Abstract
We previously showed that the sequential, but not simultaneous, culture of endothelial cells (ECs), fibroblasts (FBs), and cardiomyocytes (CMs) resulted in elongated, beating cardiac organoids. We hypothesized that the expression of Cx43 and contractile function are mediated by vascular endothelial growth factor (VEGF) released by nonmyocytes during the preculture period. Cardiac organoids (∼200 μm diameter) were cultivated in microchannels to enable rapid screening. Three experimental groups were formed: (i) Simultaneous Preculture (ECs+FBs for 48 h, followed by CMs), (ii) Sequential Preculture (ECs for 24 h, FBs for 24 h, followed by CMs), and (iii) Simultaneous Triculture (ECs+FBs+CMs). Controls included CMs only, FBs only, and ECs only groups, and preculture with ECs only or FBs only. The highest VEGF levels were found in the Preculture groups [Simultaneous Preculture, 8.9±2.7 ng/(mL·h−1); Sequential Preculture, 16.6±3.4 ng/(mL·h−1)], as compared with Simultaneous Triculture where VEGF was not detectable, as shown by enzyme-linked immunosorbent assay. Analytical flow cytometry showed that VEGFR2 was expressed by ECs (86%±2 VEGFR2+), FBs (44%±1 VEGFR2+), and CMs (49%±2 VEGFR2+), showing that all three cell types were capable of responding to changes in VEGF. Addition of anti-VEGF neutralizing IgG (0.4 μg/mL) to Simultaneous Preculture resulted in 3-fold decrease in Cx43 mRNA and 1.5-fold decrease in Cx43 protein, while Simultaneous Triculture supplemented with VEGF ligand (30 ng/mL) had a threefold increase in Cx43 mRNA and a twofold increase in Cx43 protein. Addition of a small molecule inhibitor of the VEGFR2 receptor (19.4 nM) to Sequential Preculture caused a 1.4-fold decrease in Cx43 mRNA and a 4.1-fold decrease in Cx43 protein. Cx43 was localized within CMs, and not within FBs or ECs. Enriched CM organoids and Sequential Preculture organoids grown in the presence of VEGFR2 inhibitor displayed low levels of Cx43 and poor functional properties. Taken together, these results suggest that endogenous VEGF-VEGFR2 signaling enhanced Cx43 expression and cardiac function in engineered cardiac organoids.
Introduction
Cardiomyocytes (CMs) are responsible for generation of contractile force,1,2 but are only one of the three major cell populations found in the heart. The remaining cells, or nonmyocytes (fibroblasts [FBs] and endothelial cells [ECs]), play important roles in matrix deposition, vascularization, and paracrine signaling.2,3 We previously showed that preculture of ECs and FBs prior to seeding CMs resulted in beating cardiac organoids resembling myofibers.4 In contrast, the same ratios of CMs, FBs, and ECs cultured simultaneously (“Simultaneous Triculture”) resulted in nonfunctional organoids lacking expression of the key gap junctional marker Connexin-43 (Cx43).4 Addition of conditioned medium from precultured organoids to organoids engineered from CMs alone also improved the functional properties and viability of the organoids,4 suggesting a role of factors secreted by nonmyocytes on cardiac organoid function.
Connexins are a conserved family of transmembrane proteins that assemble into gap junctions, allowing for intercellular communication and direct exchange of small molecules, ions, and second messengers between cells.5 Cx43 (43 kDa) is found abundantly in the heart, enabling coupling between adjacent CMs. FBs can also couple to CMs through Cx43 as well as Connexin-45 (Cx45) and can therefore transmit electrophysiological gradients to contractile myocytes, though they cannot exert contractile force on their own.6,7 ECs also express Cx438 and enhance Cx43 expression in CMs when grown in co-culture.9
Vascular endothelial growth factor (VEGF) signaling has been implicated in the upregulation of Cx43 expression in CMs,10 but the precise mechanism of action has not been extensively studied. In one study, it was demonstrated that uniaxial stretch-induced upregulation of Cx43 expression could be blocked by anti-VEGF antibody.10 In a different study, transgenic mice expressing only one of the three isoforms of VEGF also showed markedly reduced Cx43 expression and impaired cardiac function and angiogenesis compared with the wild-type mice.11
We hypothesized that VEGF-A165, an abundant isoform of VEGF with affinity for heparin-like domains, is secreted by FBs and ECs during preculture, resulting in upregulation of Cx43 and improved cardiac function. To test this hypothesis, we investigated the secretion of VEGF in different types of monocultures and cocultures of CMs, ECs, and FBs, and the related effects on the presence of Cx43 and functional properties of engineered cardiac organoids. We made several interesting observations: (i) VEGF is secreted at higher concentrations during preculture of nonmyocytes than during Simultaneous Triculture of all three cell types, (ii) the source of VEGF is the nonmyocytes, and (iii) VEGF-VEGFR2 binding affects expression of Cx43 at both the transcriptional and translational levels. We also observed that VEGFR2 was expressed in all three cell types (ECs, FBs, and CMs) and that Cx43 was mostly associated with CMs. Finally, we demonstrated, through manipulation of VEGF expression and electrical field stimulation testing, the existence of a causal relationship between VEGF-VEGFR2 signaling, Cx43 mRNA/protein expression levels, and cardiac contractile function.
Methods
Microfabrication of poly(ethylene glycol) templates
The technique for poly(ethylene glycol) microchannel fabrication has been previously described.4 Please see Supplementary Data for details.
Cells
Neonatal rat heart cells were isolated using serial collagenase digestions as previously described.4,12,13 Neonatal rat cardiac FBs were obtained from the adherent cell fraction in the neonatal rat heart isolate after 1 h of preplating. The cells remaining in suspension after 2×1 h preplating steps were termed Enriched CMs. Neonatal rat microvascular ECs were obtained from adherent cells during preplating by trypsinization and magnetic activated cell sorting for cells positive for the EC surface marker CD31. Detailed methods are described in Supplementary Data.
Experimental design
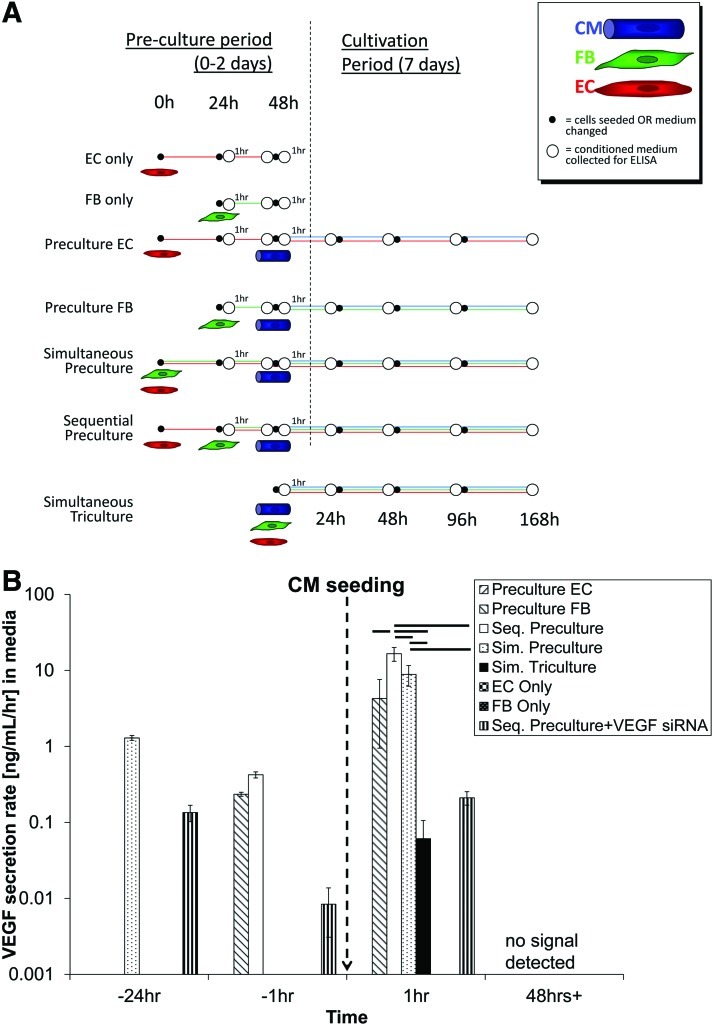
The experimental groups investigated to identify the possible sources and targets of VEGF signaling are summarized in Table 1. Three coculture groups were formed: (i) Simultaneous Preculture, as in our earlier work,4,14 where FBs and ECs were simultaneously seeded and cultured for 2 days prior to CM seeding; (ii) Sequential Preculture, as in our more recent work,15 where ECs were first seeded for 24 h to allow cord formation, followed by FBs 24 h later to stabilize the cords, and CMs after an additional 24 h; (3) Simultaneous Triculture,4,13 where all three cell types (ECs, FBs, and CMs) were seeded simultaneously and cultured together. Organoids consisting of ECs, or FBs or Enriched CMs served as monoculture controls, while organoids precultured with ECs (Preculture ECs) or FBs (Preculture FBs) served as coculture controls. Organoids were cultivated for up to 7 days in CM/FB medium (see Supplementary Data) alone or in medium supplemented with either VEGF ligand, or VEGF neutralizing antibody or VEGFR2 inhibitor. The culture medium was collected 1 h prior to and 1 h following all cell seeding steps/medium changes for enzyme-linked immunosorbent assay (ELISA) analysis (Fig. 1A). Total mRNA and protein were harvested at 1 h and 24 h, respectively, following CM seeding. Immunofluorescence microscopy was conducted on organoids cultivated for 72 h following CM seeding.
Table 1.
Cell Seeding Numbers and Percentages for Experimental Groups
| Experimental group | Number of ECs (%EC)/preculture time+cultivation time | Number of FBs (%FB)/preculture time+cultivation time | Number of CMs (%CM)/cultivation time |
|---|---|---|---|
| ECs only | 94000 (100%)/1+7 days | 0 | 0 |
| FBs only | 0 | 26000 (100%)/1+7 days | 0 |
| Enriched CMs | 0 | 0 | 80000 (100%)/7 days |
| Preculture ECs | 120000 (60%)/2+7 days | 0 | 80000 (40%)/7 days |
| Preculture FBs | 0 | 120000 (60%)/1+7 days | 80000 (40%)/7 days |
| Simultaneous Preculture | 94000 (47%)/2+7 days | 26000 (13%)/2+7 days | 80000 (40%)/7 days |
| Sequential Preculture | 16000 (8%)/2+7 days | 64000 (32%)/1+7 days | 120000 (60%)/7 days |
| Simultaneous Triculture | 94000 (47%)/0+7 days | 26000 (13%)/0+7 days | 80000 (40%)/7 days |
Organoids were cultivated as monocultures (ECs only, FBs only, and Enriched CMs), coculture controls (Preculture with only ECs, Preculture with only FBs), or as tricultures (Preculture/Tricuture). Two types of Preculture groups were used: Simultaneous Preculture, where the nonmyocytes (ECs and FBs) were simultaneously seeded and co-cultured for 2 days prior to addition of the CMs; and Sequential Preculture, where the ECs were seeded and cultivated for 24 h, followed by FBs for an additional 24 h, prior to CM seeding. In Simultaneous Triculture, ECs, FBs, and CMs were all seeded at the same instant. The inclusion of monoculture and coculture controls allowed for determination of which cell types were secreting the most endogenous VEGF by ELISA, while the inclusion of both Sequential and Simultaneous Preculture groups was useful in determining whether conditions known to support vascular cord formation would enable even greater VEGF secretion than Preculture without cords.
ECs, endothelial cells; FBs, fibroblasts; CMs, cardiomyocytes; VEGF, vascular endothelial growth factor; ELISA, enzyme-linked immunosorbent assay.
FIG. 1.
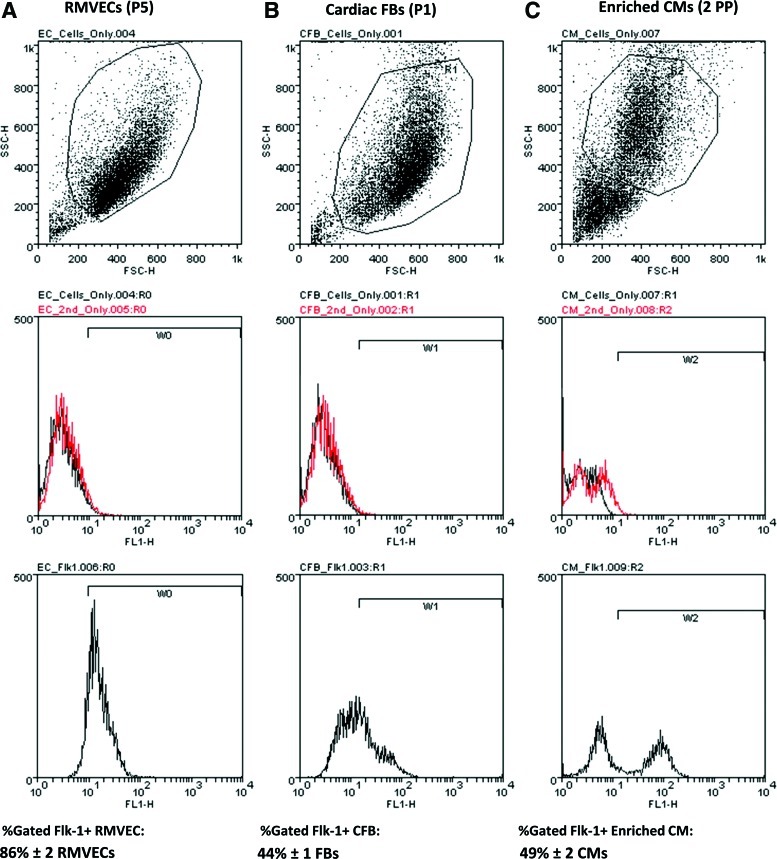
Endogenous vascular endothelial growth factor (VEGF) secretion. (A) Time points for collection of culture medium and enzyme-linked immunosorbent assay (ELISA) analysis of endogenous VEGF levels were chosen to determine the amount of VEGF present prior to or after seeding steps or medium changes, including the point at which Enriched cardiomyocytes (CMs) were seeded in the various Preculture and Triculture groups. Control groups included endothelial cells (EC) only, fibroblasts (FB) only, and Preculture with EC only or Preculture with FB only (Preculture ECs/Preculture FBs). (B) Bulk VEGF secretion rate in [ng/(mL·h)]. Graph shown in log scale. In general, VEGF secretion rate was found to be largest in the Preculture groups compared with Simultaneous Triculture and peaked maximally at 1 h after seeding CM. Within the Preculture groups, Sequential Preculture of ECs followed by FBs and then by CMs (Sequential Preculture) had the highest VEGF secretion rate compared with Simultaneous Preculture where ECs+FBs were seeded together followed by CMs (Simultaneous Preculture) or Preculture with only FBs or ECs (Preculture ECs/Preculture FBs). As expected, low VEGF levels were found in the Sequential Preculture using cells with siRNA VEGF knockdown (Seq. Preculture+VEGF siRNA). Note: No VEGF was detected in organoids having only FBs or ECs (ECs only, FBs only). n=4/group. Horizontal lines represent statistically significant difference (p<0.05) between the two groups at the ends of the line. Color images available online at www.liebertonline.com/tea
VEGF ligand addition and VEGF antibody neutralization experiments
We aimed to measure the effects of neutralizing endogenously secreted VEGF on Cx43 expression in cardiac organoids, as well as to measure the Cx43 levels after stimulating organoids with exogenously added VEGF. Accordingly, Simultaneous Preculture organoids were grown in CM/FB medium (see Supplementary Data) supplemented with VEGF neutralizing antibody (Anti-rat VEGF Antibody, AF564; R&D Systems) at a concentration of 0.4 μg/mL, corresponding to twice the neutralization dose50 (ND50). Simultaneous Tricultures were grown in CM/FB medium with or without VEGF ligand (VEGF-A165; R&D Systems; Cat. No. 293-VE) at a concentration of 30 ng/mL, corresponding to more than three times the Kd of VEGF-VEGFR2 binding.16 These groups were labeled as “Simultaneous Preculture+VEGF Ab” and “Simultaneous Triculture+VEGF,” while control groups were labeled “Simultaneous Preculture” and “Simultaneous Triculture,” respectively. In these experiments, the starting percentages of ECs, FBs, and CMs were 47%, 13%, and 40%, respectively, ensuring that the cultivation conditions were identical to our earlier work.4,14 The organoids were cultivated for either (i) 1 h, followed by RNA extraction by TRIzol and quantitative polymerase chain reaction (QPCR), or (ii) 24 h, followed by protein extraction for Western blotting, ensuring that the appropriate timescale for maximal transcriptional/translational changes in Cx43 mRNA/protein was captured.
VEGFR2 small molecule inhibitor/VEGF ligand addition studies
To test the effect of inhibiting the VEGF receptor VEGFR2 on Cx43 mRNA and protein levels, we used a small molecule kinase inhibitor of VEGFR2 (Cat. No. CB676489; Calbiochem; 19.4 nM). The inhibitor is an adenosine triphosphate (ATP)-competitive, reversible inhibitor with an inhibitory concentration50 (IC50) of 19.4 nM, the concentration at which 50% of the VEGFR2 receptors are inhibited. The effect of the inhibitor concentration (2.4−310 nM) on viability of Enriched CMs (live/dead staining) was also studied. Sequential Preculture organoids as well as Enriched CM organoids were cultivated in the presence or absence of VEGFR2 inhibitor (denoted “I,” 19.4 nM) and in the presence or absence of VEGF ligand (denoted “V,” 30 ng/mL) in four possible combinations: (−V −I), (+V −I), (−V +I), and (+V +I). The organoids were sampled after 1 h for mRNA isolation and QPCR analysis, after 24 h for protein isolation and Western blotting analysis, or after 72 h for PFA fixation, cryosectioning, and immunofluorescence microcopy.
siRNA knockdown of VEGF in sequential preculture
Primary ECs, FBs, and CMs were transfected upon individual monolayer culture with a custom siRNA-designed (Ambion/Life Technologies) targeting VEGF-A knockdown with the following sequence: 5′-CCAAAGAAAGAUAGAACAAtt (21 bp)—sense; 5′-UUGUUCUAUCUUUCUUUGGtc (21 bp)—antisense, as described in detail in Supplementary Methods. After 24 h, the cells were used to cultivate Sequential Preculture organoids as described under “Experimental Design”.
Assessments
VEGF-ELISA was performed according to manufacturer's protocols (Peprotech, Quebec; Cat. No. 900-K99). Cryosectioning and immunofluorescence staining for Cx43 and cell-specific markers cardiac troponin T (cTnT) for CMs, VE-cadherin for ECs, and vimentin for nonmyocytes (FBs+ECs) were performed as in our previous work.4,13 QPCR was performed to quantify Cx43 mRNA expression using GAPDH as a housekeeping gene.14 Western blotting was performed using commercially available reagents, antibodies for Cx43 and GAPDH, and precast gels according to a standard protocol. Live cell analytical flow cytometry was performed to quantify VEGFR2 expression in ECs, FBs, and CMs using a protocol previously described.4 Electrical field stimulation was used to determine the effect of inhibiting VEGF signaling on cardiac function using methods we described previously.4,13,14,17 Detailed procedures are described in Supplementary Data.
Statistical data analysis
Data are presented as average±standard deviation. Statistically significant differences, unless otherwise indicated, are denoted with horizontal bars or asterisks. All statistical calculations were performed using SigmaStat 3.0 (SPSS, Inc.). One-way analysis of variance (ANOVA) in conjunction with the Holm-Sidak post hoc test was used to compare three or more groups or two groups versus control, while t-test was used for pairwise comparisons. Normality of data and equality of variance were checked for all comparisons. Where normality failed, one-way ANOVA on ranks was performed using a Mann-Whitney test. Where equality of variance failed, ANOVA on ranks in conjunction with Dunn's test and multiple pairwise comparisons was performed. A p-value < 0.05 was considered statistically significant for all tests.
Results
VEGF secretion rates were higher in preculture than in simultaneous culture
Our first aim was to test whether VEGF levels were higher in the Preculture groups compared with Simultaneous Triculture group at key time points coinciding with culture medium change or cell seeding (Fig. 1A). Endogenous VEGF secretion rates were initially low [<2 ng/mL/h] prior to CM seeding (Fig. 1B), and rapidly increased 1 h after CM seeding in all Preculture groups as compared with Simultaneous Triculture group, where the secretion rate was below detectable limits. Within the Preculture groups, Sequential Preculture had the highest VEGF secretion rate at the time of CM seeding [16.6 ng/mL/h; Fig. 1B] compared with Simultaneous Preculture [8.9 ng/mL/h; Fig. 1B], or Preculture with only FBs or ECs (Preculture FBs, 4.3 ng/mL/h; Preculture ECs, 0 ng/mL/h; Fig. 1B). We could not detect any VEGF in organoids formed through seeding of only one cell type (FBs or ECs; Fig. 1B). By 48 h post-CM seeding, endogenous VEGF was below detectable limits in all groups.
Expression of VEGFR2 in ECs, FBs, and CMs
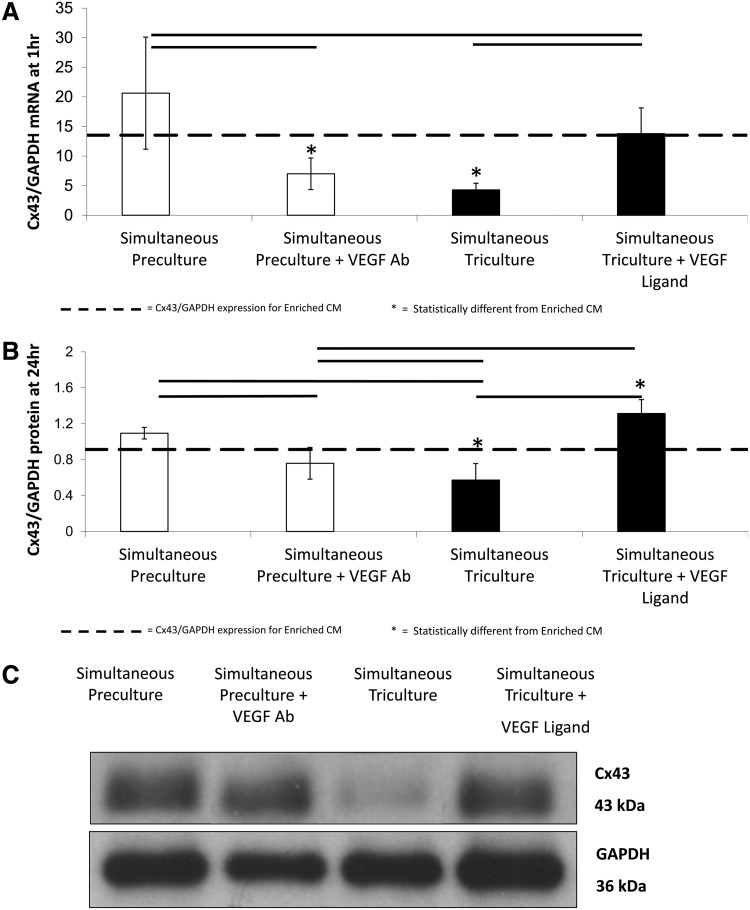
Flow cytometry indicated that rat microvascular ECs displayed the highest percentage of VEGFR2+ cells (86%±2%), as expected (Fig. 2). Cardiac FBs and CMs also showed evidence of VEGFR2+ cells, but the percentages were lower (FBs: 44%±1%, CMs: 49%±2%) (Fig. 2).
FIG. 2.
VEGFR2 expression. Analytical flow cytometry for VEGFR2 expression was performed on live rat microvascular endothelial cells [RMVECs, (A)], neonatal cardiac fibroblasts [CFBs, (B)] passaged once, and Enriched CMs [enriched by two preplating steps on adherent tissue culture flasks, (C)]. Scatter plots (top row) show the size distribution and granularity of the various cell types as well as gating out of debris and smaller red blood cells. Histograms show unstained controls (middle row, black outline) and controls stained with only secondary antibody (fluorescein isothiocyanate Goat Anti-Rabbit, 1:25). Histograms of cells stained with primary antibody (Rabbit Anti-Flk-1, 1:25) and secondary antibody are shown in the bottom row. Color images available online at www.liebertonline.com/tea
Cx43 mRNA is maximally upregulated within 1 h of VEGF stimulation
To determine the optimal time point for mRNA isolation after VEGF stimulation, we performed a time course experiment on CM monolayers grown in medium containing a nonlimiting concentration of VEGF (800 ng/mL) and measured Cx43 mRNA levels at various time points between 0 and 12 h (see Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/tea). Cx43 mRNA was shown to be maximally upregulated within 1 h of stimulation with VEGF, after which basal mRNA levels were again reached between 4 and 12 h after stimulation (Supplementary Fig. S1). All subsequent QPCR evaluations were therefore performed using mRNA harvested at the 1-h time point.
Neutralizing secreted VEGF in precultured organoids lowers Cx43 mRNA and protein expression
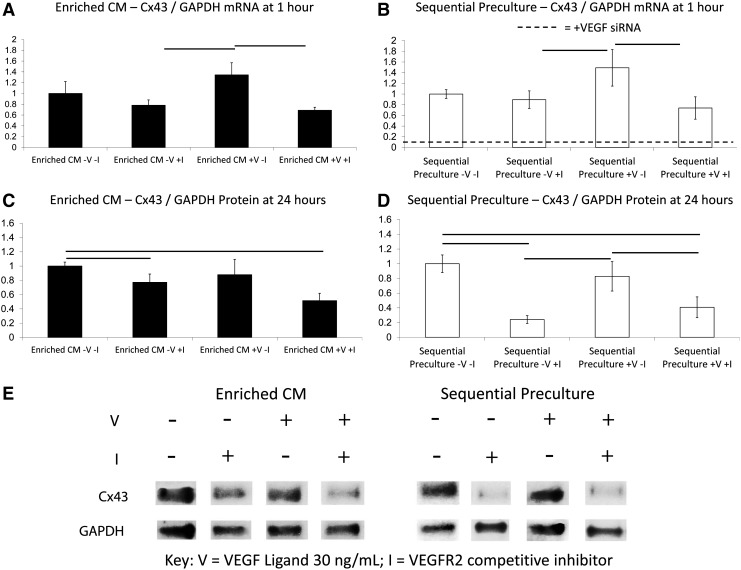
To test the effect of neutralizing endogenously secreted VEGF ligand, we added a VEGF neutralizing antibody (0.4 μg/mL) to Simultaneous Preculture organoids. After 1 h of treatment, Cx43 mRNA levels decreased by nearly threefold in the Simultaneous Preculture group compared with the Simultaneous Preculture control (Fig. 3A, white bars). Western blotting indicated that Cx43 protein levels in Simultaneous Preculture were higher than for Enriched CMs (Fig. 3B, white bars) while in Simultaneous Preculture with the VEGF Ab, the protein levels dropped by ∼1.5-fold to a value lower than Enriched CMs (white bars/dashed line, Fig. 3B).
FIG. 3.
Effects of VEGF neutralizing antibody and VEGF addition on Connexin 43 mRNA and protein levels in cardiac organoids. Simultaneous Preculture organoids were cultivated by simultaneously co-culturing the nonmyocytes (FBs and ECs) for 2 days prior to seeding of Enriched CMs in the absence (Simultaneous Preculture, white bars) or presence of Anti-VEGF neutralizing IgG at 0.4 μg/mL (Simultaneous Preculture+VEGF Ab, white bars). Simultaneous Triculture organoids, where all three cell types were seeded at the same time, were grown in medium alone (Simultaneous Triculture, black bars) or medium containing VEGF Ligand at 30 ng/mL (Simultaneous Triculture+VEGF Ligand, black bars). Enriched CM organoids served as a baseline (dashed lines). (A) A decrease in Cx43 mRNA levels was observed in the Simultaneous Preculture+VEGF Ab by QPCR, n=3/group. (B, C) Western blotting for Cx43 (43 kDa) and GAPDH (36 kDa), n=4/group. (B) Band densitometry analysis showed that Cx43 protein levels were downregulated relative to the control and baseline conditions in Simultaneous Preculture supplemented with Anti-VEGF neutralizing IgG, while for Simultaneous Triculture, Cx43 protein levels were increased relative to the control and baseline conditions when supplemented with VEGF ligand. (C) Representative image showing band intensities for GAPDH and Cx43 protein. Horizontal lines represent statistically significant difference (p<0.05) between the two groups at the ends of the line. * indicates significant difference compared with the dashed line, which represents the value for Enriched CMs.
Addition of exogenous VEGF ligand to Simultaneous Triculture organoids increases Cx43 mRNA and protein expression
We also added VEGF ligand (30 ng/mL) to Simultaneous Triculture to test the effect of VEGF supplementation in cardiac organoids with low levels of endogenous VEGF. The Simultaneous Triculture+VEGF Ligand group had threefold higher Cx43 mRNA levels than the Simultaneous Triculture control (Fig. 3A, black bars). In fact, Cx43 transcript levels in Simultaneous Triculture+VEGF Ligand were comparable to those of Simultaneous Preculture alone as well as those found in Enriched CMs (dashed line, Fig. 3A). Western blotting showed that in Simultaneous Triculture+VEGF Ligand organoids, the Cx43 protein levels also increased by more than twofold compared with the VEGF-free Simultaneous Triculture controls (Fig. 3B, black bars).
The VEGFR2 inhibitor is not cytotoxic at its inhibitory concentration
We evaluated the cytotoxicity of a small molecule, competitive inhibitor of VEGFR2 inhibitor (IC50=19.4 nM). Cell viability was quantified over inhibitor concentrations ranging from 2.4 nM to 310 nM, revealing no cytotoxicity in CM monolayers grown at concentrations up to 77 nM, above which we saw a greater number of dead cells (Supplementary Fig. S2, red nuclei). We therefore used a concentration of 19.4 nM in all of our VEGFR2 inhibition studies to prevent cytotoxicity and nonspecificity (see the Discussion section).
Inhibition of the VEGFR2 receptor downregulates Cx43 mRNA and protein expression
To test the effect of inhibiting the VEGFR2 receptor on Cx43 expression, we cultivated organoids based on Sequential Preculture, using Enriched CMs as a baseline, in the presence or absence of the VEGFR2 inhibitor (I) as well as in the presence or absence of VEGF ligand (V). Addition of VEGF alone to Enriched CMs (+V −I, third bar, Fig. 4A) resulted in mRNA levels comparable to the control (Enriched CMs −V −I) but significantly higher than both of the inhibited groups (−V +I and +V +I, Fig. 4A). Notably, Enriched CM organoids grown in the presence of both VEGF ligand and the VEGFR2 inhibitor (+V +I, Fig. 4A) showed a 1.4-fold drop in Cx43 mRNA levels that was comparable to the inhibited case (−V +I, Fig. 4A). In the Sequential Preculture group, a similar trend was seen, with a 1.5-fold increase in Cx43 mRNA in the presence of VEGF alone (+V −I, Fig. 4B) that was significantly higher than both the inhibited cases (−V +I and +V +I, Fig. 4B).
FIG. 4.
Effects of VEGFR2 inhibition on Cx43 mRNA and protein levels in cardiac organoids. (A, B) VEGF Ligand (V, 30 ng/mL) or VEGFR2 inhibitor (I, 19.4 nM) was added to organoids based on either Enriched CMs or Sequential Preculture. In the presence of the inhibitor alone (−V +I), Cx43 mRNA levels were lowered in Enriched CMs (A, black bars) and Sequential Preculture (B, white bars). Addition of exogenous VEGF ligand alone (+V −I) at 30 ng/mL improved Cx43 mRNA expression in both Enriched CMs and Sequential Preculture but these levels were not significant relative to the control (−V −I). Importantly, addition of VEGF ligand to inhibited organoids (+V +I) did not improve Cx43 mRNA levels, which were comparable to mRNA levels for organoids grown in the inhibitor alone (−V +I). n=3/group. Dashed line in (B) represents the Cx43 mRNA expression for Sequential Preculture group using cells with siRNA VEGF knockdown. (C, D) Western blotting for Cx43 protein normalized to a GAPDH loading control. n=3/group. Horizontal lines represent statistically significant difference (p<0.05) between the two groups at the ends of the line. (E) Representative blots showing the amount of protein in each lane.
Enriched CM organoids grown for 24 h in the presence of VEGFR2 inhibitor alone (−V +I, Fig. 4C) also expressed 1.3-fold less Cx43 protein relative to the control, while the presence of VEGF ligand alone (+V −I, Fig. 4C) resulted in Cx43 protein levels that were comparable to the control but significantly higher than both the inhibited cases (−V+I and +V +I, Fig. 4C). Addition of both VEGF ligand and the inhibitor in combination (+V +I, Fig. 4C) had a similar effect to adding the inhibitor alone, causing a statistically significant twofold downregulation in Cx43 protein. A similar trend in Cx43 protein levels was seen in Sequential Preculture (Fig. 4D, white bars), but the changes were more dramatic, with a 4.1-fold decrease in Cx43 protein levels in the presence of inhibitor alone (−V +I, Fig. 4D) and a 2.5-fold decrease in Cx43 protein in the presence of both the inhibitor and VEGF ligand (+V +I, Fig. 4D). Interestingly, addition of VEGF ligand in the absence of VEGFR2 inhibitor to Sequential Preculture organoids did not increase Cx43 protein levels above those of the control (+V −I, Fig. 4D), suggesting that endogenous VEGF levels were already somehow optimized in this condition. This was in contrast to the Simultaneous Triculture case, where addition of exogenous VEGF increased both Cx43 mRNA and protein levels (Simultaneous Triculture+VEGF Ligand; Fig. 3A, B, black bars).
We measured VEGF secretion rate in the Sequential Preculture siRNA group and found it to be significantly lower than Sequential Preculture without siRNA knockdown (Fig. 1B). We also found that the siRNA Sequential Preculture group had significantly lower level of Cx43 expression than other groups (Fig. 4B), thus supporting the link between autocrine VEGF secretion and upregulation of Cx43 in preculture.
Cx43 is found primarily in CMs, and not in FBs or ECs
Organoids based on Enriched CMs alone stained abundantly for both cTnT (Fig. 5, green intracellular staining) and Cx43 (Fig. 5, red punctate staining) and the Cx43 was associated with cTnT+ cells (Fig. 5A, left). However, the cTnT+ cells were rounded in the Enriched CM groups and did not exhibit evidence of striations, indicative of an immature phenotype. Cx43 was found in the areas not positive for VE-cad or Vim; that is, the CMs (Fig. 5A, center and right). Similar results were obtained when performing double immunofluorescence staining on Sequential Preculture organoids (Fig. 5B), where Cx43 was found primarily in the vicinity of cTnT+ cells (Fig. 5B, left). Moreover, cells in Sequential Preculture organoids were much more elongated and cTnT+ cells showed evidence of muscle striations, consistent with a differentiated phenotype.
FIG. 5.
Localization of Cx43. Frozen sections of Enriched CMs and Sequential Preculture organoids grown for 72 h were fluorescently labeled with antibodies for Cx43 (red) as well as the cell-specific markers cardiac troponin T (cTnT, left, green, CMs), VE-cadherin (VE-cad, center, green, ECs), and Vimentin (Vim, right, green, ECs+FBs). 4′,6-Diamidino-2-phenylindole was used as a nuclear counter stain (blue). (A, left) cTnT was found abundantly in Enriched CM organoids and was always associated with Cx43 (red). However, cTnT+ cells were much more rounded in Enriched CM organoids compared with the Sequential Preculture organoids, in which cell elongation was marked and striations were visible (B, left). (A, center) VE-cadherin staining (green) was sparse and primarily not associated with Cx43 (red) in either Enriched CMs or Sequential Preculture (B, center). (A, right) Vimentin staining (green) was not found to be co-localized with Cx43 (red) in either Enriched CM organoids or Sequential Preculture (B, right). Scale bars are 25 μm in all panels and insets. Color images available online at www.liebertonline.com/tea
Cx43 is diminished in the presence of VEGFR2 inhibitor
Consistent with our Western blotting data, we identified higher levels of Cx43 by immunostaining in the control (−V −I, Fig. 6A) and VEGF ligand only–supplemented Enriched CM organoids (+V −I, Fig. 6A) compared with the groups with the inhibitor present (−V +I and +V +I). Similar effects of the inhibitor on Cx43 immunostaining were observed in the Sequential Preculture groups (Fig. 6B). In addition, cTnT staining was more disorganized in both Sequential Preculture groups cultivated with the inhibitor, while the control and VEGF-ligand-supplemented groups showed evidence of cell elongation and muscle striations (Fig. 6B, top and bottom).
FIG. 6.
Effect of the VEGFR2 inhibitor on Cx43 in cardiac organoids. Enriched CMs or Sequential Preculture organoids grown in the presence or absence of VEGF Ligand (V) or VEGFR2 Inhibitor (I) for 72 h were double immunostained for Cx43 (red) and cTnT (green). (A) Enriched CM organoids grown without either inhibitor or VEGF ligand (−V −I) as well as organoids grown in the presence of VEGF ligand alone (+V −I) expressed similar levels of Cx43 protein, but the presence of VEGFR2 inhibitor (−V +I) visibly diminished the Cx43 protein expression. Exogenous VEGF ligand added to the inhibited organoids (+V +I) did not improve Cx43 levels. In all inhibited groups, cTnT was disorganized and diminished. (B) Sequential Preculture organoids grown in the absence of VEGF ligand/VEGFR2 inhibitor (−V −I) or in the presence of VEGF ligand alone (+V −I) showed robust Cx43 expression and evidence of cell elongation and muscle striations in cTnT+ cells. This was in stark contrast to organoids grown in VEGFR2 inhibitor (−V +I and +V +I) that displayed diminished Cx43, a rounded morphology, disorganized cTnT expression, and absence of striations. Scale bars are 25 μm in all panels and insets. Color images available online at www.liebertonline.com/tea
Addition of VEGFR2 inhibitor during cultivation negatively affects contractile properties of cardiac organoids
Functional testing was performed to measure the effect of VEGF stimulation/VEGFR2 inhibition on the excitation threshold (ET) and maximum capture rate (MCR) of engineered organoids (see Supplementary Data). Low values of ET and high values of MCR are desirable in this assessment as they point to improved coupling between CMs. Enriched CM-based organoids supplemented with VEGFR2 inhibitor had compromised functional properties, displaying an elevated ET (−V +I and +V +I, Fig. 7A, top left) compared with the inhibitor-free controls. Addition of VEGF ligand alone did not appear to lower the ET in Enriched CM organoids relative to the control (+V −I vs. −V −I, Fig. 7A). The MCR was significantly lower in the presence of the inhibitor alone (−V +I, Fig. 7A, top right) compared with the VEGF-ligand-supplemented groups (both +V −I and +V +I, Fig. 7A, top right), but not appreciably different relative to the control (−V −I). For Sequential Preculture, the addition of VEGF alone (+V −I, Fig. 7B) showed a statistically significant drop in ET relative to the inhibited groups, both of which had a high ET and a low MCR (−V +I and +V +I, Fig. 7B, bottom). In the group containing both the inhibitor as well as the VEGF ligand (+V +I, Fig. 7B, right most bar), the MCR was significantly lower than both the control (−V −I, Fig. 7B, bottom right) and the group containing VEGF ligand alone (+V −I, Fig. 7B, bottom right).
FIG. 7.
Effect of the VEGFR2 inhibitor on electromechanical properties of cardiac organoids. Organoids based on Enriched CMs and Sequential Preculture were grown for 72 h in the presence or absence of VEGF ligand (V) or a VEGFR2 inhibitor (I). Excitation threshold (ET, V/cm, left) and maximum capture rate (MCR, Hz, right) were measured to assess electrical excitability in the presence or absence of inhibitor or VEGF. In general, all inhibited Enriched CM organoids exhibited poorer contractile function with higher ETs (A, left) and lower MCR (A, right), as did all inhibited Sequential Preculture organoids (B, left/right). Addition of VEGF ligand alone (+V −I) maintained ET and MCR at values comparable with or better than the control (−V −I), but was ineffective in improving the ET/MCR in the presence of the inhibitor (+V +I). n=3–5/group. Success rate of beating organoid formation, defined as the number of samples that could be induced to beat divided by the total number of samples tested during functional testing, was reported under the ET graph for each group. In the case of Enriched CM organoids, the success rate was always 1, indicating all samples could be induced to contract. However, for Sequential Preculture, the success rate was only 1 in the control (−V −I) and VEGF-ligand-supplemented group (+V −I). The inhibited groups had success rates lower than 1, suggesting that fewer samples were able to contract in the presence of the inhibitor, even in the presence of VEGF ligand. Horizontal lines represent statistically significant difference (p<0.05) between the two groups at the ends of the line.
Another important measure of contractile function in the organoids was the ratio of samples that could be induced to beat synchronously divided by the total number of samples we tested (Fig. 7). While all Enriched CM organoids had success rates of 1.0, this was not the case with Sequential Preculture, where the inhibited groups (−V +I and +V +I, Fig. 7) had success rates of 0.5 and 0.6, indicating poorer electrical coupling of the cells.
Discussion
The main goal of this study was to elucidate the mechanisms responsible for improved functional assembly of cardiac organoids by preculture with nonmyocytes. In our previous work4 we determined that although conditioned media from EC/FB produced increase in Cx43 immunostaining in cardiac organoids, the most robust improvement in functional properties was observed in Preculture, thus motivating detailed studies of Preculture groups here and omitting the conditioned media group. We found that VEGF was rapidly released immediately after CM seeding in the Simultaneous Preculture and Sequential Preculture groups, in contrast to Simultaneous Triculture where no VEGF could be detected, and that blocking endogenous VEGF or VEGFR2 resulted in transcriptional and translational downregulation of Cx43. Conversely, supplementation with VEGF ligand resulted in transcriptional and translational upregulation of Cx43 and improved cardiac function. We also found that all three cell types used in our studies (ECs, FBs, and CMs) expressed VEGFR2 but Cx43 expression was localized to CM alone. These findings suggest a putative mechanism whereby VEGF, secreted by nonmyocytes during the Preculture period, may be stored and released to bind with VEGFR2 on CMs, which, in turn, respond by synthesizing Cx43 mRNAs and proteins to improve cell–cell communication and organoid function. This proposed mechanism is consistent with our previous observations that showed that conditioned medium from Simultaneous Preculture organoids also improved the morphology, cell density, and viability of organoids based on Enriched CMs,4 which suggested to us early on that a soluble factor might be regulating these effects. We also previously observed EC clustering, lack of contractile function, and lack of Cx43 expression in Simultaneous Triculture,4 consistent with lower VEGF levels,11 which motivated us to evaluate endogenous VEGF secretion under different cell culture conditions.
We did not investigate the effect of Sequential Preculture in order of FBs, ECs, and CMs, since we attempted to capture a developmentally relevant situation whereby endothelial progenitors appear before the appearance of other cardiac cell types.18
Since the Kd of VEGF-VEGFR2 binding is ∼10 ng/mL [16], and the average VEGF secretion rates detected in the Preculture groups were of the same order of magnitude [between 4.3 ng/(mL·h−1) and 16.6 ng/(mL·h−1)], the success of preculture may be in its ability to maintain VEGF at concentrations at or above the threshold required for signaling with VEGFR2. Addition of exogenous soluble VEGF to the Sequential Preculture organoids did not result in an increase in Cx43 expression. It is possible that this is because VEGF levels are already high in this condition, and thus, VEGFR2 receptors may be saturated and additional exogenous VEGF may not improve Cx43 levels beyond baseline levels for this condition.
All three cell types seeded into the organoids (ECs, FBs, and CMs) expressed VEGFR2, indicating that they could respond to changes in the VEGF concentration. We observed a bimodal distribution when performing flow cytometry for VEGFR2 on neonatal rat CMs, with 49% of the Enriched CMs displaying a VEGFR2+ phenotype. However, this VEGFR2+ subpopulation may have contained both myocytes and nonmyocytes, since the purity of Enriched CMs after two preplates is only ∼81%.4 Thus, even if we assume the remaining 19% of nonmyocytes to have contributed to this shift, then only 49%–19%=∼30% of the CMs were VEGFR2+. Nonetheless, this still implies the existence of a definite VEGFR2+ CM subpopulation capable of responding to changes in VEGF, including potentially synthesizing connexin mRNAs and proteins.
Regulation of Cx43 at the transcriptional (mRNA) and translational (protein) levels was shown through VEGF blocking and supplementation experiments. The choice of the 1-h time point for measurement of Cx43 mRNA was based on a half-life of typical mRNA around 30 min–1 h. Others have shown that Cx43 mRNA peaks early on after mechanical stimulation of CMs (i.e., minutes to a couple of hours).19,20 Indeed, our time course experiments confirmed this time frame for maximal Cx43 mRNA upregulation after VEGF stimulation (Supplementary Fig. S1). For Western blotting studies, however, total protein was isolated from the organoids at 24 h posttreatment with VEGF ligand or its inhibitors. This was based on published reports that stated that the half-life of Cx43 protein is short (1–2 h); however, it may continue to be synthesized after stimulation for up to 24 h before reaching steady-state levels.19–22
We chose a concentration of the neutralizing antibody (0.4 μg/mL) that was twice the ND50 required to neutralize a half of the VEGF ligand at a concentration of 30 ng/mL. We reasoned that this concentration would be sufficient to ensure that the majority of endogenously secreted VEGF ligands were not available for signaling, based on our ELISA data, where the maximum endogenous secretion rate we detected was 16.6 ng/(mL·h−1). In Simultaneous Triculture, we also observed significant increases in Cx43 expression at both the transcriptional and translational levels when supplementing the organoids with soluble VEGF-A165 at 30 ng/mL. We chose this concentration since it was three times higher than the Kd of VEGF-VEGFR2 binding,16 which ensured that we were not below the threshold for signaling.
To assess the contribution of VEGF-VEGFR2 binding to Cx43 regulation, we cultivated organoids in the presence of a small molecule, ATP-competitive VEGFR2 inhibitor. We did not use concentrations above the IC50 of 19.4 nM in our experiments, since the inhibitor is not specific to VEGFR2 alone, and has been reported to inhibit other receptor tyrosine kinases at higher concentrations (PDGFRβ IC50=34 nM, VEGFR3 IC50=190 nM, etc.). Still, this ensured that at least 50% of the VEGFR2 receptors would not be able to participate in VEGF signaling. Dose–response experiments also confirmed that no cytotoxicity was observed at 19.4 nM (Supplementary Fig. S2), which further motivated us to use this concentration. Interestingly, the inhibitor was effective in downregulating Cx43 mRNA and protein levels even in the presence of a high concentration of exogenously added VEGF ligand of 30 ng/mL (all +V +I groups). This was likely due to the fact that the inhibitor, although reversible, was an ATP-competitive inhibitor, meaning that the ATP binding pocket would still have been inaccessible in at least 50% of the receptors at a concentration of 19.4 nM, even in the presence of an excess of exogenous VEGF ligand.
A higher ET and a lower MCR (Fig. 7) in all groups containing the inhibitor were consistent with lower levels of Cx43 mRNA/protein observed in these groups (Fig. 4). The worsened contractile properties may be indicative of diminished capacity for functional coupling of CMs through gap junctions. It is unlikely that the worsened contractile properties were due to cell death since our inhibitor dose–response studies ruled out this possibility (Supplementary Fig. S2).
VEGF-VEGFR2 signaling is known to influence a myriad of cellular responses by regulating several downstream effectors. One of these downstream effectors is mitogen-activated protein kinase (MAPK), known for its regulation of cell cycle genes, such as c-myc.23,24 MAPK signaling has also been shown to affect Cx43 expression.5,25 It is possible, therefore, that VEGF-VEGFR2 binding regulates Cx43 expression by way of the MAPK pathway and its downstream targets. Another possibility, as hypothesized by Pimentel et al., is that VEGF may inactivate the degradatory pathways for Cx43 mRNAs and proteins.10 The complexity and large number of possible pathways underscore the need for further work in order to fully elucidate the precise downstream targets of VEGF-VEGFR2 binding that may regulate Cx43 expression.
Our data strongly point at the possible causal relationship between VEGF-VEGFR2 signaling and Cx43 mRNA/protein regulation. We also propose that the VEGF may be secreted by nonmyocytes and/or stored in the ECM for later release. This signaling mechanism represents a novel finding with potentially useful applications for tissue engineering. For example, engineered cardiac tissues based on multiple cell populations may benefit from the addition of VEGF ligand to culture medium to aid in promoting cardiac differentiation and development and to ensure gap junctional expression is robustly maintained. Alternatively, biomaterials can be designed to provide sustained or bolus release of VEGF, coinciding with crucial cell seeding time points. Most importantly, the use of Preculture as a cell seeding methodology itself presents a method of ensuring that endogenously secreted VEGF is maintained at physiologically relevant levels capable of improving gap junction/cell–cell communication and, in turn, proper cardiac contractile function.
While our results indicated that VEGF is secreted early on after CM seeding, there is very little endogenous VEGF secretion later on, suggesting that other signaling mediators and growth factors may be important in maintaining Cx43 expression throughout the 7-day cultivation period. While we focused primarily on VEGF for our studies, it should be noted that there are also other soluble factors worthy of investigation besides VEGF, such as Angiotensin II, Angiopoietin 1, Endothelin-1, neuroreglin, nitric oxide, and transforming growth factor-β,26 which could have additional, equally important roles and may lead to potentially undiscovered mechanisms regulating cardiac function during the tissue engineering process. Additionally, factors that are important in early cardiac development, such as fibroblast growth factor, bone morphogenetic protein, and Wnt may also guide the development of functional cardiac tissues in vitro and should be investigated in further studies. In future studies, it is also necessary to scale up the cell compositions to the millimeter-size cardiac tissue relevant for implantation and demonstrate the success of precultured patches in improving cardiac function in vivo.
Conclusions
Mechanistic studies were carried out to elucidate the role of VEGF-VEGFR2 signaling on Cx43 expression in engineered cardiac tissues based on Sequential Preculture, Simultaneous Preculture, Simultaneous Triculture, and Enriched CMs. These studies revealed that VEGF secreted by nonmyocytes modulated the Cx43 levels both at the transcriptional and translational levels. VEGF secretion peaked immediately upon seeding of CMs and was highest in the Sequential Preculture group. Blockage of the endogenous pool of secreted VEGF in Simultaneous Preculture through a neutralizing antibody resulted in significant downregulation of Cx43 mRNA and protein (shown through QPCR and Western blotting data). Meanwhile addition of VEGF ligand to Simultaneous Triculture resulted in upregulation of Cx43 mRNA and protein to levels comparable with Simultaneous Preculture. We found that all three cell types (ECs, FBs, and CMs) expressed the VEGFR2 receptor by analytical flow cytometry, but the majority of Cx43 protein was expressed by CMs as shown by double staining of Cx43 with cell-specific markers. Inhibition of the VEGFR2 receptor in Sequential Preculture and Enriched CMs using a small molecule inhibitor also resulted in downregulation of Cx43 mRNA transcripts and protein as shown through QPCR, Western blotting, and double immunofluorescence staining. Contractile function was also compromised in VEGFR2-inhibited organoids, which had poor functional properties (overall higher ET and lower success rate). The data presented here may provide new insights into VEGF-VEGFR2-Cx43 signaling dynamics during the tissue engineering process. Our findings may aid in the design and implementation of novel therapies for cardiac tissue engineering and regeneration.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the following funding sources: National Institutes of Health (R01HL076485, P41 EB002520), NSERC Strategic Grant (STPGP 381002-09), NSERC-CIHR Collaborative Health Research Grant (CHRPJ 385981-10), NSERC Discovery Grant (RGPIN 326982-10), and Discovery Accelerator Supplement (RGPAS 396125-10), Ontario Centres of Excellence International Scholarship, the Ontario Graduate Scholarship in Science and Technology (OGSST) and the Ontario Graduate Scholarship (OGS).
Disclosure Statement
No competing financial interests exist.
References
- 1.Nag A.C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41. [PubMed] [Google Scholar]
- 2.Banerjee I. Yekkala K. Borg T.K. Baudino T.A. Dynamic Interactions between Myocytes, Fibroblasts, and Extracellular Matrix. Ann N Y Acad Sci. 2006;1080:76. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 3.Kuzuya M. Kinsella J.L. Induction of endothelial cell differentiation in vitro by fibroblast-derived soluble factors. Exp Cell Res. 1994;215:310. doi: 10.1006/excr.1994.1347. [DOI] [PubMed] [Google Scholar]
- 4.Iyer R.K. Chiu L.L. Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A. 2009;89:616. doi: 10.1002/jbm.a.32014. [DOI] [PubMed] [Google Scholar]
- 5.Warn-Cramer B.J. Cottrell G.T. Burt J.M. Lau A.F. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- 6.Gaudesius G. Miragoli M. Thomas S.P. Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circulation Research. 2003;93:421. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 7.Kohl P. Camelliti P. Burton F.L. Smith G.L. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol. 2005;38:45. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 8.Little T.L. Beyer E.C. Duling B.R. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:H729. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 9.Narmoneva D.A. Vukmirovic R. Davis M.E. Kamm R.D. Lee R.T. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pimentel R.C. Yamada K.A. Kleber A.G. Saffitz J.E. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90:671. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P. Ng Y.S. Nuyens D. Theilmeier G. Brusselmans K. Cornelissen I., et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 12.Radisic M. Euloth M. Yang L. Langer R. Freed L.E. Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 13.Iyer R.K. Chui J. Radisic M. Spatiotemporal tracking of cells in tissue-engineered cardiac organoids. J Tissue Eng Regen Med. 2009;3:196. doi: 10.1002/term.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu L.L. Iyer R.K. King J.P. Radisic M. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng Part A. 2011;17:1465. doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer R.K. Chui L.L.Y. Vunjak-Novakovic G. Radisic M. Biofabrication enables efficient interrogation of sequential culture of endothelial cells, fibroblasts and cardiomyocytes for cardiac tissue engineering. Biofabrication. 2012 doi: 10.1088/1758-5082/4/3/035002. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 17.Radisic M. Park H. Shing H. Consi T. Schoen F.J. Langer R., et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratajska A. Torry R.J. Kitten G.T. Kolker S.J. Tomanek R.J. Modulation of cell migration and vessel formation by vascular endothelial growth factor and basic fibroblast growth factor in cultured embryonic heart. Dev Dyn. 1995;203:399. doi: 10.1002/aja.1002030403. [DOI] [PubMed] [Google Scholar]
- 19.Cowan D.B. Lye S.J. Langille B.L. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82:786. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 20.Wang T.L. Tseng Y.Z. Chang H. Regulation of connexin 43 gene expression by cyclical mechanical stretch in neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2000;267:551. doi: 10.1006/bbrc.1999.1988. [DOI] [PubMed] [Google Scholar]
- 21.Salameh A. Krautblatter S. Baessler S. Karl S. Rojas Gomez D. Dhein S., et al. Signal transduction and transcriptional control of cardiac connexin43 up-regulation after alpha 1-adrenoceptor stimulation. J Pharmacol Exp Ther. 2008;326:315. doi: 10.1124/jpet.108.136663. [DOI] [PubMed] [Google Scholar]
- 22.Shyu K.G. Chen C.C. Wang B.W. Kuan P. Angiotensin II receptor antagonist blocks the expression of connexin43 induced by cyclical mechanical stretch in cultured neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:691. doi: 10.1006/jmcc.2000.1333. [DOI] [PubMed] [Google Scholar]
- 23.Doanes A.M. Hegland D.D. Sethi R. Kovesdi I. Bruder J.T. Finkel T. VEGF stimulates MAPK through a pathway that is unique for receptor tyrosine kinases. Biochem Biophys Res Commun. 1999;255:545. doi: 10.1006/bbrc.1999.0227. [DOI] [PubMed] [Google Scholar]
- 24.Seko Y. Takahashi N. Tobe K. Ueki K. Kadowaki T. Yazaki Y. Vascular endothelial growth factor (VEGF) activates Raf-1, mitogen-activated protein (MAP) kinases, and S6 kinase (p90rsk) in cultured rat cardiac myocytes. J Cell Physiol. 1998;175:239. doi: 10.1002/(SICI)1097-4652(199806)175:3<239::AID-JCP1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Warn-Cramer B.J. Lampe P.D. Kurata W.E. Kanemitsu M.Y. Loo L.W. Eckhart W., et al. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem. 1996;271:3779. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 26.Brutsaert D.L. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.