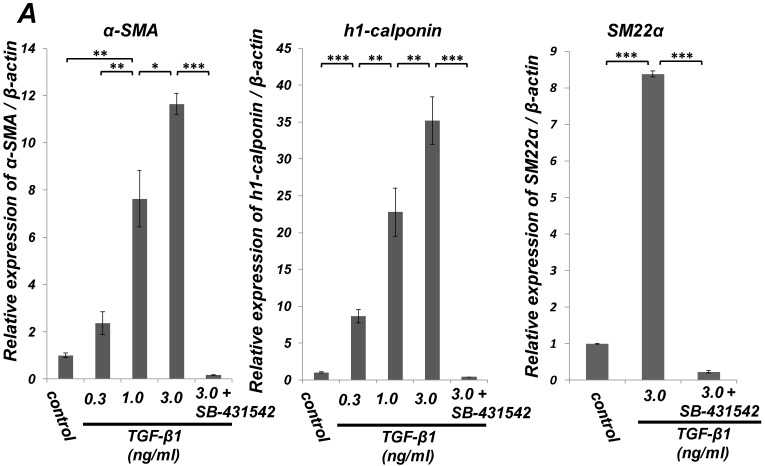
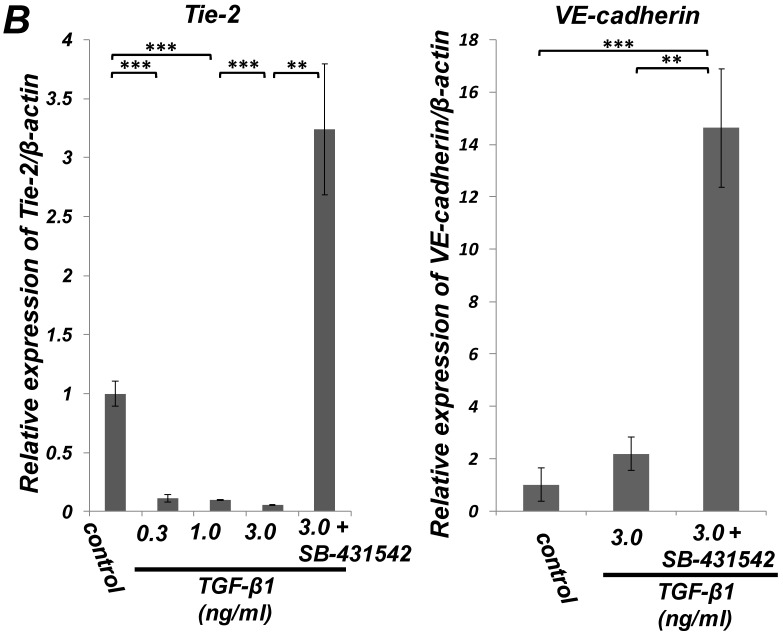
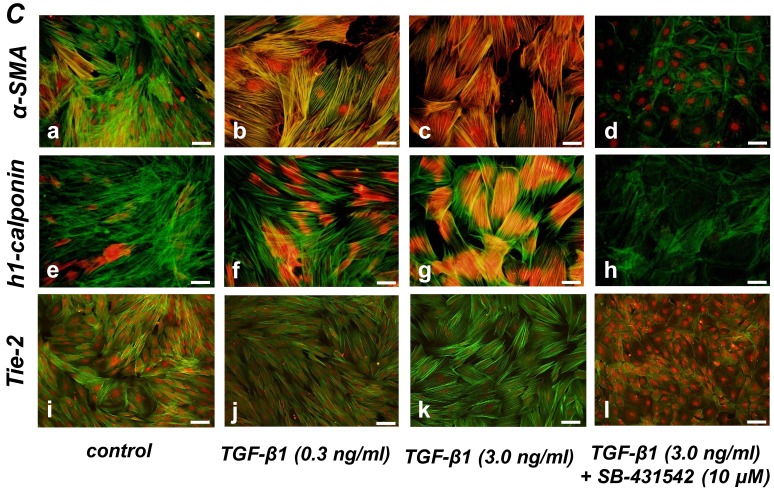
Quantitative RT-PCR (qRT-PCR) analysis revealed that mRNA expression of the SMC markers α-SMA, h1-calponin and SM22α was upregulated by TGF-β1 (Figure 2A). SB-431542 (10 μM) suppressed the TGF-β1-induced upregulation of SMC markers expression. On the other hand, TGF-β1 downregulated the expression level of EC marker Tie-2 in a dose-dependent manner (Figure 2B, left). The inhibitory effect of TGF-β1 on Tie-2 expression was also suppressed by SB-431542 (10 μM). In contrast, TGF-β1 did not affect the status of EC marker VE-cadherin expression (Figure 2B, right). Intriguingly, SB-431542 (10 μM) significantly upregulated the expression level of VE-cadherin, implicating the existence of a TGF-β autocrine loop on downregulating the level of VE-cadherin expression in the PDL-derived EPC-like fibroblastic cell culture (Figure 2B, right). Immunocytochemical analysis revealed that high-dose treatment with TGF-β1 (3 ng/mL) highly induced the protein expression of the SMC markers α-SMA and h1-calponin in SCDC2 cells, whereas the low-dose treatment (0.3 ng/mL) did moderately (Figure 2C, a-c and e-g, respectively). SB-431542 (10 μM) suppressed TGF-β1-induced upregulation of SMC markers expression (Figure 2C, d and h). Contrastingly, TGF-β1 (0.3-3 ng/mL) suppressed Tie-2 expression in SCDC2 cells (Figures 2C, i-k). In addition, SB-431542 (10 μM) significantly suppressed TGF-β1-induced inhibition of Tie-2 expression (Figure 2C, l).
Figure 2.
TGF-β1 induced expression of SMC markers (α-SMA, h1-calponin and SM22α) but suppresses expression of EC marker (Tie-2) in PDL-derived EPC-like fibroblastic cells. In A and B, SCDC2 cells were cultured for 3 days in growth medium supplemented with 5% FBS in the presence of the indicated concentrations of TGF-β1. Some of the cells were treated with the TGF-β receptor inhibitor SB-431542 (10 μM), which was added to the culture 30 min before the TGF-β administration. The relative mRNA expression levels of SMC markers (α-SMA, h1-calponin and SM22α) (A) and EC markers (Tie-2 and VE-cadherin) (B) in the cells were analyzed by qRT-PCR as described in Materials and Methods. Data represent the mean ± SD (n = 3). *P <0.05, **P <0.02, and ***P <0.01 were considered significant. In C, cells were cultured for 3 days in growth medium supplemented with 5% FBS as a control (a, e, and i) or in medium with 0.3 or 3 ng/mL TGF-β1 (b, c, f, g, j, and k). Some of the cells were treated with the TGF-β receptor inhibitor SB-431542 (10 μM), which was added to the culture 30 min before the TGF-β (3 ng/mL) administration (d, h, and l). The cells were immunostained with anti-α-SMA (a-d) (red), anti-h1-calponin (e-h) (red), and anti-Tie-2 antibodies (i-l) (red), and then labeled with Alexa Fluor® 488 phalloidin (green) as described in Materials and Methods. Scale bar, 50 μm.
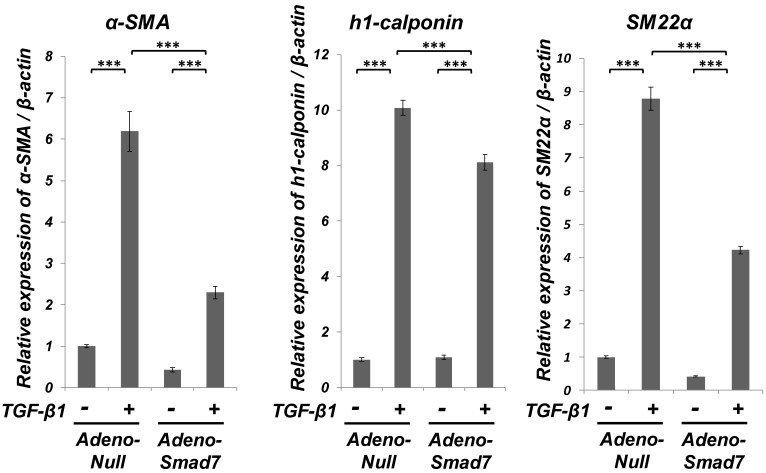
Smad7 partially suppressed the TGF-β1-induced upregulation of SMC markers (α-SMA, h1-calponin and SM22α) expression but did not the TGF-β1-induced downregulation of EC marker (Tie-2) expression of ligament-derived EPC-like fibroblastic cells
As shown in Figure 3, qRT-PCR analysis revealed that TGF-β1-induced upregulation of the SMC markers (α-SMA, h1-calponin and SM22α) mRNA expression was significantly but not completely suppressed in the Smad7-overexpressed cells. However, the overexpression of Smad7 did not reverse the TGF-β1-induced downregulation of the EC marker Tie-2 mRNA expression (data not shown). Intriguingly, TGF-β1 stimulation rapidly induced Smad7 expression in SCDC2 cells within 1 h after TGF-β1 (10 ng/mL) treatment (data not shown), implicating that Smad7 might be the TGF-β-inducible modulator of the TGF-β-induced growth inhibition or SMC differentiation. In contrast, the expression status of Smad6 mRNA did not change after TGF-β1 stimulation (data not shown).
Figure 3.
Smad7 significantly but not completely suppressed the TGF-β-induced SMC markers (α-SMA, h1-calponin and SM22α) expression in PDL-derived EPC-like fibroblastic cells. Inhibition of the TGF-β1-induced SMC markers expression by Smad7 overexpression in SCDC2 cells was evaluated by qRT-PCR as described in Materials and Methods. Firstly, SCDC2 cells were transfected with AdCMV-Smad7 or AdCMV-null and then maintained for 48 h. Then, the cells were cultured with or without TGF-β1 (3 ng/mL) for 72 h. Finally, relative mRNA expression levels of α-SMA, h1-calponin and SM22α in the cells were analyzed by qRT-PCR as described in Materials and Methods. Data represent the mean ± SD (n = 3). ***P <0.01 was considered significant.
Inhibition of p38 MAPK activity clearly suppressed the TGF-β-induced downregulation of EC marker (Tie-2) expression in ligament-derived EPC-like fibroblastic cells
We examined whether TGF-β1 affected the phosphorylation status of p38 MAPK in SCDC2 cells. As shown in Figure 4A, the phosphorylation of p38 MAPK was significantly induced at 1 h after the TGF-β1-stimulation in a dose-dependent manner. Intriguingly, SB 203580 (30 μM) clearly suppressed the TGF-β1-induced downregulation of Tie-2 mRNA expression in SCDC2 cells (Figure 4B). However, SB 203580 at the same concentration did not affect the expression status of Tie-2 in non TGF-β1-treated cells by itself (data not shown).
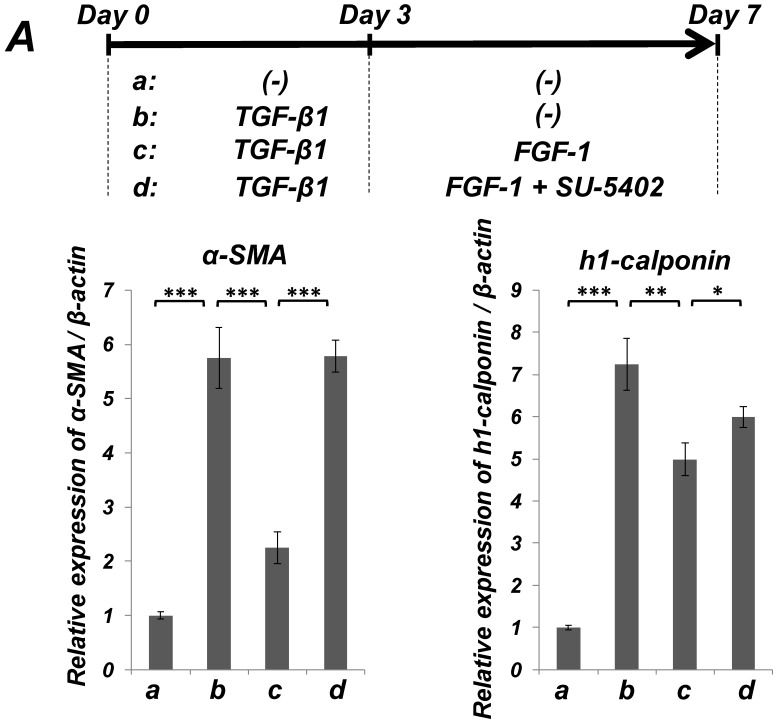
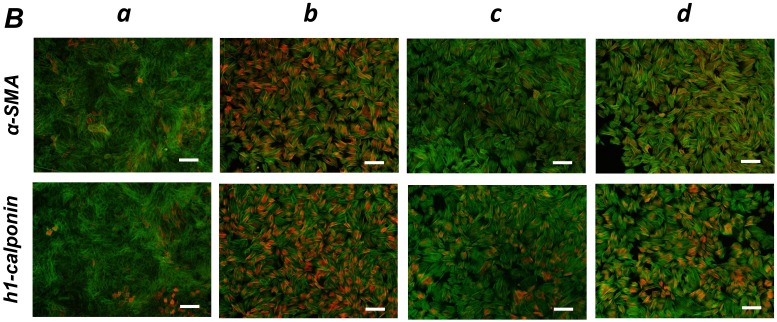
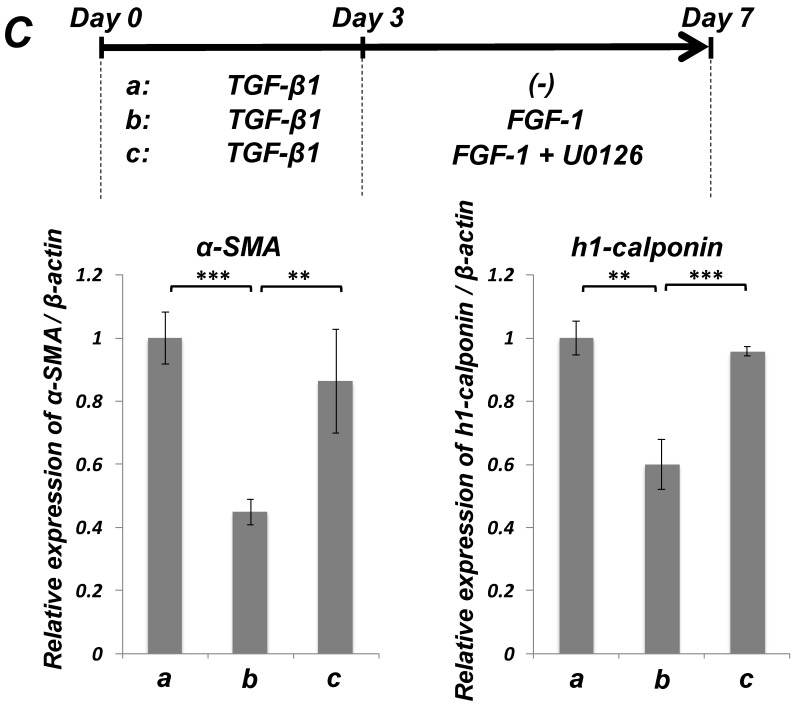
FGF stimulation partially reversed the TGF-β1-induced SMC markers expression in ligament-derived EPC-like fibroblastic cells
We previously reported that HUVE-DC differentiated into SMC-like cells through activin A-induced, Smad-dependent signaling in cultures deprived of FGF, an effect of that was reversed by FGF treatment 11. In the present study, we examined whether TGF-β1-induced SMC differentiation of ligament-derived EPC-like fibroblastic cells could be reversed by FGF-1 stimulation. qRT-PCR (Figure 5A, a-c) and immunocytochemical analyses (Figure 5B, a-c) revealed that TGF-β1 (3 ng/mL)-induced expression of α-SMA and h1-calponin was reversed by FGF-1 treatment (30 ng/mL) in SCDC2 cells. The FGF-1-induced downregulation of SMC markers expression was suppressed by the FGF receptor inhibitor SU-5402 (Figure 5A-d and B-d). In addition, a specific MEK inhibitor U0126 (10 μM) suppressed the FGF-induced downregulation of SMC markers expression in the TGF-β1-treated cells (Figure 5C). In contrast, TGF-β1-induced suppression of Tie-2 expression was not reversed by FGF-1 (data not shown).
Figure 5.
FGF-stimulation reversed the TGF-β-induced SMC markers expression in PDL-derived EPC-like fibroblastic cells in a MEK-dependent manner. In A, SCDC2 cells were firstly cultured for 3 days in growth medium supplemented with 5% FBS in the absence (experimental course a) or presence of TGF-β1 (3 ng/mL) (experimental courses b, c, and d). Then, the cells were washed with PBS (-) and maintained in new growth medium for 4 additional days in the absence (experimental courses a and b) or presence (experimental courses c and d) of FGF-1 (30 ng/mL). Some of the FGF-stimulated cells were treated with the FGF receptor inhibitor SU-5402 (10 μM) (experimental course d). The relative mRNA expression levels of α-SMA and h1-calponin in the cells at day 7 were analyzed by qRT-PCR as described in Materials and Methods. Data represent the mean ± SD (n = 3). *P <0.05, **P <0.02, and ***P <0.01 were considered significant. In B, cells were treated with TGF-β1, FGF-1 and SU-5402 as described in A (experimental courses a, b, c, and d), and then the protein expression levels of α-SMA and h1-calponin in the cells at day 7 were analyzed by immunocytochemistry. Cells were immunostained with anti-α-SMA (red; upper panels) or anti-h1-calponin antibodies (red; lower panels). Then, the cells were labeled with Alexa Fluor® 488 phalloidin (green) as described in Materials and Methods. Scale bar, 200 μm. In C, SCDC2 cells were firstly cultured for 3 days in growth medium supplemented with 5% FBS in the presence of TGF-β1 (3 ng/mL) (experimental courses a, b and c). Then, the cells were washed with PBS (-) and maintained in new growth medium for 4 additional days in the absence (experimental course a) or presence (experimental courses b and c) of FGF-1 (30 ng/mL). Some of the FGF-stimulated cells were treated with the MEK inhibitor U0126 (10 μM), which was added to the culture 30 min before the FGF-1 administration (experimental course c). The relative mRNA expression levels of α-SMA and h1-calponin in the cells at day 7 were analyzed by qRT-PCR as described in Materials and Methods. Data represent the mean ± SD (n = 3). **P <0.02 and ***P <0.01 were considered significant.
Discussion
Yamashita et al. demonstrated that embryonic stem cell-derived FLK1-positive cells differentiated into ECs upon stimulation with vascular endothelial growth factor, whereas the cells differentiated into SMCs when stimulated with platelet-derived growth factor 29. We previously reported that FGF-1- and heparin-treated HUVE-DCs retained their ability to transdifferentiate into SMCs after stimulation with activin A, a member of the TGF-β superfamily 11, 12. Intriguingly, TGF-β1 did not induce the expression of SMC markers in HUVE-DCs. On the contrary, we demonstrated that TGF-β1 induced SMC marker expression in the PDL-derived EPC-like fibroblastic cells in this study, indicating that TGF-β1 may induce SMC differentiation of undifferentiated cells in a cell type-dependent manner. These results also suggest the existence of common progenitor cells for ECs and SMCs, the fate of which is controlled by various growth factor-stimulated signals. Intriguingly, Dudley et al. demonstrated that the tumor ECs propagated with FGF and heparin, which form vascular networks during tumor progression, were capable of differentiating into osteoblasts and chondroblasts 30. These reports suggest that combinatorial treatment with FGF and heparin results in optimal FGF-1 activity 24, and induces the proliferation of undifferentiated mesenchymal cells.
We previously reported that PDL-derived SCDC2 cells, which were propagated with FGF and heparin, obtained from a single-cell culture derived from a primary rat ligament fibroblast culture expressed EC- and SMC-specific markers in addition to mesenchymal stem cell- and ligament cell-specific markers 10. Intriguingly, the cells retained the ability to differentiate into ECs and construct a Tie-2-positive vascular structure with a continuous lumen. In this study, we demonstrated that EPC-like SCDC2 cells transdifferentiated into SMCs under stimulation with TGF-β1: TGF-β1 not only inhibited the growth of SCDC2 cells (Figure 1A), but also induced the expression of SMC markers α-SMA, h1-calponin and SM22α in the cells in a Smad signal-dependent manner (Figures 2A, C and 3). In contrast, TGF-β1 suppressed the expression of EC marker Tie-2 in the cells in a p38 MAPK-dependent manner (Figure 4). On the other hand, TGF-β1 did not suppress the expression of the other EC marker VE-cadherin, whereas SB-431542 (10 μM) significantly upregulated EC marker VE-cadherin expression (Figure 2B, right), implicating the existence of a TGF-β autocrine loop on downregulating the level of VE-cadherin expression in the PDL-derived EPC-like fibroblastic cell culture. Further investigation is necessary for the elucidation of the TGF-β autocrine loop in the SCDC2 culture. These results strongly suggested that TGF-β1 determines the status of growth and SMC differentiation of PDL-derived EPC-like fibroblastic cells via Smad- and p38 MAPK-dependent manners. Generally speaking, a proliferation is poorly consistent with a differentiation: proliferation-differentiation switches have been demonstrated in various cell types 31-33. When progenitor/stem cells differentiate into specialized cells during regeneration or reconstruction of damaged tissues, the expression of the proliferation module such as transcription factors and intracellular signals for cell growth is evenly inhibited, implicating that proliferation and differentiation modules might correspond to alternative states of the two distinct molecular networks 34.
As described earlier, I-Smads (Smad6 and Smad7) can inhibit the activation of R-Smads by competing with them for type I receptor interaction and by recruiting specific ubiquitin ligases or phosphatases to the activated receptor complex, thereby targeting it for proteosomal degradation or dephosphorylation, respectively (reviewed in 35). Smad7 is a general antagonist of the TGF-β family, whereas Smad6 specifically inhibits BMP signaling. In general, I-Smads are transcriptionally induced by TGF-β family cytokines. We confirmed that TGF-β1 stimulation rapidly induced Smad7 expression in SCDC2 cells within 1 h after TGF-β1 (10 ng/mL) treatment (data not shown). In contrast, the expression status of Smad6 mRNA did not change after TGF-β1 stimulation (data not shown), implicating that Smad7 might be a TGF-β-inducible modulator of TGF-β-induced cellular events in SCDC2 cells. In this report, the overexpression of Smad7 significantly but not completely suppressed the TGF-β1-induced upregulation of SMC markers (α-SMA, h1-calponin and SM22α) expression in SCDC2 cells (Figure 3), suggesting that the other intracellular signals than Smad-mediated signal may also play important roles on the upregulation of SMC markers expression. On the other hand, Smad7 overexpression did not suppress TGF-β1-induced downregulation of EC marker Tie-2 expression (data not shown), suggesting that the TGF-β1-induced downregulation of EC marker expression was mediated by intracellular signal transduction that were not dependent on Smad signaling. Actually, a p38 MAPK inhibitor clearly suppressed the TGF-β1-induced downregulation of EC marker expression (Figure 4). In contrast to these events, as shown in Figure 2A and B, TGF-β receptor inhibitor SB-431542 clearly suppressed the TGF-β1-induced both upregulation of SMC markers (α-SMA, h1-calponin and SM22α) expression and downregulation of EC marker (Tie-2) expression in SCDC2 cells, suggesting that SB-431542 attenuated the TGF-β1-induced signaling upstream of both Smad- and p38 MAPK-mediated pathways at the receptor level.
Three TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3) have been identified in mammals. In most cases, these isoforms exhibit similar functional properties and regulate various cellular activities including cell growth and differentiation (reviewed in 36). We evaluated the effects of TGF-β2 and TGF-β3 on the cell growth, the expression of SMC markers (α-SMA and h1-calponin) and that of the EC marker (Tie-2) in SCDC2 cells. We found that TGF-β2 (Supplementary Material: Figure S1A) and TGF-β3 (Supplementary Material: Figure S1B) similar to TGF-β1 (Figure 1A) inhibited the growth of SCDC2 cells in a p38 MAPK-independent manner. In addition, TGF-β2 (Supplementary Material: Figure S2A) and TGF-β3 (Supplementary Material: Figure S2B) similar to TGF-β1 (Figure 2) upregulated the expression levels of SMC markers and downregulated the expression level of EC marker expression. Thus, all the three TGF-β isoforms similarly inhibited the growth of PDL-derived EPC-like fibroblastic cells and induced the SMC-transdifferentiation of the cells.
In the present study, we demonstrated that FGF-1 stimulation reversed TGF-β1-induced SMC markers expression of SCDC2 cells in a MEK-dependent manner (Fig. 5), suggesting that the TGF-β1 might not induce terminal SMC differentiation of the EPC-like fibroblastic cells. Thus, TGF-β1 induced the SMC-differentiation of the cells possibly at the early stage of the translineage commitment. Papetti et al. reported that FGF-2 antagonizes the TGF-β-mediated induction of retinal pericyte α-SMA expression 37. This report suggested that the cross-talk between TGF-β and FGF signals might play an important role for the decision of cellular fate of the ligament-derived EPC-like fibroblastic cells in the vascularization process. In contrast, FGF stimulation did not reverse the TGF-β-induced downregulation of Tie-2 expression (data not shown), implicating that FGF might not completely reverse the TGF-β-induced SMC differentiation of the PDL-derived EPC-like fibroblastic cells.
In conclusion, PDL-derived EPC-like fibroblastic cells were growth-arrested and retained the ability to transdifferentiate into SMCs after TGF-β stimulation: TGF-β not only negatively controls the growth of PDL-derived EPC-like fibroblastic cells via a Smad-dependent manner but also positively controls the SMC-differentiation of the cells possibly at the early stage of the translineage commitment via Smad- and p38 MAPK-dependent manners. Thus, TGF-β must be a key molecule in controlling the translineage commitment of PDL-derived EPC-like fibroblastic cells into SMCs. This is a first report for elucidation of the TGF-β-induced Smad- and non-Smad-mediated signal transduction mechanisms to control the growth and differentiation of PDL-derived EPC-like fibroblastic cells.
Recently, vascular system has been reported to supply stem/progenitor cells from bone marrow (reviewed in 38). Our findings provide new insights into the establishment of new cell therapeutic methods for PDL regeneration by constructing a vascular network composed of PDL fibroblast-like cell-derived ECs and SMCs for nutrient and stem/progenitor cells delivery to the damaged PDL tissue.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research [grant nos. 22791935 (to N.O.), 18592239 (to T. H.), 23592896 (to A.I.), and 19791370 (to N.C.)] of the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Open Research Project and High-Tech Research Project of the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Grant-in-Aid for Strategic Medical Science Research Center from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, 2010-2014; and the Keiryokai Research Foundation [grant nos. 100 (to N.C.), 2008, and 106 (to A.I.), 2009].
Author's contributions
M. Yoshida and N. Okubo were equally responsible for the design, analysis, and writing of the manuscript. N. Chosa and T. Hasegawa performed data collection and analysis. M. Ibi, M. Kamo and S. Kyakumoto participated in the analysis and discussion of the results. A. Ishisaki contributed to the study design and the analysis and discussion of the results.
Supplementary Material
Fig.S1 - TGF-β2 and TGF-β3 inhibit the growth of PDL-derived EPC-like fibroblastic cells. Fig.S2 - TGF-β2 and TGF-β3 induce the expression of SMC markers (α-SMA, h1-calponin) but suppress the expression of EC marker (Tie-2) in PDL-derived EPC-like fibroblastic cells.
References
- 1.Hou LT, Yaeger JA. Cloning and characterization of human gingival and periodontal ligament fibroblasts. J Periodontol. 1993;64:1209–1218. doi: 10.1902/jop.1993.64.12.1209. [DOI] [PubMed] [Google Scholar]
- 2.McCulloch CA. Progenitor cell populations in the periodontal ligament of mice. Anat Rec. 1985;211:258–262. doi: 10.1002/ar.1092110305. [DOI] [PubMed] [Google Scholar]
- 3.Pitaru S, Pritzki A, Bar-Kana I. et al. Bone morphogenetic protein 2 induces the expression of cementum attachment protein in human periodontal ligament clones. Connect Tissue Res. 2002;43:257–264. doi: 10.1080/03008200290001276. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Yamamoto M, Tamura M. et al. Effects of growth/differentiation factor-5 on human periodontal ligament cells. J Periodontal Res. 2003;38:597–605. doi: 10.1034/j.1600-0765.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 5.Trubiani O, Isgro A, Zini N. et al. Functional interleukin-7/interleukin-7Ralpha, and SDF-1alpha/CXCR4 are expressed by human periodontal ligament derived mesenchymal stem cells. J Cell Physiol. 2008;214:706–713. doi: 10.1002/jcp.21266. [DOI] [PubMed] [Google Scholar]
- 6.Shi S, Bartold PM, Miura M. et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 7.Seo BM, Miura M, Gronthos S. et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 8.Ibi M, Ishisaki A, Yamamoto M. et al. Establishment of cell lines that exhibit pluripotency from miniature swine periodontal ligaments. Arch Oral Biol. 2007;52:1002–1008. doi: 10.1016/j.archoralbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Shirai K, Ishisaki A, Kaku T. et al. Multipotency of clonal cells derived from swine periodontal ligament and differential regulation by fibroblast growth factor and bone morphogenetic protein. J Periodontal Res. 2009;44:788–795. doi: 10.1111/j.1600-0765.2008.01140.x. [DOI] [PubMed] [Google Scholar]
- 10.Okubo N, Ishisaki A, Iizuka T. et al. Vascular cell-like potential of undifferentiated ligament fibroblasts to construct vascular cell-specific marker-positive blood vessel structures in a PI3K-activation-dependent manner. J Vasc Res. 2010;47:369–383. doi: 10.1159/000277724. [DOI] [PubMed] [Google Scholar]
- 11.Ishisaki A, Hayashi H, Li AJ. et al. Human umbilical vein endothelium-derived cells retain potential to differentiate into smooth muscle-like cells. J Biol Chem. 2003;278:1303–1309. doi: 10.1074/jbc.M207329200. [DOI] [PubMed] [Google Scholar]
- 12.Ishisaki A, Tsunobuchi H, Nakajima K. et al. Possible involvement of protein kinase C activation in differentiation of human umbilical vein endothelium-derived cell into smooth muscle-like cell. Biol Cell. 2004;96:499–508. doi: 10.1016/j.biolcel.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Wu MY, Hill CS. TGF-β superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Goumans MJ, Liu Z, ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, Landström M, Moustakas A. Mechanism of TGF-β signaling to growth arrest, apoptosis, and epithelial-mensenchymal transition. Curr Opin Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Feng XH. Regulation of TGF-β signaling by protein phosphatases. Biochem J. 2010;430:191–198. doi: 10.1042/BJ20100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meulmeester E, ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 18.Song B, Estrada KD, Lyons KM. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20:379–388. doi: 10.1016/j.cytogfr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Zhao YD, Gibbons M. et al. Tgfbeta signaling directly induces Arf promoter remodeling by a mechanism involving Smads 2/3 and p38 MAPK. J Biol Chem. 2010;285:35654–35664. doi: 10.1074/jbc.M110.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Pauw MT, Van den Bos T, Everts V. et al. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor beta1 release of periodontal ligament and gingival fibroblasts. J Periodontol. 2000;71:31–43. doi: 10.1902/jop.2000.71.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Symons AL, Bartold PM. et al. Expression of transforming growth factor-beta 1 (TGF-β1) in the developing periodontium of rats. J Dent Res. 1998;77:1708–1716. doi: 10.1177/00220345980770090701. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Maeda H, Tomokiyo A. et al. Effects of TGF-β1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell Tissue Res. 2010;342:233–242. doi: 10.1007/s00441-010-1037-x. [DOI] [PubMed] [Google Scholar]
- 24.Harmer NJ. Insights into the role of heparan sulphate in fibroblast growth factor signaling. Biochem Soc Trans. 2006;34:442–445. doi: 10.1042/BST0340442. [DOI] [PubMed] [Google Scholar]
- 25.Nakao A, Afrakhte M, Morén A. et al. Identification of Smad7, a TGF-beta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 26.Fujii M, Takeda K, Imamura T. et al. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakao A, Fujii M, Matsumura R. et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inman GJ, Nicolás FJ, Callahan JF. et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita J, Itoh H, Hirashima M. et al. Flk1 positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 30.Dudley AC, Khan ZA, Shih SC. et al. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14:201–211. doi: 10.1016/j.ccr.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JF, Mandel EM, Thomson JM. et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti L, Sipione S, Magrassi L. et al. Shc signaling in differentiating neural progenitor cells. Nat Neurosci. 2001;4:579–586. doi: 10.1038/88395. [DOI] [PubMed] [Google Scholar]
- 33.Dugan LL, Kim JS, Zhang Y. et al. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 34.Xia K, Xue H, Dong D. et al. Identification of the proliferation/differentiation switch in the cellular network of multicellular organisms. PLoS Comput Biol. 2006;2:1483–1497. doi: 10.1371/journal.pcbi.0020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan X, Liu Z, Chen U. Regulation of TGF-β signaling by Smad7. Acta Biochim Biophys Sin. 2009;41:263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo X, Chen S-Y. Transforming growth factor-β and smooth muscle differentiation. World J Biol Chem. 2012;3:41–52. doi: 10.4331/wjbc.v3.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papetti M, Shujath J, Riley KN. et al. FGF-2 antagonizes the TGF-β1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for Myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- 38.Sordi V. Mesenchymal stem cells homing capacity. Transplantation. 2009;87:S42–45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig.S1 - TGF-β2 and TGF-β3 inhibit the growth of PDL-derived EPC-like fibroblastic cells. Fig.S2 - TGF-β2 and TGF-β3 induce the expression of SMC markers (α-SMA, h1-calponin) but suppress the expression of EC marker (Tie-2) in PDL-derived EPC-like fibroblastic cells.