Abstract
The pleiotropic cytokine interleukin-1β (IL-1β) can promote physiological cell migration, as well as cancer cell invasion and metastasis. Its role in human trophoblast invasion, however, has not been satisfactorily answered since direct, indirect as well as no effects on trophoblast motility have been published. Therefore, the role of IL-1β has been re-evaluated by exclusively using human primary trophoblast model systems. Immunofluorescence of first trimester placentae indicated IL-1 receptor 1 (IL-1R1) protein expression in first trimester villous cytotrophoblasts (vCTB) and extravillous trophoblasts (EVT). The latter expressed higher mRNA levels of the receptor as shown by comparative gene chip data of vCTB and EVT. Similarly, Western blot analyses and immunofluorescence revealed a time- and differentiation-dependent increase of IL-1R1 in primary EVT seeded on fibronectin. IL-1β dose-dependently elevated migration of isolated first trimester EVT through fibronectin-coated transwells, which was inhibited in the presence of IL-1R antagonist (IL-1Ra), whereas proliferation of these cells was not affected. Similarly, the interleukin did not alter proliferation of vCTB and cell column trophoblasts in floating villi of early pregnancy, but promoted migration in villous explant cultures seeded on collagen I. Western blot analyses of supernatants of primary EVT and first trimester villous explant cultures revealed IL-1β induced secretion of urokinase plasminogen activator (uPA), plasminogen activator inhibitor (PAI)-1 and PAI-2, which was diminished upon combined IL-1β/IL-1Ra treatment. In conclusion, these data suggest that IL-1β directly promotes trophoblast motility of first trimester EVT involving the uPA/PAI system.
Keywords: Placenta, Trophoblast, Migration, IL-1β
1. Introduction
Invasion of human extravillous trophoblasts (EVT) into maternal uterine tissues is essential for a successful pregnancy. Differentiated EVT originate from placental anchoring villi attaching to the uterine epithelial surface and develop into two distinct cell populations, the interstitial cytotrophoblasts (iCTB) and the endovascular cytotrophoblasts (eCTB). Whereas iCTB invade the uterine decidua and migrate towards the spiral arteries, eCTB directly enter these vessels and adopt a vascular adhesion phenotype [1]. Remodelling of the spiral arteries, which is thought to be initiated by uterine NK (uNK) cells, is a critical step in human placentation requiring the coordinated actions of iCTB and eCTB for completion of the process [2]. Transformation of the uterine vessels involves replacement of maternal endothelial cells as well as trophoblast-mediated apoptosis of vascular smooth muscle cells provoking disruption of the vascular wall [3]. Conversion of the vessels into dilated conduits is thought to reduce contractility, pressure and rate of blood flow into the intervillous space supporting a constant delivery of oxygen and nutrients to the developing fetus [4]. Failures in this vascular transformation process are associated with the development of gestational diseases such as severe forms of intrauterine growth restriction and preeclampsia [5]. Fluctuations in oxygen concentrations may result in hypoxia and reoxygenation of the placental villi provoking stress-mediated secretion of cytokines and microparticles into the maternal circulation finally causing the clinical symptoms of preeclampsia [6,7]. However, abnormal trophoblast invasion might also play a role in early pregnancy complications for example miscarriages [4].
Whereas molecular mechanisms controlling formation of anchoring villi, cell column proliferation and EVT differentiation are largely unknown, numerous growth factors, chemokines and cytokines expressed at the fetal–maternal interface were shown to increase EVT invasion [8–10]. These factors act through prominent signalling cascades, such as PI3K/AKT, Raf/MEK/ERK, Rho/ROCK or Wnt/β-catenin signalling [11]. However, trophoblast invasion is a finely tuned process which is negatively affected by a variety of factors, for example tumour necrosis factor alpha (TNFα), transforming growth factor beta (TGFβ), endostatin or interleukin-10 (IL-10), secreted from decidual stromal cells, uNK cells or macrophages [9]. To downregulate the invasive capacity of EVT, trophoblast-derived proteases such as matrix metalloproteinases (MMP) and urokinase plasminogen activator (uPA) are thought to be inhibited by tissue inhibitors of metalloproteinases (TIMP) and plasminogen activator inhibitors (PAI) secreted from uterine cell types [8]. Hence, despite its similarity to cancer cell invasion, trophoblast invasion is precisely controlled in a time- and distance-dependent manner.
Another factor, interleukin-1 beta (IL-1β), qualifies as a regulator of EVT function since it is released from different uterine cells, blastocysts and cultured trophoblasts [9,12,13]. Indeed, IL-1β secreted from blastocysts was suggested to promote decidualisation and implantation [14] in agreement with the assumption that the latter has characteristics of an inflammatory response. However, IL-1β may have beneficial as well as adverse effects on reproductive cells likely depending on its local concentration and the particular cell type. Aberrant IL-1β levels were shown to be associated with a variety of gestational diseases such as preeclampsia, preterm labour or spontaneous abortion [15]. The adverse effects of the cytokine might be due to abnormal matrix degradation and IL-1β-dependent recruitment of dendritic cells and macrophages into the decidua [16]. With respect to primary trophoblast cell invasion direct effects of IL-1β on MMP expression and invasion have been observed [17], whereas others postulated an indirect role of the cytokine in trophoblast motility by stimulating secretion of chemotactic IL-8 from endometrial epithelial cells [18]. Interestingly, invasion of the trophoblastic cell line SGHPL-4 representing EVT was not affected in the presence of IL-1β [19]. Therefore, IL-1 receptor 1 (IL-1R1) expression, IL-1β-dependent proliferation and migration as well as protease secretion were re-evaluated by exclusively using different primary trophoblast model systems, i.e. primary EVT as well as floating and matrix-attached first trimester villous explant cultures.
2. Materials and methods
2.1. Tissue collection
Placental tissues of early pregnancy (between the 6th and 12th week of gestation, n = 93) were obtained from legal abortions of uncomplicated pregnancies. Utilisation of tissues was approved by the ethics committee of the Medical University of Vienna requiring informed consent.
2.2. Preparation and cultivation of primary EVT
Primary EVT were isolated from first trimester placentae between the 6th and 12th week of gestation according to a modified protocol of Tarrade et al. [20] by additionally using Percoll density gradient centrifugation (10%–70%; Pharmacia, Uppsala, Sweden). Briefly, placentae were washed in ice-cold PBS and kept overnight in DMEM/Ham's F-12 medium (Invitrogen, Carlsbad, CA), supplemented with 0.05 mg/ml gentamicin (Invitrogen) and 2.5 μg/ml fungizone (Invitrogen). Villous tips were scraped with a scalpel blade and digested two times in 0.125% trypsin (Invitrogen) and 12.5 mg/ml DNase I (Sigma–Aldrich, St.Louis, Missouri) in 10% HBSS–Mg/Ca-free medium (Sigma–Aldrich) for 30 min at 37 °C without agitation. Subsequently, cells were pooled, filtered through a 70 μm cell strainer (Greiner Bio-One GmbH, Frickenhausen, Germany) and the cell suspension was separated by Percoll density gradient centrifugation (1175 g, 24 min, 4 °C). Isolated cells (between 35% and 50% Percoll layer) were cultivated on fibronectin-coated (20 μg/ml, BD Biosciences, Bedford, MA) wells in DMEM/Ham's F-12 medium, supplemented with 10% FCS and 0.05 mg/ml gentamicin as mentioned [21]. For characterisation 1 × 105 cells were seeded on fibronectin-coated Lab-Tek chamber slides (NalgeNunc International, Penfield, New York) and analysed by fluorescence microscopy using primary antibodies against cytokeratin-7 (CTK-7, monoclonal mouse, clone OV-TL 12/30, 8.3 μg/ml; Dako, Glostrup, Denmark) and vimentin (polyclonal rabbit, 20 μg/ml, GeneTex Inc., Irvine, CA). Alexa Fluor 488 (anti-mouse) and Alexa Fluor 568 (anti-rabbit) were used as secondary antibodies (2 μg/ml, Molecular Probes, Invitrogen). Isolated cells were mostly CTK-7-positive (97%), largely vimentin-negative, and expressed markers of EVT such as integrin α1 and integrin α5β1 in a differentiation-dependent manner as mentioned [21].
2.3. Immunofluorescence of first trimester placentae and primary EVT
First trimester placental (n = 4) and decidua basalis tissues (n = 3) were embedded in paraffin and sectioned on a microtome, as described [22]. Briefly, after fixation in 4% paraformaldehyde for 24 h at 4 °C, tissue samples were dehydrated and embedded in paraffin (Merck, New Jersey). Serial sections were prepared (3 μm), deparaffinised and heated in a microwave (2 × 5 min, 850 W). Blocking procedures, antibody stainings and immunofluorescence were performed as mentioned [23]. The sections were incubated with primary antibodies against IL-1R1 (monoclonal mouse, clone 40101, 10 μg/ml, LifeSpan Biosciences) and pan-keratin (KRT, cytokeratin wide-spectrum antibody, polyclonal rabbit, 1:100, GeneTex Inc.), followed by secondary Alexa Fluor 488 anti-mouse and Alexa Fluor 568 anti-rabbit antibodies. Irrelevant, isotype- and concentration-matched antibodies were used as controls. Nuclei were visualised by 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/ml, Roche, Mannhein, Germany). The stained sections were embedded in Fluoromount-G (SouthernBiotech, Birmingham, AL) and pictures were taken at a 200 fold magnification on a fluorescence microscope (Olympus BX50, Hamburg, Germany). 1 × 105 primary EVT (n = 3) were cultured on fibronectin-coated chamber slides for 24 or 48 h and stained with primary antibodies against IL-1R1 and integrin α1 (ITGA1, monoclonal mouse, clone FB12, 2 μg/ml; Chemicon, Millipore). Secondary antibodies and isotypes controls were used as described above.
2.4. Migration of primary EVT
Migration of primary EVT (n = 3, duplicates) was performed by using fibronectin-coated 12 μm pore transwell inserts (Millicell®, Millipore, Billerica, MA) as recently mentioned [21]. Briefly, fibronectin (0.5 μg/ml) was added to the upper side of the inserts, which were incubated for 30 min at 37 °C and subsequently placed into 24-well plates containing 300 μl DMEM/Ham's F-12 medium with 10% FCS and 0.05 mg/ml gentamicin. 150,000 primary EVT, resuspended in DMEM/Ham's F-12 medium with 0.1% FCS, were incubated with human recombinant IL-1β (1 ng/ml or 10 ng/ml, R&D Systems Inc., Minneapolis, MN) and/or human recombinant IL-1Ra (40 ng/ml, R&D Systems Inc.) and added to the fibronectin-coated inserts. After 48 h of incubation, cells were washed in PBS, fixed in ice-cold methanol for 10 min and again washed in PBS. Subsequently, non-invaded cells on the upper side of each insert were removed by a cotton swab and membranes were cut out with a scalpel blade. Cells were stained with primary anti-CTK-7 and secondary Alexa Fluor 488 anti-mouse antibodies together with DAPI. Finally, membranes were placed on glass slides and embedded in Fluoromount-G. Five non-overlapping pictures of each membrane representing approximately 40–50% of the overall surface were taken (40 fold magnification), cells were digitally counted by using the imaging software CellP (Olympus, Hamburg, Germany). Motility was evaluated as the percentage of CTK-7-positive cells compared to untreated control.
2.5. Migration in first trimester villous explant cultures
Pieces of villous tissue of five different first trimester placentae between the 7th and 12th week of gestation were dissected under the microscope and cultivated on collagen I (BD Biosciences)-coated dishes, allowing for trophoblast outgrowth and migration as described elsewhere [21,22]. After adherence to the matrix (4–7 h), villous explants were covered with DMEM/Ham's F-12 medium, supplemented with 0.05 mg/ml gentamicin and further incubated for 48 h in the absence or presence of human recombinant IL-1β (10 ng/ml). To determine the migratory capacity of controls (n = 60) and IL-1β-treated (n = 60) placental explants, the area of outgrowth was digitally photographed and analysed by using the imaging software CellP as previously done [21,24].
2.6. Proliferation of first trimester floating villi and primary EVT
To study the effects of IL-1β on proliferation of vCTB and cell column trophoblasts, 5-bromo-2′-deoxy-uridine (BrdU) Labelling and Detection Kit III (Roche Diagnostics, Vienna, Austria) was used as previously mentioned [22,24]. Briefly, dissected villi of three different first trimester placentae were cultured in DMEM/Ham's F-12 medium, supplemented with 0.05 mg/ml gentamicin and 10 μM BrdU in the absence (n = 45) or presence (n = 45) of human recombinant IL-1β (10 ng/ml) and/or human recombinant IL-1Ra (40 ng/ml) for 24 h. Subsequently, 15 placental villi of each condition were simultaneously embedded in paraffin and processed as described in Section 2.3. Incorporated BrdU was detected by an anti-BrdU antibody (monoclonal mouse, clone Bu20a, 2.6 μg/ml, Dako). The sections were counterstained with KRT and DAPI; secondary antibodies were used as described above. Trophoblast proliferation was quantified as the percentage of BrdU-labelled nuclei of KRT-positive vCTB relative to the overall number of DAPI-positive nuclei of vCTB. Between 500 and 600 nuclei of controls and stimulated conditions each were counted. Analogously, the ratio of BrdU/DAPI-labelled cell column trophoblasts in controls (300 nuclei) and IL-1β-stimulated villi (300 nuclei) was determined. Primary EVT (1 × 105; n = 3) were seeded in duplicates on fibronectin-coated chamber slides and incubated with 10 μM BrdU in the absence or presence of human recombinant IL-1β (10 ng/ml). After 24 h, cells were fixed in ethanol-fixans (15 mM glycine per 70% ethanol) for 20 min at −20 °C and stained as described above. Five non-overlapping pictures (100 fold magnification) of each condition were taken and the ratio of BrdU/DAPI was evaluated.
2.7. Western blot analyses
Protein preparation and Western blot analyses were performed using standard protocols as recently described [23]. Briefly, supernatants were collected from primary EVT cultivated for 48 h in DMEM/Ham's F-12 medium with 10% FCS in the absence or presence of human recombinant IL-1β (10 ng/ml) and/or human recombinant IL-1Ra (40 ng/ml). Cell debris was removed after centrifugation (1500 rpm, 5 min) and supernatants were concentrated using Ultrafree-MC filter tubes (Millipore, Billerica, MA) according to the manufacturer's instructions. EVT were trypsinised for 5 min (0.25% Trypsin-EDTA, Gibco, Invitrogen) and counted using a multi-channel electronic cell counter (CASY-I, Schärfe Systems, Reutlingen, Germany). Supernatants were normalised to cell numbers, treated with protein extraction buffer (final concentration of 63 mM Tris.Cl, pH 6.8, 2% SDS, 0.1% β-mercaptoethanol, 10% glycerol, 0.2 mg/ml bromophenol blue) and separated on 10% SDS/PAA gels. PageRuler Plus (ThermoFisher Scientific, Waltham, MA) was used as molecular size marker. Proteins were transferred onto PVDF membranes (Hybond-P, Amersham, Pharmacia Biotech, Piscataway, NJ) and after blocking in 5% non-fat dried milk in TBS-T, membranes were incubated with the following primary antibodies, diluted in 5% BSA in TBS-T: uPA (monoclonal mouse, clone 204212, 1 μg/ml, 55 kDa-precursor, 35 kDa-active enzyme, R&D Systems Inc.), PAI-1 (monoclonal mouse, 1 μg/ml, 47 kDa, Oncogene) and PAI-2 (monoclonal mouse, clone HD-PAI-2 22.1, 2 μg/ml, 48 kDa, American Diagnostica GmbH). After washing in TBS-T, membranes were incubated with secondary anti-mouse Ig horseradish peroxidase-linked antibodies (132 ng/ml, GE Healthcare, Little Chalfont, UK) and developed for 5 min by using Amersham ECL Prime Western blotting reagent (GE Healthcare). Protein quantification was done by densitometrical scanning using AlphaView software (Alpha Innotech, San Leandro, CA). For evaluation of cellular IL-1R1 expression, protein extracts of EVT isolated after 48 h of cultivation on fibronectin were analysed as previously mentioned [21]. GAPDH (1 μg/ml, Ambion) was used as loading control.
2.8. Statistics
Statistical analyses were performed using SPSS 18 (SPSS Inc., Chicago, IL) using Student's paired t-test. Gaussian distribution and equality of variances were examined with Kolmogorov–Smirnov test and Levene's test, respectively. A p-value <0.05 was considered statistically significant.
3. Results
3.1. Expression of IL-1R1 is associated with EVT differentiation
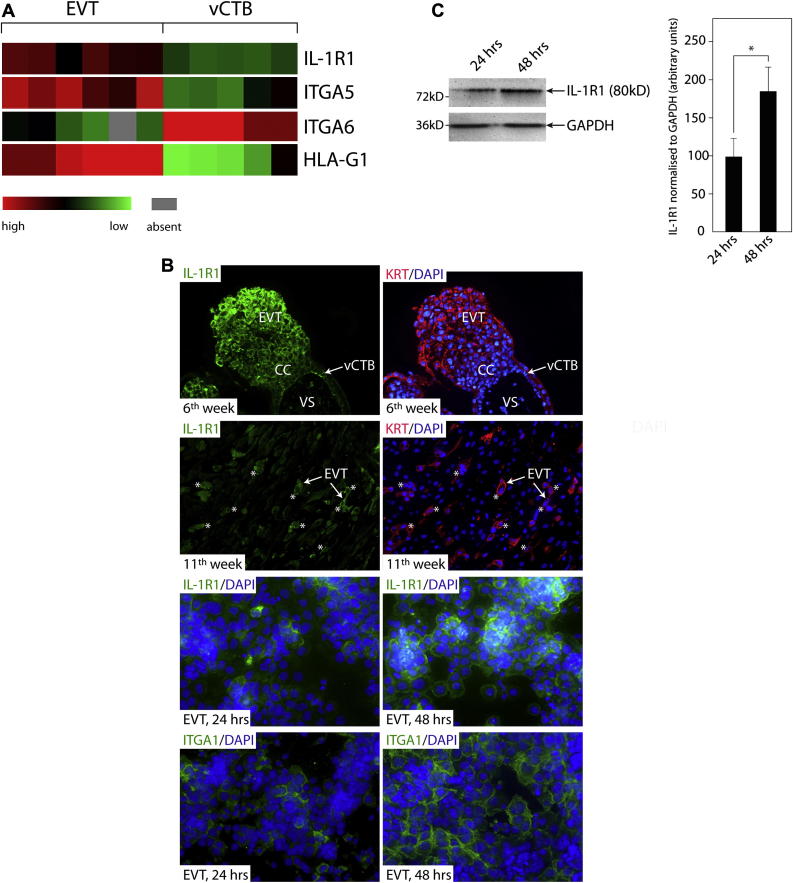
To gain insights into the expression pattern of IL-1R1 in different trophoblast subpopulations, evaluation of previously published gene chip data, immunofluorescence of first trimester placental tissues and primary EVT, as well as Western blot analyses were performed (Fig. 1). Recently, mRNA expression profiles of trypsin-isolated first trimester vCTB (n = 5) and primary EVT, isolated from differentiating villous explant cultures seeded on Matrigel (n = 6) were established [25]. Transcript levels of IL-1R1, integrins (α5, α6) and HLA-G1 in vCTB and primary EVT cell pools are depicted (Fig. 1A). Whereas IL-1β mRNA was absent from EVT (data not shown), IL-1R1 transcripts were expressed at higher levels in EVT similar to the differentiation-associated genes integrin α5 and HLA-G1. Accordingly, immunofluorescence of primary EVT revealed that the number of IL-1R1-expressing cells time-dependently increased upon cultivation on fibronectin concomitant with the differentiation-dependent elevation of the EVT marker integrin α1 (Fig. 1B) as previously noticed [21]. Similarly, immunofluorescence of first trimester placental tissue sections (6th week) indicated elevated IL-1R1 protein in distal regions of the cell column compared to vCTB and proximal cell column trophoblasts (Fig. 1B). Accordingly, EVT which have migrated into decidua basalis (11th week) also positively stained for IL-1R1 (Fig. 1B). Western blot analyses revealed that total IL-1R1 protein expression increased upon in vitro differentiation of primary EVT cultivated on fibronectin (Fig. 1C).
Fig. 1.
Expression of IL-1R1 in different human trophoblast subtypes. (A) Expression of IL-1R1 mRNA in 6 different primary EVT and 5 different vCTB cell pools measured by gene chip analyses. Markers of EVT differentiation integrin α5 (ITGA5) and HLA-G1 were elevated in isolated EVT cell pools whereas the vCTB-associated gene integrin α6 (ITGA6) was decreased. (B) IL-1R1 immunofluorescence in first trimester placental tissue (6th week), decidua basalis (11th week) and in first trimester primary EVT (n = 3) cultured for 24 and 48 h on fibronectin. Pan-keratin (KRT) antibodies were used to depict trophoblasts in placental and decidual tissue sections. Four different first trimester placentae, and three different decidua basalis regions were analysed. Representative pictures at a 200 fold (tissue sections) and 400 fold (primary EVT) magnification are shown. Areas of villous stroma (VS), cell column (CC), extravillous trophoblast (EVT) and villous cytotrophoblast (vCTB) are indicated. KRT-positive EVT expressing IL-1R1 in decidual tissue are marked by *. Integrin α1 (ITGA1) was used to monitor matrix-dependent differentiation of primary EVT. (C) Western blot analyses of IL-1R1 in primary EVT cultivated for 24 and 48 h on fibronectin. GAPDH was used as a loading control. A representative example is shown. IL-1R1 signals were normalised to GAPDH protein expression using densitometrical scanning. Bar graphs represent mean values ± S.D. of three different experiments, * indicates p < 0.05.
3.2. IL-1β promotes motility of EVT
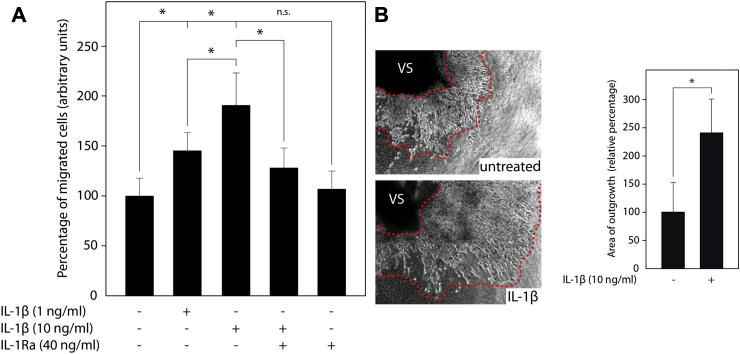
To analyse the effects of IL-1β on trophoblast migration, transwell assays with primary EVT were performed. Recombinant IL-1β dose-dependently increased trophoblast motility which was significantly inhibited in the presence of IL-1Ra (Fig. 2A). However, the antagonist did not influence basal migration of EVT suggesting the absence of autocrine IL-1β signalling. Moreover, the cytokine also stimulated migration of EVT in first trimester villous explant cultures on collagen I (Fig. 2B).
Fig. 2.
IL-1β-dependent EVT motility. (A) Migration of first trimester primary EVT through fibronectin-coated transwells in the absence or presence of human recombinant IL-1β and/or IL-1Ra. Invasion of CTK-7-positive trophoblasts was evaluated as described in Materials and methods. Bar graphs represent mean values ± S.D. of three experiments performed in duplicates. * indicates p < 0.05; ns, not significant; (B) IL-1β induced migration of primary EVT in first trimester villous explant cultures cultivated on collagen I. Representative pictures are shown (200 fold magnification). Migration of trophoblasts was analysed as mentioned in Materials and methods. Bar graphs represent mean values ± S.D. of untreated (n = 60) and IL-1β-stimulated (n = 60) villous explants isolated from five different placentae. * indicates p < 0.05.
3.3. IL-1β does not affect proliferation of vCTB, cell column trophoblasts and EVT
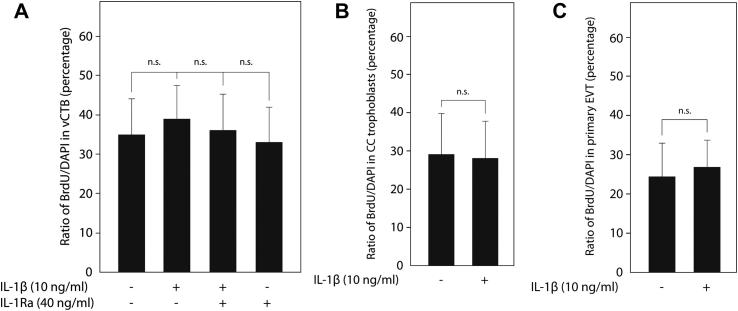
To test whether the effects of IL-1β on EVT invasion and migration could be partly explained by increased cell growth, proliferation of vCTB, cell column trophoblasts and EVT was analysed in floating villous explant cultures and primary cell isolates, respectively, using BrdU-labelling (Fig. 3). Evaluation of the BrdU/DAPI ratio in KRT-positive vCTB (Fig. 3A) and cell columns of villous explant cultures (Fig. 3B), however, revealed that IL-1β did not alter the proliferative capacity of these trophoblast subtypes. Similarly, the cytokine did not affect proliferation of trypsin-isolated primary EVT (Fig. 3C).
Fig. 3.
Effects of IL-1β on proliferation of vCTB, cell column trophoblasts and EVT. BrdU-labelling of floating villous explant cultures and isolated EVT, detection, and calculation of the BrdU/DAPI ratio were done as mentioned in Materials and methods. To measure proliferation of vCTB and cell column trophoblasts, 45 explants isolated from three different placentae were utilised per condition. (A) Proliferation of vCTB in villous explant cultures in the absence or presence of IL-1β and/or IL-1Ra. Bar graphs represent mean values ± S.D. of 500–600 nuclei analysed per condition. ns, not significant compared to untreated controls. (B) Proliferation of cell column trophoblasts in villous explant cultures in the absence or presence of IL-1β. Bars indicate ± S.D. ns, not significant. (C) Proliferation of isolated primary EVT in the absence or presence of IL-1β. Bar graphs represent mean values ± S.D. of three different experiments/preparations performed in duplicates. ns, not significant.
3.4. IL-1β induces uPA and PAI secretion in first trimester villous explant cultures and EVT
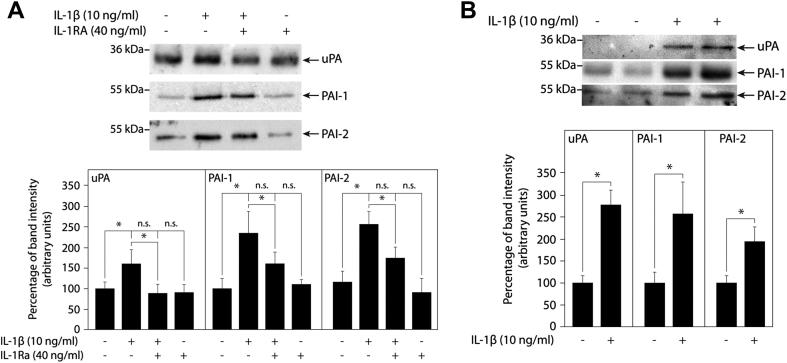
IL-1 has been well described as an inducer of the gelatinases MMP-2 and MMP-9 in different trophoblast models [17,26–28]. However, the effects of the cytokine on other protease systems of trophoblasts, such as the uPA/PAI system, are largely unknown. Hence, IL-1β-dependent expression of uPA, PAI-1 and PAI-2 was analysed in supernatants of the different trophoblast model systems (Fig. 4). Western blot analyses of first trimester villous explant cultures seeded on collagen I revealed that IL-1β induced secretion of uPA, PAI-1 and PAI-2, which was partly inhibited in the presence of IL-1Ra (Fig. 4A). Similarly, treatment of different primary EVT isolations with the cytokine provoked accumulation of uPA, PAI-1 and PAI-2 in the supernatants (Fig. 4B).
Fig. 4.
IL-1β-stimulated secretion of uPA and PAI proteins in first trimester villous explant cultures and primary EVT. Western blot analyses of supernatants and quantification were done as described in Materials and methods. (A) Western blots showing IL-1β-dependent expression of uPA, PAI-1 and PAI-2 in first trimester villous explant cultures in the absence or presence of IL-1Ra. Bar graphs show mean values ± S.D. of untreated and IL-1β- and/or IL-1Ra-stimulated explant cultures isolated from three different placentae, n = 36 for each condition; ns, not significant, * indicates p < 0.05. (B) Western blot analyses of uPA, PAI-1 and PAI-2 in primary EVT after 48 h of IL-1β treatment. Two different EVT cell pools are shown. Bar graphs represent mean values ± S.D. of three different primary EVT cell isolations, * indicates p < 0.05.
4. Discussion
The cytokine IL-1 is a well-known regulator of acute and chronic inflammatory processes [29,30]. The IL-1 protein family originally consisted of two agonists, IL-1α and IL-1β, both recognizing IL-1R1 with high affinity [30]. With the identification of novel genes such as IL-33, IL-36 or IL-38, the IL-1 family has expanded to eleven different ligands and nine distinct receptors including co-receptors, decoy receptors, inhibitory receptors and binding proteins [29]. Signalling through IL-1 involves binding of the ligand to the IL-1R1 chain, recruitment of IL-1R accessory protein (IL-1RAcP), interaction of the cytoplasmic Toll-IL-1-like receptor domain with the adaptor protein MyD88 and subsequent activation of the NFκB pathway and kinases such as IL-1R1-associated kinase (IRAK) and mitogen-activated protein kinase (MAPK) [31]. IL-1-dependent signalling can be inhibited by IL-1Ra which blocks dimerisation of IL-1R1 and IL-1RAcP upon binding to IL-1R1 [29].
Interestingly, accumulating evidence suggests that IL-1 also plays a crucial role in implantation and reproductive success [32]. Indeed, pre-implantation embryos were shown to produce IL-1 at maximal levels at the time of implantation and high concentrations of the cytokine correlated with successful implantation rates after IVF treatment [33–35]. Additionally, IL-1 was shown to regulate human in vitro decidualisation and to provoke secretion of chemokines and other factors from decidual stromal and uNK cells [32]. Along those lines, published data suggest that IL-1 also controls biological properties of placental trophoblasts. Besides stimulation of human chorionic gonadotrophin (hCG) secretion [36] and regulation of adhesion molecules [37], the cytokine has been well described as an inducer of MMP-2, MMP-3 and MMP-9 in JEG-3 cells, first trimester trophoblasts and trophoblastic cell lines [17,19,27,28].
IL-1 is likely released from decidual uNK cells, macrophages and stromal cells suggesting paracrine effects on the invasive trophoblast [38,39]. Moreover, stimulation of primary trophoblasts with inflammatory LPS or hypoxia induced secretion of immunoreactive IL-1 [17,40]. However, it remains controversial whether the cytokine is also released from trophoblasts under physiological conditions and could therefore act in an autocrine manner. Previous studies suggested IL-1 expression in vCTB of placental sections as well as basal secretion from first trimester trophoblasts prepared by Kliman's method [17,41]. In contrast, others proposed that IL-1 protein is absent in freshly isolated trophoblast preparations but rapidly induced upon in vitro cultivation suggesting artificial activation [12]. Along those lines, IL-1α and IL-1β mRNA were absent in three out of six and six out of six different EVT cell pools, respectively, measured by gene chip analyses (data not shown, accessible through GEO profiles using the URL published in [25]). EVT analysed in this comparative gene expression study have not been isolated by enzymatic digestion, but by mechanical separation after outgrowth of first trimester villous explant cultures in serum-free medium [25]. Hence, abnormal activation of IL-1 may only occur upon trypsinisation of placental tissue and/or addition of serum for cultivation. In agreement with that, the gene chip analyses indicated weak expression of IL-1α mRNA in five out of six vCTB cell pools prepared by the enzymatic Kliman method. Furthermore, the migration assays of primary EVT performed here do not support autocrine production of IL-1, since IL-1Ra did not reduce their basal motility.
Whereas IL-1 is either absent or very weakly expressed in trophoblasts, expression of IL-1R1 is prominent in EVT. Here, we show for the first time that IL-1R1 protein expression increases in distally located EVT of the cell column as well as in primary EVT differentiating on extracellular matrix in vitro. Its expression is maintained in EVT contacting stromal cells within the decidua basalis suggesting paracrine responsiveness. In agreement with the fact that IL-1R1 is rather weakly expressed in villous trophoblasts and proximal cell columns, the cytokine did neither affect proliferation of vCTB and cell column trophoblasts in floating villous explant cultures nor cell growth of primary EVT. On the other hand, upregulation of IL-1R1 during EVT differentiation could indicate a role of IL-1 in trophoblast invasion. Indeed, IL-1-dependent increase of trophoblast motility was noticed in primary EVT and in villous explant cultures seeded on collagen I. Besides the well-known IL-1-induced expression of MMPs [19,27,28,42], the proteolytic activity of uPA could also be involved since IL-1/IL-1R1-dependent accumulation of the enzyme was observed in supernatants of primary EVT and villous explant cultures. Elevated expression of uPA and PAI proteins in the presence of IL-1 has also been described in many different human cell types including endometrial stromal cells [43] and was shown to be associated with increased invasion of various cancer cell types [44–46]. On the other hand, uPA may promote motility independently of its protease activity, since the non-catalytic domain of uPA was shown to stimulate trophoblast migration and signalling upon interaction with urokinase-type plasminogen activator receptor [47].
With respect to trophoblast invasion, an indirect role of IL-1 through stimulation of IL-8 secretion from endometrial epithelial cells has been described [18]. Although this mechanism might contribute to IL-1-dependent implantation and early invasion, data obtained from the two different primary trophoblast cell models shown here confirm a direct, positive role of IL-1 in trophoblast motility as previously suggested [17]. In contrast, trophoblastic SGHPL-4 cells did not show increased invasion upon IL-1 treatment despite elevated MMP expression [19]. Hence, caution has to be taken when studies with trophoblastic cell lines are interpreted in the context of physiological trophoblast invasion. This is also reflected by the fact that the cytokine exerts differential effects on cell growth of primary and tumorigenic trophoblasts. Whereas IL-1-dependent decrease of JAR cell proliferation [48] and IL-1β-induced expansion of AC-1M88 spheroids [49] were noticed, proliferation was unaffected in our different primary trophoblast cell models.
Despite the fact that IL-1 increased motility of primary trophoblasts in vitro, the importance of IL-1 as a physiological regulator of trophoblast invasion remains uncertain. Whereas low levels of IL-1 may control implantation and early trophoblast invasion processes, continuous expression of the pro-inflammatory cytokine may have detrimental effects on pregnancy. Indeed, some authors believe that pregnancy is associated with a decrease of pro-inflammatory and T helper (Th1) cytokines such as IL-1 and TNFα and induction of anti-inflammatory Th2 cytokines which might reduce aberrant inflammation, damage and allograft rejection of the fetus [50]. However, considering different immunological phases of pregnancy and complex interactions between uterine cell types, the concept of Th1/Th2 shift is probably too simplistic and remains a matter of discussion [51–54]. Hence, elevated concentrations of active IL-1 may not necessarily be harmful for the placenta and progression of gestation. Bearing this in mind, a physiological role of IL-1 in trophoblast invasion at later times of pregnancy cannot be excluded. On the other hand, the bioactivity of IL-1 secreted from decidual stromal cells in vitro and in vivo is either absent or very low [39]. Also, uNK cells and macrophages may secrete the active cytokine mainly under inflammatory conditions. Indeed, exceeding levels of IL-1 and other Th1 cytokines were shown to be associated with various pregnancy diseases such as recurrent spontaneous abortion, preterm rupture of membranes, preterm labour and preeclampsia [15].
Although the inflammatory response provoked by IL-1 is considered as an adverse effect of the cytokine, one could also argue that it is important for immunosurveillance of gestation [55]. Upon infection bioactive IL-1 could be quickly released from its precursor, bind to EVTs and other uterine cell types via IL-1R1 and induce abnormal levels of uPA and MMPs. The enhanced proteolytic activity might then cause abnormal matrix degradation within the decidua impairing physiological trophoblast migration and inducing placental abruption. Such a mechanism would protect the mother from spreading of an infection, even at the expense of fetal survival. This evolutionary strategy might be still of importance in countries where women have limited access to anti-infective therapies.
Acknowledgements
The study is funded by grant P-22687-B13 of the Austrian Science Funds, Vienna, Austria. V. Fock is supported by grant Nr. 13955 of the “Jubiläumsfonds” of the Austrian National Bank.
References
- 1.Zhou Y., Fisher S.J., Janatpour M., Genbacev O., Dejana E., Wheelock M. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Harris L.K. IFPA Gabor Than award lecture: transformation of the spiral arteries in human pregnancy: key events in the remodelling timeline. Placenta. 2011;32(Suppl. 2):S154–S158. doi: 10.1016/j.placenta.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Burton G.J., Jauniaux E., Charnock-Jones D.S. Human early placental development: potential roles of the endometrial glands. Placenta. 2007;28(Suppl. A):S64–S69. doi: 10.1016/j.placenta.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pijnenborg R., Anthony J., Davey D.A., Rees A., Tiltman A., Vercruysse L. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98(7):648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 6.Burton G.J., Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redman C.W., Sargent I.L. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(Suppl. A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Bischof P., Meisser A., Campana A. Paracrine and autocrine regulators of trophoblast invasion–a review. Placenta. 2000;21(Suppl. A):S55–S60. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- 9.Knöfler M., Pollheimer J.I.F.P.A. Award in placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33(Suppl.):S55–S62. doi: 10.1016/j.placenta.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lala P.K., Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24(6):575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 11.Knöfler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54(2–3):269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauma S.W., Walsh S.W., Nestler J.E., Turner T.T. Interleukin-1 is induced in the human placenta by endotoxin and isolation procedures for trophoblasts. J Clin Endocrinol Metab. 1992;75(3):951–955. doi: 10.1210/jcem.75.3.1517391. [DOI] [PubMed] [Google Scholar]
- 13.Salamonsen L.A., Dimitriadis E., Robb L. Cytokines in implantation. Semin Reprod Med. 2000;18(3):299–310. doi: 10.1055/s-2000-12567. [DOI] [PubMed] [Google Scholar]
- 14.Fazleabas A.T., Kim J.J., Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment–a review. Placenta. 2004;25(Suppl. A):S26–S31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Romero R., Gotsch F., Pineles B., Kusanovic J.P. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood C.J., Huang S.J., Krikun G., Caze R., Rahman M., Buchwalder L.F. Decidual hemostasis, inflammation, and angiogenesis in pre-eclampsia. Semin Thromb Hemost. 2011;37(2):158–164. doi: 10.1055/s-0030-1270344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Librach C.L., Feigenbaum S.L., Bass K.E., Cui T.Y., Verastas N., Sadovsky Y. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269(25):17125–17131. [PubMed] [Google Scholar]
- 18.Hirota Y., Osuga Y., Hasegawa A., Kodama A., Tajima T., Hamasaki K. Interleukin (IL)-1beta stimulates migration and survival of first-trimester villous cytotrophoblast cells through endometrial epithelial cell-derived IL-8. Endocrinology. 2009;150(1):350–356. doi: 10.1210/en.2008-0264. [DOI] [PubMed] [Google Scholar]
- 19.Husslein H., Haider S., Meinhardt G., Prast J., Sonderegger S., Knöfler M. Expression, regulation and functional characterization of matrix metalloproteinase-3 of human trophoblast. Placenta. 2009;30(3):284–291. doi: 10.1016/j.placenta.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarrade A., Lai Kuen R., Malassine A., Tricottet V., Blain P., Vidaud M. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001;81(9):1199–1211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 21.Pollheimer J., Haslinger P., Fock V., Prast J., Saleh L., Biadasiewicz K. Endostatin suppresses IGF-II-mediated signaling and invasion of human extravillous trophoblasts. Endocrinology. 2011;152(11):4431–4442. doi: 10.1210/en.2011-1196. [DOI] [PubMed] [Google Scholar]
- 22.Bauer S., Pollheimer J., Hartmann J., Husslein P., Aplin J.D., Knöfler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89(2):812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 23.Biadasiewicz K., Sonderegger S., Haslinger P., Haider S., Saleh L., Fiala C. Transcription factor AP-2alpha promotes EGF-dependent invasion of human trophoblast. Endocrinology. 2011;152(4):1458–1469. doi: 10.1210/en.2010-0936. [DOI] [PubMed] [Google Scholar]
- 24.Prast J., Saleh L., Husslein H., Sonderegger S., Helmer H., Knöfler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149(3):979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilban M., Haslinger P., Prast J., Klinglmuller F., Woelfel T., Haider S. Identification of novel trophoblast invasion-related genes: heme oxygenase-1 controls motility via peroxisome proliferator-activated receptor gamma. Endocrinology. 2009;150(2):1000–1013. doi: 10.1210/en.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontana V.A., Sanchez M., Cebral E., Calvo J.C. Interleukin-1 beta regulates metalloproteinase activity and leptin secretion in a cytotrophoblast model. Biocell. 2010;34(1):37–43. [PubMed] [Google Scholar]
- 27.Karmakar S., Das C. Regulation of trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod Immunol. 2002;48(4):210–219. doi: 10.1034/j.1600-0897.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 28.Meisser A., Chardonnens D., Campana A., Bischof P. Effects of tumour necrosis factor-alpha, interleukin-1 alpha, macrophage colony stimulating factor and transforming growth factor beta on trophoblastic matrix metalloproteinases. Mol Hum Reprod. 1999;5(3):252–260. doi: 10.1093/molehr/5.3.252. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dower S.K., Kronheim S.R., Hopp T.P., Cantrell M., Deeley M., Gillis S. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986;324(6094):266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- 31.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3(105):cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 32.Dimitriadis E., White C.A., Jones R.L., Salamonsen L.A. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11(6):613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 33.Karagouni E.E., Chryssikopoulos A., Mantzavinos T., Kanakas N., Dotsika E.N. Interleukin-1beta and interleukin-1alpha may affect the implantation rate of patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 1998;70(3):553–559. doi: 10.1016/s0015-0282(98)00243-x. [DOI] [PubMed] [Google Scholar]
- 34.Krussel J.S., Simon C., Rubio M.C., Pape A.R., Wen Y., Huang H.Y. Expression of interleukin-1 system mRNA in single blastomeres from human preimplantation embryos. Hum Reprod. 1998;13(8):2206–2211. doi: 10.1093/humrep/13.8.2206. [DOI] [PubMed] [Google Scholar]
- 35.Sheth K.V., Roca G.L., al-Sedairy S.T., Parhar R.S., Hamilton C.J., al-Abdul Jabbar F. Prediction of successful embryo implantation by measuring interleukin-1-alpha and immunosuppressive factor(s) in preimplantation embryo culture fluid. Fertil Steril. 1991;55(5):952–957. doi: 10.1016/s0015-0282(16)54305-2. [DOI] [PubMed] [Google Scholar]
- 36.Steele G.L., Currie W.D., Leung E.H., Yuen B.H., Leung P.C. Rapid stimulation of human chorionic gonadotropin secretion by interleukin-1 beta from perifused first trimester trophoblast. J Clin Endocrinol Metab. 1992;75(3):783–788. doi: 10.1210/jcem.75.3.1517367. [DOI] [PubMed] [Google Scholar]
- 37.Karmakar S., Das C. Modulation of ezrin and E-cadherin expression by IL-1beta and TGF-beta1 in human trophoblasts. J Reprod Immunol. 2004;64(1–2):9–29. doi: 10.1016/j.jri.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Jokhi P.P., King A., Loke Y.W. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine. 1997;9(2):126–137. doi: 10.1006/cyto.1996.0146. [DOI] [PubMed] [Google Scholar]
- 39.White C.A., Dimitriadis E., Sharkey A.M., Stoikos C.J., Salamonsen L.A. Interleukin 1 beta is induced by interleukin 11 during decidualization of human endometrial stromal cells, but is not released in a bioactive form. J Reprod Immunol. 2007;73(1):28–38. doi: 10.1016/j.jri.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Benyo D.F., Miles T.M., Conrad K.P. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82(5):1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- 41.Simon C., Frances A., Piquette G., Hendrickson M., Milki A., Polan M.L. Interleukin-1 system in the materno-trophoblast unit in human implantation: immunohistochemical evidence for autocrine/paracrine function. J Clin Endocrinol Metab. 1994;78(4):847–854. doi: 10.1210/jcem.78.4.8157710. [DOI] [PubMed] [Google Scholar]
- 42.Librach C.L., Werb Z., Fitzgerald M.L., Chiu K., Corwin N.M., Esteves R.A. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung H.W., Wen Y., Ahn J.J., Moon H.S., Polan M.L. Interleukin-1beta regulates urokinase plasminogen activator (u-PA), u-PA receptor, soluble u-PA receptor, and plasminogen activator inhibitor-1 messenger ribonucleic acid expression in cultured human endometrial stromal cells. J Clin Endocrinol Metab. 2001;86(3):1332–1340. doi: 10.1210/jcem.86.3.7335. [DOI] [PubMed] [Google Scholar]
- 44.Bryan L., Paugh B.S., Kapitonov D., Wilczynska K.M., Alvarez S.M., Singh S.K. Sphingosine-1-phosphate and interleukin-1 independently regulate plasminogen activator inhibitor-1 and urokinase-type plasminogen activator receptor expression in glioblastoma cells: implications for invasiveness. Mol Cancer Res. 2008;6(9):1469–1477. doi: 10.1158/1541-7786.MCR-08-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng C.Y., Hsieh H.L., Sun C.C., Lin C.C., Luo S.F., Yang C.M. IL-1 beta induces urokinase-plasminogen activator expression and cell migration through PKC alpha, JNK1/2, and NF-kappaB in A549 cells. J Cell Physiol. 2009;219(1):183–193. doi: 10.1002/jcp.21669. [DOI] [PubMed] [Google Scholar]
- 46.Sawai H., Okada Y., Funahashi H., Matsuo Y., Takahashi H., Takeyama H. Interleukin-1alpha enhances the aggressive behavior of pancreatic cancer cells by regulating the alpha6beta1-integrin and urokinase plasminogen activator receptor expression. BMC Cell Biol. 2006;7:8. doi: 10.1186/1471-2121-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Chakraborty C., Graham C.H., Barbin Y.P., Dixon S.J., Lala P.K. Noncatalytic domain of uPA stimulates human extravillous trophoblast migration by using phospholipase C, phosphatidylinositol 3-kinase and mitogen-activated protein kinase. Exp Cell Res. 2003;286(1):138–151. doi: 10.1016/s0014-4827(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 48.Nilkaeo A., Bhuvanath S. Interleukin-1 modulation of human placental trophoblast proliferation. Mediators Inflamm. 2006;2006(2):79359. doi: 10.1155/MI/2006/79359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez M., Neufeld J., Reimann K., Wittmann S., Samalecos A., Wolf A. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1 beta. Mol Hum Reprod. 2011;17(7):421–433. doi: 10.1093/molehr/gar015. [DOI] [PubMed] [Google Scholar]
- 50.Wegmann T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 51.Mor G., Cardenas I., Abrahams V., Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sargent I.L., Borzychowski A.M., Redman C.W. NK cells and pre-eclampsia. J Reprod Immunol. 2007;76(1–2):40–44. doi: 10.1016/j.jri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Chaouat G., Ledee-Bataille N., Dubanchet S., Zourbas S., Sandra O., Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134(2):93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 54.Saito S., Nakashima A., Shima T., Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilczynski J.R. Th1/Th2 cytokines balance–yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):136–143. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]