Abstract
Mesenchymal stem cells have been given particular attention in tissue regeneration research due to their multipotency and proliferative activity. In this study, we investigated the possibility of epithelial differentiation of rabbit adipose-derived stem cells (rASCs) in an in vitro 3D culture system. The experimental procedure was performed with different contributing factors including all-trans retinoic acid (ATRA), epidermal growth factor (EGF), keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and hydrocortisone in air–liquid interface culture, for modulating proliferation and providing a synergistic effect on epithelial differentiation of rASCs. After induction, immunofluorescence staining, western blot analysis, flow cytometry analysis, and quantitative real-time polymerase chain reaction assay have been performed to detect the expression of epithelial-specific markers and mesenchymal marker alpha-smooth muscle actin (α-SMA). The growth pattern and viability of cells were evaluated by transmission electron microscopy and Hoechst 33258 assay, respectively. After treated with optimized induction medium (including 2.5 μM ATRA, 20 ng/mL EGF, 10 ng/mL KGF, 10 ng/mL HGF, and 0.5 μg/mL hydrocortisone), rASCs were observed to display a stratified epithelial-like morphology, with the expression of cytokeratin 19 and cytokeratin 13 in 63.69%±2.63% and 22.17%±1.51%, respectively, and the relative expression level of cytokeratin 19 increased to 3.152 compared with 0.151 before induction. The expression of α-SMA decreased to 19.40%±1.45% after induction, but almost no expression of involucrin was detected. The results showed that the establishment of an epithelial-specific microenvironment may be a feasible way for epithelial differentiation of ASCs in vitro, and provided an alternative for research on epithelium regeneration.
Introduction
As a basal component of the body, epithelial tissue plays a critical role in chemical secretion, permeability control, and waste excretion in addition to providing physical support. The deficiency of epithelial tissue caused by trauma, infection, or tumor in multiple organs such as respiratory system and urogenital system provides difficulties for clinical treatment. In the current research of urethral reconstruction, the limited amount of urothelium tissue has been considered as a main cause of the high failure rate of reconstruction. But the cellular senescence in epithelium of early passage in vitro culture is inevitable, which results in a lack of sufficient cells to meet clinical demands. In the past few years, a large number of studies have focused on epithelium regeneration with tissue engineering and stem cell technology.1–3
Mesenchymal stem cells have been extensively applied as a possible alternative for tissue repair or cell differentiation research. Among which, bone marrow-derived stem cells (BMSCs) are multipotent and have been shown to be capable of in vitro differentiation to functional epithelial-like cells under the stimulation of multiple growth factors,4 coculture with cells of epithelial lineage,5,6 or a 3D biomimetic culture environment.7
Compared with BMSCs, adipose-derived stem cells (ASCs) have the advantage of being harvested in abundant quantity and causing less trauma to the donor site. So the research question arises as to whether ASCs could differentiate into epithelial lineage, for solving the problem of limited amount of urothelium tissue for reconstruction research. In the study, we investigated whether the morphological changes toward epithelium and epithelial phenotypes expression of ASCs could be observed under appropriate in vitro conditions, and also various doses of contributing factors were tried to figure out whether the induction effect was dose dependent.
Being derived from the mesenchyme-like BMSCs, ASCs are generally thought to have the characteristics of self-renewal and multipotency also. Recent works have shown under appropriate conditions, the effects of all-trans retinoic acid (ATRA)8 or epidermal growth factor (EGF)9 on modulating proliferation and differentiation may contribute to the expression of initial epithelial phenotypes in ASCs. Besides, keratinocyte growth factor (KGF)10 and hepatocyte growth factor (HGF)11 are known to be involved in epithelial differentiation and proliferation, and further, HGF may also stimulate motility and morphogenic changes in different epithelial cell types.12,13 Inspired by the above findings, we attempted to induce epithelial differentiation of rASCs with the synergistic effect of ATRA, EGF, KGF, and HGF in an air–liquid interface (ALI) culture system, for the preliminary trial of ASCs as a substitute for urothelium in urethral tissue engineering.
In the current study, the intent is to investigate the feasibility and effectiveness of using multiple contributing factors in ALI culture system to induce rASCs into epithelial lineage. The induction was performed in the presence of basal medium (BM) alone or in combination with multiple agents including ATRA, growth factors, and hydrocortisone in ALI culture, after which proteinic and genetic analysis of the epithelial phenotypes (cytokeratin 19, an early epithelial marker; cytokeratin 13, an epithelial marker mainly expressed in mucosal epithelium; and involucrin, a terminal epithelial marker) and alpha-smooth muscle actin (α-SMA), and detections of the growth pattern and viability of cells have been performed for a full-scale assessment. The results demonstrated that under the epithelial-specific microenvironment, rASCs were observed to display a stratified epithelial-like morphology, and they acquire epithelial phenotypes by the expression of epithelial-specific proteins.
Materials and Methods
Isolation and culture of rabbit ASCs in vitro
The adipose tissues were obtained from the dorsocervical subcutaneous region of New Zealand rabbits. All the experimental protocols were approved by the Animal Care and Use Committee in our institution. The isolation and culture of rASCs were performed as previously described.14,15 Briefly, after rinsing in 0.25% chloromycetin and phosphate-buffered saline (PBS) three times each, the fresh adipose tissues were cut into small pieces, then treated with 0.10% collagenase I (Worthington Biochemical Corp.) under shaking at 37°C for 60 min. After digestion, the collagenase I was neutralized with low-glucose Dulbecco's modified Eagle's medium (LG-DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), and the suspension was filtered through a 200-μm nylon mesh to remove the undigested tissue and then centrifuged at 1200 g for 10 min. The pellet was resuspended in LG-DMEM supplemented with 10% FBS. The cells were cultivated at a density of 4×104 cells/cm2, and the media were changed every 3 days. Nonadherent cells were removed at the first medium change. After culturing for 7–9 days, the cell colonies with a characteristic spindle shape reached 70%–80% confluence and were then passaged with trypsin-EDTA. rASCs of passage 3 were used for the study.
rASCs of passage 3 were used for surface immunophenotype characterization via flow cytometry analysis. CD marker profile including CD13 (Abcam), CD29 (Chemicon, Temecula, CA), CD31 (Abcam), CD44 (Serotec, Oxford, UK), CD45 (Serotec), CD49d (Serotec), CD90 (Abcam), and CD105 (Abcam) was examined for the characterization of isolated cells. The results showed expression of CD13 (95.90%), CD90 (80.11%), CD44 (87.34%), CD105 (36.14%), CD49d (20.71%), and CD29 (79.35%), which are considered as the markers of mesenchymal stem cells. Moreover, no expression of the hematopoietic lineage markers CD31 (3.11%) and CD45 (0.90%) were observed in the isolated cells.
Epithelial differentiation of rASCs
To evaluate epithelial differentiation with different conditions, rASCs (passage 3) were cultured in the following four conditions, and the isolated rabbit urothelial cells (rUCs, passage 3) were cultured as a positive control: (1) rASCs group: rASCs, LG-DMEM supplemented with 10% FBS, under 2D monolayer culture condition; (2) BM group: rASCs, LG-DMEM supplemented with 2% FBS (BM), under ALI culture condition (described in detail below); (3) RHE-treated group: rASCs, LG-DMEM supplemented with 2% FBS, 2.5 μM ATRA (Sigma-Aldrich), 20 ng/mL EGF (Peprotech, Inc.), and 0.5 μg/mL hydrocortisone (Sigma-Aldrich), under ALI culture condition; (4) RHEHK-treated group: rASCs, LG-DMEM supplemented with 2% FBS, 2.5 μM ATRA, 20 ng/mL EGF, 10 ng/mL HGF (Peprotech, Inc.), 10 ng/mL KGF (Peprotech, Inc.), and 0.5 μg/mL hydrocortisone, under ALI culture condition; and (5) rUCs group: rUCs, keratocyte serum-free medium (KSFM), under ALI culture condition. The details of experimental groups with different culture conditions were listed in Table 1.
Table 1.
Experimental Groups with Different Culture Conditions
| Components of medium | Culture mode | |
|---|---|---|
| rASCs group | LG-DMEM supplemented with 10% FBS. | 2D monolayer culture condition |
| BM group | LG-DMEM supplemented with 2% FBS. | ALI culture condition |
| RHE-treated group | LG-DMEM supplemented with 2% FBS, 2.5 μM ATRA, 20 ng/mL EGF, and 0.5 μg/mL hydrocortisone. | ALI culture condition |
| RHEHK-treated group | LG-DMEM supplemented with 2% FBS, 2.5 μM ATRA, 20 ng/mL EGF, 10 ng/mL HGF, 10 ng/mL KGF, and 0.5 μg/mL hydrocortisone. | ALI culture condition |
| rUCs group (Positive control) | KSFM. | ALI culture condition |
rASCs, rabbit adipose-derived stem cells; ATRA, all-trans retinoic acid; EGF, epidermal growth factor; KGF, keratinocyte growth factor; HGF, hepatocyte growth factor; ALI, air–liquid interface; LG-DMEM, low-glucose Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; rUCs, rabbit urothelial cells; BM, basal medium; KSFM, keratocyte serum-free medium.
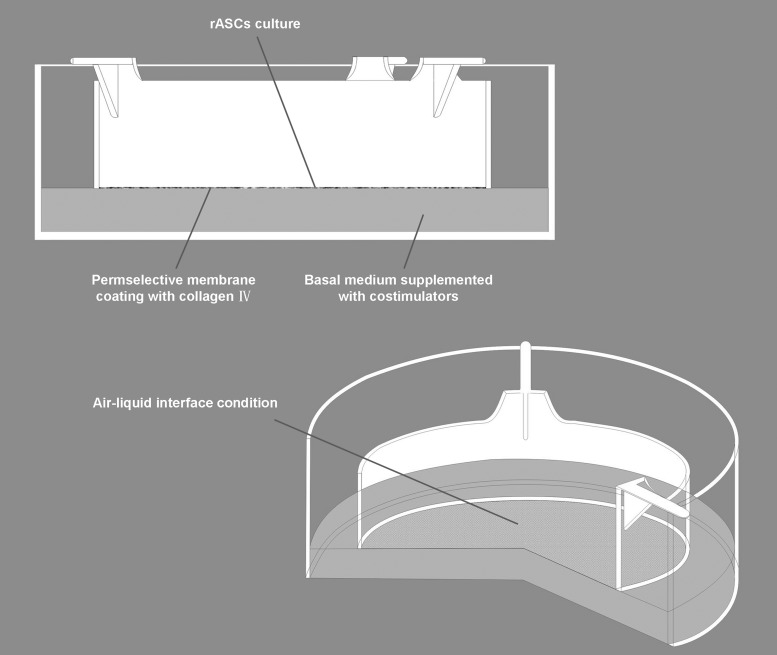
A 3D culture system was established to provide an epithelial-specific microenvironment for epithelial differentiation of rASCs in vivo. In the system, rASCs were seeded on the upper side of the membrane of a Millicell insert (1.0 μm pore size; Millipore Co.) coated with 0.10% collagen type IV (Sigma-Aldrich; Fig. 1). To create an ALI culture condition, the inducing medium in the basolateral compartment was raised to reach the level of the membrane, and then the cells were exposed to the air with 5% CO2 with 95% relative humidity while fed from the medium underneath.
FIG. 1.
Diagrammatic view of the 3D culture system. rASCs were seeded on the upper side of the membrane of a Millicell insert coated with 0.10% collagen type IV. To create an ALI culture system, the inducing medium in basolateral compartment was raised to reach the level of the membrane, then the cells were exposed to the air with 5% CO2 with 95% relative humidity while fed from the medium underneath. rASCs, rabbit adipose-derived stem cells; ALI, air–liquid interface.
A seeding density of 3×104 cells/cm2 was applied for the induction. The culture media were changed every 2 days. In the 3D culture environment, the cells were cultured submerged for 2 days in the BM after seeding, then cultured at ALI with inducing medium (Fig. 1; rUCs were cultured with KSFM consistently). The cells have not been passaged during the induction phase, for the purpose of imitating the epithelial-specific microenvironment in vivo and avoiding destruction of the layered structure of cells. After 12 days from the initial inducing, characterization of cells was performed. And during the prophase study, various doses of contributing factors including ATRA, EGF, HGF, and KGF have been tried to investigate whether the induction effect was dose dependent and confirm the ultimate doses for induction.
Immunofluorescence staining and analysis of transmission electron microscopy
Immunofluorescence was performed on the cells (fixed with 4% paraformaldehyde for 15 min) with the following primary monoclonal antibodies: anticytokeratin 19 (1:150, ab77983; Abcam), anticytokeratin 13 (1:100, sc-57003; Santa Cruz), anti-involucrin (1:100, sc-56555; Santa Cruz), and anti-α-SMA (1:100, ab7817; Abcam). After permeabilization with 0.5% Triton X-100 for 20 min at room temperature and incubation with the primary antibody for 60 min at 37°C, the specimens were washed with PBS thrice and incubated with secondary antibody (Alexa Fluor 488 goat anti-mouse IgG, 1:500; Invitrogen) for 30 min at 37°C. Cell nucleus were stained with Hoechst 33258. The specimens were examined with a fluorescence microscope (Nikon 80i; Nikon). In the assay, the negative and blank control were used to eliminate potential cross-reactivity with the rabbit proteins (with antiepithelial specific antigen including anticytokeratin 19, anticytokeratin 13, and anti-involucrin; undifferentiated rASCs were treated in the same manner as negative control, the primary antibodies were replaced by PBS as blank control; with anti-α-SMA, rUCs were treated in the same manner as negative control, the primary antibody were replaced by PBS as blank control).
The immunofluorescence staining was performed on cells attached to the membrane of Millicell inserts. The membrane was cut into 1.0×1.0 cm2 each after fixation and permeabilization, then for incubation with the primary antibody and secondary antibody in the 24-well plates. Before being viewed with the microscope, the samples were put onto glass slides, covered with coverslips.
Transmission electron microscopy examination (CM 120; Philips) was performed with membrane specimens with seeded cells (except group 1, in which cells were cultured in a standard six-well plate). The samples were fixed with 2% glutaraldehyde, postfixed with 1% osmium tetraoxide, stained with 0.5% uranyl acetate, and dehydrated with acetone. After being embedded in resin, the membranes were cut in cross sections to observe whether a multilayered structure of ASCs formed in the 3D culture system.
Flow cytometry analysis
For flow cytometry analysis, the cells were detached with trypsin-EDTA (0.25% trypsin and 0.02% EDTA) from the membrane of Millicell insert, then fixed in 2% paraformaldehyde for 30 min, and incubated with 0.5% Triton X-100 for 20 min at room temperature for permeabilization. After washing with PBS, the cells were incubated with the following primary antibodies: anticytokeratin 19 (Abcam), anticytokeratin 13 (Santa Cruz), anti-involucrin (Santa Cruz), and anti-α-SMA (Abcam) at 4°C overnight, then further washed and incubated with secondary antibody (Alexa Fluor 488 goat anti-mouse IgG; Invitrogen) for 30 min at room temperature. After three washes, analysis was performed on a FACSCalibur (BD Biosciences). An isotype-matching (IgG1) control was used in the experiment. In addition, CD marker profile was examined for the characterization of rASCs as mentioned above.
Quantitative real-time polymerase chain reaction assay
Quantitative real-time polymerase chain reaction (PCR) (TaqMan) was performed for the quantification of cytokeratin 19. Total RNA from the cell samples was extracted using TRIzol reagent (Invitrogen), then reverse transcribed into complementary DNA using PrimeScript RT reagent kit (TaKaRa Biotechnology). Amplification was carried out in a GeneAmp PCR System 9600 thermal cycler (Applied Biosystems) for 35 cycles of 95°C for 30 s, 65°C for 30 s, and 72°C for 30 s. The PCR products were then analyzed by agarose gel electrophoresis; isolated PCR fragments were ligated into pMD19T (TaKaRa Biotechnology) and transformed into TOP10 competent Escherichia coli cells. Positive clones were selected for the isolation of recombinant plasmids. The cloned targets were then purified and used for standard curve dilutions for each PCR run consisting of three replicates (StepOne Real-Time PCR System; Applied Biosystems). PCR runs were accepted when the standard curve correlation coefficient was ≥0.99.
Copy number calculations were based on DNA extraction volume and final elution volumes and the number of replicates tested. Samples were extracted and eluted in equal quantities, so the amount of samples used for PCR (5 μL) was multiplied and divided by the number of replicates to obtain a final measurement expressed as DNA copies per milliliter (copies/mL).
TaqMan probes were used for the quantification of the target gene. 18S ribosomal RNA (18S rRNA) was used as an endogenous quality control. The primers and TaqMan probe sequences of cytokeratin 19 and 18S rRNA were as follows: cytokeratin 19 (Forward) 5′TCAACGACCGCCTGGCCTCC3′; (Reverse) 5′GGTCAGCTCATCCAGCACCCTGC3′ (amplicon size: 323 bp); (Probe) 5′FAM-ACCTGGACAAGGTGCGYGCYCTGGA3′-TAMRO; 18S rRNA (Forward) 5′AGTCGCCGTGCCTACCAT3′; (Reverse) 5′CGGGTCGGGAGTGGGTAAT3′ (amplicon size: 129 bp); (Probe) 5′FAM-CGCCTGCTGCCTTCCTTGGATGTG3′-TAMRO (Shanghai Sango).
Western blot analysis
Western blot analysis was performed to measure the relative expression level of cytokeratin 19 and cytokeratin 13 in rASCs treated with various doses of contributing factors during the prophase study, and the relative expression level of cytokeratin 19, cytokeratin 13, involucrin, and α-SMA in each group after induction. Lysates of the cell samples were prepared by extracting proteins with the lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1.0 mM phenylmethanesulfonyl fluoride). Proteins were size fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). After blocking with Tris-buffered saline with 0.1% Tween-20 (TBST) containing 5% nonfat dry milk and 2% bovine serum albumin for 1 h at room temperature, the membranes were incubated with primary antibodies at 4°C overnight and subsequently with IRDye 800-conjugated goat anti-mouse secondary antibody (Rockland) for 1 h at room temperature. Proteins were visualized by the Odyssey Infrared Imaging System (LI-COR Biosciences). Anti-GAPDH antibody was used as a protein loading control. The results were quantified using Gel-Pro Analyzer (Version 4.5) software and expressed as the ratio of cytokeratin 19, cytokeratin 13, involucrin, or α-SMA to GAPDH.
Cell proliferation assay
For cell proliferation assay, rASCs were plated at a density of 3×103 cells/cm2 in six-well plates. Cells at indicated time points were crushed for full lysis with proteinase K (Sigma-Aldrich) at 56°C overnight. The resulting mixture was subjected to centrifugation and aliquots of the supernatants after mixing with 160 μL Hoechst 33258 dye solution (0.1 μg/mL; Sigma-Aldrich) were transferred to black flat-bottomed 96-well plates (Corning Costar). DNA content was quantified spectrofluorometrically using a Varioskan multimode detection reader (Thermo Electron) at a wavelength of 465 nm (the emission wavelength of 360 nm) by correlating with a DNA standard curve that was generated by lysing serial dilutions of a known concentration of rASCs.
Statistical analysis
Data were presented as mean±standard deviation from 12 animals, and in each group the experiments were performed in triplicate. Statistical analysis was performed by one-way ANOVA, and independent-samples T tests assuming equal variance were performed for other assays using SPSS 11.0 software (SPSS, Inc.). A p-value of less than 0.05 was considered statistically significant.
Results
Effect of various doses of contributing factors on epithelial differentiation of rASCs
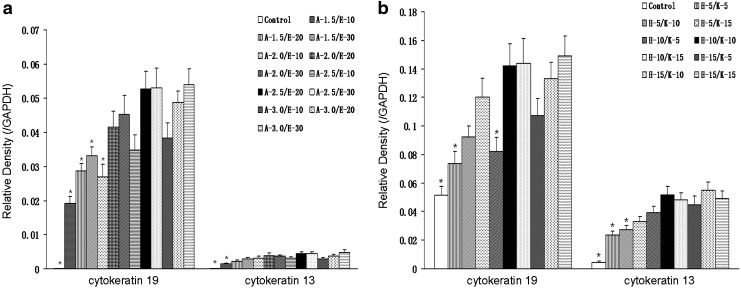
The expression of cytokeratin 19 and cytokeratin 13 in rASCs treated with various doses of contributing factors including ATRA, EGF, HGF, and KGF in ALI culture was detected by western blot analysis, and normalized to GAPDH. As shown in Figure 2a, the synergistic effect of various doses of ATRA (1.5, 2.0, 2.5, and 3.0 μM, respectively) plus EGF (10, 20, and 30 ng/mL, respectively) was evaluated. Keeping a constant dose of EGF, ATRA at a dose of 2.5 μM had a higher expression of cytokeratin 19 compared with that at 1.5 and 2.0 μM, respectively, but no significant enhancement of cytokeratin 19 expression was detected with further increase to 3.0 μM. Then combined with 2.5 μM ATRA, cytokeratin 19 expression was impressively enhanced when the dose of EGF was increased to 20 ng/mL, and the effect was only slightly enhanced with further increase in EGF to 30 ng/mL. The expression of cytokeratin 13 was weak in rASCs treated with ATRA plus EGF, but without HGF and KGF. A similar tendency of cytokeratin 13 expression was observed with various doses of ATRA and EGF, compared with that of cytokeratin 19. It was shown that rASCs treated with 2.5 μM ATRA plus 20 ng/mL EGF had a relatively high cytokeratin 13 expression, and with further increase in doses of the agents, the effect had no significant change. Thus, the doses of ATRA and EGF were fixed to 2.5 μM and 20 ng/mL, respectively.
FIG. 2.
Effect of various doses of contributing factors (ATRA, EGF, HGF, and KGF) on epithelial differentiation of rASCs in ALI culture determined by western blot analysis. (a) Expression of epithelial-specific genes (the relative intensity of cytokeratin 19 and cytokeratin 13, expressed as the ratio of cytokeratin 19 or cytokeratin 13 to GAPDH) in rASCs treated with various doses of ATRA and EGF. Control refers to without ATRA and EGF. A-1.5: ATRA 1.5 μM; A-2.0: ATRA 2.0 μM; A-2.5: ATRA 2.5 μM; A-3.0: ATRA 3.0 μM; E-10: EGF 10 ng/mL; E-20: EGF 20 ng/mL; E-30: EGF 30 ng/mL. *p<0.05 compared with A-2.5/E-20. n=3. (b) Expression of epithelial-specific genes (the relative intensity of cytokeratin 19 and cytokeratin 13) in rASCs treated with 2.5 μM ATRA+20 ng/mL EGF+various doses of HGF and KGF. Control refers to with 2.5 μM ATRA+20 ng/mL EGF, but without HGF and KGF. H-5: HGF 5 ng/mL; H-10: HGF 10 ng/mL; H-15: HGF 15 ng/mL; K-5: KGF 5 ng/mL; K-10: KGF 10 ng/mL; K-15: KGF 15 ng/mL. *p<0.05 compared with H-10/K-10. n=3. ATRA, all-trans retinoic acid; EGF, epidermal growth factor; KGF, keratinocyte growth factor; HGF, hepatocyte growth factor.
Combined with 2.5 μM ATRA plus 20 ng/mL EGF, the synergistic effect of various doses of HGF (5, 10, and 15 ng/mL, respectively) plus KGF (5, 10, and 15 ng/mL, respectively) on rASCs was evaluated (Fig. 2b). Keeping a constant dose of KGF, HGF at a dose of 10 ng/mL had a higher expression of cytokeratin 19 and cytokeratin 13 compared with that at 5 ng/mL, and with further increase to 15 ng/mL, no significant enhancement of effect was detected. Then combined with 10 ng/mL HGF, KGF at a dose of 10 ng/mL had a significant enhancement of cytokeratin expression compared with that at 5 ng/mL. With further increase in KGF to 15 ng/mL, cytokeratin 19 expression was enhanced slightly, but cytokeratin 13 expression was decreased. Hence, we chose 2.5 μM ATRA plus 20 ng/mL EGF plus 10 ng/mL HGF plus 10 ng/mL KGF to induce epithelial differentiation of rASCs in the following study.
Morphological changes of rASCs differentiated to epithelial lineage
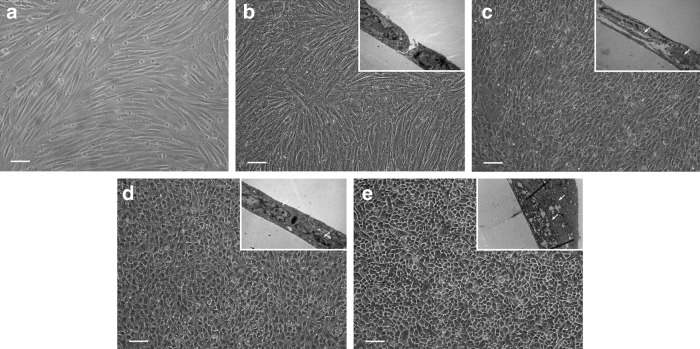
After culturing in different conditions for 7–10 days, rASCs treated either with RHE medium or RHEHK medium were observed to exhibit morphological changes toward a polygonal cell shape under phase contrast microscopy, in contrast, rASCs cultured in 2D monolayer culture or in ALI culture but without stimulators remained in an undifferentiated state with a spindle cell shape. On day 12, the morphology changes of rASCs were more significant, especially the cells cultured in RHEHK medium acquired an epithelial-like morphology (Fig. 3).
FIG. 3.
Morphological characterization of rASCs under different culture conditions assessed by phase contrast microscopy and transmission electron microscopy. Transmission electron microscopy examination shown in the inset of the images. rASCs treated with normal growth medium in 2D monolayer culture (a), with basal medium in ALI culture (b), with RHE medium in ALI culture (c), and with RHEHK medium in ALI culture (d), and rUCs of passage 3 in ALI culture as a positive control (e). After 12 days culture, a stratified epithelial-like morphology of rASCs was observed after treatment with inducing mediums (c, d), especially with the treatment of RHEHK medium (d). Scale bars: 100 μm. Arrows: tight junctions between the cells; rUCs, rabbit urothelial cells.
Transmission electron microscopy examination was performed on day 12. Cell proliferation in a stratified structure was detected in the RHE-treated group (Fig. 3c) and the RHEHK-treated group (Fig. 3d), which was similar to the epithelial morphology of rUCs (Fig. 3e). However, in the BM group rASCs maintained a monolayer growth profile, while stratified structure was observed occasionally (Fig. 3b).
Differentiation of rASCs toward epithelial phenotypes
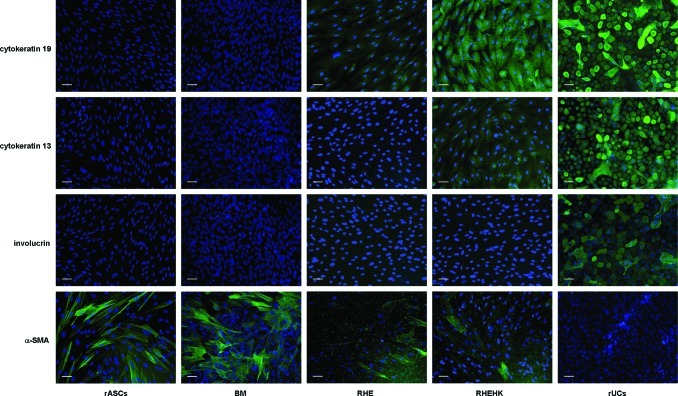
Immunofluorescence analysis was performed to assess the epithelial differentiation of rASCs after induction (compared with the negative and blank control, no significant cross-reactivity with the rabbit proteins was observed). As shown in Figure 4, when treated with RHE medium, weak expression of cytokeratin 19 (an early epithelial marker) could be detected in rASCs, but no expression of cytokeratin 13 (an epithelial marker mainly expressed in mucosal epithelium) or involucrin (a terminal epithelial marker) could be detected. Whereas, the expression of cytokeratin 19 was notably enhanced and weak cytokeratin 13 expression could be detected in the cells treated with RHEHK medium, still almost no expression of involucrin was detected. In addition, no expression of cytokeratin 19, cytokeratin 13, or involucrin could be observed in the undifferentiated rASCs cultured in 2D monolayer culture or with BM. Further, reduced expression of α-SMA was observed in rASCs treated with RHE medium and RHEHK medium, compared with the undifferentiated cells. As a positive control, expression of the epithelial markers mentioned above was examined in rUCs.
FIG. 4.
Immunofluorescence staining of rASCs cultured under different conditions for 12 days. rUCs were set as the positive control. Scale bars: 50 μm. Color images available online at www.liebertpub.com/tea
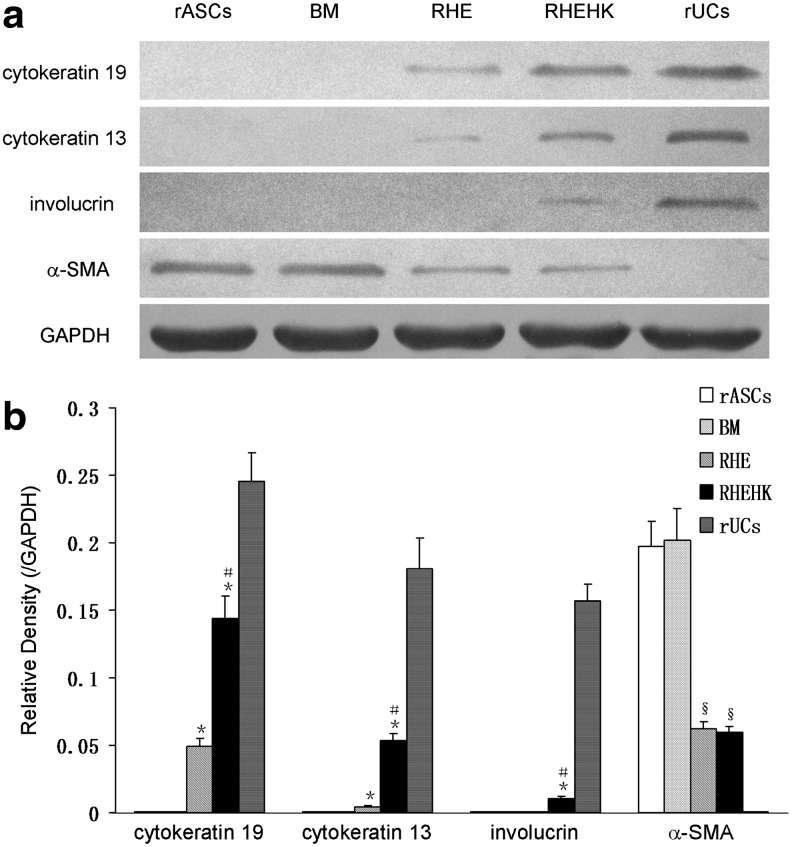
Western blotting was used for relative quantitative analysis of cytokeratin 19, cytokeratin 13, involucrin, and α-SMA (Fig. 5a, b). Consistent with the results of the immunofluorescence staining, weak expression of cytokeratin 19 could be observed in rASCs treated with RHE medium. And with RHEHK medium, the expression of the early epithelial marker was more significant enhancement. A similar increase in cytokeratin 13 expression was observed in the RHEHK-treated group compared with that in the RHE-treated group, while a baseline expression of involucrin was observed in the RHEHK-treated group. Further, quantitative real-time PCR was performed to ascertain the expression changes of cytokeratin 19 at the transcript level by normalizing the number of cytokeratin 19 DNA copies per milliliter to that of 18S rRNA in different groups. In comparison with the undifferentiated cells in the rASCs group (0.051), the relative expression levels of cytokeratin 19 in the RHE-treated group and RHEHK-treated group increased to 1.681 and 3.152, respectively (Fig. 6 and Table 2).
FIG. 5.
(a) Expression of epithelial-specific genes (cytokeratin 19, cytokeratin 13, and involucrin) and α-SMA in rASCs cultured under different conditions determined by western blot analysis. (b) Histograms show the relative intensity of cytokeratin 19, cytokeratin 13, involucrin, and α-SMA normalized to GAPDH (expressed as the ratio of cytokeratin 19, cytokeratin 13, involucrin, or α-SMA to GAPDH). It was revealed that the expression of epithelial-specific markers were enhanced in rASCs treated with RHE medium (cytokeratin 19 and cytokeratin 13) and RHEHK medium (cytokeratin 19, cytokeratin 13, and involucrin) compared with the undifferentiated cells in rASCs group, while the expression of α-SMA was decreased in both RHE-treated and RHEHK-treated group compared with that in the rASCs group. Further, more significant enhancement in cytokeratin 19 and cytokeratin 13 expression in the RHEHK-treated group was observed compared with that in the RHE-treated group. *p<0.01 compared with rASCs group; §p<0.05 compared with rASCs group; #p<0.05 compared with RHE-treated group. n=3. α-SMA, alpha-smooth muscle actin.
FIG. 6.
Detection of cytokeratin 19 in rASCs after culturing under different conditions by quantitative real-time polymerase chain reaction assay. Color images available online at www.liebertpub.com/tea
Table 2.
Relative Expression Levels of Cytokeratin 19 Measured by Real-Time Polymerase Chain Reaction (Taqman) Assay
| rASCs | BM | RHE | RHEHK | rUCs | |
|---|---|---|---|---|---|
| Cytokeratin 19 (mean, copies/mL) | 2.331E+04 | 4.092E+04 | 6.017E+05 | 1.242E+06 | 3.087E+06 |
| 18S rRNA (mean, copies/mL) | 4.58E+05 | 4.06E+05 | 3.58E+05 | 3.94E+05 | 3.87E+05 |
| Cytokeratin 19 (copies/mL)/18S rRNA (copies/mL) | 0.051 | 0.101 | 1.681a | 3.152a,b | 7.977 |
p<0.05 compared with rASCs group.
p<0.05 compared with RHE-treated group (n=3).
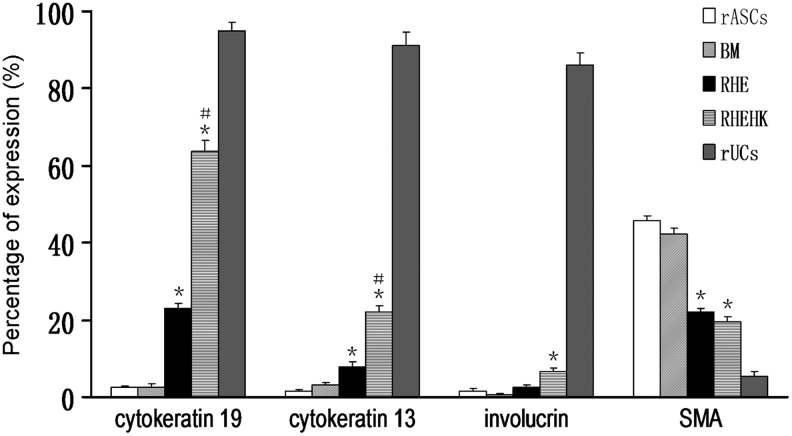
Flow cytometry analysis was carried out to analyze the proportion of cells expressing cytokeratin 19, cytokeratin 13, involucrin, and α-SMA. As shown in Table 3 and Figure 7, percentage of cells expressing cytokeratin 19 and cytokeratin 13 in the RHEHK-treated group reached 63.69%±2.63% and 22.17%±1.51%, compared with the undifferentiated cells in the rASCs group (cytokeratin 19: 2.37%±0.37%; cytokeratin 13: 1.46%±0.39%), whereas the involucrin expression remained with no remarkable enhancement after induction (rASCs group: 1.72%±0.51%; RHEHK-treated group: 6.77%±0.72%).
Table 3.
Flow Cytometry Analysis of Treated Cells
| |
Percentage of expression, % |
||||
|---|---|---|---|---|---|
| rASCs | BM | RHE | RHEHK | rUCs | |
| cytokeratin 19 | 2.37±0.37 | 2.64±0.74 | 23.08±1.31 | 63.69±2.63 | 94.71±2.27 |
| cytokeratin 13 | 1.46±0.39 | 3.28±0.44 | 7.93±1.22 | 22.17±1.51 | 91.10±3.42 |
| involucrin | 1.72±0.51 | 0.62±0.32 | 2.52±0.67 | 6.77±0.72 | 86.14±2.91 |
| α-SMA | 45.72±1.28 | 42.17±1.74 | 22.04±0.83 | 19.40±1.45 | 5.29±1.39 |
α-SMA, alpha-smooth muscle actin.
FIG. 7.
Percentage of cells expressing cytokeratin 19, cytokeratin 13, involucrin, and α-SMA after culturing under different conditions for 12 days determined by flow cytometry analysis. The increase in cytokeratin 19-positive and cytokeratin 13-positive cells, and decrease in α-SMA-positive cells was observed in both RHE-treated- and RHEHK-treated group, compared with that in the rASCs group. *p<0.05 compared with rASCs group; #p<0.05 compared with RHE-treated group. n=3.
By Hoechst 33258 assay, the cell numbers in the RHE-treated group and RHEHK-treated group were observed to keep on increasing after seeding, reached a peak at days 7 and 6, respectively, and began to decrease afterward, which were similar to the trend of rASCs' curve in undifferentiated state (Fig. 8). And the slight decrease in proliferation rate of the inducing groups might be caused by the low-serum culture compared with that of the rASCs group (BM group, RHE-treated group, and RHEHK-treated group: 2% FBS; rASCs group: 10% FBS).
FIG. 8.
Proliferation of rASCs cultured under different conditions determined by DNA assay using Hoechst 33258 dye. rASCs group was cultured with LG-DMEM+10% FBS; BM group: LG-DMEM+2% FBS; RHE-treated group: LG-DMEM+2% FBS+2.5 μM ATRA+20 ng/mL EGF+0.5 μg/mL hydrocortisone; and RHEHK-treated group: LG-DMEM+2% FBS+2.5 μM ATRA+20 ng/mL EGF+10 ng/mL KGF+10 ng/mL HGF+0.5 μg/mL hydrocortisone. LG-DMEM, low-glucose Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; BM, basal medium.
Discussion
Due to the multipotency and proliferative activity, mesenchymal stem cells have been given particular attention in studies of tissue regeneration. As shown in literature, ASCs can promote tissue recovery through the localized secretion of angiogenic cytokines such as HGF and VEGF.16,17 Compared with BMSCs, ASCs have the advantage of being harvested in abundant quantity and causing less trauma to the donor site. So we suggest that ASCs may be a potential substitute for urothelium for the research of urethral reconstruction. Herein, we showed that with the combined induction by multiple agents including ATRA, EGF, HGF, KGF, and hydrocortisone in ALI culture, rASCs were observed to display characteristics of a stratified epithelial-like morphology, with expression of cytokeratin 19 and weak cytokeratin 13, which demonstrated the epithelial differentiation potential of rASCs in an epithelial-specific microenvironment in vitro.
From early studies, ASCs have been shown to be capable of differentiating toward epithelium with the characteristic of initial epithelial phenotypes including keratin filament proteins expression.8,9,18 Based on the endeavor of predecessors, we built up an induction system of 3D construction resembling epithelial tissue in vivo functionally, imitating the autocrine/paracrine effects on epithelial proliferation and differentiation. Proteinic and genetic analysis of the epithelial phenotypes, and detections of the growth pattern and viability of cells have been performed for a full-scale assessment after induction.
The literature revealed that the role of a tissue-specific microenvironment, in this case an ALI culture system, is critical but not the only factor in epithelialization of mesenchymal stem cells in vitro.7,19 The combined effect of biomolecular supplements on cells is another factor of importance for the differentiation into epithelial lineage. Including retinoic acid,8,20 vitamin D,7,21 triiodothyronine,7,22 and epithelium-related growth factors,4 various factors have been found to contribute to the keratin expression and epithelial differentiation of mesenchymal stem cells.
Retinoids play essential roles in cellular differentiation, proliferation, and apoptosis during embryonic development. Physiological control of retinoids is mediated by binding to nuclear receptors: retinoic acid receptors or retinoic X receptors.23 As an active metabolite of Vitamin A, ATRA is a potent and effective inducer of cell differentiation. Several transcription factors act “downstream” of ATRA and mediate the transcriptional activation of the ATRA secondary response genes for regulating ATRA's differentiation-inducing effects in stem cells and other cell types.24 A previous report has shown that retinoic acid can control differentiation and proliferation of epithelium.25 In the study reported by Brzoska et al., ATRA was added to the DMEM supplemented with 10% FBS for epithelial differentiation of human ASCs in vitro, and an increase in cytokeratin 18 expression and a reduced expression of vimentin were observed after induction for 10 days.8
In this study, ATRA was added with several costimulators including hydrocortisone and multiple growth factors in a 3D culture system to imitate the autocrine/paracrine modulation of proliferation and differentiation process of epithelial cells in vivo. Hydrocortisone has been noted to be capable of promoting keratinocyte growth.22 However, current data on the effects of growth factors on ASCs are limited. EGF's multiple cellular actions are mediated by binding to EGF receptor, followed by receptor dimerization, autophosphorylation, and recruitment of kinase substrates, leading to proliferation26,27 and modulates the differentiation potential28,29 of ASCs. And with the synergistic effect of other factors including retinoic acid,9 hydrocortisone,7 literature has shown that EGF may contribute to epithelial differentiation of ASCs. In addition, with stimulation of conditioned medium from injured proximal tubular epithelial cells (PTC), significant phosphorylation of ERK1/ERK2 was detected in human ASCs, and cell morphology changed to an epithelial-like monolayer with cytokeratin 18 expression, which may due to soluble factors secreted by the impaired PTC.18
It was noted that direct exposure to the air may be a contributing factor to epithelialization and stratification of cells,7,30 which we observed in the rUCs group also (Fig. 3e). According to Long et al., the creating of an air-medium interface was a requisite additional signal to optimize the epithelial phenotype and surface localization.9 In the presence of ATRA, hydrocortisone, and EGF (Fig. 3c, d), the formation of stratified epithelial-like morphology of rASCs was observed that may be due to the effects of the factors in ALI culture, whereas the rASCs remained in an undifferentiated state with a monolayer structure in the BM group (Fig. 3b). And from the work of Schneider et al., in a local microenvironment resembling the in vivo situation, the mesenchymal stem cells showed an upregulation of paracrine and biosynthetic activity,31 indicating the ASCs secretion of bioactive factors that facilitated epithelial differentiation besides exogenous stimulators in our culture system.
In the study, the initiation of cytokeratin 19 expression in rASCs was detected after stimulation with RHE medium. As an early marker of epithelial differentiation, the expression of cytokeratin 19 indicated that under ALI culture condition, rASCs could acquire preliminary epithelial phenotype by stimulation with ATRA, hydrocortisone, and suitable growth factors.
Further, after stimulation with RHE medium supplemented with KGF and HGF (RHEHK medium), the expression of cytokeratin 19 in rASCs was notably enhanced. As shown in TaqMan PCR assay, the relative expression levels of cytokeratin 19 in the RHEHK-treated group increased to 1.88-fold compared with that in the RHE-treated group (RHE: 1.681 and RHEHK: 3.152). The mean percentage of positive cells expressing cytokeratin 19 in the RHEHK-treated group reached 63.69%±2.63% compared with 23.08%±1.31% in the RHE-treated group. In addition, no significant change of cytokeratin 19 expression was observed when the dose of EGF was increased to 30 and 40 ng/mL, respectively, in RHE medium but absence of KGF and HGF (data not shown). KGF is known to be involved in epithelial differentiation and proliferation and may contribute to epithelial repair in an autocrine manner.10 On the other hand, HGF can induce mesenchyme to epithelium conversion as a potent pleiotropic mediator.11,32 In a recent study, it was found that treatment of undifferentiated ASCs with EGF led to increased levels of endogenous HGF secretion.16 We suggest that the combination of the agents mentioned above causes synergistic stimulation of epithelial differentiation on rASCs, but not just an accumulation effect, whereas the expression of epithelial markers was almost undetected in the presence of KGF or HGF alone in ALI culture (data not shown).
Cytokeratin 13 is considered as a mucosal-specific keratin that is usually expressed in the suprabasal layers of noncornified stratified epithelium and absent in epidermis and adnexal structures.33–35 We chose cytokeratin 13 for the characterization of rASCs after induction to determine whether mucosal differentiation could be found under the moisture condition at ALI (5% CO2 with 95% relative humidity) similar to the mucosal microenvironment of gastrointestinal and urogenital systems. Based on the weak expression of cytokeratin 13 in the RHEHK-treated group, we suggest that the 3D culture system with ALI culture may contribute to the mucosal differentiation of rASCs. Further, the reduced expression of the mesenchymal marker α-SMA indicated the changes in intrinsic phenotype of rASCs after induction. And according to the result of Hoechst 33258 assay, no significant decrease in cell proliferation and viability was observed after induction. But concerning the failed expression of involucrin, the differentiated rASCs was far from being regarded as functional epithelium.
The induction effect of ATRA and multiple growth factors on rASCs was found to show a positive correlation with the doses when remained at relatively low level (Fig. 2a, b). With further increase in doses, the induction effect reached a plateau period, and no significant enhancement was shown, which we suggested might be due to the fact that the binding of contributing factors to corresponding receptors in rASCs nearly reached saturation point.
Studies on epithelium regeneration have been given increasing attention due to the deficiency of epithelial tissue in trauma, inflammation, and cancers. The cellular senescence in epithelium of early passage in vitro results in a lack of sufficient cells to meet clinical demands. Therefore, considering the epithelial differentiation potential and abundant source, ASCs may be an ideal substitute for further research of urethral reconstruction.
Herein, the study was performed to investigate the epithelial differentiation potential of ASCs just from the characteristic of structural changes, because detection of functional effects was difficult in vitro. So we chose rabbit ASCs rather than human ASCs for induction, for the purpose of investigating the functional effects of differentiated ASCs in vivo by the reconstruction of urethral defect in the animals with rASC-seeded bladder acellular matrix grafts in the next step. Besides, considering that rASCs of passage 3 have been used for the induction, the conclusion reached from the study is restricted, and whether ASCs of high passage (for example, passage 7 or 8) are the potential source of seed cells for epithelium regeneration cannot be learned from the current data, which may be accomplished in future investigation if possible.
Conclusion
Based on the literature and our work, we suggest that the establishment of an in vitro epithelial-specific microenvironment is a feasible way for epithelial differentiation of ASCs. In this experimental setting, the initial epithelial characterization of rASCs was induced through imitating the autocrine/paracrine effects on epithelial proliferation and differentiation in vivo with multiple agents in ALI culture. This proved the multidifferentiation potential of ASCs as mesenchymal stem cells and provided an alternative in epithelium lesion and regeneration research.
Acknowledgments
This study was supported by the Natural Science Foundation of China (grant no. 81170641).
Disclosure Statement
No competing financial interests exist.
References
- 1.Buchholz D.E. Hikita S.T. Rowland T.J. Friedrich A.M. Hinman C.R. Johnson L.V. Clegg D.O. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 2.Ho J.H. Ma W.H. Tseng T.C. Chen Y.F. Chen M.H. Lee O.K. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A. 2011;17:255. doi: 10.1089/ten.TEA.2010.0106. [DOI] [PubMed] [Google Scholar]
- 3.Ji S.Z. Xiao S.C. Luo P.F. Huang G.F. Wang G.Y. Zhu S.H. Wu M.J. Xia Z.F. An epidermal stem cells niche microenvironment created by engineered human amniotic membrane. Biomaterials. 2011;32:7801. doi: 10.1016/j.biomaterials.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 4.Păunescu V. Deak E. Herman D. Siska I.R. Tănasie G. Bunu C. Anghel S. Tatu C.A. Oprea T.I. Henschler R. Rüster B. Bistrian R. Seifried E. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med. 2007;11:502. doi: 10.1111/j.1582-4934.2007.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popov B.V. Serikov V.B. Petrov N.S. Izusova T.V. Gupta N. Matthay M.A. Lung epithelial cells induce endodermal differentiation in mouse mesenchymal bone marrow stem cells by paracrine mechanism. Tissue Eng. 2007;13:2441. doi: 10.1089/ten.2007.0001. [DOI] [PubMed] [Google Scholar]
- 6.Gu S. Xing C. Han J. Tso M.O. Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99. [PMC free article] [PubMed] [Google Scholar]
- 7.Ma K. Laco F. Ramakrishna S. Liao S. Chan C.K. Differentiation of bone marrow-derived mesenchymal stem cells into multi-layered epidermis-like cells in 3D organotypic coculture. Biomaterials. 2009;30:3251. doi: 10.1016/j.biomaterials.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Brzoska M. Geiger H. Gauer S. Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142. doi: 10.1016/j.bbrc.2005.02.141. [DOI] [PubMed] [Google Scholar]
- 9.Long J.L. Zuk P. Berke G.S. Chhetri D.K. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope. 2010;120:125. doi: 10.1002/lary.20719. [DOI] [PubMed] [Google Scholar]
- 10.Beyer T.A. Werner S. Dickson C. Grose R. Fibroblast growth factor 22 and its potential role during skin development and repair. Exp Cell Res. 2003;287:228. doi: 10.1016/s0014-4827(03)00139-3. [DOI] [PubMed] [Google Scholar]
- 11.Castagnino P. Lorenzi M.V. Yeh J. Breckenridge D. Sakata H. Munz B. Werner S. Bottaro D.P. Neu differentiation factor/heregulin induction by hepatocyte and keratinocyte growth factors. Oncogene. 2000;19:640. doi: 10.1038/sj.onc.1203357. [DOI] [PubMed] [Google Scholar]
- 12.Zarnegar R. Michalopoulos G.K. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo M.J. Suh S.Y. Bae Y.C. Jung J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 14.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrigoni E. Lopa S. de Girolamo L. Stanco D. Brini A.T. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res. 2009;338:401. doi: 10.1007/s00441-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 16.Kilroy G.E. Foster S.J. Wu X. Ruiz J. Sherwood S. Heifetz A. Ludlow J.W. Stricker D.M. Potiny S. Green P. Halvorsen Y.D. Cheatham B. Storms R.W. Gimble J.M. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 17.Miranville A. Heeschen C. Sengenès C. Curat C.A. Busse R. Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 18.Baer P.C. Bereiter-Hahn J. Missler C. Brzoska M. Schubert R. Gauer S. Geiger H. Conditioned medium from renal tubular epithelial cells initiates differentiation of human mesenchymal stem cells. Cell Prolif. 2009;42:29. doi: 10.1111/j.1365-2184.2008.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider R.K. Neuss S. Stainforth R. Laddach N. Bovi M. Knuechel R. Perez-Bouza A. Three-dimensional epidermis-like growth of human mesenchymal stem cells on dermal equivalents: contribution to tissue organization by adaptation of myofibroblastic phenotype and function. Differentiation. 2008;76:156. doi: 10.1111/j.1432-0436.2007.00204.x. [DOI] [PubMed] [Google Scholar]
- 20.Baer P.C. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20:1805. doi: 10.1089/scd.2011.0086. [DOI] [PubMed] [Google Scholar]
- 21.Bikle D.D. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 22.Formanek M. Millesi W. Willheim M. Scheiner O. Kornfehl J. Optimized growth medium for primary culture of human oral keratinocytes. Int J Oral Maxillofac Surg. 1996;25:157. doi: 10.1016/s0901-5027(96)80064-6. [DOI] [PubMed] [Google Scholar]
- 23.Ross A.C. Cellular metabolism and activation of retinoids: roles of cellular retinoid-binding proteins. FASEB J. 1993;7:317. doi: 10.1096/fasebj.7.2.8440409. [DOI] [PubMed] [Google Scholar]
- 24.Gudas L.J. Wagner J.A. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226:322. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rexer B.N. Zheng W.L. Ong D.E. Retinoic acid biosynthesis by normal human breast epithelium is via aldehyde dehydrogenase 6, absent in MCF-7 cells. Cancer Res. 2001;61:7065. [PubMed] [Google Scholar]
- 26.Baer P.C. Schubert R. Bereiter-Hahn J. Plösser M. Geiger H. Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur J Cell Biol. 2009;88:273. doi: 10.1016/j.ejcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Schubert R. Geiger H. Zielen S. Baer P.C. Simultaneous detection of ERK-, p38-, and JNK-MAPK phosphorylation in human adipose-derived stem cells using the cytometric bead array technology. J Immunol Methods. 2009;350:200. doi: 10.1016/j.jim.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Hauner H. Röhrig K. Petruschke T. Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur J Clin Invest. 1995;25:90. doi: 10.1111/j.1365-2362.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 29.Tamama K. Fan V.H. Griffith L.G. Blair H.C. Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- 30.Gangatirkar P. Paquet-Fifield S. Li A. Rossi R. Kaur P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protoc. 2007;2:178. doi: 10.1038/nprot.2006.448. [DOI] [PubMed] [Google Scholar]
- 31.Schneider R.K. Anraths J. Kramann R. Bornemann J. Bovi M. Knüchel R. Neuss S. The role of biomaterials in the direction of mesenchymal stem cell properties and extracellular matrix remodelling in dermal tissue engineering. Biomaterials. 2010;31:7948. doi: 10.1016/j.biomaterials.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Kopp J.B. Hepatocyte growth factor: mesenchymal signal for epithelial homeostasis. Kidney Int. 1998;54:1392. doi: 10.1046/j.1523-1755.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- 34.Moll R. Divo M. Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waseem A. Alam Y. Dogan B. White K.N. Leigh I.M. Waseem N.H. Isolation, sequence and expression of the gene encoding human keratin 13. Gene. 1998;215:269. doi: 10.1016/s0378-1119(98)00297-2. [DOI] [PubMed] [Google Scholar]