Abstract
Dynamic loading and perfusion culture environments alone are known to enhance cartilage extracellular matrix (ECM) production in dedifferentiated articular chondrocytes. In this study, we explored whether a combination of these factors would enhance these processes over a free-swelling (FS) condition using adult human articular chondrocytes embedded in 2% alginate. The alginate constructs were placed into a bioreactor for perfusion (P) only (100 μL/per minute) or perfusion and dynamic compressive loading (PL) culture (20% for 1 h, at 0.5 Hz), each day. Control FS alginate gels were maintained in six-well static culture. Gene expression analysis was conducted on days 7 and 14, while cell viability, immunostaining, and mechanical property testing were performed on day 14 only. Total glycosaminoglycan (GAG) content and GAG synthesis were assessed after 14 days. Col2a1 mRNA expression levels were significantly higher (at least threefold; p<0.05) in both bioreactor conditions compared with FS by days 7 and 14. For all gene studies, no significant differences were seen between P and PL treatments. Aggrecan mRNA levels were not significantly altered in any condition although both GAG/DNA and 35S GAG incorporation studies indicated higher GAG retention and synthesis in the FS treatment. Collagen type II protein deposition was low in all samples, link protein distribution was more diffuse in FS condition, and aggrecan deposition was located in the outer regions of the alginate constructs in both bioreactor conditions, yet more uniformly in the FS condition. Catabolic gene expression (matrix metalloproteinase 3 [MMP3] and inducible nitric oxide synthase [iNOS]) was higher in bioreactor conditions compared with FS, although iNOS expression levels decreased to approximately fourfold less than the FS condition by day 14. Our data indicate that conditions created in the bioreactor enhanced both anabolic and catabolic responses, similar to other loading studies. Perfusion was sufficient alone to promote this dual response. PL increased the deposition of aggrecan surrounding cells compared with the other conditions; however, overall low GAG retention in the bioreactor system was likely due to both perfusion and catabolic conditions created. Optimal conditions, which permit appropriate anabolic and catabolic processes for accumulation of ECM and tissue remodeling for neocartilage development, specifically for humans, are needed.
Introduction
Numerous bioreactor systems have been specifically developed to create conditions for studies of chondrogenesis and neocartilage formation, or toward the production of cartilage grafts for eventual implantation and repair of articular defects. Apart from creating desirable environments (pH, pO2, and CO2, temperature, and nutrient supply), most bioreactor systems for engineering cartilage are constructed to include the means to biophysically stimulate the developing tissue or provide means to enhance medium exchange through perfusion (see reviews in Refs.1,2). These latter two parameters are generally thought to be significant factors for the enhancement of neocartilage formation and engineered tissue quality.
In a majority of studies, mechanical stimulation, whether applied through direct mechanical compression3–11 or via hydrostatic means,12,13 has improved extracellular matrix (ECM) deposition (principally proteoglycans) and the mechanical properties of the engineered cartilage. While dynamic compressive load may stimulate ECM production, this stimulus may also reduce accumulation or increase loss of newly secreted ECM into the medium in comparison to free-swelling (FS) or unloaded conditions.14–16
Perfusion of medium has been used to enhance cell seeding of porous scaffolds,17–20 and, in comparison with static cultures, perfusion can increase glycosaminoglycan (GAG) synthesis and deposition by chondrocytes and mesenchymal stem cells.1,17,19,21–24 Similar to compressive load, reports of reduced ECM accumulation have been observed as a consequence of perfusion, which appears to move newly synthesized proteins into the medium.25,26
Few studies examined the combined effects of loading and medium perfusion. Seidel et al.27 used a perfused chamber combined with mechanical loading, which led to increased GAG deposition. However, they did not report on the individual effects of perfusion and loading on ECM formation. Davisson et al.28 showed that perfusion alone was sufficient to increase stiffness of engineered neocartilage (bovine chondrocytes seeded on polyglycolic acid felts) and comparable with perfused and compressed (10% offset and±5% dynamic strain oscillations at 0.001 Hz). Most recently, Tran et al.29 utilized a commercially available bioreactor system capable of perfusion and loading. In comparison to the FS condition, the biomechanical and biochemical properties of the scaffold-free engineered porcine cartilage were enhanced. No difference was found between the perfused or jointly perfused and loaded neotissues.
In this current study, we employed the same bioreactor system used by Tran et al.29 Mature human chondrocytes synthesize matrix components at lower rates than the young animal cells used in the majority of previous studies. Further, combined perfusion and dynamic compression might have a synergistic effect on scaffold-based tissue engineering. We therefore encapsulated human articular chondrocytes in alginate hydrogels and subjected them to FS culture conditions for comparison with encapsulated cells in perfusion only (P) or perfusion and dynamic loading (PL) cultures and characterized neocartilage tissues produced after 14 days. We assessed cellular response to perfusion and loading by measuring expression of anabolic and catabolic genes, biochemical assays, radioisotope uptake, immunohistochemistry (IHC), and mechanical properties. Our hypothesis was that a combination of perfusion and dynamic loading would increase the anabolic response relative to the catabolic response resulting in a net increase in synthesis of matrix components.
Methods
Tissues and cell culture
Human articular cartilage was obtained from tissue banks (approved by Scripps institutional review board). Chondrocytes were isolated via enzymatic digestion as described previously30 and cultured in monolayer for one passage. Nine donors were utilized (age range: 14–55 years, mean age: 31.2±14.0 years; two women and seven men). Cartilage was graded macroscopically according to the International Cartilage Repair Society map and using a modified Collins grading system.31,32 Full details of age, gender, and osteoarthritic grade for each donor are presented in Table 1.
Table 1.
Donor Age, Gender, and Osteoarthritic Grades
| Donor | Age (years) | Gender | OA gradea |
|---|---|---|---|
| 1 | 14 | Male | Grade 0 |
| 2 | 19 | Female | Grade 0 |
| 3 | 19 | Male | Grade 0 |
| 4 | 27 | Male | Grade 0 |
| 5 | 30 | Male | Grade 1 |
| 6 | 30 | Male | Grade 1 |
| 7 | 37 | Male | Grade 0 |
| 8 | 50 | Male | Grade 1 |
| 9 | 55 | Female | Grade 3 |
Preparation of alginate
Alginate (PRONOVA UP LVG; Novamatrix, Sandvika, Norway) was weighed and dissolved in HBSS to create a 2% mix, which was continuously stirred at 4°C for 2–4 h. The dissolved alginate was filter sterilized through a 0.2-μm filter with filling bell (Mediakap-50; Spectrum Laboratories, Inc., Rancho Dominguez, CA) connected to a Masterflex L/S head pump (Cole-Parmer, Vernon Hills, IL).
Overview of bioreactor system
A bioreactor capable of perfusion and dynamic loading of the same sample (Fig. 1) was used in these studies (DynaGen® Series; Tissue Growth Technologies [TGT], Minnetonka, NM). The system permits cyclic compressive strain stimulation and simultaneous culture medium perfusion in nine different wells. The bioreactor was outfitted with a load cell (FUTEK, Irvine, CA; Fig. 1A) that is connected to a control box (TGT) and laptop computer with purpose-built software program (TGT). Perfusion is achieved using a Masterflex peristaltic pump system (Masterflex L/S; Cole Parmer; Fig. 1A).
FIG. 1.
DynaGen® bioreactor system. (A) Whole system setup with loading cell and chamber, peristaltic pump, and medium reservoir. (B) Open culture chamber with nine wells and removable loading platens. (C) Captured image of software to track and control loading parameters. Color images available online at www.liebertpub.com/tea
Cell preparation and bioreactor conditions used in this present study
Human chondrocytes were suspended in the 2% alginate solution at a density of 8×106 cells per mL. The alginate–cell suspension was pipetted into a polysulfone casting frame, 1×4 wide and 2.4-mm thick, sandwiched between Whatman 3-mm filter paper held in place by stainless steel mesh and clamps and subsequently placed into a beaker containing sterile CaCl2 (120 mM; Sigma Chemical, St. Louis, MO) for at least 45 min to polymerize the alginate.33 Alginate discs were created using 6-mm dermal biopsy punches and rinsed several times in culture media (Dulbecco's modified Eagle's medium [DMEM] with 10% calf serum, 1% penicillin/streptomycin, and 30 μg/mL ascorbic acid). The discs were precultured for 24–48 h in DMEM before being transferred into chondrogenic medium (Fig. 1) used in the bioreactor and for FS conditions.
A minimum of 12 alginate disks were made per experiment. Nine disks were added to the bioreactor, where three to four gels were placed into wells for perfusion only (loading platens above the appropriate wells were removed) and five to six gels were placed in the well for both perfusion and loading. The remaining three disks were maintained in 7 mL chondrogenic medium (Fig. 1) to represent the “free-swelling” control condition. The continuous perfusion flow rate in each bioreactor well was controlled at a rate of 0.1 mL per minute and was applied during the whole duration of the experiment of 7 or 14 days, respectively. This rate was chosen based on cell viability assessments where higher rates of perfusion lead to decreased cell viability (data not shown). The total media volume used for the bioreactor was 70 mL and was changed once a week. The FS disc medium was changed every 3–4 days.
Measuring gel heights for calculation of 20% loading was performed using a set of digital calipers. Gels for loading were chosen to be within 0.05 mm of each other to ensure a relatively uniform loading among all gels within the bioreactor chambers. The loading regime consisted of 20% dynamic compressive loading (of the gel height) using a sine wave at 0.5 Hz for 1 h per day. Motor movement, platen displacement, as well as the applied force were monitored (Fig. 1C).
The chondrogenic medium used consisted of DMEM (Cellgro, Manassas, VA), ITS+1 (Sigma; i.e., 10 mg/mL insulin, 5.5 mg/mL transferrin, 5 ng/mL selenium, 0.5 mg/mL bovine serum albumin, and 4.7 mg/mL linoleic acid), 1.25 mg/mL human serum albumin (Bayer, Leverkusen, Germany), 10−7 M dexamethasone (Sigma), 0.1 mM ascorbic acid 2-phosphate (Sigma), and penicillin/streptomycin/gentamycin (Gibco, Carlsbad, CA).34 This mix was filter sterilized through a 0.22-μm filter. An end concentration of 10 ng/mL of TGFβ1 (PeproTech, Rocky Hill, NJ) was added to the medium immediately prior to use for all conditions.
Viability assessment
At least two half gels were selected from each condition for viability assessment. The sections were transferred into phosphate-buffered saline (PBS) containing calcein AM and ethidium homodimer-1 (Invitrogen, Carlsbad, CA), incubated at 37°C for 30 min as previously described for cartilage sections.35 The fluorescence generated from each reagent was assessed on a confocal microscope (LSM-510; Zeiss, Jena, Germany). Viability was assessed using a script written in MATLAB software (MathWorks, Natick, MA), configured to enumerate green (live) and red (dead) cell signals. Viability is reported as a percentage of live cells.
Quantitative real-time polymerase chain reaction
Following culture, alginate-embedded chondrocytes were washed once in PBS and dissolved with 500 μL of 50 mM ethylenediaminetetraacetic acid (EDTA) in 2.5% typsin-EDTA solution at room temperature for 10 min. The released cells were collected by centrifugation at 1500 rpm for 3 min. The pelleted cells were washed in PBS before lysis in RLT buffer used in the RNAeasy® mini kit (Qiagen, Hilden, Germany) for total RNA extraction.
First-strand cDNA synthesis was performed using total RNA as a template according to the manufacturer's protocols (Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction (RT-PCR) was performed using prevalidated TaqMan® gene expression reagents (Applied Biosystems) for the detection of Col1a1, Col2a1, Col6a1, Col10a1, aggrecan, proteoglycan (PRG4), chemokine (C-C motif) ligand 20 (CCL20), matrix metalloproteinase 3 (MMP3), inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), and tumor necrosis factor α (TNFα), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Expression levels were normalized to GAPDH using the ΔCt method as previously reported.36
Biochemical analyses
Total GAG was assessed according to a previously reported protocol adapted for assessing alginate samples with the 1,9-dimethylmethylene blue (DMMB) method.37 Briefly, the dissolved alginate-cell suspension was incubated in digestion buffer (55 mM sodium citrate, 150 mM sodium chloride, 5 mM cysteine hydrochloride, 5 mM EDTA, and 0.56 units/mL papain; Sigma) at 60°C overnight. DMMB was used at pH 1.5 to avoid unspecific interaction between the dye and alginate (seen at higher pH levels37), and the samples were measured at a wavelength of 595 nM. The standard curve was prepared with shark chondroitin 6-sulfate (Sigma). Total GAG was calculated relative to DNA content from the same lysate using the CyQUANT® assay (Invitrogen).
Histology
For assessment of GAG produced, paraffin-embedded sections were prepared for acriflavine staining.38 IHC analyses were conducted to examine protein deposition of collagen type II (II-II6B3), link protein (9/30/8-A-4), and aggrecan (12/21/1-C-6). All three antibodies were obtained from the Hybridoma Bank, University of Iowa. Species-matching isotype controls were also used in parallel.
Mechanical testing
Testing of mechanical properties of the gels was conducted by using a custom-built device consisting of two miniature brushless servo actuators (SMAC, Carlsbad, CA), one 50 g load cell (FUTEK) with steel plunger having a flat surface for compression, and LabVIEW (National Instruments, Austin, TX) software for movement control and data acquisition on a laptop. The gels were placed between two 100-μm-thick cover slips and loaded into the test chamber. The gel height was measured using the internal linear encoder of the SMAC (1 μm accuracy). A 5% of original height step compression was applied to the gel subsequently and the force was monitored and recorded. The gel was allowed to equilibrate for 2 min and then another 5% step compression was applied. The step compression was applied a total of four times, resulting in a net compression of 20%. Young's modulus was calculated from a linear fit to the equilibrium force at each 5% compression level.39
Statistical analysis
Cycle numbers for the expression of each gene (in triplicate) obtained from RT-PCR were corrected to GAPDH levels and used in a repeated measures analysis using R (R Development Core Team, www.r-project.org) to assess statistical significance between the gene expression levels for each treatment group. p-Values <0.05 were considered significant. We anticipated an experimental threshold of measuring a 1.5- to 2-fold difference in normalized gene expression between groups. Given the standard deviations in gene expression (of Col2a1, Aggrecan, Col6a1, PRG4, and CCL20) at 14 days, a sample size of nine would be therefore sufficient to detect a twofold or greater difference in gene expression with a p-value of 0.05 and a power of >80%.
Results
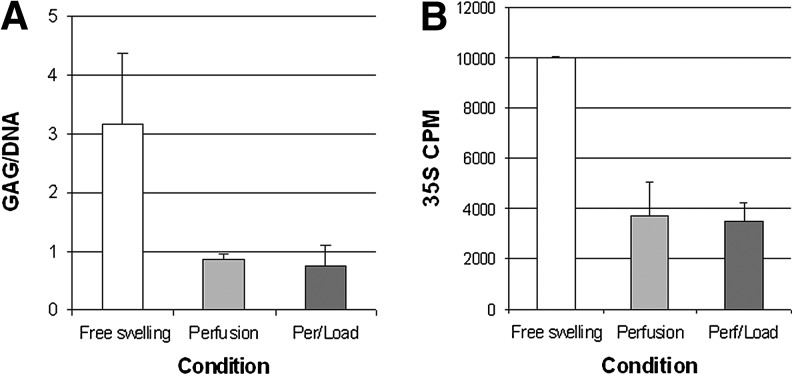
No significant differences in gel stiffness were detected between any of the treatment groups after 14 days of culture. Col2a1 mRNA expression levels were at least threefold higher (p<0.05) in both bioreactor conditions compared with FS by days 7 and 14 (Fig. 2A). For all genes studied, no significant differences were seen between P only and PL samples. Aggrecan mRNA levels were not significantly altered in any condition (Fig. 2B), although both GAG/DNA and 35S GAG incorporation studies indicated higher GAG retention and synthesis in the FS treatment (Fig. 3A and B). A trend of higher Col6a1 expression in the P group compared with PL was observed (Fig. 2C). PRG4 RNA levels were higher in the bioreactor conditions compared with FS by day 7, but no significant difference was noted by day 14 (Fig. 2D). On day 7, PL treatment led to higher PRG4 expression levels (∼12-fold) compared with approximately fourfold in P. For most samples, Col10a1 was not detectable, or only detectable after >31 cycles; however, Col10a1 gene expression levels in bioreactor samples were between 5- and 12-fold lower compared with FS (Fig. 2E). Col1a1 expression levels were reduced twofold in the PL condition at day 7, but no significant difference was found after 14 days (Fig. 2F).
FIG. 2.
Anabolic gene expression profiles of human articular chondrocytes embedded in alginate for 7 and 14 days in perfusion (P) and perfusion/loaded (PL) conditions relative to free swelling controls (FS; dotted line). Total mRNA expression levels for (A) Col2a1, (B) Aggrecan, (C) Col6a1, (D) PRG4, (E) Col10a1 (note: for most samples, Col10a1 was not detectable or only after >31 cycles), and (F) Col1a1.
FIG. 3.
Biochemical assessments for each culture condition. (A) Glycosaminoglycan (GAG)/DNA (μg/μg) ratios; (B) total 35Sulfate GAG incorporation.
The response by the chondrocytes to the conditions studied here after 14 days of culture appears not to be age but rather donor dependent. The expression levels of Col2a1, Aggrecan, and Col6a1 after 14 days in P or PL condition (relative to FS) in five donors spanning 27–55 years of age are presented in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea).
Catabolic gene expression levels show that MMP3 expression was fourfold higher in both P and PL compared with FS by day 7, which increased ten-fold by day 14 (Fig. 4A). Compared with FS, iNOS expression levels were four- to sevenfold higher in P and PL by day 7, respectively. By day 14, iNOS levels had decreased to approximately twofold less than that of the FS condition (Fig. 4B). On day 7, CCL20 mRNA levels were higher in the bioreactor conditions (Fig. 4C). Also, a greater than fourfold increase in CCL20 expression was seen in P compared with twofold in PL (Fig. 4C). By day 14, CCL20 mRNA levels were similar in all conditions. Both IL-1β and TNFα were not detected in any samples in all conditions tested in this study.
FIG. 4.
Catabolic gene expression levels of (A) matrix metalloproteinase 3 (MMP3), (B) inducible nitric oxide synthase (iNOS), and (C) chemokine (C-C motif) ligand 20 (CCL20) by chondrocytes embedded in alginate for 7 and 14 days in P and PL conditions relative to FS controls (FS).
Cell viability >80% was maintained in all conditions (Fig. 5A–C). IHC indicated an overall low deposition of collagen type II in all conditions (Fig. 5D–F), although some groups of cells in the bioreactor condition were clearly positive, supporting gene expression observations (Fig. 2). Link protein IHC showed a more intense stain surrounding the cells in all conditions (Fig. 5G–I), although a greater distribution in the intercellular region was seen in the FS gels (Fig. 5G). Aggrecan staining appeared most intense surrounding most cells in the PL treatment (Fig. 5I) and a slightly lower frequency of cells in the P condition (Fig. 5K). However, for many samples, aggrecan staining was mainly detected in cells on the peripheral regions of gels in both P and PL conditions (Supplementary Fig. S2). Chondrocytes in the FS condition showed positive aggrecan staining throughout the gel, although less intense compared with positive cells in the P and PL conditions (Fig. 5J). The more uniform deposition of aggrecan observed throughout the gel, together with the link protein staining patterns, supports the higher GAG levels detected in the biochemical and 35S assessments (Fig. 3). In our hands, alcian blue, safranin O/fast green, and toluidine blue all stained alginate in a nonspecific or false positive manner (tested also on alginate gels without cells). Acriflavine staining (Fig. 5M–O) appeared similar to both aggrecan and link protein immunostaining patterns.
FIG. 5.
Viability assessment, immunohistochemistry (IHC), and acriflavine staining of 14-day-old alginate gels containing human articular chondrocytes in FS, bioreactor P, and PL conditions. Live–dead cell viability images (10×) using confocal microscopy (a–c). IHC staining for collagen type II (d–f), link protein (g–i), and aggrecan (j–l). Acriflavine staining (m–o). (Magnification of IHC and acriflavine images: 40×). Color images available online at www.liebertpub.com/tea
Discussion
Balancing anabolic and catabolic processes is crucial in maintaining cartilage homeostatsis40 and it is beginning to be recognized that these processes are important in regenerating functional tissue. An appropriate application of dynamic mechanical stimulation is one way to initiate both processes.41 Co-induction of anabolic and catabolic responses in our study is consistent with other studies examining dynamic loading of cartilage explants42 and engineered tissues.41,43,44 Interestingly, our data indicate that perfusion alone can induce both anabolic and catabolic responses in human chondrocytes, similar to observations by Tran et al.29 employing porcine chondrocytes. This effect forces us to reexamine the requirement of dynamic stimulation. However, from the IHC data in the present study, greater aggrecan protein deposition was seen in the PL treatments.
This current study highlights the complexity of identifying optimal conditions that enhance neocartilage production in human cells. It is clear from our study that gene expression profiles and the final distribution of protein deposition do not correlate in a predictable manner. Aggrecan mRNA levels were not significantly altered in any condition, yet higher GAG and 35S incorporation were noted in the FS condition. Conversely, IHC staining shows significant deposition of aggrecan immediately surrounding the cells in the PL treatments, although more restricted to the peripheral regions in many samples. Increased MMP3 gene expression levels in the bioreactor treatments (P and PL) suggest increased MMP3 enzymatic activity. Since aggrecan and collagen types II, IX, X, and XI are substrates for MMP3,45,46 it is possible that the GAGs produced in the bioreactor conditions were both degraded and then removed under perfusion conditions.25,47 De Croos et al.4 reported increased MMP3 and MMP13 following loading. Aggrecan staining in FS indicates that most chondrocytes throughout the gel deposit aggrecan compared with chondrocytes in the P and PL conditions where many cells on the outer regions of the gel produced high-interterritorial levels of aggrecan. These differences in aggrecan distribution may be related to discrepancies seen between the gene expression and biochemical analyses.
Col2a1 mRNA expression levels were higher in both bioreactor samples, yet the accumulation of collagen type II protein was conspicuously low, except for a few clusters of cells. As noted previously, suspected increased MMP3 activity may be partly responsible for collagen degradation, although other MMPs not examined in this study may have also been enhanced and released (e.g., MMP13).
The decrease in iNOS mRNA levels in the bioreactor (compared with FS) indicates that perfusion may aid in decreasing an inflammatory response, perhaps by removing normally accumulated factors that stimulate its expression like IL-1β and TNFα,48 yet these cytokines were not detected at the mRNA level in this current study.
Both iNOS and CCL20 have been previously identified as early phase mechanoresponsive genes.49 We also see an increased expression by day 7, which indicates that the conditions created in the bioreactor were sufficient to generate a mechanoresponsive change. By 14 days, iNOS and CCL20 expression levels were similar to FS gels. The reduction of iNOS is postulated earlier; however, the reduced CCL20 may be related to its role in actin cytoskeletal rearrangements33 and linked to changes in cell phenotype during culture (i.e., re-differentiation reflected by the increased Col2a1 and decreased Col1a1 expression). The mixed results here indicate a need to optimize timing of perfusion, loading, and FS conditions. Intermittent loading regimes have significant effect on ECM production.41,50 In particular, Nicodemus and Bryant41 showed that loading and the timing of when loading is applied can dramatically influence the anabolic and catabolic activities of chondrocytes in polyethylene glycol gels. A delayed loading regime has less effect on stimulating anabolic and catabolic gene expression in comparison to using an immediate application of an intermittent loading regime. However, Khan et al.21 showed that following 2 weeks of continuous perfusion of bovine articular chondrocytes increased collagen and proteoglycan syntheses. Our FS condition showed a more uniform aggrecan and link-protein deposition; thus, a preculture approach should be tested with the system used in this current study as a possible means to improve ECM accumulation.
One feature of this current study, in relation to many previous studies, is the aspect of species and age. The literature is dominated by very promising results in young animal cells (bovine and porcine) that respond dramatically to growth factors, mechanical stimuli compression, and perfusion. In humans, however, the generally poor response in mature adult cells and the high variability make it difficult to translate animal results to patients. In the present study, we did not see a large variation in response to the conditions used based on age (see Supplementary Fig. S1), but rather a donor-specific response. This indicates that further modification of the system to aid in retaining ECM proteins is needed and may then delineate an age-related response, with respect to the passage used in the current study.
In the original seminal publication by Brittberg et al.51 and in a more recent study by Chubinskaya et al.,52 it is stated that cells being prepared for autologous chondrocyte transplantation or autologous chondrocyte implantation are cultured in monolayer for 7–21 days. The chondrocytes used in our current study are cultured for 1–2 weeks in P0 and then another 1–2 weeks at P1. Therefore, our cells are actually in culture for a comparable amount of time or even a little longer compared with the standard practice. This may reflect, in part, the more negative results observed. Thus, culturing the cells for comparably shorter times may be needed.
This study represents a first attempt to replicate positive responses to mechanical compression and perfusion that have been reported in animal cells. The negative results and high variability are in fact the challenges facing cartilage tissue engineering for clinical applications. Our observations with human cells are a reflection of the challenges inherent in cartilage tissue. Given the number of positive reports supporting mechanical compression and perfusion it is surprising that none of these have been clinically relevant. To our knowledge, no study has reported the effect of combined perfusion and dynamic compression in one system using human cells. Several studies have shown promising effects of mechanical compression and perfusion on young animal cells. However, the inability to extrapolate these results to human cells is a significant barrier for clinical translation.
In conclusion, it is clear that perfusion and perfusion with loading create conditions somewhat favorable to neocartilage formation, although the application of perfusion and dynamic stimulation requires further optimization for the accumulation of ECM proteins using human chondrocytes. The production of catabolic mediators, in particular MMPs, appears to be a normal and even required part of neotissue formation, since during this dynamic process there will be remodeling before a final tissue equilibrium is reached. Of course an excess of enzymes may be detrimental. What really matters is the end product. In that regard, it is important to continue longer-term cultures and assess the effect of various loading schemes on functional tissue development—until a steady-state is reached.
Supplementary Material
Acknowledgments
The funds for this work were provided by NIH/NIAMS P01 AG007996, NIH UL1 RR025774, and CIRM TR1-01216. Technical assistance by Nick Steklov, Peter Chen, Xian Chen, Stuart Duffy, Jonathan Gelber, and Lilo Creighton is appreciated. Special thanks to Judy Blake for copyediting. The authors would like to thank Mrs. Susan Smith D. Richardson for her generous support for our research projects. Antibodies used in this study were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, Iowa.
Disclosure Statement
No competing financial interests exist.
References
- 1.Concaro S. Gustavson F. Gatenholm P. Bioreactors for tissue engineering of cartilage. Adv Biochem Eng Biotechnol. 2009;112:125. doi: 10.1007/978-3-540-69357-4_6. [DOI] [PubMed] [Google Scholar]
- 2.Schulz R.M. Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;36:539. doi: 10.1007/s00249-007-0139-1. [DOI] [PubMed] [Google Scholar]
- 3.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 4.De Croos J.N. Dhaliwal S.S. Grynpas M.D. Pilliar R.M. Kandel R.A. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006;25:323. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Grodzinsky A.J. Levenston M.E. Jin M. Frank E.H. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 6.Kisiday J.D. Jin M. DiMicco M.A. Kurz B. Grodzinsky A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Lee D.A. Bader D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 8.Lima E.G. Mauck R.L. Han S.H. Park S. Ng K.W. Ateshian G.A., et al. Functional tissue engineering of chondral and osteochondral constructs. Biorheology. 2004;41:577. [PubMed] [Google Scholar]
- 9.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 10.Waldman S.D. Couto D.C. Grynpas M.D. Pilliar R.M. Kandel R.A. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage. 2006;14:323. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Waldman S.D. Spiteri C.G. Grynpas M.D. Pilliar R.M. Hong J. Kandel R.A. Effect of biomechanical conditioning on cartilaginous tissue formation in vitro. J Bone Joint Surg Am. 2003;85A(Suppl 2):101. doi: 10.2106/00004623-200300002-00013. [DOI] [PubMed] [Google Scholar]
- 12.Elder B.D. Athanasiou K.A. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15:43. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyland J. Wiegandt K. Goepfert C. Nagel-Heyer S. Ilinich E. Schumacher U., et al. Redifferentiation of chondrocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol Lett. 2006;28:1641. doi: 10.1007/s10529-006-9144-1. [DOI] [PubMed] [Google Scholar]
- 14.Davisson T. Kunig S. Chen A. Sah R. Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res. 2002;20:842. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 15.Hunter C.J. Mouw J.K. Levenston M.E. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage. 2004;12:117. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee C.R. Grodzinsky A.J. Spector M. Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J Biomed Mater Res A. 2003;64:560. doi: 10.1002/jbm.a.10443. [DOI] [PubMed] [Google Scholar]
- 17.Davisson T. Sah R.L. Ratcliffe A. Perfusion increases cell content and matrix synthesis in chondrocyte three-dimensional cultures. Tissue Eng. 2002;8:807. doi: 10.1089/10763270260424169. [DOI] [PubMed] [Google Scholar]
- 18.Pazzano D. Mercier K.A. Moran J.M. Fong S.S. DiBiasio D.D. Rulfs J.X., et al. Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnol Prog. 2000;16:893. doi: 10.1021/bp000082v. [DOI] [PubMed] [Google Scholar]
- 19.Santoro R. Olivares A.L. Brans G. Wirz D. Longinotti C. Lacroix D., et al. Bioreactor based engineering of large-scale human cartilage grafts for joint resurfacing. Biomaterials. 2010;31:8946. doi: 10.1016/j.biomaterials.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Wendt D. Marsano A. Jakob M. Heberer M. Martin I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol Bioeng. 2003;84:205. doi: 10.1002/bit.10759. [DOI] [PubMed] [Google Scholar]
- 21.Khan A.A. Suits J.M. Kandel R.A. Waldman S.D. The effect of continuous culture on the growth and structure of tissue-engineered cartilage. Biotechnol Prog. 2009;25:508. doi: 10.1002/btpr.108. [DOI] [PubMed] [Google Scholar]
- 22.Kreklau B. Sittinger M. Mensing M.B. Voigt C. Berger G. Burmester G.R., et al. Tissue engineering of biphasic joint cartilage transplants. Biomaterials. 1999;20:1743. doi: 10.1016/s0142-9612(99)00061-7. [DOI] [PubMed] [Google Scholar]
- 23.Sittinger M. Bujia J. Minuth W.W. Hammer C. Burmester G.R. Engineering of cartilage tissue using bioresorbable polymer carriers in perfusion culture. Biomaterials. 1994;15:451. doi: 10.1016/0142-9612(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhao F. Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnol Bioeng. 2005;91:482. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 25.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 26.Lee C.S. Gleghorn J.P. Won Choi N. Cabodi M. Stroock A.D. Bonassar L.J. Integration of layered chondrocyte-seeded alginate hydrogel scaffolds. Biomaterials. 2007;28:2987. doi: 10.1016/j.biomaterials.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 27.Seidel J.O. Pei M. Gray M.L. Langer R. Freed L.E. Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445. [PubMed] [Google Scholar]
- 28.Davisson T. Kunig S. Chen A. Sah R. Ratcliffe A. The effects of perfusion and compression on modulation of tissue engineered cartilage. Proceedings of the 48th Annual Meeting of the Orthopaedic Research Society; Dallas, TX. 2002. Poster no. 0488.27. [DOI] [PubMed] [Google Scholar]
- 29.Tran S.C. Cooley A.J. Elder S.H. Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotechnol Bioeng. 2011;108:1421. doi: 10.1002/bit.23061. [DOI] [PubMed] [Google Scholar]
- 30.Blanco F.J. Ochs R.L. Schwarz H. Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146:75. [PMC free article] [PubMed] [Google Scholar]
- 31.Brismar B.H. Wredmark T. Movin T. Leandersson J. Svensson O. Observer reliability in the arthroscopic classification of osteoarthritis of the knee. J Bone Joint Surg Br. 2002;84:42. doi: 10.1302/0301-620x.84b1.11660. [DOI] [PubMed] [Google Scholar]
- 32.Muehleman C. Bareither D. Huch K. Cole A.A. Kuettner K.E. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 33.Haudenschild D.R. Chen J. Pang N. Lotz M.K. D'Lima D.D. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum. 2010;62:191. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakob M. Demarteau O. Schafer D. Hintermann B. Dick W. Heberer M., et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Grogan S.P. Aklin B. Frenz M. Brunner T. Schaffner T. Mainil-Varlet P. In vitro model for the study of necrosis and apoptosis in native cartilage. J Pathol. 2002;198:5. doi: 10.1002/path.1169. [DOI] [PubMed] [Google Scholar]
- 36.Martin I. Jakob M. Schafer D. Dick W. Spagnoli G. Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 37.Enobakhare B.O. Bader D.L. Lee D.A. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 38.Bancroft J.D. Gamble M. Lipids. 6th. Churchill Livingstone; New York: 2007. [Google Scholar]
- 39.Korhonen R.K. Laasanen M.S. Toyras J. Rieppo J. Hirvonen J. Helminen H.J., et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 40.Manicourt D.H. Devogelaer J.P. Thonar E.J. Products of Cartilage Metabolism. Burlington: Elsevier; 2006. [Google Scholar]
- 41.Nicodemus G.D. Bryant S.J. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage. 2010;18:126. doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Blain E.J. Gilbert S.J. Wardale R.J. Capper S.J. Mason D.J. Duance V.C. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys. 2001;396:49. doi: 10.1006/abbi.2001.2575. [DOI] [PubMed] [Google Scholar]
- 43.De Croos J.N. Roughley P.J. Kandel R.A. Improved bioengineered cartilage tissue formation following cyclic compression is dependent on upregulation of MT1-MMP. J Orthop Res. 2010;28:921. doi: 10.1002/jor.21064. [DOI] [PubMed] [Google Scholar]
- 44.Raizman I. De Croos J.N. St-Pierre J.P. Pilliar R.M. Kandel R.A. Articular cartilage subpopulations respond differently to cyclic compression in vitro. Tissue Eng Part A. 2009;15:3789. doi: 10.1089/ten.TEA.2008.0530. [DOI] [PubMed] [Google Scholar]
- 45.Little C.B. Hughes C.E. Curtis C.L. Janusz M.J. Bohne R. Wang-Weigand S., et al. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 46.Wu J.J. Lark M.W. Chun L.E. Eyre D.R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991;266:5625. [PubMed] [Google Scholar]
- 47.Hsieh-Bonassera N. Pfister B. Masuda K. Sah R. Selective enhancement of matrix retention in tissue engineered cartilage in a perfusion bioreactor system. Proceedings of the 52nd Annual Meeting of the Orthopaedic Research Society; Chicago, IL. 2006. Paper no. 0045.31. [Google Scholar]
- 48.Schmidt N. Pautz A. Art J. Rauschkolb P. Jung M. Erkel G., et al. Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. Biochem Pharmacol. 2010;79:722. doi: 10.1016/j.bcp.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haudenschild D.R. Nguyen B. Chen J. D'Lima D.D. Lotz M.K. Rho kinase-dependent CCL20 induced by dynamic compression of human chondrocytes. Arthritis Rheum. 2008;58:2735. doi: 10.1002/art.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan J.C. Waldman S.D. The effect of intermittent static biaxial tensile strains on tissue engineered cartilage. Ann Biomed Eng. 2010;38:1672. doi: 10.1007/s10439-010-9917-5. [DOI] [PubMed] [Google Scholar]
- 51.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 52.Chubinskaya S. Hakimiyan A.A. Rappoport L. Yanke A. Rueger D.C. Cole B.J. Response of human chondrocytes prepared for autologous implantation to growth factors. J Knee Surg. 2008;21:192. doi: 10.1055/s-0030-1247818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.