Abstract
The Longibrachiatum Clade of Trichoderma is revised. Eight new species are described (T. aethiopicum, T. capillare, T. flagellatum, T. gillesii, T. gracile, T. pinnatum, T. saturnisporopsis, T. solani). The twenty-one species known to belong to the Longibrachiatum Clade are included in a synoptic key. Trichoderma parareesei and T. effusum are redescribed based on new collections or additional observations. Hypocrea teleomorphs are reported for T. gillesii and T. pinnatum. Previously described species are annotated.
Keywords: Hypocrea, Hypocreaceae, Hypocreales, Endophyte, Medical mycology, Revision, Systematics, Taxonomy, Soil fungi, Biogeography
Taxonomic novelties: Trichoderma aethiopicum Mulaw, Kubicek & Samuels, T. capillare Samuels & Kubicek, T. flagellatum Mulaw, Kubicek & Samuels, T. gillesii Samuels, T. gracile Samuels & Szakacs, T. pinnatum Samuels, T. saturnisporopsis Samuels & Jaklitsch, T. solani Samuels, V. Doyle & V.S. Lopez
Introduction
Before 1969 (Bisby 1939; Rifai 1969) few species were included in Trichoderma (teleomorph: Hypocrea) and even fewer species appeared in the literature. Mien Rifai (1969) was the first modern mycologist to undertake taxonomy of Trichoderma; unsurprisingly he concluded that the genus includes more than a few species. He divided the many strains that he studied among nine ‘aggregate’ species, which he acknowledged to be species complexes rather than biological species. Taxonomy of Trichoderma has gone through a remarkable transformation in the 40 years since 1969. In 1991 John Bissett (Bissett 1991a) essentially elevated Rifai’s aggregates to sectional status and between 1984 and 1991 (Bissett 1984, 1991a, b, c) he revised those sections, eventually recognizing more than 40 species, including 14 that he described as new. Today approximately 150 species are recognized, and most of them were described after 2000, many as anamorphs of Hypocrea species. Prior to 1969 almost all Trichoderma species reported in the literature were identified as T. viride (teleomorph Hypocrea rufa) but today this species is understood to be an uncommon species in the Northern Hemisphere (Jaklitsch et al. 2006).
Trichoderma longibrachiatum and T. pseudokoningii were two of the aggregate species that Rifai (1969) included in the genus. Bissett (1984) included both in sect. Longibrachiatum and then (Bissett 1991c) he corrected the taxonomy of one species and added another to make a total of five species in the section. Members of the Longibrachiatum Clade of Trichoderma are best known as producers of cellulose hydrolyzing enzymes (particularly T. reesei, Harman and Kubicek 1998; Kubicek et al. 2009), as cause of opportunistic infections of man and animals (Kuhls et al. 1999; Kredics et al. 2003), and for their association with wet building materials (Thrane et al. 2001). In the mid 1990’s molecular phylogenetic techniques applied to hyphomycetes challenged traditional species concepts based on morphology. Kuhls et al. (1997) and Samuels et al. (1998) combined DNA sequencing with phenotype in a revision of Trichoderma sect. Longibrachiatum. They demonstrated that the section is monophyletic, accepted most of Bissett’s (1984) species and doubled the number of species to ten. For the first time they included species based on teleomorph (Hypocrea, Hypocreaceae, Hypocreales) collections in what they termed the ‘Hypocrea schweinitzii complex’. Subsequent molecular phylogenetic analyses have supported this complex as the Longibrachiatum Clade of Trichoderma (e.g. Samuels 2006) and resulted in recognition of three more species (Bissett et al. 2003; Atanasova et al. 2010).
Kuhls’ et al. (1997) molecular revision of the Longibrachiatum Clade was based on sequences of the internal transcribed spacer region of ribosomal RNA (ITS 1+2), a region now known to be too highly conserved to separate many closely related species (Gazis et al. 2011). Since that time additional genes have been developed for use in systematics and the current standard for species recognition is based on phylogenetic analysis of multiple unlinked loci (genealogical concordance phylogenetic species recognition, GCPSR, Taylor et al. 2000). Druzhinina et al. (2012) applied GCPSR and the 4x concept (Birky et al. 2010) to a collection of 113 strains belonging to the Longibrachiatum Clade and found 24 phylogenetic species. The analysis of Druzhinina et al. (2012) supported the taxonomy proposed for the Longibrachiatum Clade by Gams and Bissett (1998), Samuels et al. (1998) and subsequent authors (Bissett et al. 2003; Atanasova et al. 2010) while revealing the existence of 12 undescribed phylogenetic species.
In the present work we revise the taxonomy of the Longibrachiatum Clade of Trichoderma following the molecular phylogenetic analysis of Druzhinina et al. (2012).
Materials and methods
Trichoderma strains were independently received by the Kubicek and Samuels labs from colleagues in several countries or from personal collecting. Hypocrea teleomorphs of Trichoderma species were collected in Australia, New Zealand, Sri Lanka, Canary Islands (La Palma) and Isle de la Réunion in the Indian Ocean; cultures derived from these collections were made by isolating solitary ascospores using a micromanipulator or a platinum needle on cornmeal agar (Difco or Sigma) + 2% dextrose (CMD). Strains described below as T. flagellatum were isolated from surface sterilized roots of Coffea arabica and T. solani originated in surface sterilized potato tubers.
Growth rates were determined on PDA (potato dextrose agar, Difco) and SNA (Nirenberg 1976, without filter paper) at 15, 20, 25, 30 and 35°C in darkness (with intermittent light when they were measured at intervals of 24 h). To prepare inoculum, cultures were incubated at 25°C for a few days on cornmeal agar (Difco) with 2% glucose (CMD) or on SNA. The inoculum was placed at 10–15 mm distance from the edge of the plate. It should be noted that different brands of PDA can give different colony characteristics (Jaklitsch 2009). Measurements were made at intervals of 24 h until 96 h. Colony characters were taken from colonies incubated on PDA and SNA at 25°C with alternating cool white fluorescent light and darkness (12 h/12 h) after 7–10 day; these conditions are referred to in descriptions as ‘under light’. Typically there is little intra-species variation. Measurements are reported as mean plus and minus standard deviation with extremes in brackets; the 95% confidence of the means (95% ci) is reported in cases of multiple collections for a species. Statistics were computed using Systat 10© (Wilkinson 2000). Continuous measurements (dimensions of conidia, phialides etc.) and appearance of conidiophores and conidial pustules are determined from colonies incubated 7–10 day at 25°C under light conditions described above, usually from SNA but when conidia do not form on SNA, characters are taken from CMD, less frequently on cornmeal agar without added glucose. Thirty units of each character are measured from all available cultures of each species, except where noted. In some images Helicon Focus (http://www.heliconsoft.com/heliconfocus.html) was used to provide depth of field.
The present work derives from the phylogenetic analysis of Druzhinina et al. (2012). To facilitate the location of species in the phylogenetic context a modified version of their phylogenetic tree is given as Fig. 1. Details on methods used to produce the tree, strains and their GenBank numbers are provided in that work. Representative cultures of all species are deposited in Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS) or the American Type Culture Collection, Manassas, VA, U.S.A. (ATCC).
Fig. 1.
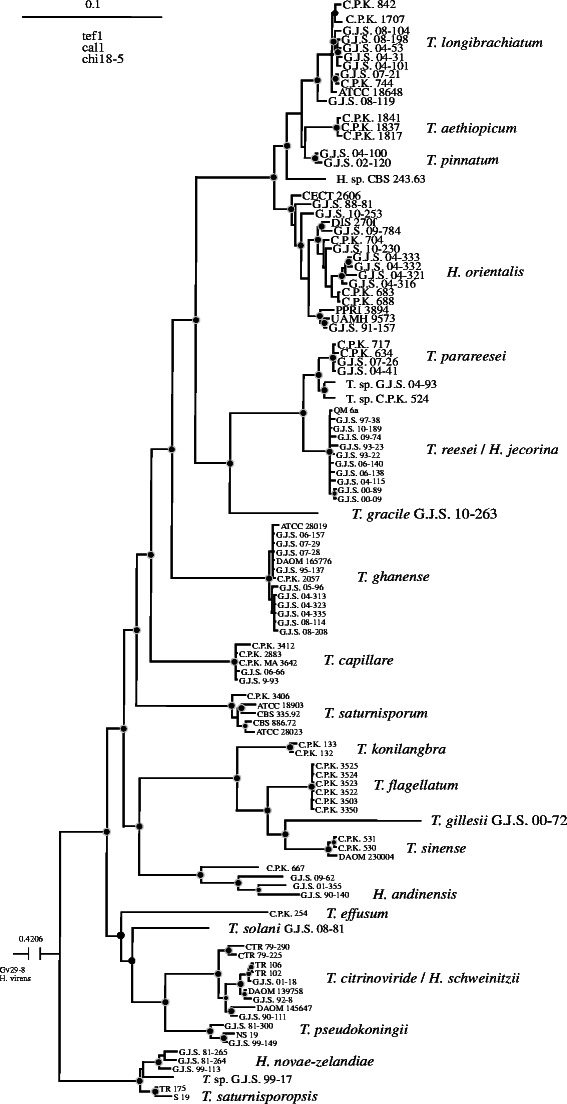
Bayesian phylogram obtained from the concatenated alignment of tef1, cal1 and chi18-5 loci. See Druzhinina et al. (2012) for details
The Longibrachiatum Clade of Trichoderma
Colonies typically growing well and sporulating at ≥ 35°C; a diffusing yellow pigment often forming on PDA (Figs. 2, 3). Conidiophores forming in the scant aerial mycelium and in small, cottony pustules (‘shrubs’; Jaklitsch 2009, 2011) within which long, plumose conidiophores often visible; sterile hairs present or not in pustules. Conidiophores typically comprising a strongly developed central axis from which phialides arise singly over several levels below the tip; phialides held in whorls in addition to solitary phialides in some species; often a single phialide terminating a basal cell with a short, spur-like phialide arising as an outgrowth of the basal cell at the septum (‘intercalary phialide’, Samuels et al. 1998). Phialides typically lageniform to nearly cylindrical, often hooked or sinuous. Conidia typically ellipsoidal to oblong, smooth, less frequently subglobose or roughened to tuberculate. Teleomorphs Hypocrea; stromata a shade of brown or dark gray to black; ostiolar areas in brown stromata often green in lactic acid; part-ascospores subglobose, hyaline, roughened; lignicolous.
Fig. 2.
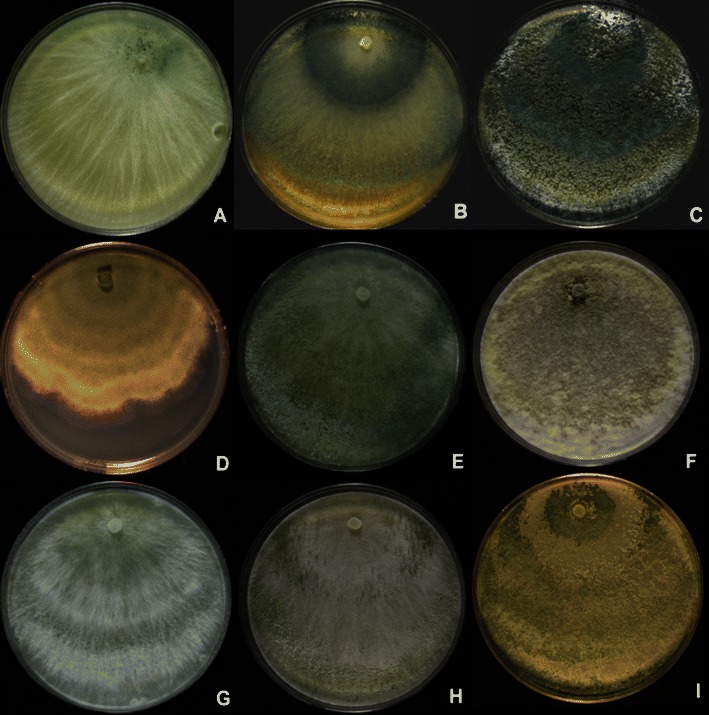
Longibrachiatum Clade. Cultures grown on PDA. a, b T. aethiopicum, G.J.S. 10–165. c T. capillare, G.J.S. 10–170. d T. effusum, DAOM 230007. e, f T. flagellatum, G.J.S. 10–162. g, h T. gracile, G.J.S. 10–263, just beginning to sporulate. i. G.J.S. 99–17. All grown 1 week at 25°C under light, except b, e, h, which were grown 1 week at 35°C in darkness with intermittent light. Note the increased sporulation in colonies grown at 35°C when compared to the same strain grown at 25°C (b vs. a, e vs. f)
Fig. 3.
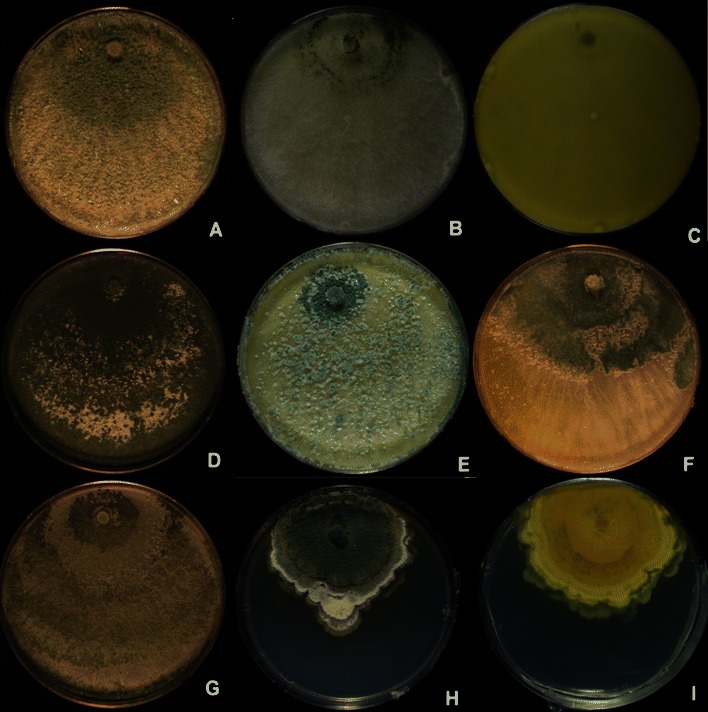
Longibrachiatum Clade. Cultures grown on PDA. a–c Hypocrea orientalis (a G.J.S. 06–317, b G.J.S. 04–321, c G.J.S. 04–316, reverse showing diffusing yellow pigment). d T. parareesei G.J.S. 04–41. e, f T. pinnatum (e G.J.S. 04–100, f G.J.S. 02–120). g T. saturnisporopsis Tr 175. h, i T. solani G.J.S. 08–81 (h colony from above, i colony reverse)
Synoptic key to members of the Longibrachiatum Clade of Trichoderma
(An asterisk (*) signifies that a species occurs in more than one lead of a character)
| Species | Known distribution |
| 1. T. aethiopicum | East Africa |
| 2. H. andinensis | Venezuela, high elevation |
| 3. T. capillare | Europe, Vietnam, Taiwan |
| 4. T. citrinoviride | North and South Temperate |
| 5. T. effusum | India, high elevation |
| 6. T. flagellatum | Ethiopia |
| 7. T. ghanense | West Africa, America, South East Asia, Europe, Australia |
| 8. T. gillesii | Indian Ocean |
| 9. T. gracile | Malaysia |
| 10. T. konilangbra | East Africa, high elevation |
| 11. T. longibrachiatum | cosmopolitan/predominantly tropical |
| 12. H. novae-zelandiae | New Zealand |
| 13. H. orientalis | pantropical, subtropical |
| 14. T. parareesei | pantropical, subtropical |
| 15. T. pinnatum | Sri Lanka/Vietnam |
| 16. T. pseudokoningii | Australasia, rare elsewhere |
| 17. T. reesei | pantropical |
| 18. T. saturnisporopsis | USA (Oregon), Europe (Sardinia) |
| 19. T. saturnisporum | USA, Mexico, South Africa, Europe |
| 20. T. sinense | Taiwan |
| 21. T. solani | México |
-
I.
COLONY CHARACTERS
Growth on PDA within 72 h at 25°C
Typically filling a 9-cm-diam Petri plate: 1, 3*, 7, 11*, 13–15*, 17*, 18, 19*
Typically not filling a 9-cm-diam Petri plate, colony radius averaging 30–60 mm: 2–6, 8–13, 15*, 16, 17*, 19–21
Growth on PDA within 72 h at 35°C
Typically filling a 9 cm diam PDA Petri plate: 1–3, 6–8, 11, 13–19*
Typically not filling a 9-cm-diam Petri plate, colony radius often 60 mm or less: 4, 5, 9, 10, 12, 16*, 19*–21
Growth on SNA within 72 h at 25°C
Typically filling a 9 cm diam Petri plate: 2*
Typically averaging > 30 and < 50 mm: 1, 3, 6, 8, 9*, 10, 11*, 13, 14*–17, 18*, 19*, 20*
Typically averaging > 50 and < 65 mm: 2*, 7, 11*
Typically averaging < 30 mm: 4, 14*, 19*, 20*, 21, G.J.S. 99-17*
Growth on SNA within 72 h at 35°C
Typically filling a 9-cm-diam Petri plate: 2, 7, 13, 16*
Typically not filling a 9-cm-diam Petri plate, colony radius 40–65 mm: 1, 3, 4*, 6*, 8, 9, 11, 14, 15*, 16*, 17, 19*
Typically not filling a 9-cm-diam Petri plate, colony radius 20–40 mm: 4*, 6*, 15*, 18, 19*, 20, G.J.S. 99–17
Typically not filling a 9-cm-diam Petri plate, colony radius < 10 mm: 5, 12, 21
Diffusing pigment on PDA within 72 h at 25–35°C in darkness
Diffusing yellow pigment: 1, 3 (pale yellow), 4, 5* (olivaceous), 6, 9–11, 12 (pale yellow), 13*–15, 16 (pale yellow), 17, 18* (pale yellow), 19*–21
No diffusing yellow pigment: 2, 5*, 7, 8, 13*, 18* (pale yellow), 19*
-
II.
CONIDIAL CHARACTERS
Conidium ornamentation
Roughened: 3*
Tuberculate: 7*, 18, 19
Smooth: 1, 2, 3*, 4–7*, 8–17, 20, 21
Conidium average length
< 3 μm: 21
>5 μm: 5, 7*
4–5 μm: 2, 6*, 7* (G.J.S. 05–96), 11*, 13, 14, 15*, 16, 17*–19, G.J.S. 99–17
3–4 μm: 1, 3, 4, 6*, 8–10, 11*, 12, 15*, 17*, 20
Conidium average width
< 2.5 μm: 1, 2*, 4*, 6, 7*-9, 12, 21
2.5–3.0 μm: 2*(G.J.S. 09–62), 3*, 4*, 5*, 7*, 10, 11, 13*-17, 19*
3.0–3.5 μm: 3*, 5*, 7*, 13*, 18, 19*, CBS 243.63
Conidium average L/W
≤ 1.3: 1*, 3, 7*, 17*, 19*, 20*, 21
≥1.3–1.7: 1*, 4, 6*–8*, 9, 10–12, 13*–15, 17*, 18*, 19*, 20*
> 1.7: 2, 5, 6*–8*, 13*, 16
-
III.
PHIALIDE CHARACTERS
Arrangement
Phialides arising singly along the main axis of the conidiophores and along branches from the main axis; whorls of phialides not dominating: 1, 3*–5, 7, 9, 11, 13–15, 17, 20
Whorls of phialides conspicuous, common; solitary phialides not arising over a long distance of the main conidiophores axis or its branches: 2, 3*, 6, 8, 10, 12, 16, 18, 19, 21
Frequency of intercalary phialides
Common: 1, 4–6, 9, 11, 13, 14, 17
Infrequent or not formed: 2, 3, 7, 8, 10, 12, 15, 16, 18–21
Ratio of phialides length to the width of its supporting cell
≤2.5: 2, 6, 8*, 10, 18*
2.6–3.0: 3, 4, 7, 8*, 9, 11–17, 18*–21
≥3.3: 1, 5, 18*
-
IV.
BRANCHING OF CONIDIOPHORES
The typical conidiophore comprises a more or less distinct central axis from which solitary phialides arise over the terminal part and branches arise within about five layers of phialides. The lateral branches usually increase in length with distance from the tip and, like the main axis, produce solitary phialides: 1–4, 6–12*, 13–17, 20, 21
No distinct central axis is formed or central axis poorly formed and no regularly repeating pattern can be seen in the conidiophores: 5, 12*, 18, 19
-
V.
HAIRS ARISING FROM PUSTULES
Present and easily seen: 3, 4, 6–8, 10, 12, 16, 18, 19
Absent or inconspicuous: 1, 2, 5, 9, 11, 13–15, 17, 20, 21
TAXONOMY
1. Trichoderma aethiopicum Mulaw, Kubicek et Samuels, sp. nov. Figs. 2a, b and 4.
Fig. 4.
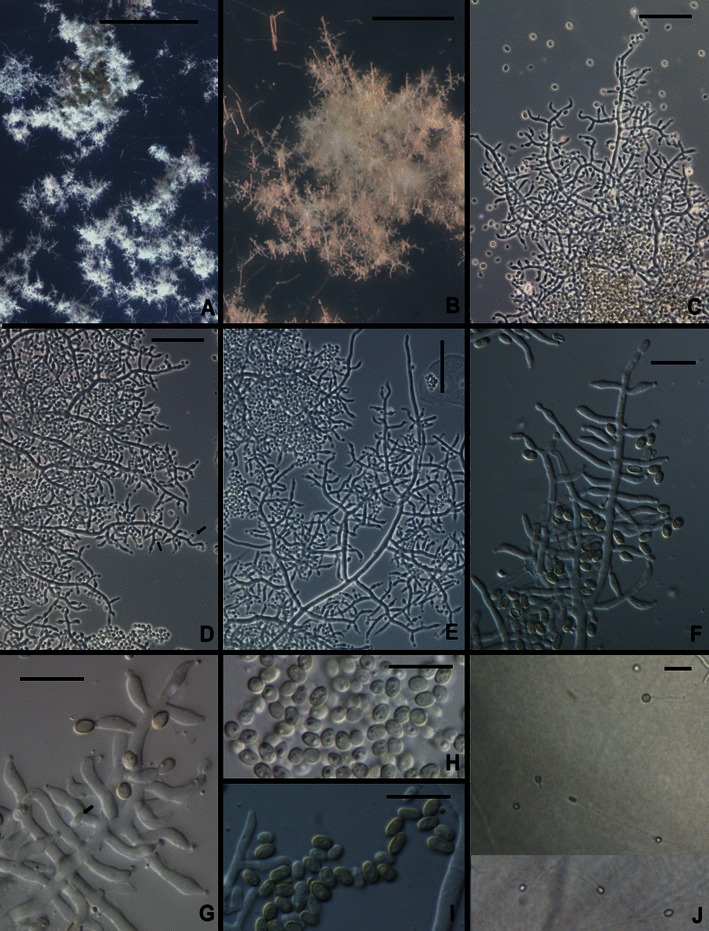
Trichoderma aethiopicum. a, b Pustules on SNA. c–g Conidiophores from SNA (Arrows in d, g show intercalary phialides). h, i Conidia. j Chlamydospores. All from SNA. a, b, d, e, h, j from G.J.S. 10–167; c, g from 10 to 166; f, i from G.J.S. 10–165. Scale bars: a = 0.5 mm, b = 100 μm, c–e, j = 20 μm, f–i = 10 μm
MycoBank MB 563902
Trichodermati longibrachiato Rifai et T. pinnato Samuels simile sed ob conidiorum longitudinis ad latitudinem rationem majorem, 1.4–1.5, distinguendum.
Holotypus: BPI 882291.
Optimum temperature for growth on PDA and SNA 25–35°C; after 96 h in darkness with intermittent light colony on PDA and SNA completely filling a 9-cm-diam Petri plate. Conidia forming within 24 h at 35°C and after 48 h at 25 and 30°C on PDA in darkness (only sparingly produced on PDA incubated 1 week under light); diffusing yellow pigment forming at 25, 30 and 35°C within 24 h; surface mycelium disposed in rays; at 35°C conidia covering nearly the entire colony. Conidia remaining white for a long time, slowly becoming dark green. Colonies grown on SNA in darkness with intermittent light forming conidia within 72–96 h at 30 and 35°C; conidia forming at 25°C in light within 10 day. On SNA conidia forming in minute pustules, < 0.25 mm diam, individual conidiophores visible within pustules; pustules formed of intertwined hyphae. Conidiophores terminating the ends of hyphae in pustules, typically comprising a long axis with phialides produced directly or shorter or longer branches arising from the conidiophore and producing phialides directly or rebranching, new branches producing phialides directly. Sterile hairs not formed. Intercalary phialides common (Fig. 4d, g). Phialides (n = 90) cylindrical to lageniform, (3.0–)5.7–9.5(−12.7) μm long, (1.7–)2.2–2.7(−3.2) μm at the widest point, L/W (1.2–)2.2–4.2(−6.2), (1.0–)1.5–2.0(−2.5) μm wide at the base, arising from a cell (1.5–)1.7–2.5(−3.7) μm wide. Conidia (n = 90) broadly ellipsoidal to nearly oblong, (2.5–)3.0–4.0(−4.5) × (1.7–)2.0–2.5(−3.0) μm, L/W (1.0–)1.2–1.7(−2.3) (95% ci: 3.3–3.5 × 2.2–2.3 μm, L/W 1.4–1.5), becoming dark green, smooth. Chlamydospores abundant, subglobose, terminal and intercalary, 5–10 μm diam.
Etymology: ‘aethiopicum’ refers to the country where this species was first discovered, Ethiopia.
Habitat: Soil
Known distribution: Ethiopia.
Holotype: Ethiopia, Welega Prov., isolated from soil under coffee, date unknown, T. Mulaw (BPI 882291; ex-type culture C.P.K. 1837 = G.J.S. 10–166 = CBS 130628). tef1 = EU401615, cal1 = EU401483, chi18-5 = EU401534, rbp2 = HM182986.
Additional cultures examined: Ethiopia, Harerga, isolated from soil under coffee, date unknown, T. Mulaw (C.P.K. 1841 = G.J.S. 10–167. Sequences: tef1 = EU401616, cal1 = EU401484, chi18-5 = EU401535); Jimma, isolated from soil under coffee, date unknown, T. Mulaw (CBS 130627 = C.P.K. 1817 = G.J.S. 10–165. Sequences: tef1 = EU401614, cal1 = EU401482, chi18-5 = EU401533).
Comments: Trichoderma aethiopicum is a member of a clade that includes T. longibrachiatum, H. orientalis, the new species T. pinnatum, and the strain CBS 243.63. The two common species in this clade, T. longibrachiatum and H. orientalis, are pantropical, whereas the other species in the clade appear to be Paleotropical/Australasian endemics. Trichoderma aethiopicum is known only from three strains isolated from soil under coffee in Ethiopia. There is no practical way to distinguish most of these species on the basis of their physical phenotype, although conidia of T. aethiopicum have a somewhat larger length/width ratio than T. longibrachiatum or H. orientalis. Strain CBS 243.63 (Fig. 5) has larger conidia than any of the members of this clade [(3.7–)4.7–7.7(−10.2) × (2.0–)2.7–3.5(−3.7) μm]. This strain was derived from ascospores of a Hypocrea collection made early in the 1960’s in New Zealand by J.M. Dingley and sent to J. Webster in the UK; that collection cannot be located. The culture appears to be degenerated. While this strain clearly represents a distinct lineage within the Longibrachiatum/Orientalis subclade, we are not confident that we can adequately characterize it. We deposit sequences in GenBank in the hope that the species will be recognized in the future.
Fig. 5.
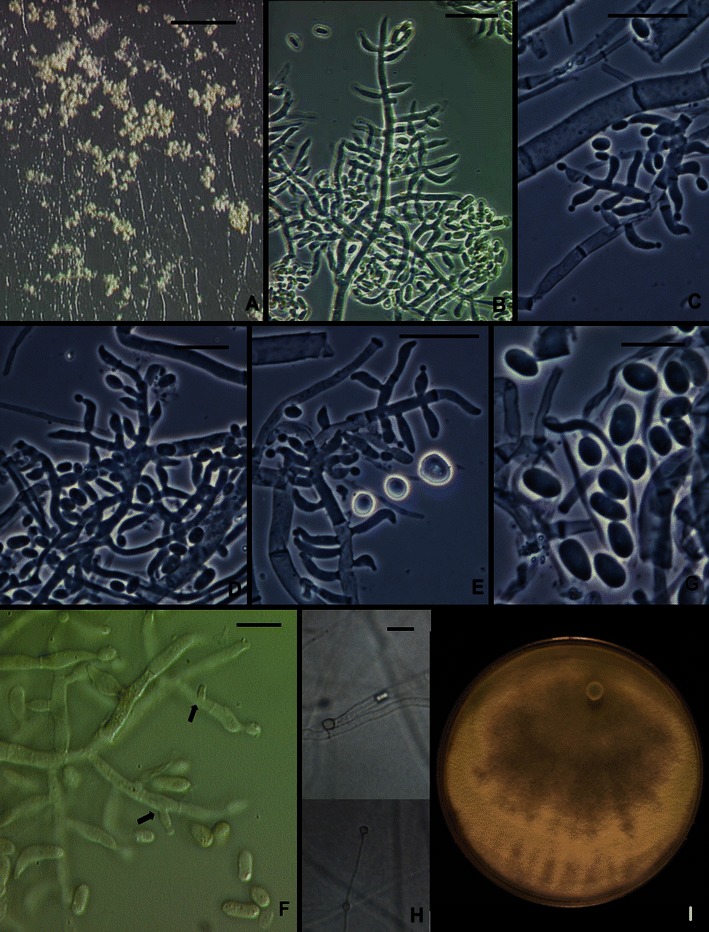
Trichoderma sp. CBS 243.63. a Pustules from CMD. b–e, f Conidiophores and phialides. f, g Conidia. Intercalary phialides indicated by arrows. h. Chlamydospores. i. Colony 1 week on PDA under light just beginning to sporulate. b, f from CMD; b–e, g, h from SNA. Scale bars: a = 2 mm, b–e, h = 20 μm. g = 10 μm
2. Hypocrea andinensis Samuels & O. Petrini in Samuels et al., Stud. Mycol. 41: 13 (1998).
Anamorph: Trichoderma sp.
Ex-type strain: G.J.S. 90–140 = CBS 354.97 = ATCC 208857
Typical sequences: ITS X93957, tef1 AY956321
This species was described (Samuels et al. 1998) based on a single perithecial collection made in the Venezuelan Andes at an elevation of 2,300 m. Since then we have examined soil cultures from Saudi Arabia (G.J.S. 01–355), Amazonian Peru (G.J.S. 09–62, San Martín State) and Hawaii (C.P.K. 667) that form a well-supported clade with the ex-type culture of H. andinensis within the Longibrachiatum Clade, which could lead to the conclusion that they represent one species (Druzhinina et al. 2012). However, considering the individual branch lengths and following the 4x rule of Birky et al. (2010), Druzhinina et al. (2012) suggested that each of these strains represents a distinct phylogenetic species. Strains C.P.K. 667 and G.J.S. 01–355 were lost before observations of their morphology could be made. The two remaining strains are morphologically typical of the Longibrachiatum Clade but differ from each other in detail. Conidia of G.J.S. 09–62 are wider than those of the ex-type strain of H. andinensis (respectively 4.5 ± 0.3 × 3.0 ± 0.2 μm, L/W = 1.5 ± 0.2, n = 30; 4.5 ± 0.5 × 2.2 ± 0.2 μm, L/W 2.2 ± 0.3, n = 30). In the absence of additional strains of these closely related phylogenetic species, we refrain from proposing a taxonomy for the undescribed species of the H. andinensis clade and H. andinensis remains known only from a single collection.
3. Trichoderma capillare Samuels et Kubicek, sp. nov. Figs. 2c and 6.
Fig. 6.
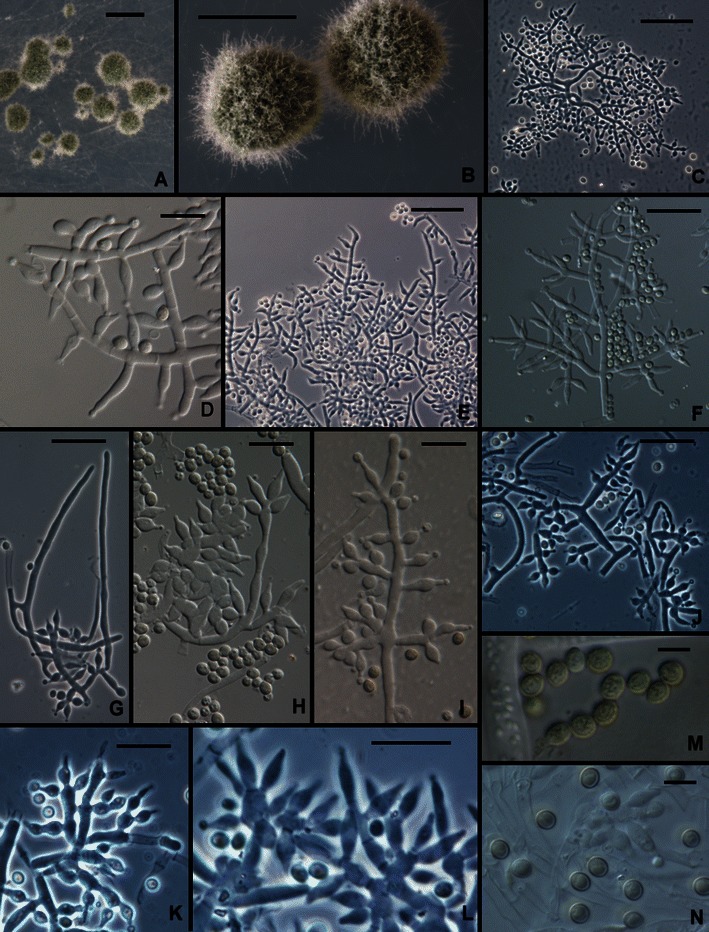
Trichoderma capillare. a, b Pustules (Hairs seen in b). c–l Conidiophores (Hairs seen in g, m). n Conidia. All from SNA except M, which is from CMD. a–c, g–i from G.J.S. 10–170; d, e from G.J.S. 06–66; f, j–l, n from G.J.S. 10–169; m from ATCC 20898. Scale bars: a, b = 0.5 mm; c, e–f, j, k = 20 μm; d, h, i, l–n = 10 μm
MycoBank MB 563903
Trichodermati saturnisporo simile sed ob conidia subglobosa vel late ellipsoidea, (2.2–)2.7–4.0(−4.5) × (1.7–)2.5–3.5(−4.0) μm differt.
Holotypus: BPI 882292
Optimum temperature for growth on PDA and SNA 25–35°C; after 96 h in darkness with intermittent light colony on PDA and SNA completely or nearly completely filling a 9-cm-diam Petri plate, only slightly slower at 20°C. Conidia and sometimes a very pale diffusing yellow pigment forming within 48 h at 25–35°C in colonies grown on PDA in darkness with intermittent light; on SNA conidia appearing somewhat later, within 72–96 h at 25–35°C. Colonies grown on PDA 1 week at 25°C under light producing conidia in dense, confluent pustules over the entire colony surface; conidia dark green to gray-green (except G.J.S. 99–3 where conidia are white). Colonies grown on SNA 1 week at 25°C under light producing dark green to gray-green conidia in scattered, pulvinate, 0.5–1.5 mm diam pustules. Individual conidiophores not visible within pustules; pustules formed of intertwined hyphae. Conidiophores arising from hyphae within pustules, highly variable in form; commonly fertile branches producing solitary phialides, intercalary phialides infrequent; often conidiophores producing fertile branches laterally with branches terminating in whorls of a few phialides; sometimes fertile branches lacking any obvious pattern, cells of fertile branches sometimes vesiculose and producing numerous phialides. Hairs arising as outgrowths of the hyphae of the pustule, conspicuous or not, septate, flexuous, sterile. Phialides lageniform, nearly cylindrical or conspicuously swollen below the middle, straight or less frequently asymmetric or hooked, (4.0–)6.5–10.5(−14.0) μm long, (2.2–)3.0–3.5(−4.5) μm at the widest point, base (1.0–)2.2–3.2 μm wide, L/W (1.5–)1.6–3.2(−5.5) (n = 120), arising from a cell (1.7–)2.2–3.5(−4.5) μm wide. Conidia subglobose to broadly ellipsoidal, (2.2–)2.7–4.0(−4.5) × (1.7–)2.5–3.5(−4.0) μm, L/W (0.9–)1.0–1.4(−1.6) (n = 120; 95% ci: 3.3–3.5 × 2.9–3.0 μm, L/W 1.1–1.2), green, roughened, less frequently smooth. Chlamydospores not observed.
Etymology:’capillare’ refers to the fine hairs arising from the conidial pustules.
Habitat: soil; isolated once from an Agaricus farm (Hungary).
Known distribution: USA (NY), Colombia, Europe (Austria, Hungary), Vietnam, Taiwan (C.P.K. 3412; morphology not assessed).
Holotype: Hungary, from Agaricus farm in cellar, C.P.K. 2883 (BPI 882292, live ex-type culture G.J.S. 10–170 = CBS 130629. Sequences: tef1 = JN182283, cal1 = JN182293, chi18-5 = JN182304, rpb2 = JN182312).
Additional cultures examined: Austria, Niederösterreich, Mannswörth, soil under Salix sp.; C.P.K. 885 = MA 3642 = G.J.S. 10–169. Sequences: tef1 = JN182277, cal1 = JN182289, chi18-5 = JN182303. USA. New York, Ontario County, Cornell Vegetable Farms, soil, ATCC 20898 = CBS 130672 = G.J.S. 99–3. Sequences: tef1 = JN175584, cal1 = JN175411, chi18-5 = JN175470, rpb2 = JN175529. Vietnam, soil, Le Dinh Don, CBS 130500 = G.J.S. 06–66. Sequences: tef1 = JN175585, chi18-5 = JN175471, rpb2 = JN175530.
Comments: The ex-type strain of this species was reported by Hatvani et al. (2007). Strain ATCC 20898, isolated from soil in New York State, is highly unusual in producing white conidia in pustules that very slowly turn green. It was cited by Smith et al., as T. viride, for biological control of Phytophthora spp. (U.S. Patent 4196557, 26 Feb 1991).
This species was cited by Wuczkowski et al. 2003 (as MA 3642, Trichoderma sp.). The subglobose, roughened conidia and often irregular branching pattern characterize this species. Hoyos-Carvajal et al. (2009) isolated this species from soil in Colombia (Guajira, San Juan).
There are no obvious close relatives for this species in the Longibrachiatum Clade (Druzhinina et al. 2012). Trichoderma capillare is unusual in the Longibrachiatum Clade for its branching pattern, which tends to be more random than in T. longibrachiatum, the frequent arrangement of phialides in divergent whorls, and for the roughened and broadly ellipsoidal to subglobose conidia. It differs from the somewhat distantly related T. saturnisporum in which conidia are ellipsoidal and tuberculate, the ornamentation typically appearing as blisters (Samuels et al. 1998).
4. Trichoderma citrinoviride Bissett, Can. J. Bot. 62: 926 (1984).
Teleomorph: Hypocrea schweinitzii (Fr.) Sacc., Syll. Fung. 2: 522 (1883).
Ex-type culture: DAOM 172792 = CBS 258.85
Typical sequences: ITS Z31017, tef1 EU280036
Bissett (1991c) distinguished between T. citrinoviride and the anamorph of H. schweinitzii based on morphology, however molecular phylogenetic analyses (Kuhls et al. 1997; Druzhinina et al. 2012) did not support a separation and Samuels et al. (1998) could not confirm a difference in phenotype between strains derived from H. schweinitzii and Trichoderma strains, including the ex-type culture of T. citrinoviride. Samuels et al. (1998) redescribed the Trichoderma and Hypocrea morphs. The teleomorph is only known from North America and Europe (Samuels et al. 1998; Jaklitsch 2011). Species having equally black or very dark stromata are H. novae-zelandiae and T. pseudokoningii, both with primarily Australasian distribution. While T. citrinoviride is isolated from a diversity of substrata around the world (Turner et al. 1997), it appears to be more common in soil isolations in temperate countries. Hoyos-Carvajal et al. (2009) did not report it from Colombia or adjacent countries and we did not find it in soils from extensive isolations made in Amazonian Peru or from Cameroon (Samuels and Arevalo, unpubl.; Samuels and Tondje, unpubl.), but it was detected in a riparian forest in south temperate Uruguay (Turner et al. 1997). Blaszczyk et al. (2011) found it to be common in forest soil, wood in forests and mushroom compost in Poland. Cellulases produced by strains identified as this species have been utilized in bioconversion (Guerra et al. 2006; Chandra et al. 2009a, b, 2010) but the species is capable of growing and sporulating at human body temperature and thus extreme care must be taken if its conidia are to be mass-produced.
For a description see Bissett (1984, 1991c), Gams and Bissett (1998), Samuels et al. (1998), and http://nt.ars-grin.gov/taxadescriptions/keys/trichodermaindex.cfm.
5. Trichoderma effusum Bissett, Kubicek & Szakacs, Can. J. Bot. 81: 575 (2003). Figures 2d and 7.
Fig. 7.
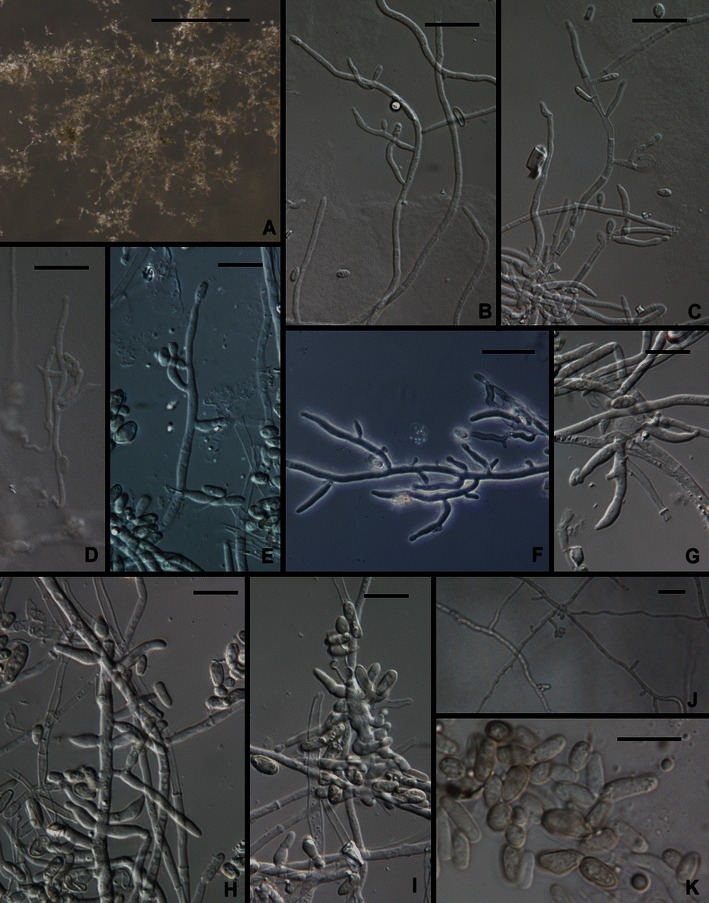
Trichoderma effusum. a–i Conidiophores. j Phialides and aphanophialides in immersed hyphae. k Conidia. All from SNA. All from DAOM 230007. Scale bars: a = 0.5 mm; b–e, g–i, k = 10 μm; f, j = 20 μm
Teleomorph: none known
Ex-type culture: DAOM 230007 = TUB F-354
Typical sequences: ITS AF149858, tef1 AF510432
This species is known only from a single soil isolation made at an elevation of 2,800 m in the Himalayan Mountains of India (Kullnig et al. 2000, as T. sp. 2 or Trichoderma sp. TUB F-354). Although gross colony characters on PDA are typical of Trichoderma the morphology of this species is atypical in the genus in the production of ‘aphanophialides’ (Gams 1971), short spur-like phialidic openings formed on hyphae (Fig. 7c, f, g), the lack of any extensively and regularly branched conidiophore, conidia that are much larger than usual in the genus, and in the production of conidia from hyphae immersed in agar. The arrangement of solitary, more or less cylindrical phialides along hyphae is at least reminiscent of other members of the Longibrachiatum Clade.
Trichoderma effusum forms a clade with T. citrinoviride, T. pseudokoningii and the new species T. solani (Druzhinina et al. 2012).
Following is a redescription of the species based on reexamination of the ex-type culture (DAOM 230007): Optimum temperature for growth on PDA and SNA 25–30°C, after 96 h in darkness with intermittent light colony radius on PDA 30–35 mm, on SNA ca. 15 mm. Colony radius at 35°C after 72 h on PDA 24 mm, on SNA 8 mm. On PDA conidia forming within 24–48 h at 25–35°C in a continuous lawn with faint concentric rings, colony appearing velvety, no pustules observed; conidia darker in the center, approx. 28D5 (grayish green) fading to nearly white at the margin, colony reverse olivaceous yellow, no distinctive odor; on SNA colony margin deeply dissected, conidia sparingly produced within 72–96 h, conidiophores arising directly from the surface of the agar and conidia also formed from phialides formed along hyphae submerged in the agar. Conidia slowly turning pale green, held in wet heads. Differentiated conidiophores not observed on SNA; conidia produced from solitary phialides arising from erect or immersed hyphae; phialides closely or distantly spaced. Phialides lageniform to ampulliform, at most only slightly swollen in the middle, straight or hooked, often reduced to short pegs (aphanophialides, Fig. 7c, f, g, j) along hyphae, sometimes aphanophialides forming in the cell subtending a phialide, (4.5–)6.5–13.0(−24) μm long, (2.2–)2.5–3.5(−4.0) μm at the widest point, L/W = (1.7–)3.6–5.0(−7.8), base (1.5–)2.0–3.5(−5.0) μm, arising from a cell (2.0–)(2.0–)2.5–3.2(−3.7) μm Conidia ellipsoidal to nearly oblong, (4.5–)5.0–7.7(−9.7) × (2.5–)2.7–3.5(−4.0) μm, L/W = (1.2–)1.5–2.7(−3.5), green, smooth. Chlamydospores not observed.
6. Trichoderma flagellatum Mulaw, Kubicek et Samuels, sp. nov. Figs. 2e, f and 8.
Fig. 8.
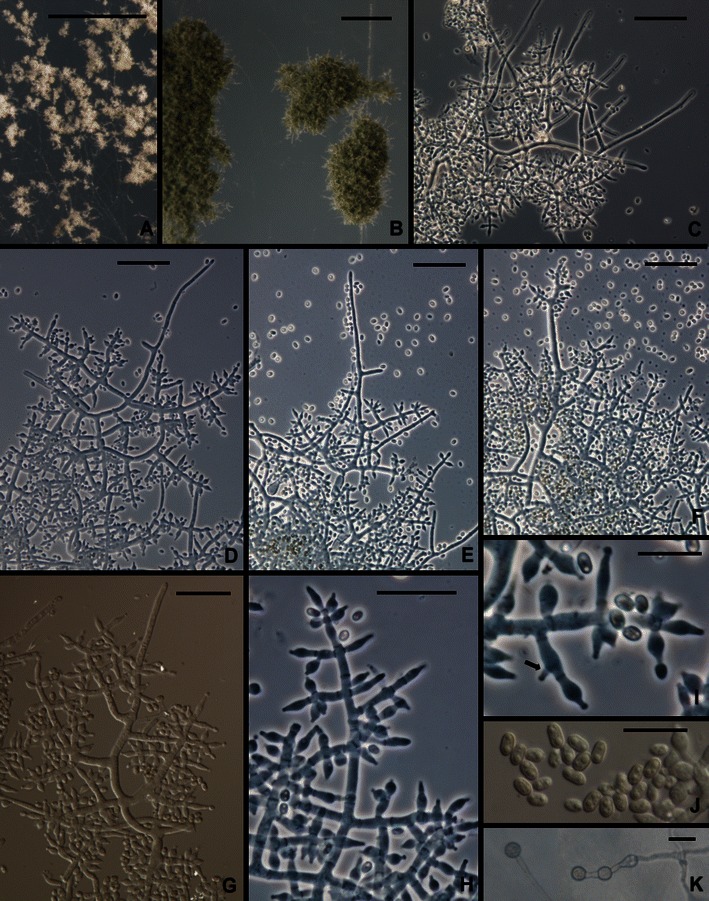
Trichoderma flagellatum. a, b. Pustules. c–h Conidiophores. Hairs visible in c–e, g, i Phialides (Arrow shows an intercalary phialide). j Conidia. k Chlamydospores. All from SNA. a from G.J.S. 10–156; b from G.J.S. 10–163; c, e, f, g, j from G.J.S. 10–164; d from G.J.S. 10–162; h, i, k from G.J.S. 10–161. Scale bars: a, b = 0.5 mm; c–h = 20 μm; I, J = 10 μm
MycoBank MB 563904
Trichodermati konilangbrae Samuels, O. Petrini et Kubicek simile sed ob conidia angustiora, 4.0–4.2 × 2.3–2.4 μm, conidiorum longitudinis ad latitudinem rationem 1.7–1.8 differt.
Holotypus: BPI 882293
Teleomorph: none known
Optimum temperature for growth on PDA and SNA 25–35°C; after 96 h in darkness with intermittent light colony on PDA and SNA completely or nearly completely filling a 9-cm-diam Petri plate; on PDA after 96 h; slightly slower at 35°C. Conidia forming at 25 and 35°C within 48 h in darkness with intermittent light on PDA; diffusing yellow pigment forming at 30 and 35°C. Yellow pigment spreading through the medium on PDA after 1 week at 25°C under light, conidia forming over more or less of the colony surface in discrete to densely disposed pustules and the mycelium on the surface disposed in rays. Conidia gray-green. Colonies grown on SNA in darkness with intermittent light forming conidia within 48 h at 35°C; conidia forming at 25°C in light only within 1 week, mainly where the agar had been cut. On SNA conidia forming in small pustules, < ¼ mm diam, individual conidiophores visible within pustules; pustules often becoming confluent and forming continuous lawns of conidia. Pustules formed of intertwined hyphae; hyphae terminating in sterile hairs and producing conidiophores. Sterile hairs straight, projecting beyond the pustule surface, septate. Conidiophores arising laterally from intertwined hyphae, typically constituting 3–5 levels of paired fertile branches, longest fertile branches nearest the conidiophore base, solitary phialides produced near the tip; fertile branches producing phialides directly or often producing paired secondary branches; secondary branches longest near the branching point and reduced to single phialides near the tip of the conidiophore; phialides appearing to be held in whorls; intercalary phialides common (Fig. 8i). Phialides (n = 179) lageniform, (3.7–)5.0–8.0(−11.5) μm long, (2.2–)2.7–3.5(−4.9) μm at the widest point, (1.0–)1.7–2.5(−3.2) μm at the base, L/W (1.1–)1.6–2.9(−4.2), arising from a cell (1.5–)2.5–3.2(−5.0) μm wide. Conidia (n = 180) ellipsoidal to nearly oblong, (2.7–)3.0–5.0(−7.2) × (1.5–)2.0–2.7(−3.5) μm, L/W (1.2–)1.5–2.1(−2.8) (95% ci: 4.0–4.2 × 2.3–2.4 μm, L/W 1.7–1.8), green, smooth. Chlamydospores abundant, subglobose, terminal and intercalary, often in pairs.
Etymology: ‘flagellatum’ refers to the long hairs that protrude from the pustule.
Habitat: endophytic in roots of Coffea arabica.
Known distribution: Ethiopia.
Holotype: Ethiopia, locality and date not known, isolated from surface-sterilized roots of Coffea arabica, T. Mulaw (BPI 882293; ex-type culture C.P.K. 3525 = G.J.S. 10–164 = CBS 130626). Sequence: tef1 = FJ763184.
Additional cultures examined. Ethiopia, all isolated from surface-sterilized roots of Coffea arabica: C.P.K. 3334 = G.J.S. 10–156, sequences: tef1 = FJ763149, chi18-5 = JN258684, rpb2 = JN258688. C.P.K. 3503 = G.J.S. 10–158, sequence: tef1 = FJ763179. C.P.K. 3522 = G.J.S. 10–161, C.P.K. 3523 = G.J.S. 10–162 = CBS 130754, C.P.K. 3524 = G.J.S. 10–163, sequence: tef1 = FJ763183.
Additional cultures not analyzed morphologically: Ethiopia, isolated from surface-sterilized roots of Coffea arabica, C.P.K. 3350, sequences: tef1 = FJ763163, chi18-5 = JN258686. C.P.K. 3345, sequences: tef1 = FJ763158, chi18-5 = JN258685, rpb2 = JN258689.
Comments: Trichoderma flagellatum is common as an endophyte in roots of coffee in Ethiopia. It forms a clade with T. sinense, T. konilangbra and the new species T. gillesii (Druzhinina et al. 2012). These species are known only from Paleotropical/Asian areas, including East Africa (T. flagellatum, T. konilangbra), the Indian Ocean (T. gillesii) or Taiwan (T. sinense). Apart from T. sinense, which has broadly ellipsoidal to subglobose conidia, the members of this clade are morphologically all very similar. All tend to have phialides arranged in whorls and to produce whip-like sterile hairs. Trichoderma gillesii is known only from a single teleomorph collection; it is the only species in the clade that has been linked to a teleomorph and possibly is endemic to Isle de la Réunion in the Indian Ocean, although there has been little or no exploration for Hypocrea in East Africa and the Indian Ocean region. There is no practical way to separate T. flagellatum from T. gillesii; conidia of the single collection of T. gillesii are slightly narrower than those of T. flagellatum.
7. Trichoderma ghanense Yoshim. Doi, Y. Abe & J. Sugiy., Bull. Natl. Sci. Mus. Tokyo Ser. B (Bot.) 13: 3 (1987).
= Trichoderma parceramosum Bissett, Can. J. Bot. 69:2418 (1991).
≡ Trichoderma atroviride Bissett, Can. J. Bot. 62: 930 (1984), non P. Karst.
Teleomorph: none known
Ex-type culture: IAM 13109 = ATCC 208858 = G.J.S. 95–137
Typical sequences: ITS Z69588, tef1 AY937423
This species was first described from soil in Ghana (Doi et al. 1987). Bissett (1984, 1991c) described T. atroviride Bissett (non P. Karst.), later renamed as T. parceramosum (Bissett 1991c), from soils of North Carolina and Virginia. Kuhls et al. (1997) could not distinguish the ex-type strains of T. ghanense and T. parceramosum by their ITS sequences and Samuels et al. (1998) synonymized the species. This synonymy was confirmed by the multilocus analysis of Druzhinina et al. (2012). Trichoderma ghanense has not been reported frequently. Hoyos-Carvajal et al. (2009) did not report it from their survey of soil-inhabiting Trichoderma from South and Central America but we obtained several strains from soil under coffee in Peru and from natural and cultivated soils of Cameroon, Ghana and Nigeria, and a single strain isolated from peat in Italy.
A striking aspect of T. ghanense is its tuberculate conidia. As distinctive as it is, there is considerable variation in this character. In most microscope preparations many or most conidia do not have visible tubercles and typically only one or a few tubercles are seen on individual conidia. The grossly tuberculate conidia described by Doi et al. (1987) for this species are extreme. Conidia of an Italian strain (G.J.S. 05–96) are considerably smaller (4.7 ± 0.5 × 2.5 ± 0.4 μm) than is typical for the species (6.2 ± 0.8 × 3.5 ± 0.4 μm) but in the analysis of Druzhinina et al. (2012) this strain could not otherwise be distinguished within T. ghanense.
Trichoderma ghanense is typically a soil species and has not been linked to a teleomorph. We have studied Peruvian strains isolated from trees and fruits of Theobroma cacao (cacao) infected with destructive parasites, respectively Moniliophthora perniciosa (Witches’ Broom Disease) and the pseudostroma of M. roreri parasitizing cacao pods (Frosty Pod Rot).
8. Trichoderma gillesii Samuels, sp. nov. Figs. 9 and 10.
Fig. 9.
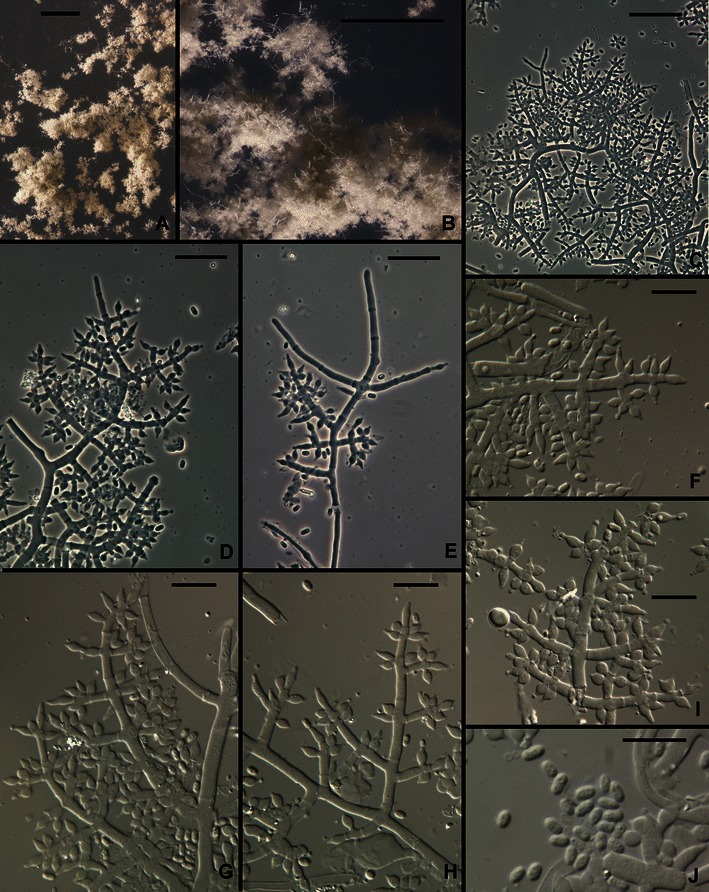
Trichoderma gillesii anamorph. a, b Pustules. c–i Conidiophores (Hairs visible in e). j Conidia. All from SNA. All from G.J.S. 00–72. Scale bars: a = 1 mm, b = 0.25 mm; c–e = 20 μm; f–i = 10 μm
Fig. 10.

Trichoderma gillesii, Hypocrea teleomorph. a, b Stroma morphology. c Stroma surface, macro view. d Stroma surface, micro view. e–g Perithecia, median longitudinal sections showing surface region and internal tissue of stroma. h, i Asci. j Part-ascospores. Note the subglobose part-ascospores in Figs. i and j All from G.J.S. 00–72. Scale bars: a, b = 1 mm; c = 0.5 mm; d, g = 20 μm; e = 50 μm, f = 100 μm; h–j = 10 μm
MycoBank MB 563905
Trichodermati sinensi Bissett, Kubicek et Szakacs simile sed ob conidia anguste ellipsoidea, 3.2–4.0 × 1.7–2.2 μm differt.
Holotypus: BPI 882294.
Teleomorph: Hypocrea sp.
Optimum temperature for growth on PDA and SNA 25–35°C; after 72 h in darkness with intermittent light colony on PDA completely or nearly completely filling a 9-cm-diam Petri plate (slightly slower at 35°C); within 96 h in darkness with intermittent light colony radius on SNA 40–50 mm (slightly faster at 35°C). Conidia forming on PDA within 48–72 h at 25–35°C in darkness with intermittent light; after 1 week on SNA at 25°C under light. No diffusing pigment noted on PDA. Colonies grown on SNA for 1 week at 25°C under light slowly producing pustules. Pustules formed of intertwined hyphae, individual conidiophores not evident, slowly turning green. Conidiophores arising from hyphae of the pustule, typically comprising a strongly developed main axis with fertile lateral branches and often terminating in a sterile terminal extension (‘hair’). Hairs conspicuous, short, stiff erect, sterile, blunt, septate. Fertile branches increasing in length from the tip of the conidiophore, often paired, rebranching to produce either solitary phialides or unicellular secondary branches; secondary branches terminating in a whorl of 3–5 divergent phialides. Intercalary phialides not seen. Phialides lageniform, nearly obovoidal, typically widest below the middle, (4.0–)4.5–7.0(−9.5) μm long, (2.2–)2.5–3.0(−3.2) μm at the widest point, base (1.2–)1.5–2.0(−3.0) μm wide, L/W (1.4–)1.5–2.5(−3.5) μm, arising from a cell (1.7–)2.0–3.0(−3.7) μm wide. Conidia ellipsoidal, (3.0–)3.2–4.0(−4.5) × (1.5–)1.7–2.2(−2.5) μm, L/W (1.4–)1.5–2.2(−2.5), green, smooth. Chlamydospores not observed.
Teleomorph: Stromata brown, discoidal, margins slightly free, 3–4 mm diam, cespitose and covering an area ca. 15 mm diam, surface plane to undulate, conforming to the surface of the substratum and adjacent stromata, ostiolar openings appearing as minute black papillae, no reaction to 3% KOH, ostiolar area greenish in lactic acid. Cells of the stroma surface in face view pseudoparenchymatous, ca. 5.5 × 4.5 μm diam, slightly thick-walled. Perithecia elliptical in section, 220–250 μm high, 130–190 μm wide, ostiolar region formed of small cells and gradually merging with the cells of the surrounding stroma surface. Stroma surface region 15–20 μm wide, cells pigmented, pseudoparenchymatous, 4–6 μm diam, walls 2–4 μm thick. Tissue between perithecia hyphal. Stroma interior below perithecia formed of degenerating, large-celled hyphae. Part-ascospores monomorphic, subglobose, distal part (2.7–)3.0–3.5(−3.7) × (2.2–)2.7–3.5 μm, proximal part (2.2–)2.7–3.5(−2.2) × (2.5–)3.0–3.2(−3.5) μm, finely spinulose, hyaline. Asci cylindrical, (43–)51–63– (67) × (3.0–)3.5–4.5(−4.7) μm, apex thickened and with a ring.
Etymology: named in honor of G. Gilles, French entrepreneur and collector of tropical Hypocreales.
Habitat: bark.
Known distribution: known only from the type locality.
Holotype: France, Isle de la Réunion, Salazie, on dead wood, 11 March 2000, G. Gilles comm F. Candoussau 690 (BPI 882294, and a dried culture ex ascospores of Hypocrea sp. BPI 842330; ex-type culture CBS 130435 = G.J.S. 00–72). Sequences: tef1 = JN175583, cal1 = JN175409, chi18-5 = JN175468, rpb2 = JN175527.
Comments: In this species there is a tendency for phialides to be held in divergent whorls. The dark brown, somewhat peltate stromata with an ostiolar area that is green in lactic acid and the subglobose Part-ascospores strongly suggest H. jecorina, the teleomorph of the pantropical species T. reesei. Trichoderma gillesii belongs in a clade with T. aethiopicum, T. konilangbra, and T. sinense. The closest relative (Druzhinina et al. 2012) of T. gillesii is T. sinense, which is known only from Taiwan and which has subglobose conidia. Trichoderma gillesii has the most narrow conidia in the clade. For a further discussion of members of this clade see T. flagellatum.
9. Trichoderma gracile Samuels et Szakacs, sp. nov. Figs. 2g, h and 11.
Fig. 11.
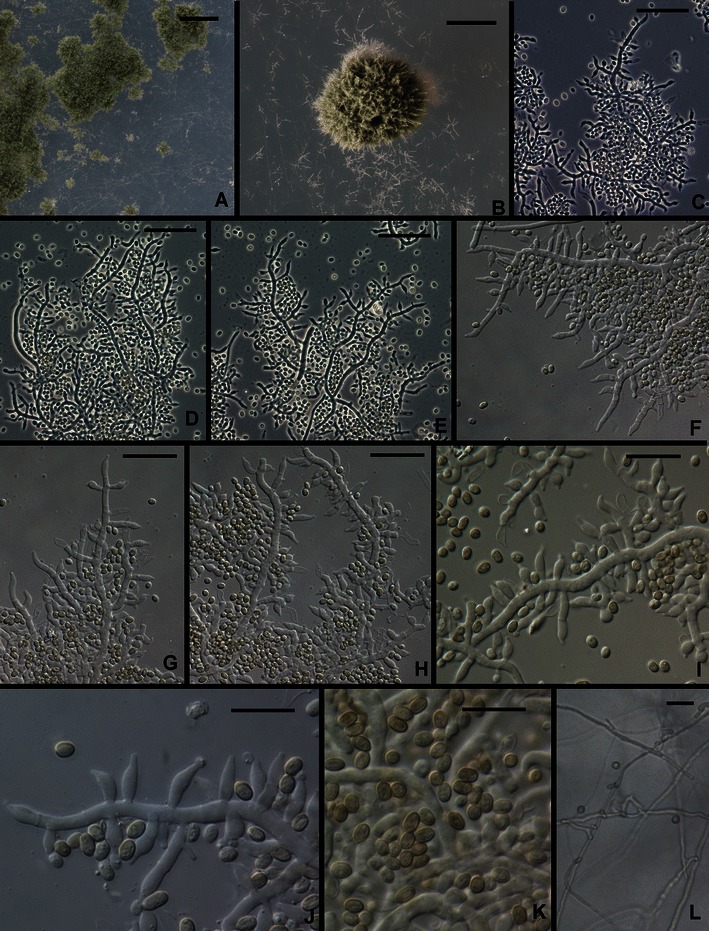
Trichoderma gracile. a, b. Pustules. c–j. Conidiophores (Arrows in e, j show intercalary phialides). k Conidia. l Chlamydospores. All from SNA. All from G.J.S. 10–263. Scale bars: a = 1 mm, b = 0.5 mm; c–h, l = 20 μm; i–k = 10 μm
MycoBank MB 563906
Trichodermati longibrachiato Rifai simile sed ob incrementum tardius, radium coloniae < 60 mm in agaro dicto PDA post 72 h ad temperaturam 35°C distinguendum.
Holotypus: BPI 882295
Teleomorph: none known
Optimum temperature for growth on PDA and SNA 25–30°C; after 96 h in darkness with intermittent light colony on PDA completely or nearly completely filling a 9-cm-diam Petri plate, somewhat slower at 25°C; within 96 h in darkness with intermittent light completely filling a 9-cm-diam Petri plate, somewhat slower at 30°C. A yellow diffusing pigment forming on PDA within 48 h at 25–35°C; conidia only appearing in colonies incubated at 35°C, on PDA after 96 h in colonies incubated in darkness (not under fluorescent light), on SNA in colonies incubated in darkness or under light. Conidial production sparse. Pustules formed on SNA gray green, 0.5–1 mm diam, hemispherical or pulvinate, with stiff, erect, terminally fertile projecting conidiophores. Individual conidiophores not visible within pustules. Pustules formed of intertwined hyphae. Conidiophores arising from hyphae of the pustule, comprising a more or less long main axis with laterally produced solitary phialides and fertile branches; solitary phialides produced over 50–75 μm of the tip of the conidiophore; fertile branches increasing with length from the tip of the conidiophore, producing solitary phialides along the length; often branches comprising a single phialide terminating a basal cell with a short, spur-like intercalary phialide formed as an outgrowth of the basal cell at the septum (Fig. 11j). Phialides (n = 30) typically lageniform, often somewhat swollen below the middle, straight, rarely hooked or sinuous, (5.0–)5.5–9.0(−11.7) μm long, (2.0–)2.5–3.2(−4.0) μm at the widest point, L/W (1.5–)1.8–3.6(−5.1), base (1.0–)1.5–2.2(−2.7) μm, arising from a cell 2.0–3.0(−3.5) μm wide. Conidia (n = 30) narrowly ellipsoidal to nearly oblong, (3.0–)3.2–3.7(−4.5) × (2.0–)2.2–2.5(−2.7) μm, L/W (1.1–)1.3–1.7(−2.0) (95% ci: 3.4–3.6 × 2.3–2.4 μm, L/W 1.4–1.6), green, smooth. Chlamydospores abundant, subglobose, terminal and intercalary.
Etymology: “gracile” refers to the slender fertile parts of conidiophores that produce solitary phialides over a relatively long distance.
Habitat: bark.
Known distribution: Malaysia, known only from the type collection.
Holotype: Malaysia, Pasir Panjang island, isolated from tree bark, date not known, G. Szakacs TUB F-2543 (BPI 882295; ex-type culture CBS 130714 = G.J.S. 10–263). Sequences: tef1 = JN175598, cal1 = JN175427, chi18-5 = JN175488, rpb2 = JN175547.
Comments: Trichoderma gracile is unusual in the Longibrachiatum Clade for its sparing production of conidia, and then typically only at high temperature and, at least on PDA, in darkness with only intermittent exposure to light. This species belongs in a clade with T. reesei and T. parareesei (Druzhinina et al. 2012).
10. Trichoderma konilangbra Samuels, O. Petrini & Kubicek, Stud. Mycol. 41: 21 (1998).
Teleomorph: none known
Ex-type culture: CBS 100808 = ATCC 208860 = IMI 378807
Typical sequences: ITS AF012763, tef1 AY937425.
This species was based on three collections isolated from three soil samples made in the Ruwenzori Mountains of Uganda at elevations of 1,700–3,400 m. We have not seen it since its original description. It belongs in a clade with T. flagellatum, T. gillesii, and T. sinense (Druzhinina et al. 2012). For a discussion of this clade see T. flagellatum.
11. Trichoderma longibrachiatum Rifai, Mycol. Pap. 116: 42 (1969).
Teleomorph: none known
Ex-type culture: ATCC 18648 = CBS 816.68
Typical sequences: ITS Z31019, tef1 AY937412
This species was redescribed and illustrated in Bissett (1984), Samuels et al. (1998), Gams and Bissett (1998) and http://nt.ars-grin.gov/taxadescriptions/keys/trichodermaindex.cfm. Although it was described originally from soil in the USA (Ohio), it is more common in tropical than temperate regions. Sperry et al. (1998) isolated it from within a continuously submerged marine sponge. Although it is sometimes reported as a mycoparasite and potential biocontrol agent (e.g. Sanchez et al. 2007), it has also been isolated from immunocompromised humans (Kuhls et al. 1997; Kredics et al. 2003). Its ability to grow at human body temperature should give caution to those who would wish to develop this species as a biocontrol agent.
Apparently T. longibrachiatum is a clonal species. Kuhls et al. (1997) and Samuels et al. (1998) noted that T. longibrachiatum and Hypocrea orientalis could not be distinguished on the basis of ITS sequences but for reasons of phenotype, they did not consider the two to represent a single species. The distinction was supported by MALDI-TOF MS by De Respinis et al. (2010) and by multilocus phylogenetic analysis and Druzhinina et al (2008) postulated that T. longibrachiatum and H. orientalis could have evolved in parallel from a common species forming two sympatric species. However in the multilocus analysis of Druzhinina et al. (2012) H. orientalis and T. longibrachiatum clearly represent a species complex within which there are several well-supported internal lineages, some of which we recognize here as distinct sister species, viz. T. aethiopicum and T. pinnatum, the latter derived from ascospores of a collection made in Sri Lanka but also isolated from soil in Vietnam. The single strain CBS 243.63, based on an ascospore culture from New Zealand, is a distinct phylogenetic lineage; however the culture appears to be degenerated and the collection from which it was made cannot be located.
12. Hypocrea novae-zelandiae Samuels & O. Petrini in Samuels et al., Stud. Mycol. 41: 25 (1998; as ‘novaezelandiae’).
Anamorph: Trichoderma sp.
Ex-type culture: G.J.S. 81–265 = CBS 639.92 = ATCC 208856
Typical sequences: ITS DQ083019, tef1 X93969
This species was based originally on two collections made in native Nothofagus forests of New Zealand (Samuels et al. 1998) and remains known only from New Zealand, where it is not uncommon.
Hypocrea novae-zelandiae occupies a basal position in the Longibrachiatum Clade (Druzhinina et al. 2012). It forms a clade with the new species T. saturnisporopsis and the phylogenetic species G.J.S. 99–17. Within this clade there are two morphologically unequivocal groups: one with ellipsoidal to oblong, smooth conidia and known only from sexual spores (H. novae-zelandiae) and one apparently clonal group having ellipsoidal, grossly tuberculate conidia (Tr 175: USA: OR; S19: Sardinia; G.J.S. 99–17: Japan). The strains having warted conidia represent two phylogenetic species that are discussed below under T. saturnisporopsis.
13. Hypocrea orientalis Samuels & O. Petrini in Samuels et al., Stud. Mycol. 41: 30 (1998). Figures 3a–c and 12.
Fig. 12.
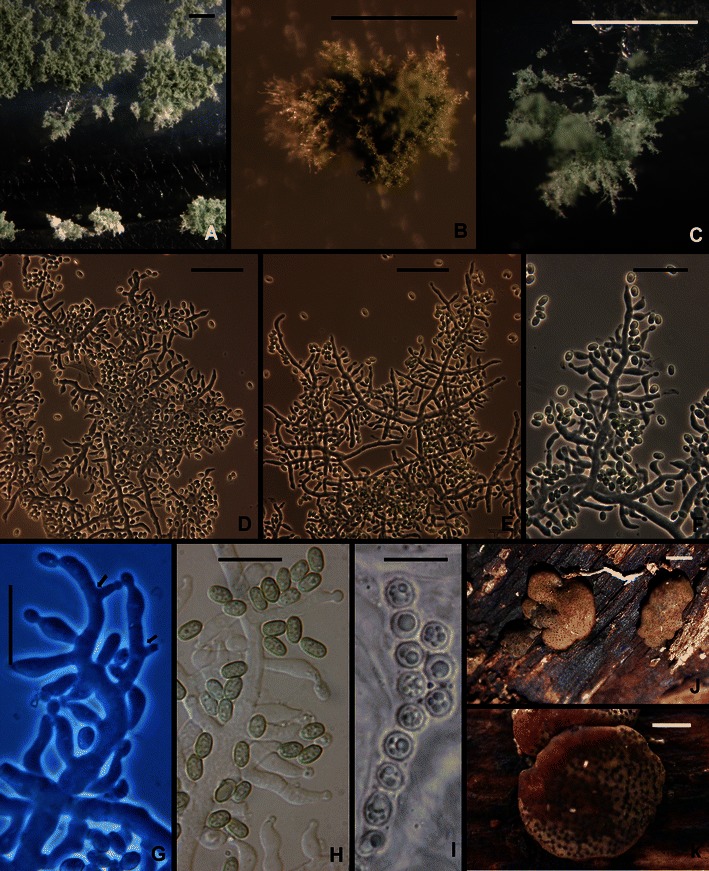
Hypocrea orientalis. a–c Pustules. d–f Conidiophores. g Phialides. arrows show intercalary phialides. h Conidia. i Part-ascospores; note the globose to subglobose shape. j, k Stromata. a–g from SNA. a, c, g from G.J.S. 04–316; b, d–f, h from DIS 270f; i–k from WU 31609. Scale bars a = 0. 5 mm; b, c = 250 μm; d–f = 20 μm; g–i = 10 μm; j, k = 1 mm
Anamorph: Trichoderma sp.
Ex-type culture: G.J.S. 88–81 = CBS 130428
Typical sequences: ITS EU401550, tef1 EU401581
This species was originally based on a single Hypocrea collection made in tropical Yunnan Province of China and until recently was known only from that collection. Samuels et al. (1998) hypothesized that this could be the teleomorph of T. longibrachiatum; but this has been disproven; see T. longibrachiatum for additional comments. In the present work we report an additional teleomorph collection from the Canary Islands (La Palma), and clonal collections from East Africa (Zambia, 1) and South America (Brazil, 2; Ecuador, 1; Peru, 19). In addition, Druzhinina et al. (2008) reported it as an anamorphic isolate from Europe (the strain G.J.S. 91–157 is from Germany, not Switzerland as reported by Druzhinina et al.), Costa Rica, South Africa, Sierra Leone and New Zealand. Hoyos-Carvajal et al. (2009) did not isolate it in their study of Trichoderma from South America. We isolated the species as an endophyte from leaves of wild Theobroma cacao in Peru as well as from soil at the base of wild cacao trees; we also found it growing in Peru on the pseudostroma of the cacao pathogen Moniliophthora roreri, cause of the destructive Frosty Pod Rot of cacao. Three of the strains reported by Druzhinina et al. (2008) were isolated from human patients, one from a child with acute lymphoblastic leukemia (provenance unknown), one from a peritoneal catheter tip (Canada, Nova Scotia) and one from the stool of a pediatric patient (provenance unknown).
Hypocrea orientalis is a member of a large clade of common, morphologically homogeneous species that includes T. longibrachiatum, T. aethiopicum, T. pinnatum and phylogenetic species CBS 243.63 (Druzhinina et al. 2012).
Following is a revised description of H. orientalis based on recent collections.
Optimum temperature for growth on PDA 25–35°C, on SNA 30–35°C; colony on PDA and SNA after 96 h in darkness with intermittent light completely or nearly completely filling a 9-cm-diam Petri plate; on PDA only slightly slower at 20°C; on SNA only slightly slower at 25°C. Conidia typically forming in concentric rings on PDA and SNA within 48 h at 20–35°C in darkness; a yellow pigment often intense, forming or not within 72 h at 20–30°C, not forming at 35°C. In colonies grown on SNA in darkness with intermittent light conidia typically beginning to form within 48 h at 25–35°C, conidia more abundant at higher than at lower temperatures. In colonies grown 1 week at 25°C under light conidial pustules forming in obscure concentric rings; hyphae of pustules more or less cottony or more dense, individual conidiophores or fascicles of conidiophores visible as ‘spikes’ or columns; hairs lacking. Pustules formed of intertwined hyphae, individual conidiophores arising along hyphae of the pustule, comprising a long central axis with 5–7 levels of solitary phialides below the tip before branching; solitary phialides arising from branches, intercalary phialides common (Fig. 12g). Phialides (n = 180) lageniform, straight or less frequently hooked, asymmetric or sinuous, (3.5–)6.2–10.5(−15.7) μm long, (2.0–)2.5–3.7(−4.5) μm at the widest point, L/W = (1.3–)1.6–3.8(−7.7), base (1.0–)1.7–2.7(−3.5) μm wide, arising from a cell (1.5–)2.5–4.0(−5.5) μm wide. Conidia (n = 180) oblong to ellipsoidal, (3.2–)3.7–6.2(−10.5) × (2.0–)2.5–3.5(−5.2) μm. L/W = (1.1–)1.3–2.5(−4.9) (95% ci: 4.9–5.2 × 2.8–3.0 μm, L/W 1.8–2.0), green, smooth. Chlamydospores typically forming on SNA, terminal and intercalary, subglobose to clavate, (4.5–)6.2–9.0(−14.0) μm diam.
Teleomorph: Stromata scattered or aggregated in small groups of 2–4, when fresh ca. 1–4 mm diam, linear aggregates up to 8 mm long, up to 1.5 mm thick; pulvinate or discoid to undulate, surface glabrous or slightly velutinous, grayish olive when immature, light brown or orange-brown to dull dark brown with olive tones, with nearly black ostiolar dots. Stromata when dry (1.0–)1.2–2.5(−3.2) × (1.0–)1.2–2.0(−2.7) mm, 0.2–0.7(−1.0) mm high (n = 20), discoid with concave top, or pulvinate, with circular, oblong or irregularly lobate outline, often margin free to a large extent (narrow attachment); starting as a yellow compacted mycelium, immature distinctly velutinous, light olive with a yellowish tone, later olive-brown, less commonly orange-brown, with delicate, more or less stellate fissures 45–110 μm long, later with distinct, even or convex black ostiolar dots (39–)48–78(−102) μm diam (n = 30), often surrounded by torn, crumbly cortex; when old collapsing to thin, rugose, dark (olive-) brown crusts. Spore deposits whitish. Ostioles apically green in lactic acid. Asci cylindrical, (74–)78–89(−93) × (5.2–)5.8–6.7(−7.0) μm, apex truncate, with an inconspicuous apical ring. Part-ascospores monomorphic, globose or subglobose; distal cell (3.2–)3.7–4.5(−4.7) × (3.5–)3.7–4.2(−4.7) μm, l/w (0.9–)1.0–1.1(−1.2) (n = 30), proximal cell (3.7–)4.0–4.7(−5.0) × (3.5–)3.7–4.5(−4.7) μm, l/w 1.0–1.2(−1.3) (n = 30), ascospore basal in the ascus typically laterally compressed, dimorphic; verrucose with warts ca. 0.5 μm long.
Known distribution: Europe (Germany), Canary Islands (La Palma), China, East Africa (Sierra Leone, Zambia), South Africa, Central America (Costa Rica), South America (Brazil, Ecuador, Peru). Teleomorph confirmed only from China and the Canary Islands.
Habitat: wood and fungi growing on it (teleomorph), soil.
The above description of the teleomorph is based on the following collection: Spain, Canarias, La Palma, Cumbre Nueva, Castanea plantation at the road LP 301, close to crossing with LP 3; on dead branches 2–10 cm thick of Castanea sativa, on wood, soc. and on Annulohypoxylon multiforme, soc. Bisporella sulfurina, Hypocrea cf. viridescens and Terana caerulea, 13 Dec 2009, W. Jaklitsch S187 (WU 31609; culture CBS 131488).
14. Trichoderma parareesei Jaklitsch, Druzhinina & Atanasova in Atanasova et al., Appl. Environ. Microbiol. 76: 7261 (2010). Figures 3d and 13.
Fig. 13.
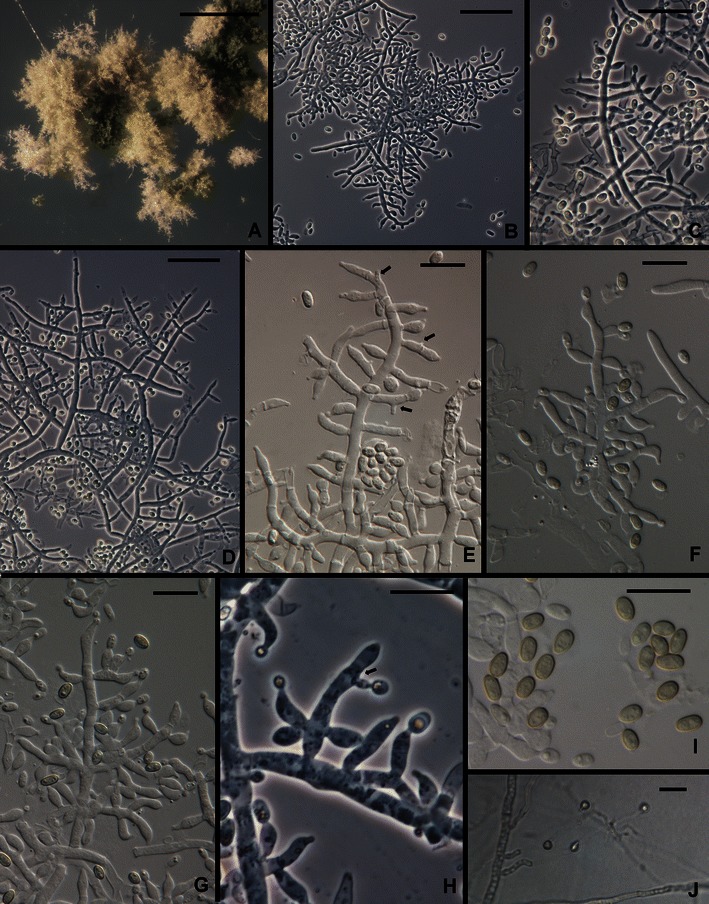
Trichoderma parareesei. a Pustules. b–h Conidiophores and phialides (Arrows in e, h show intercalary phialides). i. Conidia.. j. Chlamydospores. All from SNA. a, d, e from G.J.S. 10–168; b, f, g, i from G.J.S. 07–26; c, from G.J.S. 04–41; h, j from G.J.S. 04–250. Scale bars: a = 0.5 mm; b–d, j = 20 μm; e–i = 10 μm
Teleomorph: none known
Ex-type culture: C.P.K. 717 = CBS 125925 = TUB F-1066
Typical sequences: ITS HM466668 (G.J.S. 04–41), tef1 GQ354353
Trichoderma parareesei is sister to H. jecorina/T. reesei in a clade that includes also T. gracile (Druzhinina et al. 2012). Trichoderma parareesei is a pantropical/subtropical clonal species that shares a common ancestor with the holomorphic T. reesei (H. jecorina teleomorph).
Following is a redescription of T. parareesei based on newly discovered American collections: Optimum temperature for growth on PDA (Difco) and SNA 30–35°C; on PDA and SNA slightly faster at 35°C, completely filling a 9-cm-diam Petri plate within 48–72 h; on SNA filling a 9-cm-diam Petri within 96 h at 25–35°C. Conidia forming on PDA within 48 h at 25–35°C; on SNA within 72–96 h, rarely as early as 48 h. An often intense yellow pigment diffusing on PDA within (48–)72 h at 25–35°C. After one wk on PDA at 25°C under light a 9-cm-diam Petri plate completely filled with yellow-green conidia in a dense lawn in a few obscure concentric rings; on SNA conidia forming in a few obscure concentric rings in the aerial mycelium and in minute, often confluent, cottony pustules; individual conidiophores visible within pustules, pustules lacking sterile hairs or long protruding, terminally fertile conidiophores. Pustules formed of intertwined hyphae. Conidiophores arising along hyphae of the pustule, typically comprising a long central axis with up to several levels of solitary phialides before commencement of lateral branching; lateral branches often comprising a single cell terminated by a single phialide or up to ca. four cells in length with solitary phialides arising near the tip and single cells terminated by a solitary phialide toward the base at the main axis; intercalary phialides common (Fig. 13e, f, h). Phialides (n = 150) lageniform, swollen or not at the middle, straight, less frequently sinuous, asymmetric or hooked, (3.2–)5.7–9.0(−13.0) μm long, (2.0–)2.5–3.2(−4.0) μm at the widest point, L/W = (1.1–)2.0–3.2(−5.0), base (1.0–)1.5–2.5(−3.2) μm, arising from a cell (1.5–)2.2–3.2(−4.5) μm wide. Intercalary phialides common. Conidia (n = 191) ellipsoidal to oblong, (3.2–)3.7–4.7(−6.2) × (1.7–)2.5–3.0(−3.5) μm, L/W = (1.2–)1.4–1.8(−2.7) (95% ci: 4.1–4.2 × 2.5–2.6 μm, L/W = 1.5–1.6), green, smooth. Chlamydospores not common, subglobose to pyriform, mainly terminal.
Known distribution: Argentina (Iguaçu), Brazil, Ethiopia, Ghana, Mexico, Sri Lanka, Taiwan, Vietnam.
Habitat: soil.
Comments: This description emends somewhat the original description of T. parareesei (Atanasova et al. 2010) in that we have observed conidia to be somewhat narrower (2.8–3.2 μm in the protologue) and to have a narrower range of L/W (1.3–1.5 in the protologue). We have also observed a considerably slower growth rate on SNA in the Samuels lab for both T. reesei and T. parareesei than was recorded in the protologue. These differences possibly reflect the greater number of strains used in the present study. The conidial dimensions given in the description here include those of the two strains included in Atanasova et al. (2010). In agreement with Atanasova et al. (2010) we observed in cultures of the two species on PDA, incubated at 25°C under light that T. parareesei produced considerably more conidia than did T. reesei.
15. Trichoderma pinnatum Samuels, sp. nov. Figs. 3e, f and 14.
Fig. 14.
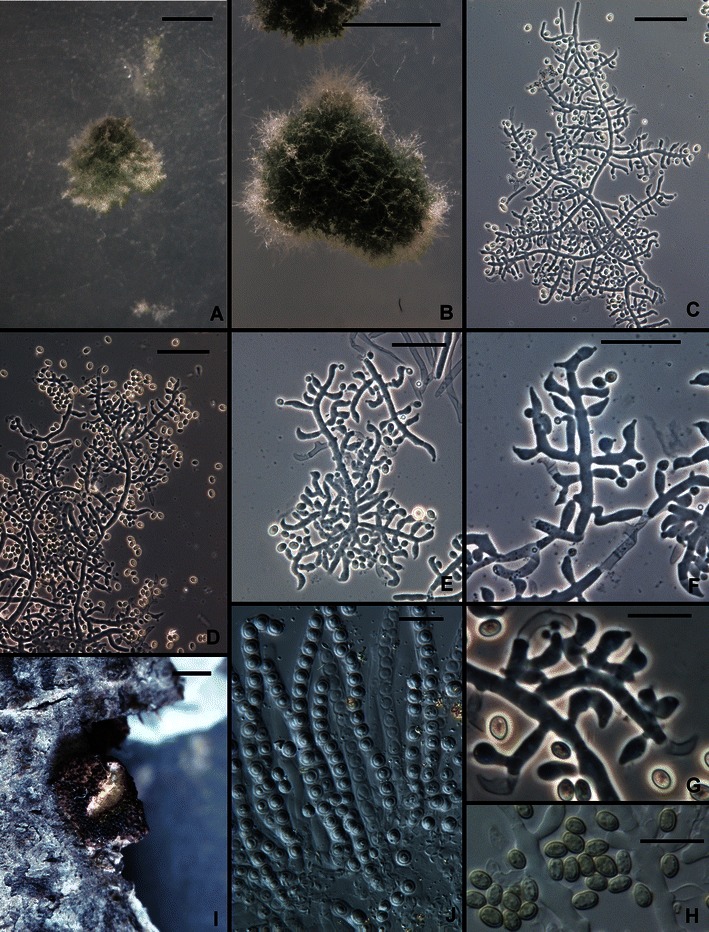
Trichoderma pinnatum. a, b Pustules. c–g Conidiophores. h Conidia. i Overmature stroma. J. Asci with subglobose part ascospores. a–h From SNA. a, c, e–j from G.J.S. 02–120; b, d from G.J.S. 04–100. Scale bars: a, b = 0.5 mm; c–f = 20 μm; g, h, j = 10 μm; i = 1 mm
MycoBank MB 563908
Trichodermati aethiopico Mulaw, Kubicek et Samuels simile sed ob conidia majora, 2.5–3.5 × 2.5–3.0 μm, differt.
Holotypus: BPI 882296
Teleomorph: Hypocrea sp.
Optimum temperature for growth on PDA 30–35°C, on SNA 30°C; on PDA after 72 h at 30–35°C in darkness with intermittent light colony completely filling a 9-cm-diam Petri plate; on SNA after 96 h at 25–30°C in darkness with intermittent light completely filling a 9-cm-diam Petri plate, slightly slower at 35°C. Conidia and a pale yellow diffusing pigment forming within 24 h at 30–35°C and within 48 h at 20–25°C in colonies grown on PDA in darkness with intermittent light; on SNA conidia appearing somewhat later, within 48 h at 30–35°C and within 72 h at 25°C. Colonies grown on PDA for 1 week at 25°C under light producing conidia in abundance in scattered blue green to dark green pustules, sometimes in concentric rings. Colonies grown on SNA for 1 week at 25°C under light producing scattered pustules; pustules hemispherical, 0.25–1 mm diam, dark green, lacking hairs. Individual conidiophores visible within pustules on SNA; pustules formed of intertwined hyphae. Conidiophores arising from hyphae within pustules, typically comprising a main axis producing solitary phialides; intercalary phialides infrequent. Phialides (n = 60) typically lageniform, straight, sinuous or hooked, (4.2–)5.5–9.0(−12.0) μm long, (2.0–)2.5–3.5(−4.2) μm at the widest point, L/W (1.3–)1.5–3.5(−5.0), base (1.2–)1.5–2.2(−2.7) μm wide, arising from a cell (1.7–)2.0–3.0(−4.0) μm wide. Conidia (n = 60) ellipsoidal, (2.2–)2.5–3.5(−5.0) × (1.7–)2.5–3.0(−3.5) μm, L/W (1.2–)1.3–1.7(−1.0) (95% ci: 3.9–4.1 × 2.6–2.7 μm, L/W 1.5–1.6), green, smooth. Chlamydospores not observed.
Teleomorph: Stromata discrete, circular, 1.0–1.5 mm diam, slightly constricted at the base, light brown, surface rugose from protruding perithecial papillae, not reacting to 3% KOH. Asci (n = 30) cylindrical, (59–)61–71(−78) × (4.0–)4.5–5.5(−6.7) μm, apex thickened and with a ring. Part-ascospores (n = 30) monomorphic, subglobose, (2.5–)3.2–3.7(−4.2) μm diam, finely warted, hyaline.
Etymology: ‘pinnatum’ refers to the more or less pinnately arranged phialides that are typical of the Longibrachiatum Clade.
Habitat: soil, teleomorph on wood.
Known distribution: Vietnam, Sri Lanka.
Holotype: Vietnam, Tp. Ho Chi Minh City, Trung Tâm Nông Lâm Ngu, from soil, 2004, Le Dinh Don T-17 (BPI 882296; ex-type culture G.J.S. 04–100 = CBS 131292). Sequences: tef1 = JN175571, czl1 = JN175395, chi18-5 = JN175453, rpb2 = JN175515.
Paratype: Sri Lanka, Southern Province, Yala National Park, Block 1, ca. 10 km NE of park headquarters, elev. 23 m, 06°21′N, 81°27′E, teleomorph on wood, 18 Dec. 2002, G.J. Samuels 9345, A. Nalim, N. Dayawansa (BPI 871415; culture G.J.S. 02–120, dead). Sequences: tef1 = JN175572, cal1 = JN175396, chi18-5 = JN175454, rpb2 = JN175516.
Comments: Trichoderma pinnatum is known only from two widely separated collections, one a Hypocrea collection from Sri Lanka and the other an isolation from soil from Vietnam. The Sri Lankan ascospore-derived culture has been lost, thus we designate the Vietnamese collection from soil as the holotype. Its closest relationships are with T. aethiopicum and T. longibrachiatum (Druzhinina et al. 2012). Within this clade conidia of T. aethiopicum and CBS 243.63 are diagnostic, the former being the smallest and the latter the largest. Trichoderma pinnatum cannot be distinguished from the common species T. longibrachiatum on the basis of morphology.
The Hypocrea collection of T. pinnatum consists of two pieces of bark and a few old stromata. The degenerated tissues of the stromata did not permit us to describe stromal anatomy. The monomorphic, subglobose Part-ascospores are typical of members of the Longibrachiatum Clade. Hypocrea jecorina, the teleomorph of T. reesei, was described from Sri Lanka, where the two morphologically similar and related species are apparently sympatric. We have not seen collections of T. reesei from Vietnam, although this species has a wide tropical distribution including Southeast Asia.
16. Trichoderma pseudokoningii Rifai, Mycol. Pap. 116: 45 (1969).
Teleomorph: Hypocrea pseudokoningii Samuels & O. Petrini, Stud. Mycol. 41: 36 (1998).
Ex-type culture: NS19 = CBS 408.91 = ATCC 208861 = DAOM 167678
Typical sequences: ITS Z31014, tef1 EU280037
Trichoderma pseudokoningii is one of the nine species aggregates proposed by Rifai (1969). It was included by Bissett (1984) in Trichoderma sect. Longibrachiatum and by Kuhls et al. (1997) and Samuels et al. (1998) in their revision of the H. schweinitzii species complex. It was redescribed by Gams and Bissett (1998) and online at http://nt.ars-grin.gov/taxadescriptions/keys/trichodermaindex.cfm. The ex-type culture of T. pseudokoningii was derived from ascospores of a Hypocrea collected in South Australia and subsequently described as Hypocrea pseudokoningii (Samuels et al. 1998). Kuhls et al. (1997) re-identified several strains that had been identified as T. pseudokoningii as T. longibrachiatum Rifai or T. citrinoviride Bissett. Trichoderma pseudokoningii is not common outside of Australasia although Samuels et al. (1998) reported individual strains isolated from soil from the USA (New Hampshire) and Sri Lanka based on their ITS sequences; perithecial collections are common in New Zealand or southern Australia. Because this species is rare outside of Australasia, the frequent reports of this species in the biological control and genomics literature are possibly based on misidentified strains.
Trichoderma pseudokoningii shares a common ancestor with T. citrinoviride in a moderately well supported clade that includes the rare species T. effusum and T. solani (Druzhinina et al. 2012). T. citrinoviride and T. pseudokoningii comprise a teleomorph and both have black, gray, or dark green to nearly black stromata. This is in contrast to most of the teleomorphs in the Longibrachiatum Clade (H. andinensis, H. jecorina/T. reesei, H. orientalis, H. novae-zelandiae, T. pinnatum, T. gillesii), which have light to dark brown stromata. Trichoderma effusum and T. solani are, morphologically, highly divergent in the Longibrachiatum Clade, dissimilar to each other and to T. citrinoviride and T. pseudokoningii. The conidiophore morphology of T. pseudokoningii is somewhat atypical in the Longibrachiatum Clade because of the tendency for phialides to be disposed in whorls.
17. Trichoderma reesei E.G. Simmons, Abstr. Second International Mycological Congress Vol. M–Z. p. 618 (1977).
Teleomorph: Hypocrea jecorina Berk. & Broome, J. Linn. Soc. Bot. 14: 112 (1873).
Ex-type culture: QM 6a = ATCC 13631 = CBS 383.78
Typical sequences: ITS Z31016 (ATCC 13631), tef1 DQ025754 (ATCC 24449, a mutant of QM 6a).
Trichoderma reesei is probably the best known species in the genus because of its extraordinary ability to produce cellulolytic and hemicellulolytic enzymes used for hydrolysis of lignocelluloses in the food and feed industry, manufacture of textiles and production of biofuels (see references in Harman and Kubicek 1998; Kubicek et al. 2009). It was originally isolated from rotting canvas fabric in the Solomon Islands in the 1940’s and until 1997 was known from only a single strain, QM 6a (Simmons 1977). It has since been found to have a wide tropical distribution where its teleomorph is common (Kubicek et al. 1996; Lieckfeldt et al. 2000). The genome of T. reesei was published by Martinez et al. (2008).
Trichoderma reesei forms a clade with T. parareesei and T. gracile, which is sister clade to the clade that includes T. longibrachiatum and H. orientalis (Druzhinina et al. 2012). There are very few morphological features to distinguish the species in these clades from each other or from the more distantly related T. citrinoviride. All fill a 9-cm-diam PDA Petri plate within 72 h at 35°C and produce diffusing yellow pigment and conidia on PDA within 48 h at 25–35°C. Trichoderma reesei tends to produce fewer conidia on PDA and SNA than the other species, and sterile hairs arise from pustules of T. citrinoviride on SNA but not the other species. Bissett (1984) synonymized T. reesei under T. longibrachiatum based on their considerable shared morphology but molecular phylogenetic analyses separate them (e.g. Kuhls et al. 1996; Druzhinina et al. 2012). Druzhinina et al. (2010) and Atanasova et al. (2010) distinguished T. parareesei from T. reesei, the former a genetically isolated, clonal sister species.
18. Trichoderma saturnisporopsis Samuels et Jaklitsch, sp. nov. Figs. 3g and 15.
Fig. 15.
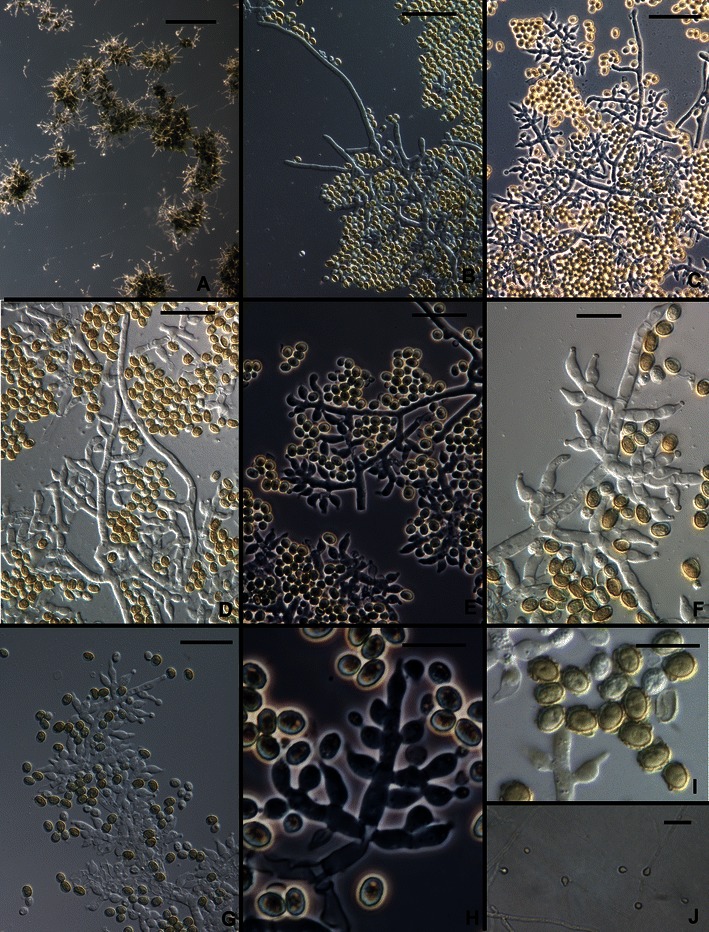
Trichoderma saturnisporopsis. a Pustules. b–h Conidiophores (hairs seen in b–d). i Conidia. j Chlamydospores. All from SNA. a–d, f, i from Tr 175; e, g, h, j from Jaklitsch S 19. Scale bars: a = 0.5 mm; b–e, g, j = 20 μm; f, h, i = 10 μm
MycoBank MB 563910
Trichodermati saturnisporo Hammill simile sed in temperatura minore (25–30°C) magis celeriter crescens. Conidia late ellipsoidea, 4.2–5.0 × 3.5–4.0 μm, tuberculata vel laevia.
Holotypus: BPI 882297
Teleomorph: none known
Optimum temperature for growth on PDA and SNA 25–30°C; after 96 h in darkness with intermittent light colony on PDA completely or nearly completely filling a 9-cm-diam Petri plate; within 96 h in darkness with intermittent light colony radius on SNA 20–25 mm (60 mm in strain TR 175). Conidia forming on PDA and SNA within 96 h at 25–35°C in darkness with intermittent light. Colonies grown on PDA for 1 week at 25°C under light producing conidia densely beginning in the center of the colony, forming concentric rings, more or less gray-green to dark green; no distinctive odor; sometimes with a pale diffusing yellow pigment. Colonies grown on SNA for 1 week at 25°C under light producing pustules in one or two concentric rings beginning in the center of the colony; pustules flat to hemispherical, becoming confluent; formed of intertwined hyphae, producing stiff, erect, straight, septate, sterile hairs with blunt ends. Conidiophores variable; sometimes comprising a rather wide discernable central axis with paired lateral branches, the branches increasing in length from the tip, each branch re-branching to produce solitary phialides or convergent or divergent whorls of phialides; the tip of the conidiophore often elongated into a sterile hair; sometimes fertile branches arising singly and at irregular intervals along hyphae of the pustule, producing mainly solitary phialides; sometimes phialides densely clustered in convergent heads at the tips of short branches of hyphae. Phialides (n = 60) lageniform to ampulliform, straight, widest below the middle, (4.0–)5.7–10.5(−14.0) μm long, (2.2–)3.0–3.7(−5.5) μm at the widest point, L/W (1.3–)1.6–3.2(−5.5), base (1.0–)1.5–2.5(−3.2) μm wide, arising from a cell (1.7–)2.2–3.2(−4.5) μm wide. Intercalary phialides rare. Conidia (n = 90) broadly ellipsoidal, (3.7–)4.2–5.0(−6.0) × (2.5–)3.2–4.0(−4.5) μm, L/W (1.0–)1.1–1.5(−1.9) (95% ci: 4.5–4.7 × 3.5–3.7 μm,. L/W 1.3–1.4), green, typically conspicuously tuberculate, less frequently tubercles few. Chlamydospores uncommon, terminal and intercalary, globose, ellipsoidal or pyriform.
Etymology: ‘saturnisporopsis’ refers to morphological similarity to T. saturnisporum.
Habitat: roots, branches.
Known distribution: USA (OR), Sardinia.
Holotype: USA, Oregon. Oregon Coast Range: 46°1′N, 123°4′W; elev. 420 m, from fumigated roots of Douglas Fir (Pseudotsuga menziesii) infected with Phellinus weirii, 1983, E. Nelson 15(BPI 882297; ex-type culture TR 175 = CBS 130751). Sequences: tef1 = JN182281, chi18-5 = JN182299, rpb2 = DQ857348. See Nelson et al. (1987), as No. 15.
Additional culture: Italy, Sardinia, at the road SP17, between junctions to Burgos and Foresta di Burgos, on a branch of Quercus virgiliana, 5 Nov. 2009, W. Jaklitsch S19 = CBS 128829. Sequences: tef1 = JN175580, cal1 = JN175404, chi18-5 = JN175463.
Comments: Colonies of T. saturnisporopsis strains S19 and TR 175 are different from each other. Most notably, colonies of strain S19 grown at 30–35°C have a highly dissected margin and relatively slow rate of growth, whereas colonies of Tr 175 have a uniform colony margin and a much faster rate of growth. The appearance of colonies in S19 grown at higher temperature suggests that it is aberrant. The description of growth rates and colony morphology is drawn mainly from TR 175.
Trichoderma saturnisporopsis belongs to a clade that includes H. novae-zelandiae and the phylogenetic species G.J.S. 99–17 (Figs. 2i and 16; Druzhinina et al. 2012). This clade is basal in the Longibrachiatum Clade. Its members differ from typical species of the Longibrachiatum Clade in the formation of divergent whorls of phialides or, in the case of phylogenetic species G.J.S. 99–17, the dense disposition of ampulliform phialides in ‘pachybasium’ type heads (Bissett 1991a). In T. saturnisporopsis and H. novae-zelandiae the formation of solitary phialides over a considerable distance of the tip of the conidiophores is infrequent, and in G.J.S. 99–17 this character is absent. Conidia of H. novae-zelandiae are typical of most species in the clade in being ellipsoidal and smooth. Conidia of T. saturnisporopsis and G.J.S. 99–17 are ellipsoidal and tuberculate, strongly reminiscent of T. saturnisporum. Trichoderma saturnisporopsis differs from G.J.S. 99–17 in the pachybasium-like heads of phialides produced in the latter. None of the members of this clade are common. Hypocrea novae-zelandiae is endemic to New Zealand, where it has only been found as its teleomorph on wood in primarily Nothofagus forests of the North and South Islands. The deviating strain G.J.S. 99–17 was isolated from soil in Japan (Kyushu). Trichoderma saturnisporopsis is known only from two widely separated Northern Hemisphere strains (TR 175: Oregon; S19: Sardinia).
Fig. 16.
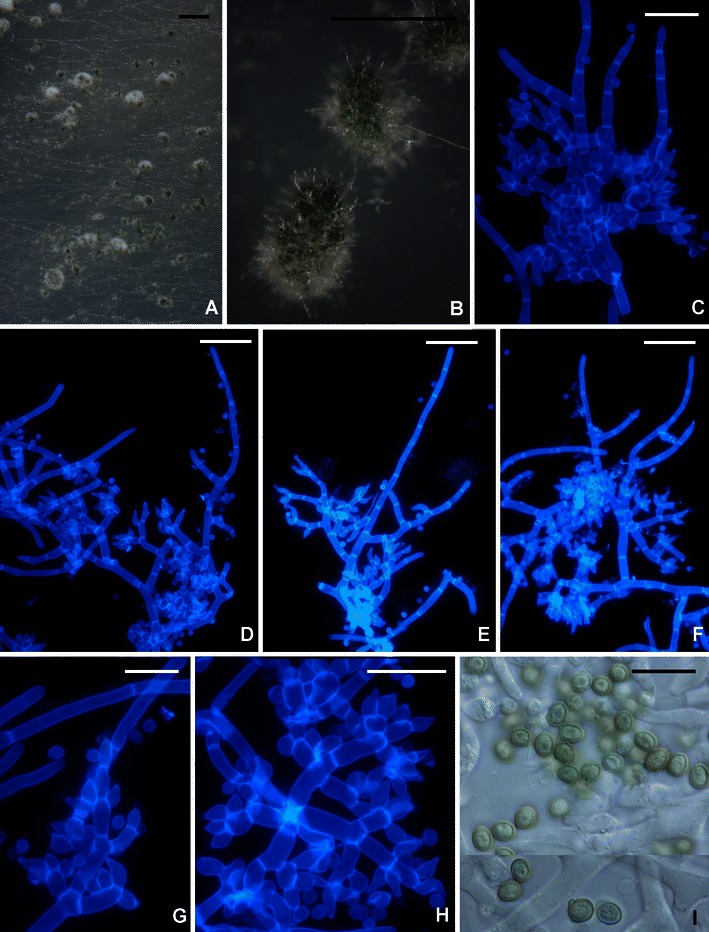
Trichoderma sp. G.J.S. 99–17. a, b Pustules. c–h Conidiophores. i Conidia. All from CMD. c–h fluorescence microscopy in calcofluor (hairs visible in b–f). Scale bars: a = 1 mm, b = 0.5 mm; c–h = 20 μm; i = 10 μm
It may be impossible to distinguish T. saturnisporopsis from T. saturnisporum on the basis of their phenotypes despite their rather wide phylogenetic separation. Both species are characterized by broadly ellipsoidal, conspicuously tuberculate conidia, irregularly branched conidiophores and poorly developed pustules that have sterile hairs and an ability to grow well at 35°C. The most conspicuous difference is that T. saturnisporopsis is better able to grow at lower temperatures (25–30°C) than T. saturnisporum, with the exception of T. saturnisporopsis strain S 19, which is overall slower than the two other known strains of T. saturnisporopsis and T. saturnisporum but has a highly dissected margin when grown at 30°C and above.
Fujimori and Okuda (1994) included strain G.J.S. 99–17 (as FP5566) in an early attempt to use molecular methods to eliminate duplicate strains from their screening for antibiotics. Because of the warted conidia, they had identified FP5566 as T. viride. Although conidia of this strain are similar to those of T. viride (Jaklitsch et al. 2006), the two species are otherwise not similar and only distantly related.
19. Trichoderma saturnisporum Hammill, Mycologia 62: 112 (1970).
Teleomorph: none known.
Ex-type culture: ATCC 18903 = CBS 330.70
Typical sequences: ITS Z48726, tef1 EU280044
Samuels et al. (1998) and Gams and Bissett (1998) redescribed this uncommon but wide-spread, (North America, Caribbean Ocean region, Europe, South Africa, Australia) clonal species. The species was originally described from Georgia. It is morphologically indistinguishable from the phylogenetically unrelated T. saturnisporopsis.
Doi et al. (1987) proposed Trichoderma sect. Saturnisporum for T. saturnisporum and T. ghanense. This section was characterized by the tuberculate conidia. Molecular phylogenetic results (Kuhls et al. 1997; Druzhinina et al. 2012) indicate that these two species belong to the Longibrachiatum Clade but despite the unusual conidial ornamentation, they are not closely related. Trichoderma saturnisporum does not have any close relationships in the Longibrachiatum Clade.
20. Trichoderma sinense Bissett, Kubicek & Szakacs in Bissett et al., Can. J. Bot. 81: 572 (2003, as ‘sinensis’).
Teleomorph: none known
Ex-type culture: DAOM 230000 = TUB F-1043
Typical sequences: ITS AF486014, tef1 AY750889 (DAOM 230004)
Trichoderma sinense is unusual in the Longibrachiatum Clade for its broadly ellipsoidal, smooth conidia, although its conidiophore branching and disposition of its phialides are typical of the clade. It is known (Bissett et al. 2003) from collections made in Taiwan and tropical China (Yunnan Province) and is possibly widespread in tropical East Asia. Druzhinina et al. (2012) included it in a clade with T. konilangbra, T. flagellatum, and T. gillesii.
21. Trichoderma solani Samuels, V. Doyle et V. S. Lopez, sp. nov. Figs. 3h, i and 17.
Fig. 17.
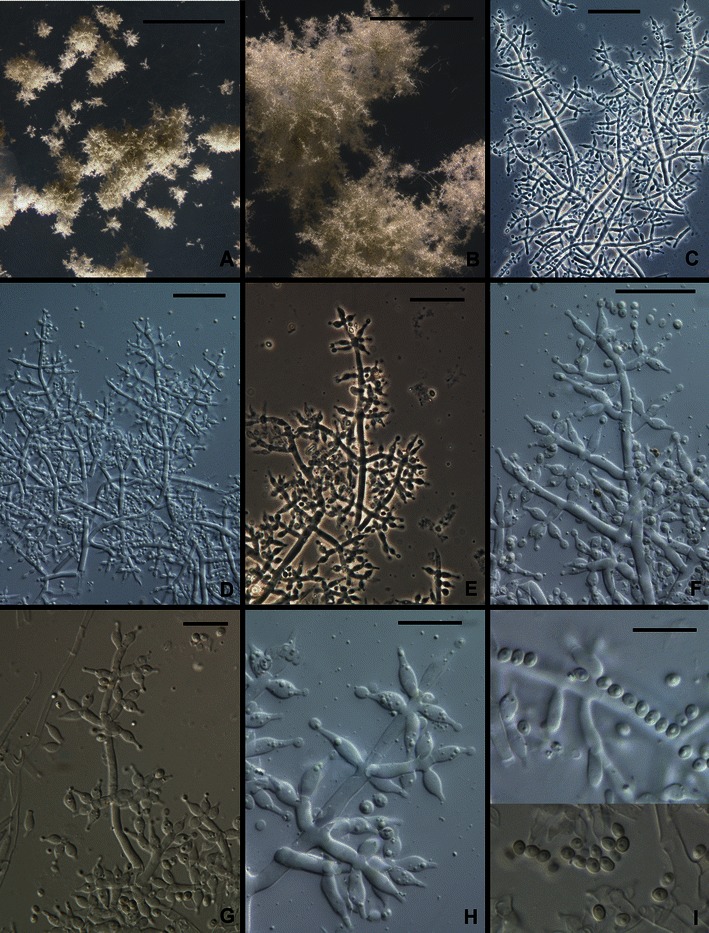
Trichoderma solani. a, b Young pustules, conidia just beginning to turn green. c–h Conidiophores. i Conidia. All from G.J.S. 88–81. Scale bars: a = 1 mm, b =250 μm, c–f = 20 μm, g–i = 10 μm
MycoBank MB 563912
Conidiophora verticillate ramosa. Phialides lageniformes, ad apicem in collula brevia constrictae. Conidia ellipsoidea, 2.5–2.7(−3.0) × 1.7–2.2 μm, laevia, atroviridia. Incrementum tardum; in agaro dicto PDA ad temperaturam 20–30°C post 96 h radius coloniae ca. 25 mm, colonia lutescens.
Holotypus: BPI 882298
Teleomorph: none known
Optimum temperature for growth on PDA 20–30°C, on SNA 25–30°C; after 96 h in darkness with intermittent light colony radius on PDA at 20–30°C ca. 25 mm, on SNA at 25–30°C 15–20 mm; at 35°C after 96 h colony radius less than 10 mm on PDA, less than 5 mm on SNA. Conidia forming on PDA within 72 h at 30°C, within 96 h at 20–25°C; diffusing yellow pigment forming on PDA within 48 h at 25–30°C. Colony on PDA after 1 week at 25°C under light with a scalloped margin; conidia forming over the whole surface of the colony in zonate rings, gray-green, surface disposed in rays; at 35°C conidia covering nearly the entire colony. Colonies grown on SNA in darkness with intermittent light sterile after 96 h; conidia forming within 1 week at 25°C under light in 1–2 mm diam, flat pustules in the center of the colony; individual conidiophores visible in pustules; pustules formed of intertwined hyphae, typically comprising a distinct central axis with frequently paired fertile lateral branches, the lateral branches distal to the tip longer than branches proximal to the tip; phialides arising directly from lateral branches, the longer lateral branches re-branching in pairs, the short secondary branches typically consisting of a single cell and terminating in a whorl of 2 or 3 phialides; intercalary phialides not seen. Phialides (n = 30) lageniform, (4.7–)5.5–8.5(−10.2) μm long, (1.7–)2.2–3.0(−4.2) μm at the widest point, L/W 1.9–3.5(−4.6), base (1.0–)1.2–2.0(−2.5) μm wide, arising from a cell (1.5–)2.0–2.5(−3.2) μm wide. Intercalary phialides not seen. Conidia (n = 30) ellipsoidal, (2.0–)2.5–2.7(−3.0) × 1.7–2.2(−2.5) μm, L/W (1.1–)1.2–1.4 (95% ci: 2.5–2.6 × 2.0–2.1 μm, L/W 1.2–1.3), dark green, smooth. Chlamydospores not observed.
Etymology: ‘solani’ refers to the host from which this species was isolated, Solanum hintonii.
Habitat: endophytic in tubers of Solanum hintonii.
Known distribution: Mexico, known only from the type locality.
Holotype: México, Estado de México, 6.5 km from junction of road from Temascaltepec towards San Pedro Tenayac, W of stream and 150 m N of the road, 19.05041 N, 100.10523 W, 25 Jul 2007, isolated as an endophyte from tubers of Solanum hintonii, V. Doyle 31t41a (BPI 882298; ex-type culture G.J.S. 08–81 = CBS 130506). Sequences: tef1 = JN175597, cal1 = JN175426, chi18-5 = JN175487, rpb2 = JN175546.
Comments: Trichoderma solani is phenotypically anomalous in the Longibrachiatum Clade because its growth rate is much slower at all temperatures, barely growing at 35°C, and for its small, broadly ellipsoidal to subglobose conidia. Druzhinina et al. (2012) found this species to be phylogenetically associated with T. effusum, T. citrinoviride and T. pseudokoningii.
Acknowledgments
Over several years cultures for this project were provided by Toru Okuda (formerly Nippon Roche) Japan; Giovanni Vanacci, University of Pisa; Harry Evans, CABI UK; Le Dinh Don, Long Nam University, Vietnam; Enrique Arevalo, ICT Peru; Andrews Akrofi, CRIG, Ghana; Sunday Agbeniyi, CRIN, Nigeria; Pierre Tondje, IRAD, Cameroon; G. Gilles and Françoise Candoussau, Pau, France; V. Doyle, The New York Botanical Garden, and V.S. Lopez, Universidad del Papaloapan, Oaxaca, México; Tomas Melgarejo, Universidad Nacional Agrararia La Molina, Lima, Peru. Orlando Petrini corrected several of the Latin descriptions. Collecting in Sri Lanka was supported by NSF grant DEB 0089474 to the Dept. of Plant Pathology, The Pennsylvania State University. Work in the lab of C.P.K. was supported by the Austrian Science Foundation (grant FWF P-19340-MOB). The financial support of W.M.J. by the Austrian Science Fund (FWF; project P22081-B17) is acknowledged. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The U.S. Department of Agriculture is an equal opportunity employer.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Atanasova L, Jaklitsch WM, Komoń-Zelazowska M, Kubicek CP, Druzhinina IS. Clonal species Trichoderma parareesei sp. nov. likely resembles the ancestor of the cellulase producer Hypocrea jecorina/T. reesei. Appl Environ Microbiol. 2010;76:7259–7267. doi: 10.1128/AEM.01184-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW, Jr, Adams J, Gemmel M, Perry J. Using population genetic theory and DNA sequences for species detection and identification in asexual organisms. PLoS One. 2010;5(5):e10609. doi: 10.1371/journal.pone.0010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby GR. Trichoderma viride Pers. ex Fries, and notes on Hypocrea. Trans Br Mycol Soc. 1939;23:149–168. doi: 10.1016/S0007-1536(39)80020-1. [DOI] [Google Scholar]
- Bissett J. A revision of the genus Trichoderma. I. Section Longibrachiatum sect. nov. Can J Bot. 1984;62:924–931. doi: 10.1139/b84-131. [DOI] [Google Scholar]
- Bissett J. A revision of the genus Trichoderma. II. Infrageneric classification. Can J Bot. 1991;69:2357–2372. doi: 10.1139/b91-297. [DOI] [Google Scholar]
- Bissett J. A revision of the genus Trichoderma. III. Section Pachybasium. Can J Bot. 1991;69:2373–2417. doi: 10.1139/b91-298. [DOI] [Google Scholar]
- Bissett J. A revision of the genus Trichoderma. IV. Additional notes on Section Longibrachiatum. Can J Bot. 1991;69:2418–2420. doi: 10.1139/b91-299. [DOI] [Google Scholar]
- Bissett J, Szakacs G, Nolan CA, Druzhinina I, Gradinger C, Kubicek CP. New species of Trichoderma fom Asia. Can J Bot. 2003;81:570–586. doi: 10.1139/b03-051. [DOI] [Google Scholar]
- Blaszczyk L, Popiel D, Chelkowski J, Koczyk G, Samuels GJ, Sobíeralski K, Silwulski (2011) Species diversity of Trichoderma in Poland. J Appl Genetics 52:233–243. doi:10.1007/s13353-011-0039-z [DOI] [PMC free article] [PubMed]
- Chandra M, Kalra A, Sangwan NS, Gaurav SS, Darokar MP, Sangwan RS. Development of a mutant of Trichoderma citrinoviride for enhanced production of cellulases. Bioresource Technol. 2009;100:1569–1662. doi: 10.1016/j.biortech.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Chandra M, Kalra A, Sharma PK, Sangwan RS. Cellulase production by six Trichoderma spp. fermented on medicinal plant processings. J Industr Microbiol Biotechnol. 2009;36:605–609. doi: 10.1007/s10295-009-0544-9. [DOI] [PubMed] [Google Scholar]
- Chandra M, Kalra A, Sharma PK, Kumar H, Sangwan RS. Optimization of cellulases production by Trichoderma citrinoviride on marc of Artemisia annua and its application for bioconversion process. Biomass Bioenergy. 2010;34:805–811. doi: 10.1016/j.biombioe.2010.01.024. [DOI] [Google Scholar]
- De Respinis S, Vogel G, Benagli C, Tonolla M, Petrini O, Samuels GJ. MALDI-TOF MS of Trichoderma: a model system for the identification of microfungi. Mycol Prog. 2010;9:79–100. doi: 10.1007/s11557-009-0621-5. [DOI] [Google Scholar]
- Doi Y, Abe Y, Sugiyama J. Trichoderma sect. Saturnisporum, sect. nov. and Trichoderma ghanense sp. nov. Bull Natl Sci Mus Tokyo Ser B (Bot) 1987;13:1–9. [Google Scholar]
- Druzhinina IS, Komoń-Zelazowska M, Kredics L, Hatvani L, Antal Z, Belayneh T, Kubicek CP. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology. 2008;154:3447–3459. doi: 10.1099/mic.0.2008/021196-0. [DOI] [PubMed] [Google Scholar]
- Druzhinina IS, Komoń-Zelazowska M, Atanasova L, Seidl V, Kubicek CP (2010) Evolution and ecophysiology of the industrial producer Hypocrea jecorina (anamorph Trichoderma reesei) and a new sympatric agamospecies related to it. PLos One 5(2):1–15. www.plosone.org [DOI] [PMC free article] [PubMed]
- Druzhinina IS, Komoń-Zelazowska M, Ismaiel A, Jaklitsch WM, Mulaw T, Samuels GJ, Kubicek CP (2012) Molecular phylogeny and species delimitation in the Longibrachiatum Clade of Trichoderma. Fung Genet Biol: In Press [DOI] [PMC free article] [PubMed]
- Fujimori F, Okuda T. Application of the random amplified polymorphic DNA using the polymerase chain reaction for efficient elimination of duplicate strains in microbial screening. I. Fungi. J Antibiot. 1994;47:173–182. doi: 10.7164/antibiotics.47.173. [DOI] [PubMed] [Google Scholar]
- Gams W. Cephalosporium-artige Schimmelpilze. Stuttgart: G. Fischer; 1971. p. 262. [Google Scholar]
- Gams W, Bissett J. Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE, editors. Trichoderma and Gliocladium. Vol. 1. Basic biology, taxonomy and genetics. London: Taylor & Francis; 1998. pp. 3–25. [Google Scholar]
- Gazis R, Rehner SR, Chaverri P (2011) Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Mol Ecol 20:3001–3013. doi:10.1111/j.1365-294X.2011.05110.x [DOI] [PubMed]
- Guerra G, Casado G, Argüelles JC, Sánchez MA, Manzano AM, Guzman T. Cellulase production with sugarcane straw by Trichoderma citrinoviride on solid bed. Sugar Tech. 2006;8:30–35. doi: 10.1007/BF02943738. [DOI] [Google Scholar]
- Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium. Vol. 2. Enzymes, biological control and commercial applications. London: Francis & Taylor; 1998. [Google Scholar]
- Hatvani L, Antal Z, Manczinger L, Szekeres A, Druzhinina IS, Kubicek CP, Nagy A, Nagy E, Vágvölgyi C, Kredics L. Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology. 2007;97:532–537. doi: 10.1094/PHYTO-97-4-0532. [DOI] [PubMed] [Google Scholar]
- Hoyos-Carvajal L, Orduz S, Bissett J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fung Genet Biol. 2009;46:61–631. doi: 10.1016/j.fgb.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. European species of Hypocrea. Part I. The green-spored species. Stud Mycol. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM. European species of Hypocrea part II: species with hyaline ascospores. Fungal Divers. 2011;48:1–250. doi: 10.1007/s13225-011-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Samuels GJ, Dodd SL, Lu B-S, Druzhinina IS. Hypocrea rufa/Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Stud Mycol. 2006;56:137–177. doi: 10.3114/sim.2006.56.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredics L, Antal Z, Dóczi I, Manczinger L, Kevei F, Nagy E. Clinical importance of the genus Trichoderma. A review. Acta Microbiol Immunol Hung. 2003;50:105–117. doi: 10.1556/AMicr.50.2003.2-3.1. [DOI] [PubMed] [Google Scholar]
- Kubicek C, Bölzlbauer UM, Kovacs W, Mach RL, Kuhls K, Lieckfeldt E, Börner T, Samuels GJ. Cellulase production by species of Trichoderma sect. Longibrachiatum and of Hypocrea species with anamorphs referable to Trichoderma sect. Longibrachiatum. Fung Genet Biol. 1996;20:105–114. doi: 10.1006/fgbi.1996.0025. [DOI] [PubMed] [Google Scholar]
- Kubicek CP, Mikus M, Schuster A, Schmoll M, Seiboth B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol Biofuels. 2009;2:19. doi: 10.1186/1754-6834-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhls K, Lieckfeldt E, Samuels GJ, Kovacs W, Meyer W, Petrini O, Gams W, Börner T, Kubicek CP. Molecular evidence that the asexual industrial fungus Trichoderma reesei is a clonal derivative of the ascomycete Hypocrea jecorina. Proc Natl Acad Sci U S A. 1996;93:7755–7760. doi: 10.1073/pnas.93.15.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhls K, Lieckfeldt E, Samuels GJ, Börner T, Meyer W, Kubicek CP. Revision of Trichoderma sect. Longibrachiatum including related teleomorphs based on analysis of ribosomal DNA internal transcribed spacer sequences. Mycologia. 1997;89:442–460. doi: 10.2307/3761038. [DOI] [Google Scholar]
- Kuhls K, Lieckfeldt E, Börner T, Guého E. Molecular reidentification of human pathogenic Trichoderma isolates as Trichoderma longibrachiatum and Trichoderma citrinoviride. Med Mycol. 1999;37:25–33. [PubMed] [Google Scholar]
- Kullnig CM, Szakacs G, Kubicek CP. Molecular identification of Trichoderma species from Russia, Siberia and the Himalaya. Mycol Res. 2000;104:1117–1125. doi: 10.1017/S0953756200002604. [DOI] [Google Scholar]
- Lieckfeldt E, Kullnig C, Samuels GJ, Kubicek CP. Sexually competent sucrose- and nitrate-assimilating strains of Hypocrea jecorina (Trichoderma reesei) from South American soils. Mycologia. 2000;92:374–380. doi: 10.2307/3761493. [DOI] [Google Scholar]
- Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, Danchin EG, Grigoriev IV, Harris P, Jackson M, Kubicek CP, Han CS, Ho I, Larrondo LF, de Leon AL, Magnuson JK, Merino S, Misra M, Nelson B, Putnam N, Robbertse B, Salamov AA, Schmoll M, Terry A, Thayer N, Westerholm-Parvinen A, Schoch CL, Yao J, Barabote R, Nelson MA, Detter C, Bruce D, Kuske CR, Xie G, Richardson P, Rokhsar DS, Lucas SM, Rubin EM, Dunn-Coleman N, Ward M, Brettin TS. Genome sequence analysis of the cellulolytic fungus Trichoderma reesei (syn. Hypocrea jecorina) reveals a surprisingly limited inventory of carbohydrate active enzymes. Nat Biotechnol. 2008;26:553–560. doi: 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Goldfarb B, Thies WG. Trichoderma species from fumigated Douglas Fir roots decayed by Phellinus weirii. Mycologia. 1987;9:370–374. doi: 10.2307/3807458. [DOI] [Google Scholar]
- Nirenberg HI. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt Biol Bundesanst Land- Forstw Berlin-Dahlem. 1976;169:1–117. [Google Scholar]
- Rifai MA. A revision of the genus Trichoderma. Mycol Pap. 1969;116:1–56. [Google Scholar]
- Samuels GJ. Trichoderma : systematics, the sexual state, and ecology. Phytopathology. 2006;96:195–206. doi: 10.1094/PHYTO-96-0195. [DOI] [PubMed] [Google Scholar]
- Samuels G, Petrini O, Kuhls K, Lieckfeldt E, Kubicek CP. The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. Stud Mycol. 1998;41:1–54. [Google Scholar]
- Sanchez V, Rebellodo O, Piscaso RM, Cardenas E, Cordova J, Gonzalez O, Samuels GJ. Trichoderma longibrachiatum: a mycoparasite of Thielaviopsis paradoxa. Mycopathologia. 2007;163:49–58. doi: 10.1007/s11046-006-0085-y. [DOI] [PubMed] [Google Scholar]
- Simmons EG (1977) Classification of some cellulose-producing Trichoderma species. Second International Mycological Congress, Abstracts Vol. M–Z, p. 618
- Sperry S, Samuels GJ, Crews P. Vertinoid polyketides from the saltwater culture of the fungus Trichoderma longibrachiatum separated from a Haliclona marine sponge. J Org Chem. 1998;63:10011–10014. doi: 10.1021/jo9808122. [DOI] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM. Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- Thrane U, Poulsen SB, Nirenberg HI, Lieckfeldt E. Identification of Trichoderma strains by image analysis of HPLC chromatograms. FEMS Microbiol Lett. 2001;203:249–255. doi: 10.1111/j.1574-6968.2001.tb10849.x. [DOI] [PubMed] [Google Scholar]
- Turner D, Kovacs W, Kuhls K, Lieckfeldt E, Peter B, Arisan-Atac I, Strauss J, Samuels GJ, Börner T, Kubicek CP. Biogeography and phenotypic variation in Trichoderma sect. Longibrachiatum. Mycol Res. 1997;101:449–459. doi: 10.1017/S0953756296002845. [DOI] [Google Scholar]
- Wilkinson L. SYSTAT© 10. Statistics I. Chicago: SPSS; 2000. [Google Scholar]
- Wuczkowski M, Druzhinina I, Gherbawy Y, Klug B, Prillinger H, Kubicek CP. Species pattern and genetic diversity of Trichoderma in a mid-European, primeval floodplain-forest. Microbiol Res. 2003;158:125–133. doi: 10.1078/0944-5013-00193. [DOI] [PubMed] [Google Scholar]


