Abstract
The use of tissue-engineered human skin equivalents (HSE) for fundamental research and industrial application requires the expansion of keratinocytes from a limited number of skin biopsies donated by adult healthy volunteers or patients. A pharmacological inhibitor of Rho-associated protein kinases, Y-27632, was recently reported to immortalize neonatal human foreskin keratinocytes. Here, we investigated the potential use of Y-27632 to expand human adult keratinocytes and evaluated its effects on HSE development and in vitro gene delivery assays. Y-27632 was found to significantly increase the life span of human adult keratinocytes (up to five to eight passages). The epidermal morphology of HSEs generated from high-passage, Y-27632-treated keratinocytes resembled the native epidermis and was improved by supplementing Y-27632 during the submerged phase of HSE development. In addition, Y-27632-treated keratinocytes responded normally to inflammatory stimuli, and could be used to generate HSEs with a psoriatic phenotype, upon stimulation with relevant cytokines. Furthermore, Y-27632 significantly enhanced both lentiviral transduction efficiency of primary adult keratinocytes and epidermal morphology of HSEs generated thereof. Our study indicates that Y-27632 is a potentially powerful tool that is used for a variety of applications of adult human keratinocytes.
Introduction
There is a growing demand for tissue-engineered in vitro skin models for industrial applications and in fundamental research. Nowadays, in vitro pharmacotoxicological testing and industrial screening of drugs are accepted as an alternative to experimental animal testing, and in vitro skin models are increasingly used to study the skin biology and pathophysiology of skin diseases. Although the development of organotypic cultures, further designated as human skin equivalents (HSEs), for reconstructive purposes has been disappointing over the last decades, there are several indications that they may find their way to the clinic. Their potential use ranges from application in chronic ulcers,1 to the treatment of patients with keratinization disorders or blistering diseases using epidermal grafts of keratinocytes derived from genetically corrected-induced pluripotent stem cells.2
Submerged keratinocyte cultures have been extensively used as in vitro skin models; however, over the last decade, HSEs have emerged as a more biologically and physiologically relevant model, as they more closely mimic the native epidermis. To meet the increasing need for HSEs, large quantities of primary human keratinocytes are required. Recently, increased cloning efficiency of neonatal human foreskin keratinocytes3 and even efficient immortalization due to increased telomerase expression and stabilization of the telomere lengths4 have been described. Both studies used a selective inhibitor of the Rho-associated coiled-coil forming protein serine/threonine kinase (ROCK), Y-27632.
The Rho/ROCK pathway is essential in a variety of cellular functions in keratinocytes, including actin cytoskeletal organization,5 keratinocyte adhesion,6 and motility.7,8 Related to skin morphogenesis, it was recently described that RhoE, a member of the Rho GTPase family, is required for epidermal morphogenesis by regulating epidermal stratification.9 RhoA, however, was found to be dispensable for skin development, as knockout mice lack a skin phenotype.8 In contrast, downstream effectors of RhoA, ROCK I and II, have been reported to regulate keratinocyte differentiation.10,11
Hitherto, studies describing ROCK inhibition by Y-27632 use HaCaT cell lines or neonatal foreskin keratinocytes. However, in HSE development, primary human adult keratinocytes are preferably used, as they generate HSEs that more closely resemble the native epidermis in structure and function.12 For the application of HSEs in reconstructive surgery or gene therapy, the expansion of primary keratinocytes isolated from a few biopsies is required to generate sufficient amounts of cells. However, the life span of primary adult keratinocytes is relatively short, and senescence is usually observed after a few passages. In the light of the emerging applications of HSEs, we anticipate a strong need for human adult keratinocytes and have, therefore, evaluated the potential of Y-27632 to prolong the life span of these cells. In addition, we thoroughly investigated the effects of Y-27632 on HSE development. Finally, we studied the potential of Y-27632 to facilitate gene delivery studies using lentiviral-transduced adult keratinocytes to generate HSEs.
Methods
Cell culture
Cells from the mouse fibroblast cell line 3T3 were cultured in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Inc.) supplemented with penicillin/streptomycin (50 IU/mL; ICN Biomedicals) and 10% calf serum with iron (Hyclone). Keratinocytes were isolated from human abdominal skin derived from donors who underwent surgery for abdominal wall correction as previously described.12 The study was conducted according to the Declaration of Helsinki principles. Keratinocytes were either stored in liquid nitrogen or directly used. Keratinocytes were cultured in Greens medium (2:1 [v/v] DMEM:Ham's F12 (both from Life Technologies, Inc.) supplemented with 10% fetal bovine serum (Hyclone), l-glutamine (4 mM; Life Technologies, Inc.), penicillin/streptomycin (50 IU/mL; Life Technologies, Inc.), adenine (24.3 μg/mL; Calbiochem), insulin (5 μg/mL; Sigma), hydrocortisone (0.4 μg/mL; Merck), triiodothyronine (1.36 ng/mL; Sigma), and cholera toxin (10−10 M; Sigma) in the presence of irradiated 3T3-J2 feeder cells. After 3 days, the medium was replaced by Greens medium containing epidermal growth factor (EGF, 10 ng/mL; Sigma). The cells were grown in the presence or absence of 10 μM Y-27632 (Sigma) as indicated and were refreshed every 2–3 days. On 90–100% confluency, the cells were subcultured by the removal of feeder cells by ethylenediaminetetraacetic acid (EDTA) (Sigma) and subsequent trypsinization with trypsin-EDTA (0.05%; Sigma), after which they were passaged 1:10 on irradiated 3T3-J2 feeder cells.
Estimation of population doubling rate
At each passage, the number of cells harvested was determined, and population doubling (PD) was calculated as follows: PD=3.32(log[number of cells harvested/number of cells seeded]).4
Analysis of cloning efficiency
To assess the effect of Y-27632 on the cloning efficiency of freshly isolated adult keratinocytes versus liquid-nitrogen-stored adult keratinocytes, the cells were seeded at different seeding densities on 3T3-J2 feeders cells and cultured as just described. The cells were grown in the presence or absence of 10 μM Y-27632 and after 3 days, the 3T3-J2 feeder cells were removed by EDTA treatment, and keratinocyte colonies were fixed using 3.7% paraformaldehyde for 20 min at room temperature. The cells were washed twice with phosphate-buffered saline (PBS) (Braun) and stained with freshly prepared 1% Rhodanile Blue (Sigma) in PBS for 15 min. Afterward, the cells were extensively washed with tap water, and photographs were taken. Quantification analysis was performed using Image J software.
Generation of HSEs
De-epidermized dermis was generated as previously reported,12 and 8-mm tissue samples were obtained using a biopsy punch. The de-epidermized dermis was placed in a 24-well transwell system and seeded with 105 keratinocytes. Liquid-nitrogen-stored primary adult keratinocytes (passage 1) or Y-27632-treated keratinocytes (passage 6 or 13) were used for experiments as indicated. After culturing the HSEs submerged for 3 days in a medium containing 5% serum, consisting of two parts of DMEM and one part of Ham's F12 medium (both from Life Technologies, Inc.) supplemented with 5% calf serum (Hyclone), 4 mM l-glutamine and 50 IU/mL penicillin or streptomycin (both from Life Technologies, Inc.), 24.3 μg/mL adenine (Calbiochem), 1 μM hydrocortisone (Merck KGaA), and 50 μg/mL ascorbic acid, 0.2 μM insulin, 1.36 ng/mL triiodothyronine, and 10−10 mM cholera toxin (all from Sigma), the HSEs were cultured at the air–liquid interface for 10 days in a medium without serum, consisting of two parts of DMEM and one part of Ham's F12 medium (both from Life Technologies, Inc.) supplemented with 4 mM l-glutamine and 50 IU/mL penicillin or streptomycin (both from Life Technologies, Inc.), 24.3 μg/mL adenine (Calbiochem), 1 mg/mL l-serine and 2 μg/mL l-carnitine (both from Sigma), bovine serum albumin lipid mix (25 μM palmitic acid, 7 μM arachidonic acid, 15 μM linoleic acid, and 0.4 μg/mL vitamin E; all from Sigma), 1 μM hydrocortisone (Merck KGaA), and 50 μg/mL ascorbic acid, 0.1 μM insulin, 1.36 ng/mL triiodothyronine, 10−10 mM cholera toxin, 5 ng/mL keratinocyte growth factor, and 2 ng/mL EGF (all from Sigma). Y-27632 was supplemented in the culture medium at indicated culture periods and concentrations.
Cytokine stimulation
Liquid-nitrogen-stored primary adult keratinocytes or Y-27632-treated adult keratinocytes (passage 6) were cultured to confluency in a keratinocyte growth medium (KGM), consisting of KBM (0.15 mM Ca2+ BioWhittaker) supplemented with ethanolamine (0.1 mM; Sigma), phosphoethanolamine (0.1 mM; Sigma), bovine pituitary extract (0.4% vol/vol; BioWhittaker) EGF (10 ng/mL; Sigma), insulin (5 μg/mL; Sigma), hydrocortisone (0.5 μg/mL; Collaborative Research), penicillin (100 U/mL; Life Technologies, Inc.), and streptomycin (100 μg/mL; Life Technologies, Inc.), with different concentrations of Y-27632. The cells were switched to KGM depleted of growth factors (bovine pituitary extract, EGF, hydrocortisone, and insulin) for 48 h. Differentiating cell cultures were either stimulated with pro-inflammatory cytokines interleukin (IL)-1α (30 ng/mL), tumor necrosis factor (TNF)-α (30 ng/mL), and interferon (IFN)-γ (500 U/mL) (all from Preprotech), or left untreated. After 48 h, the cells were harvested for mRNA isolation using the Trizol reagent (Life Technologies, Inc.).
Psoriatic skin equivalents were generated by stimulating HSEs during the last 3 days of the air–liquid interface culture with a mixture of IL-1α (10 ng/mL), TNF-α (5 ng/mL), and IL-6 (5 ng/mL) (all from Preprotech), as previously described.12
Lentiviral vector production
For the generation of recombinant lentiviral particles, we used the third-generation self-inactivating transfer vector pRLL-cPPT-PGK-GFP-PRE-SIN (kind gift from Dr. F. van de Loo, Department of Rheumatology, Radboud University Medical Centre Nijmegen. See Geurts et al.13 for details on plasmids and cells used for virus production). Packaging of VSV-G pseudotyped viruses was performed by transient transfection of 293T cells. Overall, 293T cells were seeded in a T225 flask at 1×105 cells/cm2 in DMEM (Life Technologies, Inc.) supplemented with 10% bovine cals serum (BCS, Hyclone), 100 μg/mL penicillin (Life Technologies, Inc.), 100 μg/mL streptomycin (Life Technologies, Inc.), 1 mM sodium pyruvate (Sigma), and 0.01 mM water-soluble cholesterol (Sigma). At least 1 h before transfection, the medium was replaced with 24 mL DMEM supplemented with 10% BCS, 1 mM sodium pyruvate, and 0.01 mM water-soluble cholesterol. For each flask, the following DNA mixture was prepared containing 56.7 μg transfer vector, 42.3 μg packaging vector pMDL-g/p-RRE, 20.0 μg expression vector pHIT-G, and 14.2 μg expression vector pRSV-REV in a final volume of 100 μL water. Subsequently, 1.5 mL 0.5 M CaCl2 (Sigma) was added, and 1.5 mL 2× Hepes-buffered saline solution (Sigma) was gently added under constant mixing. The formed precipitates were immediately added to the cells. Sixteen hours after transfection, the medium was refreshed with DMEM supplemented with 100 μg/mL penicillin, 100 μg streptomycin, and 1 mM sodium pyruvate. Forty-eight hours post transfection, the virus-containing supernatant was filtered through a 0.45 μm Stericup (Millipore) to remove cell debris. Recombinant lentiviral particles were concentrated to 1.5 mL and dialyzed against sterile PBS using an Amicon filter (MWCO 100 kDa, Millipore). Viral stocks were aliquoted and stored at −80°C. Viral titers were determined using an enzyme-linked immunosorbent assay kit (Abbott) targeting the viral envelope protein p24gag and expressed as ng p24/μL.
Lentiviral transduction of adult keratinocytes
Primary adult keratinocytes from three different donors were grown to 40% confluency in a 6-well plate using the KGM medium (Lonza) supplemented or not with 10 μM Y-27632. Subsequently, each well was incubated with 2 μg of enhanced green fluorescent protein (eGFP)-lentivirus for 4 h, after which the cells were washed twice with sterile PBS. After 24 h of recovery in KGM supplemented or not with 10 μM Y-27632, the cells were harvested with trypsin-EDTA and washed twice using PBS. The percentage of eGFP-positive keratinocyte was analyzed by flow cytometry (EPICS Elite flow cytometer).
To generate eGFP-transduced HSEs, 1.5×105 harvested keratinocytes were seeded onto de-epidermised dermis in 24-well inserts, and HSEs were generated as previously described with 10 μM Y-27632 supplemented during the submerged phase of the HSE culture as indicated.
Quantitative real-time polymerase chain reaction
For the skin equivalents, epidermis was separated by dispase (Roche Diagnostics) treatment for 2 h at 4°C, and total RNA was isolated from the epidermis using the Trizol reagent (Life Technologies, Inc.) and a subsequent RNeasy mini kit (Qiagen Benelux B.V.) according to the manufacturer's specifications. cDNA was generated and used for quantitative real-time PCR (qPCR), which was performed with the MyiQ Single-Colour Real-Time Detection System for quantification with Sybr Green and melting curve analysis (Bio-Rad Laboratories, Inc.), as previously described.14 Primers for KRT10, KRT14, INV, LOR, Ki-67, FLG, TGM1, LCE3E, human beta defensin-2 (hBD-2), TNF-α, IL-8, IL-1β, and the housekeeping gene human acidic ribosomal phosphoprotein P0 (RPLP0) were obtained from Biolegio (Malden). Relative mRNA expression levels of all examined genes were measured using the method described by Livak and Schmittgen.15 See Table 1 for primer sequences.
Table 1.
Primer Sequences of All Genes Examined by Real-Time Quantitative Polymerase Chain Reaction
| HUGO gene symbol | Description of gene/protein | Forward primer | Reverse primer | Ea |
|---|---|---|---|---|
| KRT10 | Keratin 10, KRT10 (K10) | tggttcaatgaaaagagcaagga | gggattgtttcaaggccagtt | 1.93 |
| KRT14 | Keratin 14, KRT14 (K14) | ggcctgctgagatcaaagactac | cactgtggctgtgagaatcttgtt | 1.93 |
| MKI67 | Ki-67 (Ki-67) | aaaccaacaaagaggaacacaaatt | gtctggagcgcagggatattc | 2.21 |
| IVL | Involucrin (IVL) | acttatttcgggtccgctaggt | gagacatgtagagggacagagtcaag | 1.93 |
| TGM1 | Transglutaminase 1 (TGM1) | cccccgcaatgagatctaca | atcctcatggtccacgtacaca | 1.99 |
| LOR | Loricrin (LOR) | aggttaagacatgaaggatttgcaa | ggcaccgatgggcttagag | 2.08 |
| FLG | Filaggrin (FLG) | acttcactgagtttcttctgatggtatt | tccagacttgagggtctttttctg | 1.89 |
| LCE3E | Late cornified envelope 3E (LCE3E) | ctgatgctgagacaagcgatctt | gatcccccacaggaaaacct | 2.20 |
| DEFB4 | Human β-defensin-2 (hBD-2) | gatgcctcttccaggtgttttt | ggatgacatatggctccactctt | 1.99 |
| IL8 | Interleukin 8 (IL-8) CXCL8 | cttggcagccttcctgattt | ttctttagcactccttggcaaaa | 2.11 |
| TNF | Tumor necrosis factor alpha (TNF) | tcttctcgaaccccgagtga | cctctgatggcaccaccag | 2.00 |
| IL1B | Interleukin 1 beta (IL1B) | aatctgtacctgtcctgcgtgtt | tgggtaatttttgggatctacactct | 2.19 |
| RPLP0 | Ribosomal phosphoprotein P0 (RPLP0) | caccattgaaatcctgagtgatgt | tgaccagcccaaaggagaag | 2.02 |
Parentheses refers to the name used in text.
E is efficiency as fold increase in fluorescence per polymerase chain reaction cycle.
Morphological and immunohistochemical analysis
HSEs were fixed in buffered 4% formalin for 4 h, processed for routine histology, and embedded in paraffin. Six micrometer sections were either stained with hematoxylin and eosin (Sigma) or processed for immunohistochemical staining using an indirect immunoperoxidase technique with avidin-biotin complex enhancement (Vectastain Laboratories). To study epidermal proliferation, an antibody directed against Ki-67 (1:50; MIB-1; Dako) was used, whereas epidermal differentiation was studied using antibodies directed against cytokeratin 10 (1:100; Sanbio), cytokeratin 14 (1:50, Novocastra), involucrin (1:50; Mon150; Sanbio), loricrin (1:500; BAbCO), filaggrin (1:200; Novocastra), transglutaminase 1 (1:200, Santa Cruz), and late cornified envelope 2 (LCE2; 1:1000, See Bergboer et al.16). The stress or psoriatic marker, hBD-2, was stained using goat anti-hBD-2 polyclonal serum (1:100; Abcam), and SKALP/elafin was stained using polyclonal antibodies as previously described.17 Detection by 3-amino-9-ethylcarbazole (Calbiochem) was followed by nuclei counterstaining with Mayer's hematoxylin solution (Sigma), and sections were mounted using glycerol gelatin (Sigma).
Statistics
Statistical significance was determined using the paired two-tailed Student's t test.
Results
Y-27632 increases the life span of primary adult keratinocytes
As previously described for freshly isolated neonatal foreskin keratinocytes,4 we cultured liquid-nitrogen-stored primary adult keratinocytes obtained from 3 mm skin biopsies and neonatal foreskin keratinocytes with or without Y-27632. The population doubling rate was calculated at each passage. Based on previous work and dose optimization studies, a 10 μM concentration of Y-26732 was used. As shown in Figure 1a, senescence was observed between days 30 and 60 of culture in all five adult keratinocyte donors cultured without Y-27632; this was in contrast to neonatal foreskin keratinocytes, which remained proliferative throughout the culture period, which indicates the profound differences between foreskin and adult keratinocytes. All adult keratinocytes cultured in the presence of Y-27632 showed a markedly increased life span, and three out of five keratinocyte donors remained proliferative for 80 days or longer. Morphologically, high-passage keratinocytes cultured with Y-27632 resembled primary keratinocytes, as they were small and homogenous in size and formed a honeycomb pattern of cohesive polygonal cells. Adult keratinocytes cultured in the absence of Y-27632 became large and heterogeneous in size with bloated cytoplasm, which are signs of senescence (Fig. 1b). Since immortalization of foreskin keratinocytes by Y-27632 due to induced telomerase expression was previously reported,4 we analyzed telomerase mRNA expression in high-passage, Y-27632-treated adult and foreskin keratinocytes; however, we were unable to detect telomerase expression in all samples (data not shown). We also studied the effect of Y-27632 on the proliferation rate of freshly isolated adult keratinocytes as compared with liquid-nitrogen-stored, primary adult keratinocytes and found no differences between both keratinocyte batches (Fig. 1c and Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/tea), suggesting that keratinocyte cultures of previously isolated and liquid-nitrogen-stored adult keratinocytes can benefit from Y-27632 treatment.
FIG. 1.
Effect of Y-27632 on life span and cloning efficiency of primary human adult keratinocytes. (a) Growth rate of human keratinocytes from one neonatal foreskin (HFK) donor or five adult (HAK1–5) donors (abdominal wall skin) cultured in the presence or absence of 10 μM Y-27632. The arrows indicate cells lines that continued to divide after 100 days in culture. The growth rate is measured as population doubling per day. (b) Images of primary adult keratinocytes (left), passage 4 adult keratinocytes cultured without Y-27632 (middle), and passage 13 adult keratinocytes cultured with Y-27632 (right). Scale bar, 100 μM. (c) Fold increase in cloning efficiency of freshly isolated keratinocytes and liquid-nitrogen-stored primary keratinocytes cultured with Y-27632 as compared with cultures without Y-27632. Error bars represent SD (n=3). SD, standard deviation.
Normal HSE can be generated from Y-27632-treated keratinocytes
To evaluate the effect of Y-27632 on HSE development, we used our three-dimensional (3D) skin model consisting of de-epidermised dermis as a matrix, and supplemented the culture medium with 10 μM Y-27632. Y-27632-treated keratinocytes (passage 6) were used to generate skin equivalents, and we compared them with liquid nitrogen-stored, primary adult keratinocytes (passage 1), with Y-27632 supplemented during different phases of the skin equivalent development. Passage 6 of adult keratinocytes generated high-quality HSEs, with a multilayered epidermis, the presence of stratum granulosum, and a basket-weave stratum corneum (Supplementary Fig. S2). Normal expression patterns of major epidermal differentiation proteins were observed, and HSEs were negative for the stress/psoriatic marker hBD-2 (Fig. 2). (See Supplementary Fig. S3a for epidermal differentiation analysis in control HSEs). Even at passage 12, Y-27632-treated adult keratinocytes still generated high-quality HSEs comparable to the native epidermis (Supplementary Fig. S3b). Furthermore, epidermal morphology appeared more regular when Y-27632 was supplemented during the submerged phase of the culture period (Supplementary Fig. S2c, d); this was in contrast to the HSEs cultured with Y-27632 during the entire culture period in which epidermal morphology was negatively affected, and deep epidermal ridges were observed (Supplementary Fig. S2e, f). Therefore, it was decided to use Y-27632 only during the submerged phase of the HSE development for further experiments.
FIG. 2.
Epidermal differentiation patterns in HSEs generated from passage 6, Y-27632-treated keratinocytes. HSEs were cultured without Y-27632 (left panel), with 10 μM Y-27632 supplemented during the submerged phase (middle panel) or during the entire culture period (right panel). HSEs show normal expression patterns for all analyzed epidermal markers: Ki-67 (proliferative cells), keratin 14 (basal keratinocytes), keratin 10 (suprabasal keratinocytes), involucrin, and transglutaminase 1, loricrin, filaggrin, and Late cornified envelope 2 (LCE2) (terminal differentiation). HSEs are negative for the stress/psoriatic marker, hBD-2. Scale bar, 100 μM. hBD-2, human beta defensin-2; HSEs, human skin equivalents.
Since we had observed that the timing of Y-27632 exposure clearly affected HSE morphology, we also addressed the possible adverse effects of high concentrations of the ROCK inhibitor on epidermal morphology. At 30 and 100 μM of Y-27632, deep epidermal ridges were observed, and at 100 μM, epidermal protrusions were seen in the stratum corneum (Fig. 3a). These morphological changes were, however, not associated with significant alterations in epidermal differentiation gene expression (Fig. 3b) or protein expression (Supplementary Fig. S4); nor were they caused by the induction of a keratinocyte inflammatory response, as all HSEs lacked hBD-2 expression (Supplementary Fig. S4). The protein expression of involucrin, however, was slightly extended toward the stratum spinosum in HSEs cultured with 30 and 100 μM of Y-27632, which may be indicative of an activated epithelium.
FIG. 3.
Y-27632 concentration-dependant changes in epidermal morphology. Deep rete ridges are observed in HSEs generated in the presence of 30 or 100 μM Y-27632, and epidermal protrusions are observed at 100 μM (a). Scale bar, 100 μM. (b) Relative mRNA expression levels of various epidermal differentiation genes in HSEs generated with 10, 30, or 100 μM Y-27632. The relative amount of mRNA in Y-27632-stimulated HSEs is normalized to the mean of unstimulated HSEs. Error bars represent SD (n=2).
In vitro psoriasis skin models can be generated from Y-27632-treated keratinocytes
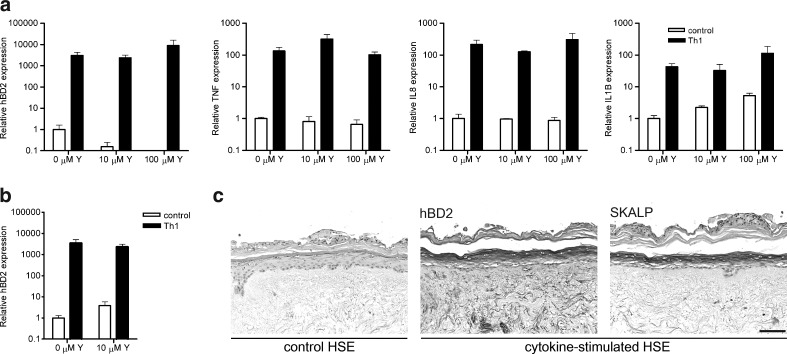
Since human primary keratinocytes are extensively used in vitro to study their function in the innate immune system, we cultured adult keratinocytes (passage 1) submerged with various concentrations of Y-27632 and used pro-inflammatory cytokines IL-1α, TNF-α, and IFN-γ to mimic an inflammatory milieu in vitro. As depicted in Fig. 4a, unstimulated (control) keratinocytes showed lower expression levels of hBD-2, while IL-1β mRNA expression levels were slightly elevated at 10 and 100 μM Y-27632. More importantly, keratinocyte activation was unaffected by Y-27632, as hBD-2, TNF-α, IL-8, and IL-1β mRNA expression levels were still significantly induced after cytokine stimuli. High-passage Y-27632-treated keratinocytes also showed a significant induction of hBD-2 when cultured with 10 μM Y-27632 (Fig. 4b). To verify this in our 3D skin model, we generated HSEs from Y-27632-treated keratinocytes and added 10 μM Y-27632 during the submerged phase of HSE development. Thereafter, HSEs were stimulated with psoriasis-associated cytokines, IL-6, IL-1α, and TNF-α during the last 3 days of air-exposed culture. The protein expression of the psoriatic markers hBD-2 and SKALP/elafin was strongly induced in cytokine-stimulated HSEs (Fig. 4c), indicating that psoriatic skin equivalents can be generated from Y-27632-treated keratinocytes.
FIG. 4.
Normal keratinocyte response to inflammatory stimuli after exposure to Y-27632. Primary adult keratinocytes (a) and passage 6 Y-27632-treated keratinocytes (b) were cultured, submerged with 10 or 100 μM Y27632, and stimulated with pro-inflammatory (Th1) cytokines IL-1α (10 ng/mL), TNF-α (50 ng/mL), and IFN-γ (500 U/mL). Relative mRNA expression levels of hBD-2, TNF-α, IL-8, and IL1-β were compared with unstimulated (control) keratinocytes. Significant induction (p<0.01) of all analyzed genes was observed after cytokine stimulation. The already low basal hBD-2 levels further decreased by 10 (p<0.05) and 100 μM (p<0.01) of Y-27632, while basal IL-1β levels significantly increased (p<0.05) at 10 and 100 μM of Y-27632. Error bars represent SD (n=3–5). (c) Immunohistochemical staining of psoriatic markers, hBD-2 and SKALP/elafin shows the strong induction of both markers in HSEs generated from Y-27632-treated keratinocytes with 10 μM Y27632 supplemented during the submerged phase of HSE development, on stimulation with psoriasis-associated cytokines IL-6 (5 ng/mL), TNF-α (5 ng/mL), and IL-1α (10 ng/mL). Scale bar, 100 μM. IL, interleukin; TNF, tumor necrosis factor, IFN, interferon.
In the experiments just described, we used keratinocytes from healthy donors to generate a psoriasis-like phenotype in vitro. We also successively used Y-27632 to prolong the life span of the keratinocytes derived from patients with skin diseases such as psoriasis or atopic dermatitis (data not shown), which could be subsequently used for HSE development.
Y-27632 facilitates gene delivery studies in human adult keratinocytes
The application of gene knockdown or overexpression techniques in HSEs is an elegant way to functionally analyze individual genes in an organ-like environment. We have observed that the proliferative capacity of high-passage adult keratinocytes after lentiviral transduction is insufficient to generate HSEs thereafter (data not shown). Therefore, we assessed whether an adult keratinocyte culture and subsequent HSE development can benefit from Y-27632 treatment in gene delivery assays. We used the eGFP lentivirus as a model system for gene transduction and generated HSEs of eGFP-transduced keratinocytes, which were cultured in the presence or absence of Y-27632, and compared gene transduction efficiency and HSE morphology. The eGFP-transduced HSEs generated in the absence of Y-27632 showed a poorly formed epidermis consisting of only a few cell layers, with no stratum granulosum and parakeratosis present in the stratum corneum. The HSEs generated from the Y-27632-treated keratinocytes with Y-27632 present during the submerged phase of the HSE development generated significantly improved skin equivalents with a multilayered epidermis, the presence of stratum granulosum, and no parakeratosis in the stratum corneum (Fig. 5a). The expression of epidermal differentiation markers in the Y-27632 treated, eGFP-transduced HSEs appeared similar to the native skin, as shown by the normal expression pattern of major epidermal differentiation proteins: keratin 10, involucrin, loricrin, and LCE2 (Fig. 5b). Importantly, the Y-27632-treated keratinocytes showed a significantly higher percentage of eGFP-transduced cells after recovery (Fig. 5c), and this was also prominent in the Y-27632-treated HSEs with the majority of keratinocytes expressing eGFP (Fig. 5d).
FIG. 5.
Lentiviral transduction of human adult keratinocytes. (a) HSEs generated from transduced keratinocytes that express eGFP driven by a PGK promoter cultured in the presence of Y-27632 show a superior epidermal morphology to transduced HSEs generated in the absence of Y-27632 (control). (b) Epidermal differentiation markers keratin 10, LCE2, involucrin, and loricrin are usually expressed in Y-27632-treated, eGFP-transduced HSEs. Flow cytometric analysis shows a significantly higher percentage of eGFP-positive cells in the Y-27632-treated keratinocytes (c). Error bars represent SD (n=3). *p<0.05. HSEs generated from the Y-27632-treated keratinocytes show eGFP expression in the majority of keratinocytes, while in the untreated lentiviral-transduced HSEs, only a few eGFP positive cells are detected (d). Scale bar, 100 μM. eGFP, enhanced green fluorescent protein; LCE2, late cornified envelope 2; PGK, human phosphoglycerate kinase promoter.
Discussion
HSE development has been extensively optimized over the last decade to generate HSEs highly comparable to the native skin. Nowadays, HSEs can be generated using a variety of dermal matrices such as animal-derived collagen matrices populated or not with human fibroblasts,18 human de-epidermised dermis,12 or human fibroblast-derived dermal equivalents.19 Large-scale HSE development is, therefore, only restricted by the limited availably of human adult keratinocytes. Here, we demonstrate an adequate solution for this problem, as Y-27632 prolongs the life span of human adult keratinocytes and improves HSE quality.
Increased cloning efficiency by Y-27632 has been described for several freshly isolated epithelial cells,20 including, human neonatal foreskin keratinocytes,3,4 human and murine prostate epithelial cells,3,21 human vaginal and cervical epithelial cells,4 and also for human embryonic stem cells.22 Here, we describe increased cloning efficiency and the prolonged life span of human adult keratinocytes, which have been stored in liquid nitrogen before Y-27632 treatment. Our findings may, therefore, have widespread applications for other institutes to expand their established cell banks.
The immortalization of human foreskin and cervical keratinocytes due to increased telomerase expression and stabilized telomere lengths was described by Chapman et al.4 We, however, were not able to detect such phenomena in our adult keratinocytes. This is probably due to the very high passage number (from passage 34 onward) in which they reported these characteristics, while we were only able to culture our human adult keratinocytes till passage 15. The differences in life span between the Y-27632-treated keratinocytes used in our study and those used by Chapman et al.4 may result from differences in the cell source (neonatal foreskin vs. adult keratinocytes). Moreover, liquid-nitrogen-stored keratinocytes might have a shorter life span than that of freshly isolated keratinocytes, although during the first passages, we observed no differences in proliferation rate between freshly isolated and liquid-nitrogen-stored keratinocytes.
It has been reported that high-passage Y-27632-treated epithelial cells display a normal cell cycle progression, karyotype, and DNA damage response.4,20 In addition, the Y-27632-treated epithelial cells are not capable of inducing tumors after a subcutaneous injection in mice.20 These data suggest that the Y-27632 treatment of epithelial cells does not result in permanent DNA damage or the induction of tumor-like characteristics. Our study shows that, at least for the biomarkers used here, the Y-27632-treated adult keratinocytes do not behave differently from control cells.
Chapman et al.4 generated organotypic cultures from Y-27632-treated foreskin keratinocytes without the addition of Y-27632 during the entire culture period. The morphology of these organotypic cultures, however, did not completely resemble the native epidermis, as a recognizable stratum granulosum was absent, and involucrin expression extended into the stratum spinosum. The latter is abnormal and only observed in the activated epidermis (e.g., psoriasis or skin injury). In addition, from our previous studies, we know that foreskin-derived HSEs show an activated phenotype as witnessed by parakeratosis and expression of the psoriasis marker SKALP/elafin.12 Here, we demonstrate that the HSEs generated from high-passage, Y-27632-treated, adult keratinocytes faithfully mimic the native epidermis, in both epidermal morphology and protein expression. We performed an elaborate protein expression analysis, and all investigated major epidermal differentiation proteins showed similar expression patterns to that of native skin. We can, however, not exclude the fact that other genes or biological processes may be affected by Y-237632 treatment. The beneficial effect of Y-27632 on HSE morphology when used during the first 3 days (submerged phase) of HSE culture might be due to increased keratinocyte survival and/or proliferation by Y-27632, resulting in a well-formed multilayered epidermis later on. Even when Y-27632 was added during the terminal differentiation phase, we were able to generate HSEs with a multilayered epidermis and a stratum granulosum, which corresponds to the finding that RhoA is dispensable for epidermal morphogenesis.8 The previously reported effects of ROCK I and II depletion on keratinocyte differentiation were obtained from submerged keratinocyte cultures6 and/or HaCaT cell lines.10 Such models are, however, considered less biologically relevant than in vitro generated HSEs. In addition, the normal terminal differentiation observed in our Y-27632-treated HSEs may be indicative of compensatory mechanisms only present in an organ-like environment to overcome ROCK depletion as a necessity in epidermal morphogenesis. The observed changes in HSE epidermal morphology when using Y-27632 throughout the HSE culture period or at high concentrations of Y-27632 might be due to the roles of the Rho/ROCK pathway in actin-cytoskeletal architecture5 or cell migration.7,8 In addition, Y-27632 has also been described to inhibit other kinases such as citron kinase, protein kinase C, and myosin light chain kinase.23 Although the potency of Y-27632 for ROCK is >100 times higher than for these kinases, the additional effects on other kinases when using Y-27632 at high concentrations cannot be completely excluded.
Primary keratinocytes are widely used to model skin inflammation in vitro, as they play pivotal roles in inflammatory skin diseases as psoriasis and atopic dermatitis (reviewed by Guttman-Yassky et al.24). We have previously described the induction of a psoriatic12,25 and atopic dermatitis26 phenotype in submerged keratinocyte cultures and HSE models. For these models, large quantities of human adult keratinocytes are required, and here, we demonstrated that the Y-27632-treated keratinocytes can be used for such purposes, as keratinocyte activation remains unaffected by Y-27632.
In vitro skin irritation and corrosion testing of new chemical compounds or drugs using HSEs is now recognized as a validated method that substitutes experimental animal use.27,28 For this purpose, also, large quantities of human primary keratinocytes are needed. Companies developing HSEs on a large scale for commercial use may, therefore, benefit from the effects of Y-27632 on adult keratinocytes reported here.
Over the last decade, small interfering RNA and transgenic techniques have made it possible to investigate gene function and signaling pathways in vitro. Only a few studies have described in vitro gene therapy in HSEs using various methods for gene delivery.29–31 Low transduction efficiency and poor epidermal morphology of transduced HSEs appears to be a common hurdle for which Y-27632 might provide a solution. Here, we have demonstrated increased lentiviral transduction efficiency in Y-27632-treated keratinocytes and, importantly, the epidermal morphology of transduced HSEs was markedly improved by Y-27632. Since Y-27632 has been described to suppress dissociation-induced apoptosis in murine prostate cells21 and increase the thaw-survival rates of stem cells after cryopreservation,22 keratinocytes might overcome transduction-induced stress when cultured with Y-27632, and Y-27632 might prevent cell death after the dissociation of transduced keratinocytes to generate HSEs.
Conclusion
We have demonstrated that Y-27632 is a powerful tool that is employed for large-scale human adult keratinocyte culture and HSE development, as high-passage adult keratinocytes generated HSEs comparable to the native epidermis. Furthermore, Y-27632 enhances lentiviral transduction efficiency and improves the epidermal morphology of transduced HSEs, thereby facilitating gene delivery studies in primary keratinocytes and providing biologically relevant applications.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Nijmegen Institute for Infection, Inflammation, and Immunity (N4i), Radboud University Nijmegen Medical Center, The Netherlands. J.S. and P.L.J.M.Z. are supported by The Alternatives to Animal Experiments Program of the ZonMW grant (number 114000084) and P.L.J.M.Z. by a Horizon Breakthrough grant from the Netherlands Genomics Initiative (number 93519004).
Disclosure Statement
No competing financial interests exist.
References
- 1.Gibbs S. van den Hoogenband H.M. Kirtschig G., et al. Autologous full-thickness skin substitute for healing chronic wounds. Br J Dermatol. 2006;155:267. doi: 10.1111/j.1365-2133.2006.07266.x. [DOI] [PubMed] [Google Scholar]
- 2.Bilousova G. Chen J. Roop D.R. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J Invest Dermatol. 2011;131:857. doi: 10.1038/jid.2010.364. [DOI] [PubMed] [Google Scholar]
- 3.Terunuma A. Limgala R.P. Park C.J. Choudhary I. Vogel J.C. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A. 2010;16:1363. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman S. Liu X. Meyers C. Schlegel R. McBride A.A. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaezi A. Bauer C. Vasioukhin V. Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 6.Tu C.L. Chang W. Bikle D.D. The calcium-sensing receptor-dependent regulation of cell–cell adhesion and keratinocyte differentiation requires Rho and filamin A. J Invest Dermatol. 2011;131:1119. doi: 10.1038/jid.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar S. Egelhoff T. Baskaran H. Insights into the roles of non-muscle myosin IIA in human keratinocyte migration. Cell Mol Bioeng. 2009;2:486. doi: 10.1007/s12195-009-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson B. Peyrollier K. Pedersen E., et al. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell. 2011;22:593. doi: 10.1091/mbc.E09-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebig T. Erasmus J. Kalaji R., et al. RhoE is required for keratinocyte differentiation and stratification. Mol Biol Cell. 2009;20:452. doi: 10.1091/mbc.E07-11-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMullan R. Lax S. Robertson V.H., et al. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003;13:2185. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 11.Lock F.E. Hotchin N.A. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS One. 2009;4:e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjabringa G. Bergers M. van Rens D. de Boer R. Lamme E. Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173:815. doi: 10.2353/ajpath.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts J. Joosten L.A. Takahashi N., et al. Computational design and application of endogenous promoters for transcriptionally targeted gene therapy for rheumatoid arthritis. Mol Ther. 2009;17:1877. doi: 10.1038/mt.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jongh G.J. Zeeuwen P.L. Kucharekova M., et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005;125:1163. doi: 10.1111/j.0022-202X.2005.23935.x. [DOI] [PubMed] [Google Scholar]
- 15.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Bergboer J.G. Tjabringa G.S. Kamsteeg M., et al. Psoriasis risk genes of the late cornified envelope-3 group are distinctly expressed compared with genes of other LCE groups. Am J Pathol. 2011;178:1470. doi: 10.1016/j.ajpath.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingens M. van Bergen B.H. Hiemstra P.S., et al. Induction of SLPI (ALP/HUSI-I) in epidermal keratinocytes. J Invest Dermatol. 1998;111:996. doi: 10.1046/j.1523-1747.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Ghalbzouri A. Gibbs S. Lamme E. Van Blitterswijk C.A. Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 2002;147:230. doi: 10.1046/j.1365-2133.2002.04871.x. [DOI] [PubMed] [Google Scholar]
- 19.El-Ghalbzouri A. Commandeur S. Rietveld M.H. Mulder A.A. Willemze R. Replacement of animal-derived collagen matrix by human fibroblast-derived dermal matrix for human skin equivalent products. Biomaterials. 2009;30:71–78. doi: 10.1016/j.biomaterials.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Liu X. Ory V. Chapman S., et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L. Valdez J.M. Zhang B. Wei L. Chang J. Xin L. ROCK inhibitor Y-27632 suppresses dissociation-induced apoptosis of murine prostate stem/progenitor cells and increases their cloning efficiency. PLoS One. 2011;6:e18271. doi: 10.1371/journal.pone.0018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthaman K. Fong C.Y. Bongso A. Effect of ROCK inhibitor Y-27632 on normal and variant human embryonic stem cells (hESCs) in vitro: its benefits in hESC expansion. Stem Cell Rev. 2010;6:86. doi: 10.1007/s12015-009-9107-8. [DOI] [PubMed] [Google Scholar]
- 23.Ishizaki T. Uehata M. Tamechika I., et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976. [PubMed] [Google Scholar]
- 24.Guttman-Yassky E. Nograles K.E. Krueger J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 25.van Ruissen F. de Jongh G.J. Zeeuwen P.L. van Erp P.E. Madsen P. Schalkwijk J. Induction of normal and psoriatic phenotypes in submerged keratinocyte cultures. J Cell Physiol. 1996;168:442. doi: 10.1002/(SICI)1097-4652(199608)168:2<442::AID-JCP23>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Kamsteeg M. Bergers M. de BR., et al. Type 2 helper T-cell cytokines induce morphologic and molecular characteristics of atopic dermatitis in human skin equivalent. Am J Pathol. 2011;178:2091. doi: 10.1016/j.ajpath.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.OECD. In vitro skin irritation: reconstructed human epidermis test method. OECD Guideline for Testing of Chemicals No. 439. www.oecdbookshop.org. Jul 22, 2004. www.oecdbookshop.org Adopted and published.
- 28.OECD. In vitro skin corrosion: human skin model test. OECD Guideline for Testing of Chemicals No. 431. Apr 13 22, 2004. www.oecdbookshop.org. Jul 13 22, 2010. www.oecdbookshop.org Adopted and published.
- 29.Hanakawa Y. Shirakata Y. Nagai H., et al. Cre-loxP adenovirus-mediated foreign gene expression in skin-equivalent keratinocytes. Br J Dermatol. 2005;152:1391. doi: 10.1111/j.1365-2133.2005.06637.x. [DOI] [PubMed] [Google Scholar]
- 30.Mildner M. Jin J. Eckhart L., et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130:2286. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- 31.Van Gele M. Geusens B. Speeckaert R., et al. Development of a 3D pigmented skin model to evaluate RNAi-induced depigmentation. Exp Dermatol. 2011;20:773–775. doi: 10.1111/j.1600-0625.2011.01319.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.