Abstract
Tissue-engineered muscle has been proposed as a solution to repair volumetric muscle defects and to restore muscle function. To achieve functional recovery, engineered muscle tissue requires integration of the host nerve. In this study, we investigated whether denervated muscle, which is analogous to tissue-engineered muscle tissue, can be reinnervated and can recover muscle function using an in vivo model of denervation followed by neurotization. The outcomes of this investigation may provide insights on the ability of tissue-engineered muscle to integrate with the host nerve and acquire normal muscle function. Eighty Lewis rats were classified into three groups: a normal control group (n=16); a denervated group in which sciatic innervations to the gastrocnemius muscle were disrupted (n=32); and a transplantation group in which the denervated gastrocnemius was repaired with a common peroneal nerve graft into the muscle (n=32). Neurofunctional behavior, including extensor postural thrust (EPT), withdrawal reflex latency (WRL), and compound muscle action potential (CMAP), as well as histological evaluations using alpha-bungarotoxin and anti-NF-200 were performed at 2, 4, 8, and 12 weeks (n=8) after surgery. We found that EPT was improved by transplantation of the nerve grafts, but the EPT values in the transplanted animals at 12 weeks postsurgery were still significantly lower than those measured for the normal control group at 4 weeks (EPT, 155.0±38.9 vs. 26.3±13.8 g, p<0.001; WRL, 2.7±2.30 vs. 8.3±5.5 s, p=0.027). In addition, CMAP latency and amplitude significantly improved with time after surgery in the transplantation group (p<0.001, one-way analysis of variance), and at 12 weeks postsurgery, CMAP latency and amplitude were not statistically different from normal control values (latency, 0.9±0.0 vs. 1.3±0.7 ms, p=0.164; amplitude, 30.2±7.0 vs. 46.4±26.9 mV, p=0.184). Histologically, regeneration of neuromuscular junctions was seen in the transplantation group. This study indicates that transplanted nerve tissue is able to regenerate neuromuscular junctions within denervated muscle, and thus the muscle can recover partial function. However, the function of the denervated muscle remains in the subnormal range even at 12 weeks after direct nerve transplantation. These results suggest that tissue-engineered muscle, which is similarly denervated, could be innervated and become functional in vivo if it is properly integrated with the host nerve.
Introduction
Volumetric muscle defects due to traumatic injury are usually repaired with vascularized muscle flaps or grafts.1–5 However, debilitation remains a problem when donor muscle tissue is unavailable, and the defect is not completely repaired.1,6 Studies in tissue engineering have devised techniques that utilize in vitro-cultured muscle cells to create muscle tissue for functional restoration in vivo.7–11 However, to achieve functional recovery, engineered muscle tissues require integration of a host nerve to facilitate a coordinated motion.12,13 It is currently unclear whether in vitro-engineered muscle constructs are able to integrate with the host nerve and develop normal contractility.
Injuries to motor neurons can lead to denervation atrophy of the associated muscle and functional impairment.14–16 A denervated muscle mass undergoes a rapid decline, and can lose up to two-thirds of its mass within 1 month.14 The motor neuron exerts a trophic influence on muscle fibers that is mediated by a number of substances and by nerve impulses themselves. This effect is thought to act through the synapse, and if this connection is disrupted by injury or disease, protein synthesis in the muscle slows and the muscle atrophies. This is manifested as a reduction in muscle fiber diameter, significant fibrosis, and replacement of muscle tissue with fat. These structural changes lead to observed reductions in electrophysiological processes and muscle function.
Several surgical methods for repairing damaged nerves have been developed in an attempt to restore and maintain proper nerve and muscle function. In some trauma cases, a transected nerve can be repaired using an end-to-end anastomosis technique, and this can prevent denervation atrophy and restore some function. However, in many cases, this type of primary nerve repair is not possible. In these cases, nerve grafts, nerve conduits, nerve transfer17 and nerve-muscle pedicles18 can be used; however, all of these techniques require that the patient has an adequate distal nerve stump that can be connected to the proximal stump either directly or through a conduit.
In patients who lack a distal nerve segment for anastomosis, direct nerve transplantation into the denervated muscle (neurotization)19–36 is indicated. This group would include those receiving a transplant of tissue-engineered muscle to repair volumetric muscle loss, because the engineered muscle does not contain host nerve tissue and thus cannot be anastomosed. In the neurotization procedure, a healthy nerve segment is taken from another site and used to repair the injured nerve site. Although this method was first developed at the beginning of the 20th century,35 its effect on muscle functionality is not fully understood.21,22,26,28 Previous studies designed to determine whether functional recovery occurs after nerve transplantation did not investigate endpoints beyond the electrophysiological recovery.20,22,24,27,33,34 In addition, many preclinical and clinical studies19,22,23,25,28–33,36,37 did not exclude the possible effects of the presence of uninjured collateral nerves on muscle function and regeneration.
Because in vitro-engineered muscle constructs are analogous to denervated muscle tissue, a denervation model can be used to study some of the potential responses of such constructs in vivo. We investigated whether denervated muscle tissue can be reinnervated by neurotization and whether this procedure has an impact on the development or recovery of muscle function. We created an in vivo model of denervation and tested whether reinnervation of the muscle using neurotization would induce recovery of the muscle function. We hypothesized that the outcomes of this investigation would provide insights into the ability of tissue-engineered muscle, if provided with sufficient nerve tissue, to integrate with host nerve and acquire the function of normal muscle tissue.
Materials and Methods
Animals
The Animal Care and Use Committee at Wake Forest University Health Sciences approved all procedures performed on animals. All experiments were conducted using 3-week-old (200–250 g) male Lewis rats. All rats had access to food and water and were inspected daily during the preoperative and the 12-week postoperative periods. Rats were divided into three groups: a normal control group (n=16), a denervation group (n=32), and a transplantation group (n=32). Animals from the normal control group were sacrificed 2 and 4 weeks postoperatively (n=8 at each time point). Animals from the two experimental groups were sacrificed at 2, 4, 8, and 12 weeks postoperatively (n=8 for each time point).
Surgical procedures
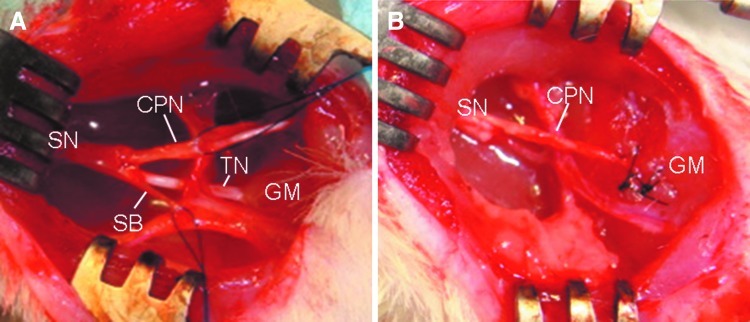
All animals were anesthetized with 3% isoflurane before the surgical procedure. An aseptic technique was used for all procedures. An incision of 10 mm was made in the posterior area of the right hind limb. In the denervation group, a 10-mm segment of the sciatic nerve, including the common peroneal nerve, tibial nerve, and sensory nerve branches, was removed. In the transplantation group, a graft created from the common peroneal nerve was imbedded into the gastrocnemius muscle (GM) after excision of the tibial nerve and sensory nerve branch (Fig. 1). The nerve graft was placed close to the native motor end plate of the GM. The epineurium of the common peroneal nerve was anastomosed microsurgically to the epimysium of the muscle with two 7–0 Pronova sutures (Ethicon). The anastomotic procedure was carried out under a dissecting microscope under sterile conditions. After repair, the muscle and skin were closed using 5–0 Vicryl sutures (Ethicon).
FIG. 1.
Diagram of the surgical site used to denervate the gastrocnemius muscle (GM) in a typical animal from the study. All animals were anesthetized with 3% isoflurane before the surgical procedure. An incision of 10 mm was made in the posterior area of the right hind limb. (A) Normal anatomy of the branches of the sciatic nerve at right popliteal area. Landmarks: sciatic nerve (SN), tibial nerve (TN), common peroneal nerve (CPN), sensory nerve branch (SB), gastrocnemius muscle (GM). In the denervation group, a 10-mm segment of the sciatic nerve (SN), including the common peroneal nerve (CPN), tibial nerve (TN) and sensory nerve branches (SB), was removed. In the transplantation group, a graft created from the common peroneal nerve was imbedded into the GM after excision of the tibial nerve and sensory nerve branch (B). The nerve graft was placed close to the native motor end plate of the GM. Color images available online at www.liebertpub.com/tea
Neurobehavioral studies
The development of postural changes of the injured limb was observed after surgery. Motor functional recovery after nerve transplantation was examined by measuring the extensor postural thrust (EPT), as proposed by Thalhammer et al.38 The entire body of the rat, with the exception of the hind limbs, was wrapped in a surgical towel. EPT was elicited by supporting the animal by the thorax and by lowering the affected hind limb to the platform of a digital balance. As the animal was lowered to the platform, it extended its hind limb in anticipation of contact made by the distal metatarsus and digits. EPT was measured as the force applied to the digital platform balance by the heel. Sensory functional recovery was evaluated by measuring nociceptive withdrawal reflex. To evaluate the withdrawal reflex latency (WRL), proposed by Masters et al.,39 a modified version of the hotplate test was used. Briefly, the rat was wrapped in a surgical towel above its midsection and then positioned such that the affected hind paw was in contact with a hot plate set to 56°C. WRL was defined as time elapsed from the onset of hotplate contact to withdrawal of the hind paw. This was measured with a stopwatch. The cutoff time for heat stimulation was 12 s, to avoid skin damage to the foot.
Electrophysiological assessment
Under anesthesia, the tibial nerve (control group) and the transplanted common peroneal nerve (transplantation group) were exposed. A disposable monopolar needle electrode (25 mm×0.45 mm, 26G; CadwellInc) was used. The ground electrode was put on the back of the animal, and stimuli were applied using a hook-shaped bipolar tungsten electrode placed 1 cm proximal to the insertion site of the common peroneal nerve into the GM. A recording electrode was placed in the midsection of the GM, and a reference electrode was placed at the distal area of the muscle. The nerve was electrically stimulated by 2.0 mA using a generator. Electrodiagnostics were performed using the Cadwell-Sierra LT EMG setup (Cadwell, Inc.). Digitalized data were stored on a personal computer, and latency and amplitude of the compound muscle action potential (CMAP) of the GM were calculated from these data.
Histological assessment
At the end of the experiment, animals were sacrificed, and the GMs were dissected. Sections were taken to include the transplanted nerve and attached muscle such that the nerve bundles and nerve terminals were parallel with the long axis of the muscle. For histological analyses, the specimens were frozen in an OCT compound (Sakura Finetek USA, Inc.). Twenty-micrometer longitudinal and transverse sections were cut on a cryotome (Frigocut) at −20°C for immunofluorescence and hematoxylin and eosin (H&E) staining. Rabbit anti-NF-200 (polyclonal 1:80; Sigma), which reacts with the 200-kDa neurofilament protein, was used to visualize axonal sprouting from the transplanted nerve at the nerve/muscle junctions. Rhodamine-conjugated goat anti-rabbitIgG antibody (1:200; Jackson-immunogens) was used as the secondary antibody. Alexa Fluor® 488-conjugated alpha-bungarotoxin (1:400; Invitrogen/Molecular Probes) was used to stain the acetylcholine receptor (AchR). After staining, slides were washed and mounted with a Vectashield (Vector)-mounting medium.
Statistical analysis
Data for the electrophysiological and functional studies are presented as mean±standard deviation. Student's t-tests were utilized for comparisons of means between groups. The recovery of muscle function as defined by the times above was analyzed using a one-way analysis of variance (ANOVA). p-values<0.05 were considered to be statistically significant. All calculations were conducted using SPSS 11.0 software (SPSS).
Results
All animals recovered uneventfully from surgery. Self-mutilation after denervation of the limb was not observed, and all surgical incisions healed without complications. At the time of implant retrieval, all transplanted nerves were intact, with no anastomotic disruptions. Grossly, there was no evidence of significant inflammatory reactions or neuromas.
Neurobehavioral study
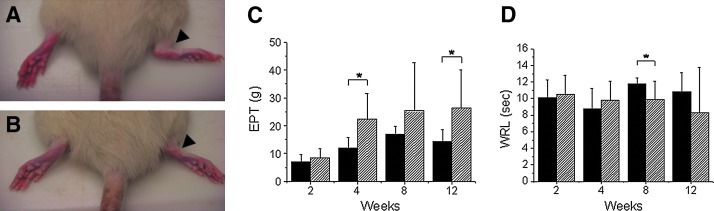
Dorsal flexion contracture of the ankle joint was observed in all animals in the denervation group, but no contracture was seen in the transplantation group (Fig. 2A, B). EPT was significantly higher in the transplantation group than in the denervation group at 4 and 12 weeks postoperatively (p=0.012 and 0.036, respectively) (Fig. 2C). In the transplantation group, EPT was significantly different between time points (p=0.024, one-way ANOVA), suggesting a trend toward functional improvement over time in this group. On post hoc analysis, a difference was identified at 12 weeks (p=0.031, Tukey HSD test). However, all EPT values were significantly lower than those seen in the normal control group (155.0±38.9 vs. 26.3±13.8 g, normal at 4 weeks vs. transplantation group at 12 weeks post-transplant, p<0.001).
FIG. 2.
(A) Image showing dorsal flexion contracture of the ankle joint (black arrow) in a Lewis rat from the denervated group. (B) Image indicating lack of contracture of the ankle joint (black arrow) in a Lewis rat receiving a nerve transplant after denervation of the gastrocnemius muscle. (C) Bar graph illustrating the values for extensor postural thrust (EPT) measured in the study animals. EPT was significantly higher in the transplantation group than in the denervation group at 4 and 12 weeks postoperatively (p=0.012 and 0.036, respectively). In the transplantation group, EPT was also significantly different between time points (p=0.024, one-way analysis of variance [ANOVA]), suggesting a trend toward functional improvement over time in this group. On post hoc analysis, a difference was identified at 12 weeks (p=0.031, Tukey HSD test). However, all EPT values were still significantly lower than those seen in the normal control group (155.0±38.9 vs. 26.3±13.8 g, normal at 4 weeks vs. transplantation group at 12 weeks post-transplant, p<0.001). The “*” symbol denotes statistically significant differences between groups. (D) Bar graph illustrating the values for withdrawal reflex latency (WRL) measured in the study animals. The WRL in the transplantation group was significantly lower than that of the denervation group at 8 weeks (p=0.036). However, there was no significant difference between the denervated and transplanted groups over time (p=0.595, one-way ANOVA). WRL values were consistently in the subnormal range, even in the transplantation group at 12 weeks postoperatively (2.7±2.30 vs. 8.3±5.5 s, normal vs. transplantation group, p=0.027). The “*” symbol denotes statistically significant differences between groups. Color images available online at www.liebertpub.com/tea
The WRL in the transplantation group was significantly lower than that of the denervation group at 8 weeks (p=0.036) (Fig. 2D). However, there was no significant difference between the denervated and transplanted groups over time (p=0.595, one-way ANOVA). WRL values were consistently in the subnormal range, even in the transplantation group at 12 weeks postoperatively (2.7±2.30 vs. 8.3±5.5 s, normal vs. transplantation group, p=0.027).
Electrophysiological study
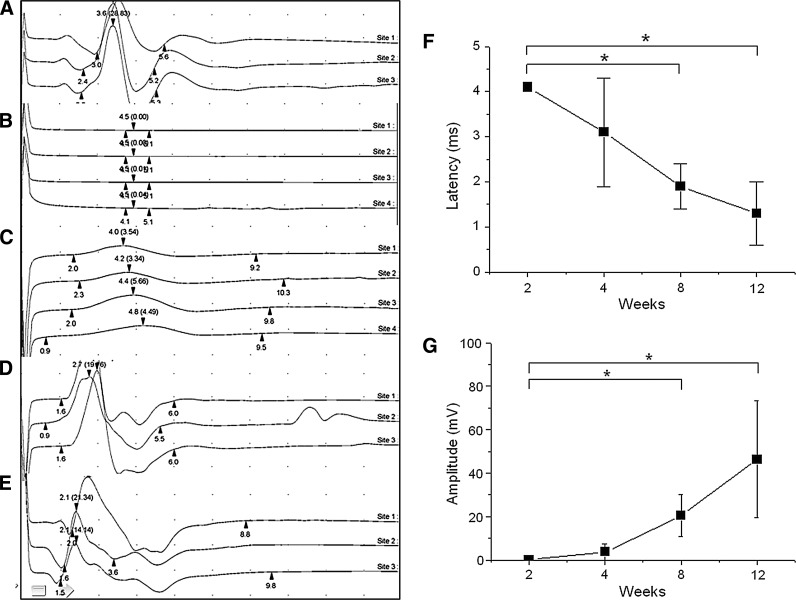
Electrophysiological stimulation studies indicated that the CMAPs of the animals in the transplantation group improved over time (Fig. 3A–E). The first visible signs of recovery occurred at 4 weeks post-transplant, when weak contractions in response to nerve stimulation could be seen. This response had improved dramatically after 8 weeks. At 12 weeks, the contractions grew in amplitude and became hard to distinguish from those measured in the normal control group. Latencies in the transplantation group decreased with time (p<0.001, one-way ANOVA) (Fig. 3F). On a post hoc analysis, differences were identified at 8 and 12 weeks (p<0.001, Tukey HSD test). However, despite the improvement seen in the transplantation group, the measured values were still significantly different from those measured in the normal control group (0.9±0.0 vs. 1.3±0.7 ms, normal vs. transplantation group, p=0.012). In addition, the CMAP amplitude measured in the transplanted animals significantly increased with time (p<0.001, one-way ANOVA) (Fig. 3G). On post hoc analysis, the largest difference was identified at 8 and 12 weeks after transplantation (p=0.042 and p<0.001, respectively, Tukey HSD test). The CMAP at 12 weeks after transplantation was not different from the normal control group (30.2±7.0 vs. 46.4±26.9 mV, normal vs. transplantation group, p=0.184).
FIG. 3.
Electrophysiological stimulation of the gastrocnemius muscle indicated that CMAPs of the animals in the transplantation group improved over time: (A) Control animal; (B) denervated animal; (C) 4 weeks post-transplant; (D) 8 weeks post-transplant; (E) 12 weeks post-transplant; (F) measured latencies decrease with time after nerve transplantation; (G) amplitude of muscle contraction increases with time after nerve transplant. (*=difference is statistically significant).
Histology
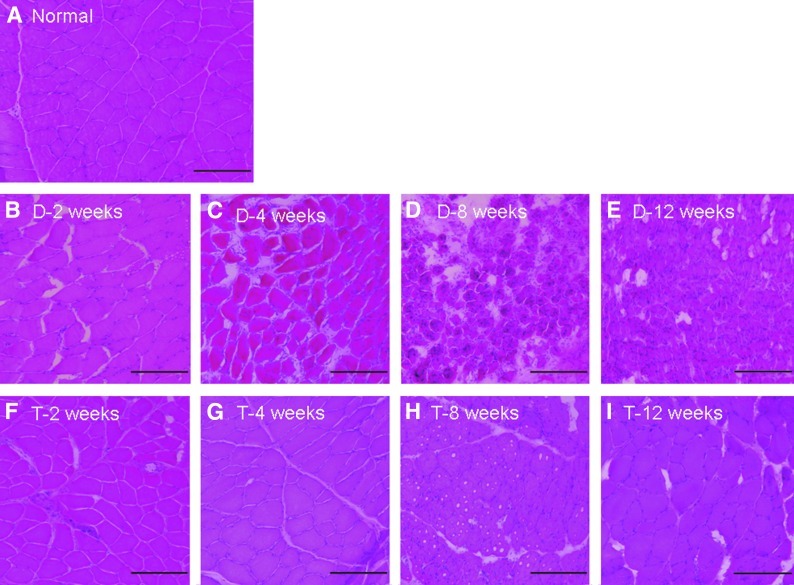
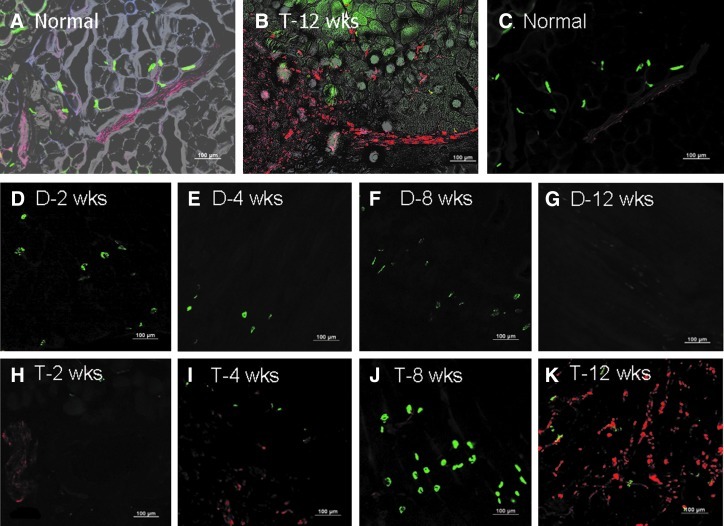
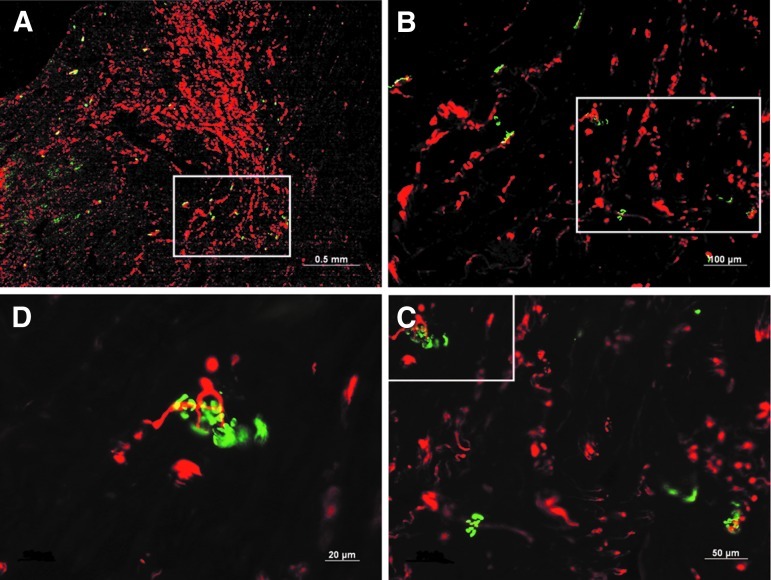
H&E staining indicated that recovery of muscle fibers in the transplantation group occurred with time, whereas in the denervation group, increased fatty infiltration, decreases in the sarcomere size, and increased nuclear packing were observed (Fig. 4). At 12 weeks, reinnervated muscle from the transplantation group did not appear to be different from the muscles in the normal control group. In the phase-contrast image of the transplantation group at 12 weeks, axonal sprouts from the proximal terminal axon grew prominently, with even distribution of axon fibers (Fig. 5A, B). In the denervation group, remnant axon filaments had disappeared almost completely, although staining for the AchR remained even at 12 weeks (Fig. 5D–G). By counting NF-positive (red) cells per field at 200× magnification, we determined that in the denervated group, visible axon filaments were reduced to 17% of control at 2 weeks, 2.1% of control at 4 weeks, and 6.4% of control at both 8 and 12 weeks after the denervation procedure. In the transplantation group, however, we found that NF staining was nearly normal at 2 weeks after transplant (85.1% of control), but then it declined to 36.1% of control by 4 weeks and 40.4% of the control by 8 weeks. Interestingly, by 12 weeks after transplantation, the NF staining in the sections had once again returned to near normal levels (87.2% of the control). This indicates that even after transplantation, there is a period in which axon filaments regress, possibly due to trauma to the transplanted nerve. However, the nerve appears to recover by 12 weeks after transplantation. Additionally, the integration of axon and the AchR improved with time (Fig. 5H–K) in the transplantation group. Clusters of AchR, adjacent to the growing sprouts, were apparent from about 8 weeks after transplantation (Fig. 6).
FIG. 4.
Histological studies. Hematoxylin and eosin (H&E) staining of normal muscle tissue is shown in (A). H&E staining indicates that recovery of muscle fibers in the transplantation group occurred with time (F–I), whereas in the denervation group, increased fatty infiltration, decreases in the sarcomere size, and increased nuclear packing were observed (B–E). At 12 weeks, reinnervated muscle from the transplantation group did not appear to be different from the muscles in the normal control group (compare A and I). Color images available online at www.liebertpub.com/tea
FIG. 5.
Rabbit anti-NF-200 (polyclonal 1:80, Sigma), which reacts with the 200-kDa neurofilament protein, was used to visualize axonal sprouting from the transplanted nerve at the nerve/muscle junctions. In the phase-contrast image of the transplantation group at 12 weeks, axonal sprouts from the proximal terminal axon grew prominently, with an even distribution of axon fibers (A and B). Alexa Fluor® 488-conjugated alpha-bungarotoxin (1:400, Invitrogen/Molecular Probes) was used to stain the acetylcholine receptor (AchR). (C) A double stained image of normal muscle shows an intact neuromuscular junction. In the denervation group, remnant axon filaments had disappeared almost completely, although staining for the AchR remained even at 12 weeks (D–G). In the transplantation group, the integration of axon and AchR improved with time (H–K).
FIG. 6.
Images illustrating clusters of the AchR, stained with Alexa Fluor 488-conjugated alpha-bungarotoxin, in the regenerating neuromuscular junction. These appeared adjacent to the growing nerve sprouts and were apparent from about 8 weeks after transplantation. Consecutive magnification views are shown in the four panels: (A) 25X, (B) 100X, (C) 200X, and (D) 400X.
Discussion
While volumetric tissue restoration for aesthetic purposes is the prime goal in repairing muscle tissue defects using tissue-engineered muscle, recovery of muscle function is essential in achieving coordinated motion and would be ideal. Innervation of engineered muscle tissue is also critical for preventing muscle tissue atrophy.40–45 As such, currently available tissue-engineered constructs consisting of cultured muscle cells, which do not contain nerve supplies and are thus analogous to denervated muscle, would need to be integrated with host nerve to become functional. It is currently unclear whether such tissue-engineered constructs can be completely innervated by host nerve and become fully functional in vivo. To that end, we used an animal model in which denervated GM represented a tissue-engineered muscle construct. Using this model, we examined whether neurotization of the denervated muscle tissue has the ability to reinnervate and recover muscle function. Using this model, we were able to determine the levels of functional recovery of muscle after direct nerve transplantation (neurotization) into denervated muscle. We show that the transplanted nerve is able to regenerate the neuromuscular junction within the muscle and leads to partial functional recovery, but that the muscle function achieved using this technique subnormal despite the fact that electrophysiological measurements indicate almost complete recovery.
Until recently, there has not been a way to quantify the functional recovery of muscle other than through measurement of the electrophysiological response, although there are some clinical methods that have been used for measurement of muscle function recovery after nerve damage.28,30–31 However, these methods did not exclude the effect of collateral nerve input into the muscle, and thus could not determine the functional result of replacing a single nerve. In the present study, both of these issues have been addressed. First, we have developed a set of tests that can objectively measure specific muscle function after nerve transplantation, and we have developed a model in which the contribution of a single nerve to functional muscle recovery can be measured. The data presented here indicate that the actual function of denervated muscle remains in the subnormal range even at 12 weeks after direct nerve transplantation, although transplantation appeared to regenerate the neuromuscular junction and provided electrophysiological recovery. In this study, the functional recovery of the GM was just 17% of normal control muscle even at 12 weeks after nerve transplantation, despite the fact that electrophysiological parameters appeared to recover. These parameters, particularly CMAP amplitude, are not related to the number of muscle fibers in each motor unit, but rather to the diameter of the muscle fibers and the density of muscle fibers comprising the motor unit closest to the electrode.31 Therefore, CMAP is dependent on the electrode location, and its recovery does not necessarily indicate recovery of normal muscular function. However, CMAP has been considered a functional indicator in a number of studies,20,22,24,27,33,34 and our study suggests that this is not the case.
In this study, the direct nerve transplantation groups did not display dorsal flexion contracture of the ankle joint, although there was no new voluntary movement in the GM. The quantitative analysis of neurofunctional recovery is a complicated issue in animal studies of reinnervation, because it is not possible to selectively observe a voluntary movement in animals. Here, we used EPT for measurement of motor function and WRL to measure the sensory function. Both of these have been validated in studies of functional recovery of sciatic nerve.38,39 In this study, the functional recovery of denervated muscle in terms of EPT and WRL was minimal, despite the fact that the CMAP amplitude was near normal after 8–12 weeks. This functional recovery was much lower than that reported in other studies (50%), which used muscle force measurement to determine recovery.21–24 In addition, the functional delay in our study was longer than 25 days as reported by Gutmann.23 It is possible that the delayed recovery observed in our study may be due to differences in methodology rather than an actual delay in muscular recovery, since muscle force or CMAP measurement produces different results than EPT measurement, which was used here.
In this study, expression of the AchR and axonal sprouting were shown to be time dependent. In the denervated muscle, even though axonal sprouting did not occur, as shown via immunohistochemistry for NF-200. In the transplanted group, however, several interesting findings were revealed using NF-200 immunohistochemistry, indicating that even in this group, the number of sprouting axons fluctuates after transplantation of a nerve segment. The regression of axonal sprouting seen between 4 and 12 weeks after transplantation may be due to trauma incurred by the nerve segment during the transplantation surgery, or it may be a result of inflammation or the wound-healing response that likely occurs within the muscle tissue itself during the postoperative period. However, the data suggest that the transplanted nerve segment is eventually able to recover from this after 12 weeks and form functional neuromuscular junctions in its new environment. Further studies are ongoing to determine the molecular and cellular mechanisms governing the process of axonal sprouting from the transplanted nerve.
In addition, it is important to note that the axonal sprouts from the proximal terminal axon grew predominantly in the vicinity of the wound, and there was a large variability in the direction of the new fibers. Direct contact of the transplanted nerve with the surface of the sarcoplasm gives trophic support for axonal regeneration and induces differentiation of a motor endplate.23,46,47 However, functional regeneration occurs when the distal nerve segment lost during degeneration is replaced, allowing reinnervation of target organs and a subsequent functional recovery.48 Therefore, our data suggest that restoration of the electrophysiological response in these animals may be associated with axonal sprouting that creates multiple endplate connections from a single motor neuron. However, this is different from normal muscle, and the change in neural structure may cause the observed delay in functional recovery.
It is also important to note that the delay of functional recovery observed in this study may be prolonged, because the surgical technique used was designed to exclude the effect of collateral sprouting by uninjured nerves. Many previous studies19,20,22,23,25,28–33,36,37 did not exclude any effects from uninjured collateral nerves in denervated muscle. In the present study, the common peroneal nerve was implanted into the GM after excision of a 10-mm segment of the tibial nerve and a sensory nerve branch. Rupp et al. have shown that electrical activity recorded in the GM after stimulation of the proximal nerve was generated by surrounding hind limb muscles unaffected by denervation.37 Although Becker et al. have shown that an active muscle movement was restored after nerve transplantation in 7 neurotized patients,30 their study could be biased by the effect of collateral nerve function, as this was a clinical study, and the collateral nerves could not be removed.
The outcome of direct nerve transplantation is dependent upon the chronicity of denervation,23,24,28,32 regeneration distance,24,27,30 distance between the nerve implantation site and the native motor endplate zone,36 patient age, quantity and quality of remaining muscle mass,28,32 status of donor nerve, and surgical technique.25,30,33 This study is minimally biased based on these factors, because the donor nerve was transplanted immediately after transection of all branches of the recipient's nerve, and the graft was placed close to the native motor endplate zone. However, this model does not address issues that may arise when implanting a 3D muscle construct into the environment of a severe traumatic injury. The structural and biochemical events after trauma may provide different results from those presented here, as the injury environment would contain a number of molecular and cellular cues (proinflammatory cytokines, growth factors, the presence of T cells, and a number of other factors) that are not present in the model described in this article. Such a complete study was beyond the scope of this article; however, creating a model that takes into account the environment of a traumatic injury would be an exciting follow-up study.
Direct nerve transplantation is an option when a primary suture, nerve transfer, or a graft is not available, although functional recovery may not be achieved for a long period after surgery.28,30,31 As we have shown, abundant axonal regrowth and normalized CMAP response do not necessarily indicate a return of the muscular function. However, we have not investigated the effect of exogenous enhancing factors, such as neurotrophic factors, on the functional recovery after nerve transplantation. It is possible that, for a faster functional recovery, the extensive branching of the nerve endings seen after transplantation could be organized into well-structured integrations using supportive tools such as these exogenous factors.
Acknowledgments
The authors wish to thank Dr. John Jackson for editorial assistance. This study was supported, in part, by a grant from the Department of Defense [Orthopaedic Trauma Research Program (W81XWH-08-1-0333)].
Disclosure Statement
No competing financial interests exist.
References
- 1.Grogan B.F. Hsu J.R. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl 1):S35. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Jarvinen T.A. Jarvinen T.L. Kaariainen M. Kalimo H. Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 3.Vekris M.D. Beris A.E. Lykissas M.G. Korompilias A.V. Vekris A.D. Soucacos P.N. Restoration of elbow function in severe brachial plexus paralysis via muscle transfers. Injury. 2008;39(Suppl 3):S15. doi: 10.1016/j.injury.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Baechler M.F. Groth A.T. Nesti L.J. Martin B.D. Soft tissue management of war wounds to the foot and ankle. Foot Ankle Clin. 2010;15:113. doi: 10.1016/j.fcl.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan C. Jiang P. Fu L. Cai P. Sun L. Zeng B. Functional reconstruction of traumatic loss of flexors in forearm with gastrocnemius myocutaneous flap transfer. Microsurgery. 2008;28:71. doi: 10.1002/micr.20449. [DOI] [PubMed] [Google Scholar]
- 6.Terada N. Takayama S. Yamada H. Seki T. Muscle repair after a transsection injury with development of a gap: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2001;35:233. doi: 10.1080/028443101750523131. [DOI] [PubMed] [Google Scholar]
- 7.Candiani G. Riboldi S.A. Sadr N. Lorenzoni S. Neuenschwander P. Montevecchi F.M., et al. Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. J Appl Biomater Biomech. 2010;8:68. [PubMed] [Google Scholar]
- 8.Deans T.L. Elisseeff J.H. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20:537. doi: 10.1016/j.copbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Khodabukus A. Paxton J.Z. Donnelly K. Baar K. Engineered muscle: a tool for studying muscle physiology and function. Exerc Sport Sci Rev. 2007;35:186. doi: 10.1097/jes.0b013e318156df01. [DOI] [PubMed] [Google Scholar]
- 10.Ladd M.R. Lee S.J. Stitzel J.D. Atala A. Yoo J.J. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials. 2011;32:1549. doi: 10.1016/j.biomaterials.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Liao H. Zhou G.Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng Part B Rev. 2009;15:319. doi: 10.1089/ten.teb.2009.0092. [DOI] [PubMed] [Google Scholar]
- 12.Skouras E. Ozsoy U. Sarikcioglu L. Angelov D.N. Intrinsic and therapeutic factors determining the recovery of motor function after peripheral nerve transection. Ann Anat. 2011;193:286. doi: 10.1016/j.aanat.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Dhawan V. Lytle I.F. Dow D.E. Huang Y.C. Brown D.L. Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng. 2007;13:2813. doi: 10.1089/ten.2007.0003. [DOI] [PubMed] [Google Scholar]
- 14.Sterne G.D. Coulton G.R. Brown R.A. Green C.J. Terenghi G. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J Cell Biol. 1997;139:709. doi: 10.1083/jcb.139.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viguie C.A. Lu D.X. Huang S.K. Rengen H. Carlson B.M. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248:346. doi: 10.1002/(SICI)1097-0185(199707)248:3<346::AID-AR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Reyes O. Sosa I. Kuffler D.P. Promoting neurological recovery following a traumatic peripheral nerve injury. P R Health Sci J. 2005;24:215. [PubMed] [Google Scholar]
- 17.Merrell G.A. Barrie K.A. Katz D.L. Wolfe S.W. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am. 2001;26:303. doi: 10.1053/jhsu.2001.21518. [DOI] [PubMed] [Google Scholar]
- 18.Toth A. Szucs A. Harasztosi C. Matesz K. Pucsok K. Miko I., et al. Intrinsic laryngeal muscle reinnervation with nerve-muscle pedicle. Otolaryngol Head Neck Surg. 2005;132:701. doi: 10.1016/j.otohns.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Emery E. Rhrich-Haddout F. Kassar-Duchossoy L. Lyoussi B. Tadie M. Horvat J.C. Motoneurons of the adult marmoset can grow axons and reform motor endplates through a peripheral nerve bridge joining the locally injured cervical spinal cord to the denervated biceps brachii muscle. J Neurosci Res. 2000;62:821. doi: 10.1002/1097-4547(20001215)62:6<821::AID-JNR9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Horvat J.C. Pecot-Dechavassine M. Mira J.C. Davarpanah Y. Formation of functional endplates by spinal axons regenerating through a peripheral nerve graft. A study in the adult rat. Brain Res Bull. 1989;22:103. doi: 10.1016/0361-9230(89)90134-2. [DOI] [PubMed] [Google Scholar]
- 21.Sakellarides H.T. Sorbie C. James L. Reinnervation of denervated muscles by nerve transplantation. Clin Orthop Relat Res. 1972;83:195. [PubMed] [Google Scholar]
- 22.Sorbie C. Porter T.L. Reinnervation of paralysed muscles by direct motor nerve implantation. An experimental study in the dog. J Bone Joint Surg Br. 1969;51:156. [PubMed] [Google Scholar]
- 23.Gutmann E. Young J.Z. The re-innervation of muscle after various periods of atrophy. J Anat. 1944;78:15. [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson A.N. Wolfe S.W. Khazzam M. Feinberg J. Ehteshami J. Doty S. Comparison of neurotization versus nerve repair in an animal model of chronically denervated muscle. J Hand Surg Am. 2008;33:1093. doi: 10.1016/j.jhsa.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noordin S. Ahmed M. Rehman R. Ahmad T. Hashmi P. Neuronal regeneration in denervated muscle following sensory and muscular neurotization. Acta Orthop. 2008;79:126. doi: 10.1080/17453670710014879. [DOI] [PubMed] [Google Scholar]
- 26.McNamara M.J. Garrett W.E., Jr. Seaber A.V. Goldner J.L. Neurorrhaphy, nerve grafting, and neurotization: a functional comparison of nerve reconstruction techniques. J Hand Surg Am. 1987;12:354. doi: 10.1016/s0363-5023(87)80003-5. [DOI] [PubMed] [Google Scholar]
- 27.Askar I. Sabuncuoglu B.T. Yormuk E. Saray A. The fate of neurotization techniques on reinnervation after denervation of the gastrocnemius muscle: an experimental study. J Reconstr Microsurg. 2001;17:347. doi: 10.1055/s-2001-16027. [DOI] [PubMed] [Google Scholar]
- 28.Brunelli G.A. Brunelli G.R. Direct muscle neurotization. J Reconstr Microsurg. 1993;9:81. doi: 10.1055/s-2007-1006656. [DOI] [PubMed] [Google Scholar]
- 29.Mackinnon S.E. McLean J.A. Hunter G.A. Direct muscle neurotization recovers gastrocnemius muscle function. J Reconstr Microsurg. 1993;9:77. doi: 10.1055/s-2007-1006655. [DOI] [PubMed] [Google Scholar]
- 30.Becker M. Lassner F. Fansa H. Mawrin C. Pallua N. Refinements in nerve to muscle neurotization. Muscle Nerve. 2002;26:362. doi: 10.1002/mus.10205. [DOI] [PubMed] [Google Scholar]
- 31.Brunelli G.A. Direct neurotization of muscles by presynaptic motoneurons. J Reconstr Microsurg. 2001;17:631. doi: 10.1055/s-2001-18873. [DOI] [PubMed] [Google Scholar]
- 32.Keilhoff G. Fansa H. Successful intramuscular neurotization is dependent on the denervation period. A histomorphological study of the gracilis muscle in rats. Muscle Nerve. 2005;31:221. doi: 10.1002/mus.20260. [DOI] [PubMed] [Google Scholar]
- 33.Yazici I. Ayhan S. Elmas C. Temucin C. Atabay K. Motor neurotization by segmental epineurectomy and implantation: lateral muscular neurotization. J Reconstr Microsurg. 2008;24:435. doi: 10.1055/s-0028-1082892. [DOI] [PubMed] [Google Scholar]
- 34.Park D.M. Shon S.K. Kim Y.J. Direct muscle neurotization in rat soleus muscle. J Reconstr Microsurg. 2000;16:379. doi: 10.1055/s-2000-7349. [DOI] [PubMed] [Google Scholar]
- 35.Erlacher P. Direct and muscular neurotization of muscles: experimental research. Am J Orthop Surg. 1915;13:22. [Google Scholar]
- 36.Payne S.H., Jr. Brushart T.M. Neurotization of the rat soleus muscle: a quantitative analysis of reinnervation. J Hand Surg Am. 1997;22:640. doi: 10.1016/S0363-5023(97)80121-9. [DOI] [PubMed] [Google Scholar]
- 37.Rupp A. Dornseifer U. Fischer A. Schmahl W. Rodenacker K. Jutting U., et al. Electrophysiologic assessment of sciatic nerve regeneration in the rat: surrounding limb muscles feature strongly in recordings from the gastrocnemius muscle. J Neurosci Methods. 2007;166:266. doi: 10.1016/j.jneumeth.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Thalhammer J.G. Vladimirova M. Bershadsky B. Strichartz G.R. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Masters D.B. Berde C.B. Dutta S.K. Griggs C.T. Hu D. Kupsky W., et al. Prolonged regional nerve blockade by controlled release of local anesthetic from a biodegradable polymer matrix. Anesthesiology. 1993;79:340. doi: 10.1097/00000542-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Jakubiec-Puka A. Kulesza-Lipka D. Kordowska J. The contractile apparatus of striated muscle in the course of atrophy and regeneration. II. Myosin and actin filaments in mature rat soleus muscle regenerating after reinnervation. Cell Tissue Res. 1982;227:641. doi: 10.1007/BF00204794. [DOI] [PubMed] [Google Scholar]
- 41.Jakubiec-Puka A. Laskowska-Bozek H. Morphological changes in rat skeletal muscle following reinnervation. Folia Histochem Cytochem (Krakow) 1977;15:333. [PubMed] [Google Scholar]
- 42.Schroder J.M. Kemme P.T. Scholz L. The fine structure of denervated and reinnervated muscle spindles: morphometric study of intrafusal muscle fibers. Acta Neuropathol. 1979;46:95. doi: 10.1007/BF00684810. [DOI] [PubMed] [Google Scholar]
- 43.Schwarting S. Schroder M. Stennert E. Goebel H.H. Morphology of denervated human facial muscles. ORL J Otorhinolaryngol Relat Spec. 1984;46:248. doi: 10.1159/000275718. [DOI] [PubMed] [Google Scholar]
- 44.Bishop B. Neural plasticity: part 3. Responses to lesions in the peripheral nervous system. Phys Ther. 1982;62:1275. doi: 10.1093/ptj/62.9.1275. [DOI] [PubMed] [Google Scholar]
- 45.Schmalbruch H. The effect of peripheral nerve injury on immature motor and sensory neurons and on muscle fibres. Possible relation to the histogenesis of Werdnig-Hoffmann disease. Rev Neurol (Paris) 1988;144:721. [PubMed] [Google Scholar]
- 46.Madison R.D. Robinson G.A. Chadaram S.R. The specificity of motor neurone regeneration (preferential reinnervation) Acta Physiol (Oxf) 2007;189:201. doi: 10.1111/j.1748-1716.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 47.Saffran B.N. Crutcher K.A. NGF-induced remodeling of mature uninjured axon collaterals. Brain Res. 1990;525:11. doi: 10.1016/0006-8993(90)91315-8. [DOI] [PubMed] [Google Scholar]
- 48.Navarro X. Vivo M. Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]