Abstract
Muscle-derived stem cells (MDSCs) are known to exhibit sexual dimorphism, by donor sex, of osteogenic, chondrogenic, and myogenic differentiation potential in vitro. Moreover, host sex differences in the myogenic capacity of MDSCs in vivo are also observed. This study investigated the role of host sex and host sex hormones in MDSC-mediated bone formation and healing. Using unaltered male, castrated male, unaltered female, and ovariectomized female mice, both MDSC-mediated ectopic bone formation and cranial defect healing were examined. Male hosts, whether unaltered or castrated, form significantly larger volumes of MDSC-mediated ectopic bone than female hosts (either unaltered or ovariectomized), and no differences in ectopic bone volume were found between hosts of the same sex. In a cranial defect healing model, similar results were found—unaltered and castrated male hosts display larger volumes of bone formed when compared with unaltered and ovariectomized female hosts. However, in this healing model, some volume differences were found between hosts of the same sex. In both models, these differences were attributed to varying rates of endochondral bone formation in male and female hosts.
Introduction
Muscle-derived stem cells (MDSCs) have been isolated from mouse skeletal muscle using a modified preplate technique.1–4 MDSCs have been shown to be multipotent, differentiating into musculoskeletal tissues such as bone, cartilage, and skeletal muscle, in addition to endothelial, neural, and hematopoietic tissues.3–9 MDSCs can undergo osteogenic differentiation with BMP4 treatment in vitro; retrovirally transduced cells can produce high amounts of BMP4, and these BMP4 transduced cells can then produce ectopic bone in skeletal muscle, as well as improve bone healing in critical-sized cranial defects.8–10
MDSCs are known to exhibit sex differences in muscle regeneration, as well as chondrogenic and osteogenic differentiation both in vivo and in vitro.11–13 MDSCs isolated from female mice have a higher regeneration efficiency when transplanted into mdx or mdx/SCID mice, a dystrophin-deficient animal model of muscular dystrophy. In mdx mice, a significant effect of host sex was also seen, with female hosts exhibiting higher regeneration.12 Male MDSCs show superior chondrogenic differentiation in a micromass culture system in vitro and are also better at repairing a created osteochondral defects.13 With regards to in vitro osteogenic differentiation after stimulation with BMP4, MDSCs isolated from males proved to be superior. Male MDSCs show more differentiation, as measured through markers of osteogenic differentiation, including staining for alkaline phosphatase (ALP), ALP activity, ALP and Runx2 gene expression, and in vivo implantation of MDSCs of both sexes showed that male MDSCs produce denser and more consistent volumes of ectopic bone, while female MDSCs produce variable volumes of ectopic bone.11
In addition to MDSCs, other progenitor or stem cells also exhibit sexual dimorphism, by donor sex, of differentiation potential. Bone marrow stem cells from human females do not decrease their differentiation potential with the advanced age of the donor, but differentiation of bone marrow stem cells from human males does change with donor age.14 Endothelial progenitor cells from male donors are less migrative and form fewer colonies.15 Bone marrow stem cells from female donors are more effective at functionally repairing cardiac ischemia than the same cells from male donors.16 Osteoprogenitors derived from female rat bone increase proliferation and differentiation in response to progesterone, while the same cells from male rats do not.17 Adipose stem cells (ASCs) isolated from donors of different sexes also exhibit divergent differentiation potential. In separate studies, male rabbit ASCs are more osteogenic than those from female donors, while female mouse ASCs are more adipogenic.18,19 Taking into account sex differences in cellular progenitor therapies to treat diseases, it will become increasingly important to consider the sex of a patient when choosing treatments. Currently, outcomes of transplantation, whether solid organ or bone marrow, are known to be affected by the sex of the donor and sex mismatch between the donor and the host.20 Future stem cell therapies should include sex considerations, of both host and donor, in order to maximize positive outcomes.
This study aimed at examining the effect of host sex, as well as sex hormones, on MDSC-mediated bone formation and cranial defect healing by utilizing four host groups: unaltered males, castrated males, unaltered females, and ovariectomized females. Using microcomputed tomography (microCT) and histological analyses, bone healing and formation were evaluated to better understand the role of host sex in MDSC bone therapies.
Materials and Methods
MDSC isolation and transduction
MDSCs were isolated using the following technique previously described.1,4 Briefly, skeletal muscle from both lower limbs of a male C57BL/6J mouse was dissected, and connective and adipose tissues were removed. After fine mincing, successive enzymatic digestions with collagenase (Sigma-Aldrich), dispase (Invitrogen) and trypsin (Invitrogen) were performed. Finally, a single cell suspension was achieved by passing a mixture of cell digest and proliferation medium through 18G, 23G, and 27G needles. Proliferation medium consisted of the following: high glucose Dulbecco's modified Eagle's medium (Invitrogen), 10% fetal bovine serum (Invitrogen), 10% horse serum (Invitrogen), 0.5% chick embryo extract (Accurate Chemical Co.), and 1% penicillin-streptomycin (Invitrogen). This cell suspension was then plated onto collagen-coated flasks. After 2 h, nonadherent cells were transferred from the previous flask to a new collagen-coated flask. After 24 h, and every 24 h for the next 5 total days, this procedure of transferring nonadherent cells to a new collagen-coated flask was repeated. The resulting cells isolated were termed MDSCs.
Based on previous development of retroviral vectors in our laboratory, a retrovirus called CLB2/4G, encoding for both BMP4 and green fluorescent protein (GFP), was applied to MDSCs in a 1:1 ratio with a proliferation medium with 8 g/mL of polybrene for 24 h.10,21 These retrovirally transduced cells were then sorted using fluorescence activated cell sorting (FACS), and only cells expressing GFP (and, therefore, only cells expressing BMP4) were used in consequent experiments. These cells were termed MDSC-B4. Average BMP4 secretion of MDSC-B4 was determined to be 168 ng/mL by 106 cells in 24 h via human BMP4 ELISA (R&D Systems).
MDSCs were cultured in proliferation media and maintained at a low confluency until sufficient numbers were reached for experiments. Stocks of transduced MDSCs were also frozen at a low passage number so that all experiments could be performed using similar cells. Frozen stocks were stored in freezing media consisting of proliferation media and 10% dimethyl sulfoxide.
Surgical procedures
For ectopic bone formation and cranial defect assays, four host animal groups were used: unaltered male, castrated male, unaltered female, and ovariectomized female C57BL/10J mice. All animals were purchased from Jackson Laboratories, and castration and ovariectomy were performed before acquisition at an age of 5 weeks. Surgeries were performed at an age of 12 weeks. After sacrifice, castration or ovariectomy was visually confirmed.
For ectopic bone formation assays, 1 day before implantation, 250,000 MDSC-B4 were placed on a 5 mm by 5 mm by 1 mm gelatin sponge and allowed to adsorb onto the surface. Proliferation medium was then added to cover the sponge, and the sponges were kept in a normal cell culture condition overnight until the time of implantation. Following sterile surgical procedures outlined in the University of Pittsburgh Institutional Animal Care & Use Committee (IACUC) Protocol #0807916, each animal was anesthetized, and bilateral incisions were made in the skin on the back of the hind limbs. A small pocket was made between the muscles and the gelatin sponge with MDSC-B4 was inserted. The incisions were then closed with sutures, and the animals were allowed to resume normal activity. Animals were euthanized at 21 and 42 days post implantation.
For cranial defect healing assays, sterile surgical procedures outlined in the University of Pittsburgh IACUC Protocol #0807916 were followed, and each animal was anesthetized, and an incision was made along the sagittal suture. To create the cranial defect, the scalp was dissected, and 5 mm trephine was used to create the circular bone defect. Just before treatment, 500,000 MDSC-B4 were mixed with sealer protein solution, and this mixture was applied to the defect simultaneously with thrombin solution. The sealer and thrombin solutions are components of the Tisseel fibrin sealant kit (Baxter Biosurgery), and total volume was ∼40uL of fibrin sealant. The incisions were then closed with sutures, and the animals were allowed to resume normal activity. Animals were sacrificed at 10 and 28 days after treatment.
MicroCT analysis
Ectopic bone formation was evaluated in all groups with a microCT scanner (VivaCT40; Scanco Medical) at 7, 14, 21, 28, 35, and 42 days post implantation. Mice were anesthetized and positioned prone in an animal scanning bed with the legs fixed in a specially designed holder. For cranial defect healing, mice were anesthetized and positioned in an animal scanning bed with the skull in a specially designed holder at 1, 14, and 28 days post treatment. For both assays, scanning was performed with the following settings: energy of 55 kVp, intensity of 145 μA, nominal resolution of 35 μm, and integration time of 200 ms. Two-dimensional image slices were obtained, and contour lines were drawn to define the volume of interest (VOI). An appropriate threshold was chosen for the bone voxels by visually matching thresholded areas to grayscale images. The threshold was kept constant throughout the analyses. This led to a three-dimensional reconstruction of the ectopic bone and provided quantitative data on bone volume (mm3), presented as mean±standard deviation. For ectopic bone formation assays, n=14 unaltered male samples, 14 castrated male samples, 14 unaltered female samples, and 16 ovariectomized female samples. Each bilateral implant was considered an independent sample. For cranial defect healing assays, n=8 unaltered male samples, 11 castrated male samples, 8 unaltered female samples, and 10 ovariectomized female samples.
Histological analysis
For both assays, after euthanasia, both the ectopic bone and surrounding muscle tissue or skulls, with the mandibles and skin removed, were dissected and placed in formalin for 24 h. The samples were then decalcified in 10% ethylenediaminetetraacetic acid (Sigma-Aldrich). Dehydration and then paraffin embedding followed, and samples were cut into five micron sections and dried.
After deparaffinization and rehydration, sections of ectopic bone tissue were stained using standard procedures for hematoxylin and eosin staining (H&E) to determine general tissue morphology. Donor cells were distinguished from host cells by staining for GFP, as all donor cells produced both GFP and BMP4. Briefly, after blocking in both 3% H2O2 in methanol and 2% horse serum in phosphate-buffered saline, biotintylated anti-GFP antibody (Vector Laboratories) was applied at a 1:1000 dilution. Then, streptavidin/HRP (R&D) was applied at a 1:50 dilution followed by the DAB Peroxidase Substrate Kit (Vector Laboratories) as per manufacturer's instructions. Hematoxylin QS (Vector Laboratories) was used as a counterstain. Resultant staining showed donor cells expressing GFP (MDSC-B4) as brown and all cells from the host as purple.
The quantification of histological stains was determined as follows: for both ectopic bone formation and cranial defect healing assays, five random 100× H&E images from one sample were analyzed with ImageJ (NIH) to determine pixel area of new bone and cartilage tissue. For cranial defect healing, the pixel area of total visible defect was also calculated. These pixel areas were then converted to a fraction of new tissue.
Statistical analysis
Sample size of 14–16 ectopic bone implants per host group and sample size of 8–11 cranial defects per host group were used for microCT analysis. Bone volume (mm3), bone/cartilage fraction of ectopic bone (%), and bone/cartilage fraction of cranial defect area (%) were reported as mean±standard deviation. Using SPSS statistical software, two-way repeated measures analysis of variance (ANOVA) with Tukey's post hoc analysis was used for ectopic bone volume, and t-tests were used for bone/cartilage fraction of ectopic bone. One-way repeated measures ANOVA with Tukey's post hoc analysis was used for total and within cranial defect bone volume, and bone/cartilage fraction of the cranial defect area.
Results
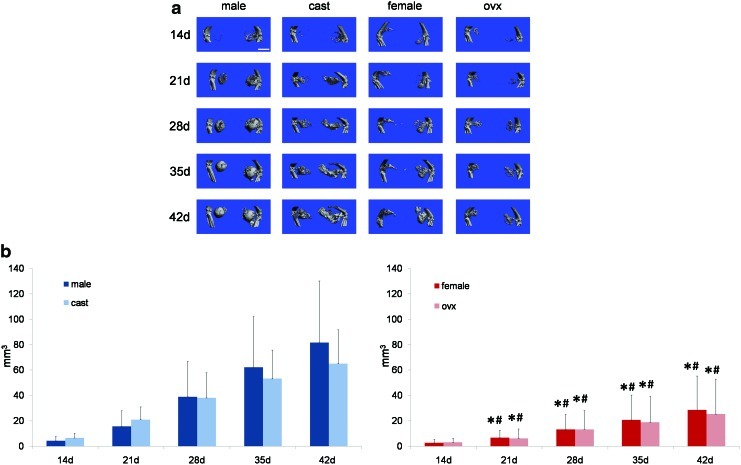
MDSC-mediated ectopic bone formation in four host groups (unaltered male, castrated male, unaltered female, and ovariectomized female) was monitored with weekly microCT scanning, and representative microCT reconstructions are shown in Figure 1a. In summary, male hosts (whether unaltered or castrated) form significantly more bone than female hosts (whether unaltered or ovariectomized) at 21, 28, 35, and 42 days post implantation of MDSC-B4 infused gelatin sponges, and this is displayed in Figure 1b. However, the volume of bone formed was not different between unaltered and castrated male hosts or between unaltered and ovariectomized female hosts. Moreover, via two-way repeated measures ANOVA, it was determined that the interaction of volume and time, indicating the rate of volume accretion over time, was significantly different between, but not within host sex groups (F=4.076, p<0.001). These volume data are summarized in Table 1.
FIG. 1.
Microcomputed tomography (microCT) reconstructions and volume quantification of ectopic bone in unaltered male, castrated male, unaltered female, and ovariectomized female hosts. (a) microCT reconstructions of ectopic bone in unaltered male, castrated male, unaltered female, and ovariectomized female hosts at 14, 21, 28, 35, and 42 days post implantation of muscle-derived stem cell (MDSC)-B4. Scale bar represents 5 mm. (b) volume of ectopic bone formed in unaltered male, castrated male, unaltered female, and ovariectomized female hosts at 14, 21, 28, 35, and 42 days post implantation of MDSC-B4. *indicates different from unaltered male at same time, #indicates different from castrated male at same time (p<0.05 for both). Color images available online at www.liebertpub.com/tea
Table 1.
Quantification of Ectopic Bone Formation Volume (mm3) in All Groups
| Male | Cast | Female | Ovx | |
|---|---|---|---|---|
| 14 days | 4.20±3.46 | 6.49±3.61 | 2.65±2.88 | 3.09±3.33 |
| 21 days | 15.51±12.53 | 20.82±10.19 | 6.71±5.91 | 6.18±7.42 |
| 28 days | 38.85±28.13 | 38.17±19.81 | 13.32±12.08 | 13.29±15.02 |
| 35 days | 62.20±40.17 | 53.20±22.49 | 20.71±19.56 | 18.85±20.54 |
| 42 days | 81.61±48.64 | 64.97±26.87 | 28.60±26.78 | 25.38±27.51 |
All values are mean±standard deviation.
Since no differences in volume or rate of ectopic bone formation were seen between hosts of the same sex, that is, unaltered and castrated male hosts or unaltered and ovariectomized female hosts, only unaltered male and female host groups were analyzed histologically and displayed in Figure 2. At 21 and 42 days post implantation of MDSC-B4 infused gelatin sponges, samples from both unaltered male and female hosts were examined using H&E, as well as GFP immunostaining to identify donor cells. Through these histological analyses, the process of endochondral ossification that resulted in ectopic bone formation was evaluated. In addition, the fates of both donor and host cells were determined.
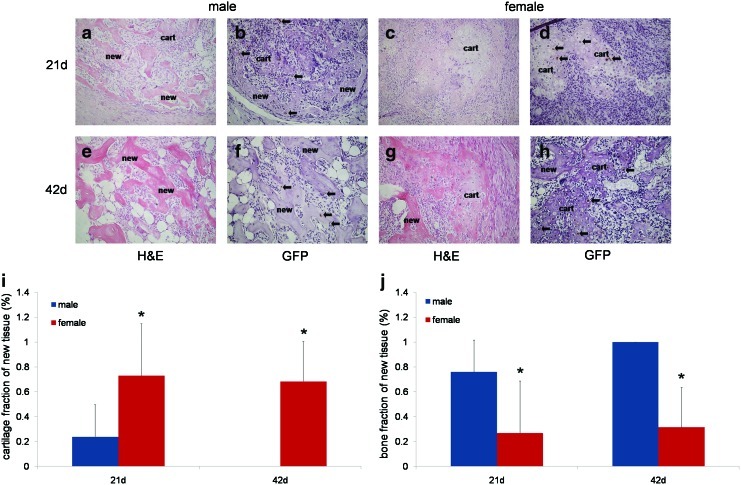
FIG. 2.
Histological analyses of ectopic endochondral bone formation in unaltered male and unaltered female hosts. (a–d) 21 days post implantation of MDSC-B4 (e–h) 42 days post implantation. Both hematoxylin and eosin (H&E) and green fluorescent protein (GFP) immunostaining are shown. “new” indicates new bone tissue formed, while “cart” indicates cartilage formed during the endochondral ossification process. (i) cartilage fraction (%) of new tissue, *indicates different from unaltered male at same time (p<0.05) (j) bone fraction (%) of new tissue, *indicates different from unaltered male at same time (p<0.05). Arrows indicate GFP positive (brown) cells. All images 200×. Color images available online at www.liebertpub.com/tea
Representative images of MDSC-mediated ectopic bone formation in both male and female hosts at 21 and 42 days post implantation are shown in Figure 2a–h. Male hosts displayed bone tissue at both 21 and 42 days, with the latter time point exclusively showed mature, lamellar bone with fat and blood cell-filled spaces, resembling marrow cavities (Fig. 2a, e). In contrast, female hosts at 21 days display large clusters of chondrocytes with no apparent bone tissue (Fig. 2c). By 42 days, a shell of bone tissue is surrounding the chondrocytes clusters, but it appears to be less mature bone than is observed in male hosts, and no marrow-like spaces are seen (Fig. 2g), indicating that male hosts undergo endochondral ossification faster than female hosts.
To determine the contribution of donor and host cells to ectopic bone formation, peroxidase-based immunostaining for GFP was performed. Donor cells (MDSC-B4) that were transduced with a retrovirus encoding for both BMP4 and GFP were stained brown, while host cells were stained purple (Fig. 2b, f, d, h). As seen in both male and female hosts, donor cells were found, but host-derived cells predominate in the ectopic bone tissue. Moreover, in both host groups, donor cells participated in all aspects of endochondral bone formation. Donor cells are observed as chondrocytes, osteoblasts, and osteocytes. However, only cells from the host fill the marrow-like spaces found in the more mature bone of male hosts at later time points (Fig. 2f). There was no marked difference between the number of donor cells present in male and female hosts at either 21 or 42 days post implantation. Therefore, it was concluded that although male and female hosts form differing amounts of bone in response to MDSC-B4 implantation, and this was due to a faster rate of endochondral bone formation in male hosts, the presence of donor cells was not a contributing factor to this rate difference.
From these histological analyses, the percentages of new tissue made up of cartilage and bone were determined (Fig. 2i, j). Female hosts, at both 21 and 42 days post MDSC-B4 implantation, displayed a larger percentage (0.73±0.42 and 0.68±0.32) of cartilage tissue in the new tissue formed, when compared with male hosts at the same time points (0.24±0.25 and 0.0±0.0), as seen in Figure 2i. Fraction of 0.0±0.0 indicates that none of the new tissue observed was cartilage. In contrast, male hosts exhibited a larger percentage of bone tissue in the new tissue formed after MDSC-B4 implantation, as seen in Figure 2j (male 21 days 0.76±0.26, 42 days 1.0±0.0 vs. female 21 days 0.27±0.42, 42 days 0.32±0.32). Again, the fraction of 1.0±0.0 indicates that all new tissue observed was bone.
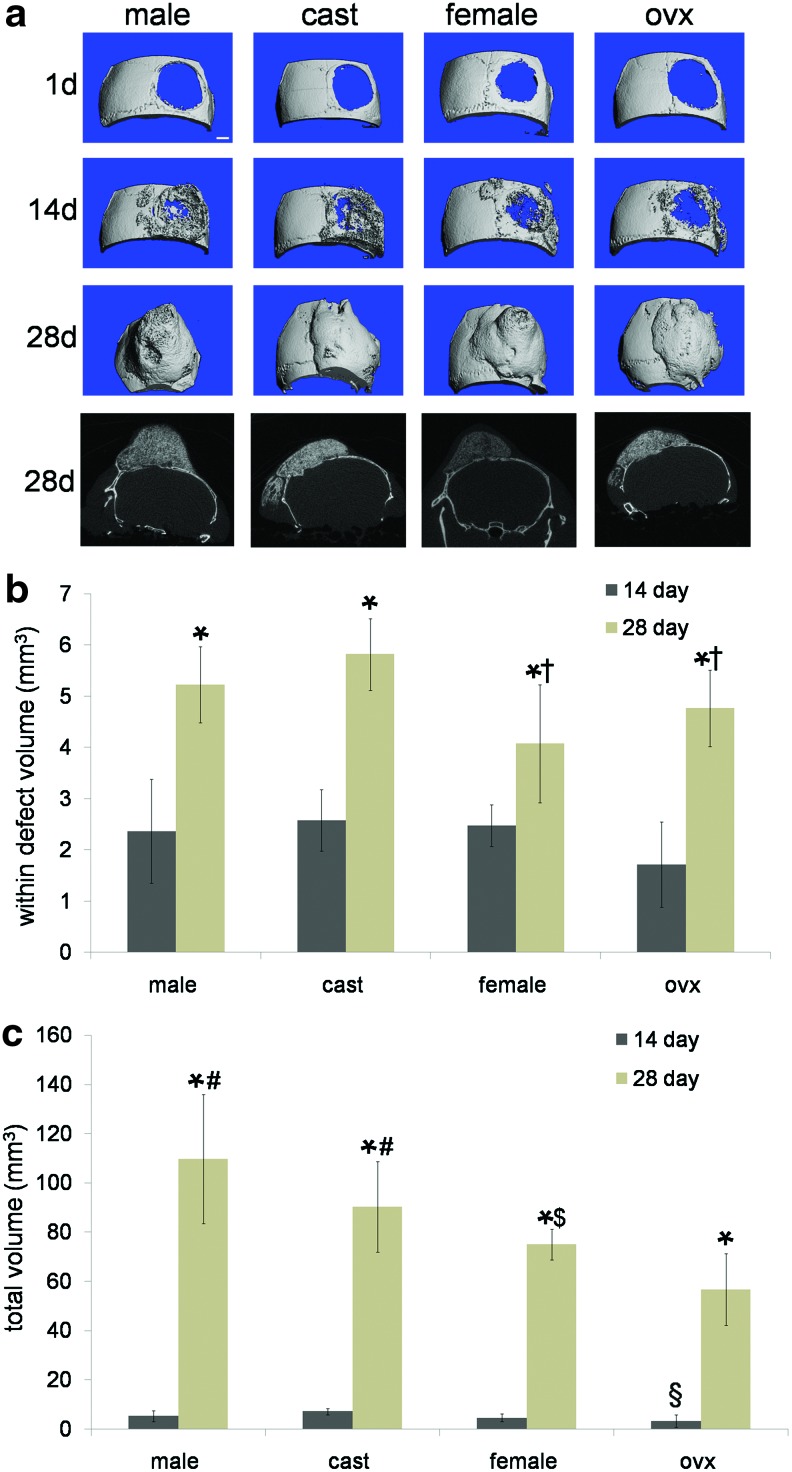
MDSC-mediated bone healing was examined in a cranial defect model, and representative microCT reconstructions of healing in four host groups (unaltered male, castrated male, unaltered female, and ovariectomized female) are shown in Figure 3a. Both within defect bone volume and total bone volume formed were quantified for each host sex group at both 14 and 28 days post treatment (Fig. 3b, c). Each host sex group displayed significantly more bone at 28 days post treatment than at 14 days, and female hosts (both unaltered female, 4.08±0.74mm3 and ovariectomized female, 4.76±1.15mm3) showed significantly less within defect bone than castrated male hosts (5.82±0.74 mm3) at 28 days (Fig. 3b). Moreover, at 28 days post treatment, total bone volume was significantly less in unaltered female hosts (75.0±26.21 mm3) when compared with unaltered male hosts (109.68±18.36 mm3); ovariectomized hosts also showed significantly less total bone volume (3.22±1.55 mm3) than castrated hosts (7.09±2.47 mm3) at 14 days post treatment with MDSC-B4, as demonstrated in Figure 3c.
FIG. 3.
microCT reconstructions and volume quantification of cranial defect healing in unaltered male, castrated male, unaltered female, and ovariectomized female hosts (a) microCT reconstructions of cranial defect healing at 1, 14, and 28 days post treatment with MDSC-B4 and representative coronal slices at 28 days. Scale bar represents 1 mm. (b) volume (mm3) of new bone formed within cranial defect space post treatment with MDSC-B4. *indicates different from same host group at 14 days, †indicates different from castrated male hosts at 28 days. (c) volume (mm3) of total new bone formed post treatment with MDSC-B4. *indicates different from same host group at 14 days, §indicates different from castrated male hosts as 14 days, #indicates different from ovariectomized female hosts at 28 days, $indicates different from unaltered male hosts at 28 days (all p<0.05). Color images available online at www.liebertpub.com/tea
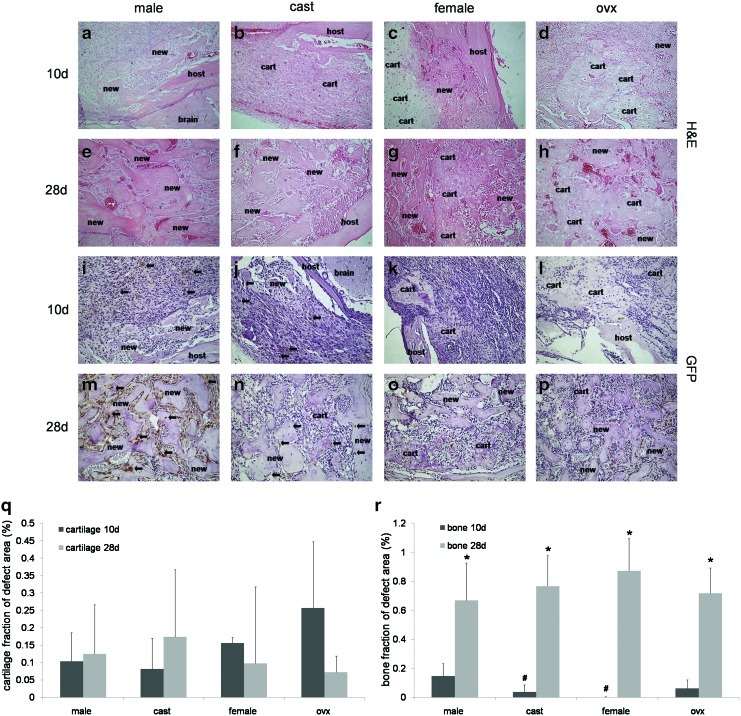
Unlike the previously described ectopic bone formation experiments, host groups of the same sex (unaltered/castrated male and unaltered/ovariectomized female) did have significant differences in some microCT measures; therefore, all four host groups were histologically examined. H&E staining (Fig. 4a–h) and GFP immunostaining (Fig. 4i–p) were performed on sections of each host sex group at various time points post treatment in order to evaluate MDSC-B4 mediated bone formation and healing of the critical size cranial defect. Representative images are shown for all groups at 10 and 28 days post treatment (Fig. 4a–d, i–l, e–h, m–p respectively).
FIG. 4.
Histological analyses of cranial defect healing in unaltered male, castrated male, unaltered female, and ovariectomized female hosts. (a–d) H&E 10 days post treatment with MDSC-B4 (e–h) H&E 28 days post treatment with MDSC-B4 (i–l) GFP immunostaining 10 days post treatment with MDSC-B4 (m–p) GFP immunostaining 28 days post treatment with MDSC-B4 (q) cartilage fraction (%) of new tissue, (r) bone fraction (%) of new tissue. *indicates different from same host group at 10 days, #indicates different from unaltered male hosts at 10 days. Arrows indicate GFP positive (brown) cells. All images 200×. Color images available online at www.liebertpub.com/tea
In all host groups at 10 days post treatment, early endochondral bone formation was observed with some cartilage, and new bone formed at the defect site (Fig. 4a–d). A trend of more cartilage in both unaltered and ovariectomized female hosts was also observed (Fig. 4c–d, q). After 28 days of treatment with MDSC-B4, male hosts, both unaltered and castrated, demonstrated endochondral bone formation at more mature stages of ossification when compared with female hosts, both unaltered and ovariectomized. At this later time point, male hosts demonstrated almost exclusively new bone formed at the cranial defect site, with little to no cartilage present; this bone was arranged into mature trabecular bone with marrow-like spaces filled with red blood cells (Fig. 4e, f). However, female hosts still displayed cartilage tissue with some new bone tissue surrounding it (Fig. 4g, h), suggesting an earlier stage of endochondral ossification. All host groups significantly increased bone tissue fraction of new tissue formed from 10 to 28 days post treatment with MDSC-B4, as displayed in Figure 4r, and at 10 days, unaltered males (0.15±0.09) demonstrated a significantly larger bone tissue fraction than both castrated males (0.04±0.05) and unaltered females (0.002±0.003), also observed in Figure 4r.
GFP immunostaining revealed the contribution of both donor and host cells to the bone formed at the cranial defect site. Representative images of all host groups at 10 and 28 days after treatment with MDSC-B4 are shown in Figure 4i–p, with donor cells stained brown and host cells stained purple. At 10 days after treatment, both donor and host cells are found in the new bone forming at the defect site in unaltered and castrated male hosts (Fig. 4i, j). In female hosts at 10 days post treatment, both donor and host cells are seen in the cartilage phase of endochondral bone formation found in both unaltered and ovariectomized hosts (Fig 4k, l). Donor cells are also found interspersed among a majority of host cells in the proliferative stage of endochondral bone formation in all groups at 10 days post treatment (Fig. 4i–l). At 28 days after treatment, donor cells are found as osteoblasts, osteocytes, and chondrocytes (Fig 4m–p). Donor cells were never observed in the marrow-like spaces present in later stages of mature bone; these cells were always derived from the host animal, as seen in Figure 4m–p. Most importantly, as in bone formation, the majority of cells participating in all stages of healing of the defect were host derived, regardless of the sex group of the host.
Discussion
Previous studies using MDSCs found significant differences in osteogenic, chondrogenic, and myogenic differentiation (both in vivo and in vitro) based on donor sex, in addition to in vivo differences in muscle repair correlated to host animal sex.11–13 The present study examined the effect of host sex and sex hormones on MDSC-mediated ectopic bone formation and healing of a bone defect. Using four host animal groups (unaltered male, castrated male, unaltered female, and ovariectomized female) in ectopic intramuscular bone formation and cranial bone defect healing models, microCT analyses and histological stains were evaluated. Male hosts, whether unaltered males or castrated males, displayed significantly more bone formed than female hosts, whether unaltered females or ovariectomized female, in ectopic bone formation assays. Similar results of more healing (larger volumes of bone) were found in MDSC-treated cranial defects in male hosts compared with female hosts. Accelerated endochondral bone formation and healing induced by MDSC-B4 treatment was observed in both groups of male hosts, and this was determined to be the reason for greater bone formation and healing when compared with female hosts.
No bone volume differences were observed between male and castrated groups or between female and ovariectomized groups, suggesting that sex hormones did not affect MDSC-mediated ectopic bone formation in this study. These results were in contrast to previous ectopic bone formation studies, utilizing demineralized bone matrix, which show sex hormone effects on bone formation in multiple species.22–24 With regard to native bone maintenance and turnover, ovariectomy is also known to decrease bone volume and quality in both rats and mice (including the C57BL strain of mice used in this study).25–29 Normal skeletal bone formation, growth, and maintenance are all known to be influenced by sex steroid hormones.30–32 However, MDSC-mediated bone formation appeared not to be influenced by ovariectomy or castration, which could be promising for translation to clinical therapies.
In this study, ectopic bone was created in the skeletal muscle of hosts of different sexes. It was observed that the majority of the cells contributing to new bone tissue are host derived, either originating from the muscle tissue itself or traveling from some other location via the circulatory system. A factor that could play a role is non hormone-related, sex-related differences in gene expression of the skeletal muscle environment surrounding and contributing to the MDSC-mediated ectopic bone formation. Of the 7367 genes expressed in murine skeletal muscle, 55.4% of these are sexually dimorphic.33 Interestingly, four of these sexually dimorphic skeletal muscle genes are also known to be involved in BMP-induced endochondral ossification.34 If the bone formation environment (the skeletal muscle in this model) is innately different between male and female hosts, this could influence MDSC-mediated bone formation.
MDSC-mediated ectopic bone formation was found to be affected by host sex, but not sex hormones; however, this model did not indicate how MDSC-mediated bone repair may correlate to bone formation. Intramuscular ectopic bone formation assays, while providing a fast and valuable measure of the efficacy of osteogenic capacity of cells, materials, and other therapies, do not fully recapitulate bone formation. Unlike developmental bone formation or long bone fracture healing, in ectopic bone formation assays, the endochondral bone formation cascade is brought about by extrinsic osteogenic factors (cells, proteins, or biomaterials) that are not normal developmental or healing signals. While these factors can, in fact, induce ectopic bone formation, a bone healing research model provides more information with regard to the clinical efficacy of osteogenic therapies. In order to determine whether host sex and/or sex hormones affect bone repair, MDSC-mediated healing of a cranial defect was evaluated in unaltered male, castrated male, unaltered female, and ovariectomized female hosts. Increased bone formation at the callus site of a long bone fracture has been correlated to fracture healing.35 Since increased MDSC-mediated ectopic bone formation was seen in male hosts compared with female hosts, it was theorized that healing of a bone fracture or defect may also be accelerated in male hosts compared with female hosts. In this bone healing study, as in ectopic bone formation studies, differences were found between male and female hosts. However, unlike ectopic bone formation studies, some measures were also different between hosts of the same sex: male and castrated male or female and ovariectomized female.
Some clinical evidence exists that sex may be considered a factor in fracture healing; however, these studies are usually retrospective studies evaluating nonunion as an outcome and do not examine the rate of healing. Female sex is associated with increased risk of nonunion in femoral, scaphoid, and mandibular fractures.36–38 No known studies have examined host sex or sex hormones in the context of a critical-sized cranial defect model. However, a few studies have investigated host sex and sex hormones in long bone fracture healing.39–42 While the study presented here does not demonstrate differences in bone defect healing between sexes, it does reveal host sex differences in the response to MDSC-B4 when applied as a bone healing therapy which is important for translational considerations.
Although some inferences can be made for translational therapies, it is important to note several limitations of the data presented. Since MDSCs from male donors have previously been shown to be more osteogenic and in order to simplify a complex set of in vivo experiments, only male donor MDSCs were used to induce bone formation or bone healing. Although not tested in the current study, it is expected that male and female hosts would have a similar response to female donor MDSC-induced bone formation and healing. In a previous study by our group, differences in host sex effect on muscle healing showed similar trends, regardless of donor sex.12 Moreover, bone formed in the cranial defect studies demonstrates overgrowth of new bone outside the defect margins; this could be due to the sustained production of BMP4 protein by retrovirally transduced MDSCs or caused by overflow of the fibrin sealant containing MDSCs around the edge of the defect. While this bone formation was not ideal, the purpose of this study was to examine the effect of host sex and host sex hormones; therefore, other variables such as cell type and delivery vehicle were kept constant. Future studies will concentrate on optimization of these variables for bone healing.
These results suggest that MDSCs, when transduced with a retrovirus encoding for BMP4, induce endochondral bone formation, in both an ectopic bone formation and a cranial bone healing model. The sex of the host, but not sex hormones, affects intramuscular ectopic bone formation; male hosts demonstrate more rapid bone formation and hence, larger volumes of ectopic bone formed than their female counterparts. Similar trends were observed in a cranial defect healing model, with male hosts forming more bone than female hosts at the defect site. However, some quantitative volume differences were found between hosts of the same sex (castrated and unaltered males or ovariectomized and unaltered females) that were not observed in an ectopic bone model. These studies, taken together, demonstrate the importance of taking host sex into account when determining cell-based osteogenic therapies. Future studies will examine the methods of modulating and controlling bone formation and healing differences known to be associated with host sex, in order to provide better bone healing therapies for both sexes.
Acknowledgments
The authors wish to acknowledge Jim Cummins, Burhan Gharaibeh, Mathieu Huard, and Zach Nelson. Funding has been provided by NIH R01 DE013420-10, the Henry J. Mankin Endowed Chair at University of Pittsburgh, and the William F. and Jean W. Donaldson Endowed Chair at Children's Hospital of Pittsburgh of UPMC.
Disclosure Statement
Johnny Huard is a paid consultant for Cook MyoSite, a company that has licensed technology developed by his laboratory. All other authors declare no conflicts of interest. No competing financial interests exist.
References
- 1.Gharaibeh B., et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 2.Jankowski R.J., et al. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.Y., et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu-Petersen Z., et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B., et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda R., et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 7.Peng H., et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H.C., et al. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone. 2004;34:982. doi: 10.1016/j.bone.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Wright V., et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 10.Peng H., et al. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9:885. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Corsi K.A., et al. The osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res. 2007;22:1592. doi: 10.1359/jbmr.070702. [DOI] [PubMed] [Google Scholar]
- 12.Deasy B.M., et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto T., et al. The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum. 2008;58:3809. doi: 10.1002/art.24125. [DOI] [PubMed] [Google Scholar]
- 14.Payne K.A. Didiano D.M. Chu C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18:705. doi: 10.1016/j.joca.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoetzer G.L., et al. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99:46. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crisostomo P.R., et al. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142:215. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Ishida Y. Heersche J.N. Progesterone stimulates proliferation and differentiation of osteoprogenitor cells in bone cell populations derived from adult female but not from adult male rats. Bone. 1997;20:17. doi: 10.1016/s8756-3282(96)00315-8. [DOI] [PubMed] [Google Scholar]
- 18.Dudas J.R., et al. Leporine-derived adipose precursor cells exhibit in vitro osteogenic potential. J Craniofac Surg. 2008;19:360. doi: 10.1097/SCS.0b013e318163e17b. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa R., et al. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem Biophys Res Commun. 2004;319:511. doi: 10.1016/j.bbrc.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Csete M. Gender issues in transplantation. Anesth Analg. 2008;107:232. doi: 10.1213/ane.0b013e318163feaf. [DOI] [PubMed] [Google Scholar]
- 21.Peng H., et al. Development of an MFG-based retroviral vector system for secretion of high levels of functionally active human BMP4. Mol Ther. 2001;4:95. doi: 10.1006/mthe.2001.0423. [DOI] [PubMed] [Google Scholar]
- 22.McMillan J., et al. Osteoinductivity of demineralized bone matrix in immunocompromised mice and rats is decreased by ovariectomy and restored by estrogen replacement. Bone. 2007;40:111. doi: 10.1016/j.bone.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Burnett C.C. Reddi A.H. Influence of estrogen and progesterone on matrix-induced endochondral bone formation. Calcif Tissue Int. 1983;35:609. doi: 10.1007/BF02405102. [DOI] [PubMed] [Google Scholar]
- 24.Kapur S.P. Reddi A.H. Influence of testosterone and dihydrotestosterone on bone-matrix induced endochondral bone formation. Calcif Tissue Int. 1989;44:108. doi: 10.1007/BF02556469. [DOI] [PubMed] [Google Scholar]
- 25.Bouxsein M.L., et al. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res. 2005;20:1085. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., et al. Short- to mid-term effects of ovariectomy on bone turnover, bone mass and bone strength in rats. Biol Pharm Bull. 2007;30:898. doi: 10.1248/bpb.30.898. [DOI] [PubMed] [Google Scholar]
- 27.Coxam V., et al. Effects of dihydrotestosterone alone and combined with estrogen on bone mineral density, bone growth, and formation rates in ovariectomized rats. Bone. 1996;19:107. doi: 10.1016/8756-3282(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 28.Boyd S.K., et al. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone. 2006;39:854. doi: 10.1016/j.bone.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Klinck J. Boyd S.K. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif Tissue Int. 2008;83:70. doi: 10.1007/s00223-008-9150-5. [DOI] [PubMed] [Google Scholar]
- 30.Riggs B.L. Khosla S. Melton L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 31.Vanderschueren D., et al. Androgens and bone. Endocr Rev. 2004;25:389. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 32.Abu E.O., et al. The localization of androgen receptors in human bone. J Clin Endocrinol Metab. 1997;82:3493. doi: 10.1210/jcem.82.10.4319. [DOI] [PubMed] [Google Scholar]
- 33.Yang X., et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy B.M., et al. A gene expression profile for endochondral bone formation: oligonucleotide microarrays establish novel connections between known genes and BMP-2-induced bone formation in mouse quadriceps. Bone. 2003;33:46. doi: 10.1016/s8756-3282(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 35.Beil F.T., et al. Effects of increased bone formation on fracture healing in mice. J Trauma. 2011;70:857. doi: 10.1097/TA.0b013e3181de3dd9. [DOI] [PubMed] [Google Scholar]
- 36.Chang M.A., et al. The outcomes and complications of 1,2-intercompartmental supraretinacular artery pedicled vascularized bone grafting of scaphoid nonunions. J Hand Surg Am. 2006;31:387. doi: 10.1016/j.jhsa.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., et al. Abnormal union of mandibular fractures: a review of 84 cases. J Oral Maxillofac Surg. 2006;64:1225. doi: 10.1016/j.joms.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Parker M.J. Raghavan R. Gurusamy K. Incidence of fracture-healing complications after femoral neck fractures. Clin Orthop Relat Res. 2007;458:175. doi: 10.1097/BLO.0b013e3180325a42. [DOI] [PubMed] [Google Scholar]
- 39.Hao Y.J., et al. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone. 2007;41:631. doi: 10.1016/j.bone.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Mehta M., et al. A 5-mm femoral defect in female but not in male rats leads to a reproducible atrophic non-union. Arch Orthop Trauma Surg. 2011;131:121. doi: 10.1007/s00402-010-1155-7. [DOI] [PubMed] [Google Scholar]
- 41.Melhus G., et al. Experimental osteoporosis induced by ovariectomy and vitamin D deficiency does not markedly affect fracture healing in rats. Acta Orthop. 2007;78:393. doi: 10.1080/17453670710013988. [DOI] [PubMed] [Google Scholar]
- 42.Strube P., et al. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone. 2009;45:1065. doi: 10.1016/j.bone.2009.08.005. [DOI] [PubMed] [Google Scholar]