Abstract
Background
Previous research suggests that cancer patients frequently experience multiple symptoms during chemotherapy; however, relationships among symptom changes are largely unknown.
Purpose
The aim of the current study was to examine daily and intraday changes and interrelationships among fatigue, depression, and objectively measured disruptions in sleep and activity during chemotherapy.
Methods
Participants were 78 women with gynecologic cancer. Fatigue, depression, sleep, and activity were assessed the week before and the week after the participants’ first three infusions.
Results
Significant changes in fatigue, depression, sleep, and activity were observed over time. Before infusions, increases in fatigue were associated with increases in depression. After infusions, increases in fatigue were associated with increases in depression and minutes awake at night, as well as decreases in daytime activity and regularity of sleep/activity patterns (ps<.05).
Conclusions
This study is among the first to track daily and intraday changes in symptoms and interrelationships during chemotherapy. Results indicate that symptoms are interrelated and return to baseline levels after infusions.
Keywords: Gynecologic cancer, Fatigue, Depression, Actigraphy, Sleep, Chemotherapy
Introduction
Fatigue, depression, and disrupted sleep and activity are increasingly recognized as some of the most common side effects of chemotherapy for cancer. Research conducted primarily in women with breast cancer suggests that the prevalence of moderate to severe fatigue during chemotherapy is 26–60% [1–3], depressive symptomatology 20–39% [4, 5], self-reported insomnia 79% [6, 7], and self-reported inactivity 40–74% [8, 9]. Objective sleep and activity, measured via wrist actigraphy, also show significant disruptions during chemotherapy [10–12]. These symptoms tend to be highly distressing to patients and are associated with reduced quality of life [8, 13–15]. For example, patients report that fatigue affects their ability to work, socialize, and enjoy life [16–20]. Accordingly, understanding and treating fatigue and other symptoms should be a high priority in cancer care and research [17, 20].
Studies examining longitudinal change in symptoms during chemotherapy have typically reported a “roller coaster” effect, in which symptoms are highest in the week following a chemotherapy infusion, then decline prior to the next infusion. For example, Savard and colleagues [21] examined objective sleep/activity patterns using actigraphy in breast cancer patients during chemotherapy. They found in the week following the first infusion that sleep/activitypatterns were significantly disrupted, then returned to pre-chemotherapy baseline levels 2 and 3 weeks after the first infusion. Sleep/activity patterns were again disrupted in the week following the fourth infusion and continued to be disrupted in the second and third weeks after the fourth infusion relative to pre-chemotherapy baseline. Similar findings have been reported for perceived sleep quality [22]. Other studies in breast cancer patients suggest a similar roller coaster pattern also holds true for fatigue and depression, which are highest in the first few days following a chemotherapy infusion, then begin to subside prior to the next infusion [22–24] until they have returned to pre-chemotherapy baseline levels [22, 25].
As this roller coaster pattern suggests, fatigue, depression, and disruptions in sleep and activity tend to co-occur during chemotherapy. Numerous studies have documented statistically significant cross-sectional correlations between symptoms [6, 14, 22, 23, 26–28]. For example, Redeker and colleagues [14] found that fatigue and depression were correlated r=.43 during chemotherapy, while fatigue and self-reported insomnia were correlated r=.26 and depression and self-reported insomnia were correlated r =.30; all correlations were statistically significant. Fatigue has been associated with greater objectively measured nighttime awakenings and less activity over a 24-h period during chemotherapy [12, 27]. Depression has also been associated with reduced activity as measured by actigraphy [12]. In addition, fatigue and depressive symptomatology were significantly higher in cancer survivors who did not meet Centers for Disease Control and Prevention guidelines for physical activity as compared to those who did [29], although data are conflicting [30].
Most studies of the relationships between fatigue, depression, sleep, and activity during chemotherapy have been cross-sectional. Less data exist regarding whether changes among symptoms are also correlated. Data regarding correlations among symptom change are important in determining whether symptoms fluctuate together over time. Available longitudinal data suggest that increases in fatigue from the first to the fourth chemotherapy infusions are associated with increases in depression, increases in objectively measured sleep, and decreases in objectively measured daytime activity in women with breast cancer [31]. In addition, depression prospectively predicted increased self-reported sleep disturbance over the course of a year in women with metastatic breast cancer [32]. However, symptoms were measured relatively infrequently in these studies [i.e., time between assessments was 63 days [31] and 4 months [32], respectively].
Despite evidence that chemotherapy is a time of high fluctuation in symptoms, very few studies have examined daily or intraday symptom change. Most studies have assessed symptoms only periodically during this time, with several weeks between assessments. Even when symptoms are assessed on a daily basis (e.g., using actigraphy, daily diaries), data are often averaged over a period of several days or more. We are aware of only two studies examining daily and intraday changes in symptoms during chemotherapy. De Jong and colleagues found that daily fatigue peaked at 3–5 days following the third chemotherapy infusion in breast cancer patients [24]. Badr and colleagues [33] found that fatigue increased over the course of the day in ovarian cancer patients undergoing chemotherapy and was significantly associated with negative mood. Data from these studies are intriguing because they suggest that there may be significant daily and intraday fluctuations in symptoms during chemotherapy. Additional research is needed to examine daily and intraday trajectories of fatigue, depression, and disruptions in sleep and activity as well as concurrent relationships between trajectories.
The goal of the current study was to examine daily and intraday changes in fatigue, depression, sleep, and activity in women undergoing platinum-based chemotherapy for gynecologic cancer. This sample was selected because fatigue, depression, and disruptions in sleep and activity are both common and severe in women with gynecologic cancer [6, 7, 29, 34–36], due in part to the arduous platinum-based chemotherapy regimens prescribed as first- and second-line treatment [37]. Symptoms were assessed in the week before and the week after the first three chemotherapy infusions. Three infusions were selected to minimize patient burden while allowing for exploration of the cumulative effect of chemotherapy on symptoms over time. We had three hypotheses: (1) daily symptoms would occur in a roller coaster pattern in which fatigue, depression, and disruptions in sleep and activity were lowest in the weeks before each infusion and highest in the weeks after each infusion, (2) baseline clinical characteristics (i.e., disease recurrence, time since surgery, disease stage, prescription of antidepressants, prescription of sleep medications) would predict symptom trajectories, and (3) there would be significant relationships among concurrent changes in symptoms. We also explored intraday change in fatigue and depression. Because these analyses were exploratory, no a priori hypotheses are offered.
Methods
Participant Eligibility and Recruitment
Women with gynecologic cancer were recruited for an IRB-approved study examining side effects of platinum-based chemotherapy. Eligibility criteria were that participants: (1) be at least 18 years of age, (2) be scheduled to receive intravenous platinum-based chemotherapy for gynecologic cancer at Moffitt Cancer Center, (3) have not been treated with chemotherapy for at least 2 months prior to recruitment, (4) be free of documented or observable psychiatric or neurologic disorders that could interfere with study participation (e.g., Parkinson’s disease, schizophrenia), (5) be able to speak and read English, and (6) provide written informed consent. Patients were recruited between September 2007 and July 2009.
Eligibility was determined by chart review and consultation with the attending physician. Eligible women were recruited and informed consent was obtained during an outpatient clinic visit before the start of chemotherapy. All participants completed a baseline demographic assessment and began actigraphic monitoring at this time. Participants continuously wore the actigraph and completed daily diaries of bedtime, rising time, fatigue, and depression until 7 days after their first infusion, when they returned the actigraph and diaries by mail. Participants were mailed fresh actigraphs and diaries at least 7 days prior to their second and third infusions and mailed them back 7 days after each infusion.
A total of 112 women were approached for study participation and 80 (71%) signed consent. Reasons for refusal included: too sick (n=2), too busy (n=16), not interested (n=7), not able to comply with actigraphy/diaries due to work or other obligations (n=4), and no reason given (n=3). Two patients who signed consent did not provide any data and thus are not included in analyses; one of these patients became ineligible due to change in treatment plans and the other elected to discontinue study participation. An additional nine patients who signed consent provided partial data before becoming ineligible or electing to discontinue study participation; to reduce potential attrition bias, their available data were included in analyses.
Measures
Demographic and Clinical Data
Age, race/ethnicity, marital status, education level, annual and household income were assessed in all participants via self-report. Cancer type, disease stage, chemotherapy regimen, days since surgery, recurrence status, prescription of antidepressants at recruitment, and prescription of sleep medications at recruitment (i.e., benzodiazepines, barbiturates, sedatives, hypnotics) were assessed in participants via medical chart review.
Daily Diaries
Participants were asked to rate their fatigue and depression at 10 a.m., 2 p.m., and 6 p.m. during assessment days. These times were selected because they occurred at regularly scheduled intervals when participants were likely to be awake. The actigraph beeped at these times to remind participants to complete their ratings. Fatigue and depression were rated on an 11-point scale (0=no fatigue/ depression at all, 10=as fatigued/depressed as I could be). To assess daily fatigue and depression, ratings were averaged for each day to produce one mean fatigue score and one mean depression score per participant per day. Participants also recorded their bedtimes and rising times during assessment days.
Objective Sleep and Activity
Wrist actigraphy was used to objectively measure sleep, activity, and sleep/activity patterns. The Actiwatch®-Score (Mini Mitter, Bend, OR) actigraph was used. Participants were asked to continuously wear the actigraph on the non-dominant wrist during assessment periods. The actigraph uses a piezoelectric accelerometer to monitor and store the degree and intensity of motion, averaging over every minute. The American Academy of Sleep Medicine has noted that actigraphy is reliable and valid for detection of sleep [38]. Actigraphy is also reliable and valid for the measurement of activity [39, 40]. In the current study, actigraphy variables were calculated in combination with patient recordings of bedtime and rising time. In accordance with recent recommendations [41], sleep was assessed with the following variables: (1) time in bed at night, (2) sleep efficiency (i.e., the percentage of time in bed spent sleeping), (3) minutes awake after sleep onset (i.e., minutes awake after an initial period of sleep), and (4) percentage of time spent sleeping during the day. Activity was assessed with the following variables: (1) daytime activity and (2) nighttime activity. Sleep/activity patterns were assessed with a dichotomy index or ratio of nighttime activity to daytime activity [42, 43]. Higher dichotomy index scores indicate greater disruptions in sleep/activity patterns.
Statistical Analysis
Analyses followed a three-stage procedure. First, changes in fatigue, depression, sleep, activity, and sleep/activity patterns were described by applying mixed models using piecewise regression [44]. Piecewise regression offers the ability to model rates of change within intervals set by the researcher [45]. Because the data fall naturally into pre-chemotherapy and post-chemotherapy intervals, piecewise regression allows for examination of differences in rates of change depending on whether symptoms were assessed before or after chemotherapy. In addition, piecewise regression enables analysis of all available data, rather than analysis of only subjects with complete data as in repeated measures ANOVA. We were interested in both linear and curvilinear (i.e., quadratic or U- or ∩-shaped) changes in symptoms over time. For each symptom, nine effects were estimated.1 The first was linear change in the symptom over time across the three infusion days (i.e., days 0, 21, and 42 in Fig. 1; “infusion number” in Table 1). Pre-chemotherapy intervals refer to days −6 to −1, 15 to 20, and 38 to 41 in Fig. 1. Effects describing the pre-chemotherapy intervals were: linear change in the symptom over time averaged across pre-chemotherapy intervals (i.e., “pre-chemo” in Table 1), quadratic change in the symptom over time averaged across the pre-chemotherapy intervals (i.e., “pre-chemo×pre-chemo”), differences in linear change in the symptom by infusion number (i.e., “infusion number×pre-chemo”), and differences in quadratic change in the symptom by infusion number (i.e., “infusion number×pre-chemo×pre-chemo”). Post-chemotherapy intervals refer to days 1 to 6, 22 to 27, and 43 to 48 in Fig. 1. Effects describing the post-chemotherapy intervals were: linear change in the symptom over time averaged across the post-chemotherapy intervals (i.e., “post-chemo” in Table 1), quadratic change in the symptom over time averaged across the post-chemotherapy intervals (i.e., “post-chemo×post-chemo”), differences in linear change in the symptom by infusion number (i.e., “infusion number×post-chemo”), and differences in quadratic change in the symptom by infusion number (i.e., “infusion number×post-chemo×post-chemo”). Piecewise regressions examining fatigue and depression also included an additional nine effects examining intraday variation in these symptoms (not shown in Table 1). These additional effects were: a main effect of intraday variation, cumulative effect of infusion number on intraday variation, change in intraday variation by linear change in symptoms during pre-chemotherapy intervals, change in intraday variation by linear change in symptoms during post-chemotherapy intervals, change in intraday variation by quadratic change in symptoms during pre-chemotherapy intervals, change in intraday variation by quadratic change in symptoms during post-chemotherapy intervals, cumulative effect of infusion number on change in intraday variation by linear change in symptoms during pre-chemotherapy intervals, cumulative effect of infusion number on change in intraday variation by linear change in symptoms during post-chemotherapy intervals, cumulative effect of infusion number on change in intraday variation by quadratic change in symptoms during pre-chemotherapy intervals, and cumulative effect of infusion number on change in intraday variation by quadratic change in symptoms during post-chemotherapy intervals. Due to partial data on days in which patients were asked to start and stop actigraphic monitoring and daily diary recording, these days were dropped from analyses, and only the periods 6 days before to 6 days after each infusion were used in analyses.
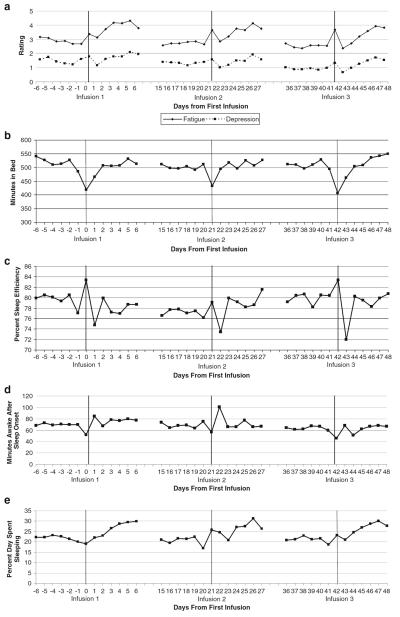
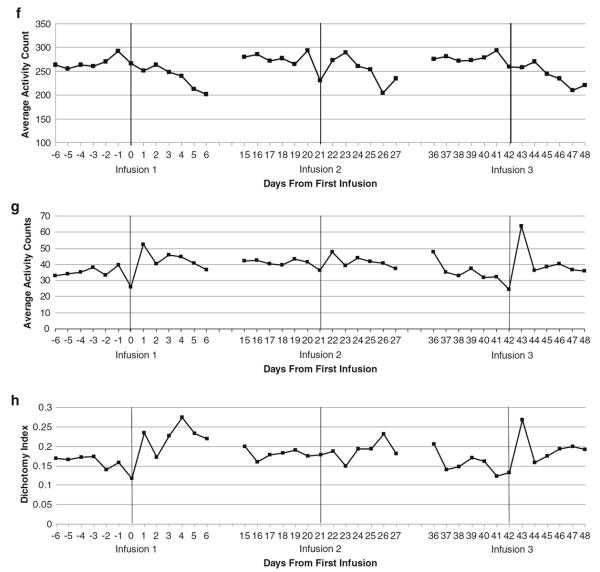
Fig. 1.
Average daily fatigue and depression during chemotherapy. b Nightly time in bed. c Nightly sleep efficiency. d Nightly minutes awake after sleep onset (WASO). e Percent of time spent sleeping during the day. f Daytime activity. g Nighttime activity. h Dichotomy index
Table 1.
Piecewise regression models examining change in daily symptoms over time
| Independent Variable | t | p | Interpretation |
|---|---|---|---|
| Dependent variable: average daily fatigue | |||
| Infusion number | −2.18 | .03 | Fatigue on infusion days decreases over time |
| Pre-chemo | 1.11 | .27 | No linear change in fatigue before infusions |
| Post-chemo | 5.47 | <.001 | Fatigue increases after infusions |
| Pre-chemo×pre-chemo | 1.44 | .15 | No quadratic change in fatigue before infusions |
| Post-chemo×post-chemo | −4.37 | <.001 | Fatigue increases, then decreases after infusions |
| Infusion number×pre-chemo | −1.37 | .17 | No differences in linear change in pre-chemotherapy fatigue by infusion number |
| Infusion number×post-chemo | −1.55 | .12 | No differences in linear change in post-chemotherapy fatigue by infusion number |
| Infusion number×pre-chemo× pre-chemo |
−1.37 | .17 | No differences in quadratic change in pre-chemotherapy fatigue by infusion number |
| Infusion number×post-chemo× post-chemo |
2.43 | .02 | Peaks in fatigue are most pronounced after the first infusion |
| Dependent variable: average daily fatigue | |||
| Infusion number | −3.93 | <.001 | Depression on infusion days decreases over time |
| Pre-chemo | 1.18 | .24 | No linear change in depression before infusions |
| Post-chemo | −.36 | .72 | No linear change in depression after infusions |
| Pre-chemo×pre-chemo | 1.25 | .22 | No quadratic change in depression before infusions |
| Post-chemo×post-Chemo | 1.26 | .21 | No quadratic change in depression after infusions |
| Infusion number×pre-chemo | −1.55 | .12 | No differences in linear change in pre-chemotherapy depression by infusion number |
| Infusion number×post-chemo | 1.54 | .12 | No differences in linear change in post-chemotherapy depression by infusion number |
| Infusion number×pre-chemo× pre-chemo |
−1.42 | .16 | No differences in quadratic change in pre-chemotherapy depression by infusion number |
| Infusion number×post-chemo× post-chemo |
−1.11 | .27 | No differences in quadratic change in post-chemotherapy depression by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | .14 | .89 | No difference in time in bed on nights prior to infusions |
| Pre-chemo | −4.15 | <.001 | Time in bed decreases before infusions |
| Post-chemo | 3.81 | <.001 | Time in bed increases after infusions |
| Pre-chemo×pre-chemo | −2.78 | .007 | Time in bed increases then decreases before infusions |
| Post-chemo×post-chemo | −2.65 | .01 | Time in bed increases then decreases after infusions |
| Infusion number×pre-chemo | −.02 | .98 | No differences in linear change in pre-chemotherapy time in bed by infusion number |
| Infusion number×post-chemo | −.16 | .88 | No differences in linear change in post-chemotherapy time in bed by infusion number |
| Infusion number×pre-chemo× pre-chemo |
−.42 | .68 | No differences in quadratic change in pre-chemotherapy time in bed by infusion number |
| Infusion number×post-chemo× post-chemo |
.72 | .47 | No differences in quadratic change in post-chemotherapy time in bed by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | .52 | .60 | No difference in sleep efficiency on nights prior to infusion |
| Pre-chemo | 1.19 | .24 | No linear change in sleep efficiency before infusions |
| Post-chemo | −1.60 | .11 | No linear change in sleep efficiency after infusions |
| Pre-chemo×pre-chemo | 1.22 | .23 | No quadratic change in sleep efficiency before infusions |
| Post-chemo×post-chemo | 1.51 | .14 | No quadratic change in sleep efficiency after infusions |
| Infusion number×pre-chemo | −.02 | .98 | No differences in linear change in pre-chemotherapy sleep efficiency by infusion number |
| Infusion number×post-chemo | .13 | .90 | No differences in linear change in post-chemotherapy sleep efficiency by infusion number |
| Infusion number×pre-chemo× pre-chemo |
−.28 | .78 | No differences in quadratic change in pre-chemotherapy sleep efficiency by infusion number |
| Infusion number×post-chemo× post-chemo |
−.02 | .98 | No differences in quadratic change in post-chemotherapy sleep efficiency by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | −1.23 | .22 | No difference in WASO on nights prior to infusion |
| Pre-chemo | −1.42 | .16 | No linear change in WASO before infusions |
| Post-chemo | 2.22 | .03 | WASO increases after infusions |
| Pre-chemo×pre-chemo | −1.09 | .28 | No quadratic change in WASO before infusions |
| Post-chemo×post-chemo | −1.59 | .12 | No quadratic change in WASO after infusions |
| Infusion number×pre-chemo | −.49 | .62 | No differences in linear change in pre-chemotherapy WASO by infusion number |
| Infusion number×post-chemo | −.80 | .42 | No differences in linear change in post-chemotherapy WASO by infusion number |
| Infusion number×pre-chemo× pre-chemo |
−.48 | .63 | No differences in quadratic change in pre-chemotherapy WASO by infusion number |
| Infusion number×post-chemo× post-chemo |
.87 | .38 | No differences in quadratic change in post-chemotherapy WASO by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | 1.60 | .11 | No difference in percent daytime sleep across infusion days |
| Pre-chemo | −.94 | .35 | No linear change in percent daytime sleep before infusions |
| Post-chemo | 3.11 | .003 | Percent daytime sleep increases after infusions |
| Pre-chemo×Pre-chemo | −.74 | .46 | No quadratic change in percent daytime sleep before infusions |
| Post-chemo×post-chemo | −1.48 | .14 | No quadratic change in percent daytime sleep after infusions |
| Infusion number×pre-chemo | 1.26 | .21 | No differences in linear change in pre-chemotherapy percent daytime sleep by infusion number |
| Infusion number×post-chemo | −1.01 | .31 | No differences in linear change in post-chemotherapy percent daytime sleep by infusion number |
| Infusion number×pre-chemo× pre-chemo |
1.19 | .23 | No differences in quadratic change in pre-chemotherapy percent daytime sleep by infusion number |
| Infusion number×post-chemo× post-chemo |
.50 | .62 | No differences in quadratic change in post-chemotherapy percent daytime sleep by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | −.67 | .50 | No difference in daytime activity infusion days |
| Pre-chemo | −.22 | .82 | No linear change in daytime activity before infusions |
| Post-chemo | .50 | .62 | No linear change in daytime activity after infusions |
| Pre-chemo×pre-chemo | −.28 | .78 | No quadratic change in daytime activity before infusions |
| Post-chemo×post-chemo | −2.12 | .04 | Average activity increases then decreases after infusions |
| Infusion number×pre-chemo | −1.17 | .24 | No differences in linear change in pre-chemotherapy daytime activity by infusion number |
| Infusion number×post-chemo | .54 | .59 | No differences in linear change in post-chemotherapy daytime activity by infusion number |
| Infusion number×pre-chemo× pre-chemo |
−1.00 | .32 | No differences in quadratic change in pre-chemotherapy daytime activity by infusion number |
| Infusion number×post-chemo× post-chemo |
−.12 | .90 | No differences in quadratic change in post-chemotherapy daytime activity by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | −.41 | .68 | No difference in nighttime activity on the nights prior to infusions |
| Pre-chemo | −1.57 | .12 | No linear change in nighttime activity before infusions |
| Post-chemo | 3.34 | .001 | Nighttime activity increases after infusions |
| Pre-chemo×pre-chemo | −1.75 | .08 | No quadratic change in nighttime activity before infusions |
| Post-chemo×post-chemo | −3.40 | .001 | Nighttime activity increases then decreases after infusions |
| Infusion number×pre-chemo | .96 | .34 | No differences in linear change in pre-chemotherapy nighttime activity by infusion number |
| Infusion number×post-chemo | −.90 | .37 | No differences in linear change in post-chemotherapy nighttime activity by infusion number |
| Infusion number×pre-chemo× pre-chemo |
1.70 | .09 | No differences in quadratic change in pre-chemotherapy nighttime activity by infusion number |
| Infusion number×post-chemo× post-chemo |
1.12 | .26 | No differences in quadratic change in post-chemotherapy nighttime activity by infusion number |
| Dependent variable: average daily fatigue | |||
| Infusion number | 1.01 | .31 | No difference in dichotomy index across infusion days |
| Pre-chemo | −1.79 | .08 | No linear change in dichotomy index before infusions |
| Post-chemo | 3.52 | <.001 | Ratio of nighttime to daytime activity increases (becomes worse) after infusions |
| Pre-chemo×pre-chemo | −1.53 | .13 | No quadratic change in dichotomy index before infusions |
| Post-chemo×post-chemo | −2.52 | .01 | Ratio of nighttime to daytime activity increases then decreases after infusions |
| Infusion number×pre-chemo | 1.49 | .14 | No differences in linear change in pre-chemotherapy dichotomy index by infusion number |
| Infusion number×post-chemo | −2.22 | .03 | Ratio of nighttime to daytime activity increases most after first infusion |
| Infusion number×pre-chemo× pre-chemo |
1.71 | .09 | No differences in quadratic change in pre-chemotherapy dichotomy index by infusion number |
| Infusion number×post-chemo× post-chemo |
1.85 | .06 | No differences in quadratic change in post-chemotherapy dichotomy index by infusion number |
Next, we examined relationships between baseline clinical predictors and changes in daily symptoms over time. We also examined correlations among daily changes in fatigue, depression, sleep, activity, and sleep/activity patterns averaged across all pre-chemotherapy intervals and across all post-chemotherapy intervals. These analyses were done by conducting the mixed models described above, but saving the random linear effects of time (i.e., slopes) in pre-chemotherapy intervals and post-chemotherapy intervals for each participant. Correlations between clinical predictors and slopes were analyzed, as well as correlations among slopes. Piecewise regressions and correlations were run using SAS 9.2 (Cary, NC).
Results
Sample Descriptives
The sample (N=78) had a mean age of 63 years (SD=11). The majority of participants were Caucasian (91%), non-Hispanic (97%), married (64%), had completed high school (89%), and had an annual household income of $40,000 a year or more (52%). Participants were diagnosed with ovarian (41%), endometrial/uterine (38%), cervical (6%), or other gynecologic cancers (14%). Nineteen percent had stage I disease, 14% stage II, 51% stage III, and 15% stage IV. All patients received platinum-based chemotherapy (91% carboplatin, 5% cisplatin, 4% oxaliplatin) as a single agent (4%) or in combination with paclitaxel (78%), docetaxel (17%), gemcitabine (9%), bevacizumab (4%), and/or topotecan (1%). Patients were a median of 45 days from surgery (range, 13–4,868), and 74% were undergoing first-line chemotherapy (i.e., had not recurred). Nineteen percent of patients were prescribed antidepressants at baseline; 32% were prescribed sleep medication.
Description of Daily Changes in Symptoms
The diary completion rate was high; participants completed 2,466 days of ratings across 3,003 possible days (82%). Prior to conducting piecewise regression analyses, all variables were tested for maximum likelihood assumptions by examining skewness. Non-normal variables were log-transformed prior to piecewise regression analyses, but these transformations did not change results. Consequently, analyses of untransformed data are presented. Daily symptom means are plotted in Fig. 1a–h. Piecewise regressions are shown in Table 1. Significant changes over time were observed in fatigue, depression, and most sleep and activity variables (i.e., statistical significance of one or more of the nine daily effects described above).
Regarding fatigue, a significant main effect of chemotherapy cycle was found, such that fatigue on infusion days was higher during earlier infusions as compared to later infusions. A significant linear effect of time was found such that daily fatigue was higher in post-chemotherapy intervals than pre-chemotherapy intervals. A significant quadratic effect of time was also found such that fatigue tended to significantly increase and then decrease following chemotherapy infusions. There was a significant interaction between chemotherapy cycle and the quadratic effect of time, such that the peak in fatigue was more pronounced after the first chemotherapy infusion. Finally, there was an intraday effect of time such that patients reported more fatigue as the day wore on (t=5.90, p<.001). Regarding depression, a significant main effect of chemotherapy cycle was found, such that depression on infusion days was higher during earlier infusions as compared to later infusions. No other linear or quadratic effects of time on depression were significant, depression was not significantly different between pre-chemotherapy and post-chemotherapy intervals, nor was there significant intraday variation in depression.
Regarding actigraphy variables, time spent in bed demonstrated linear and quadratic decreases before chemotherapy infusions, as well as linear and quadratic increases after chemotherapy infusions. No significant linear or quadratic changes were observed in sleep efficiency. Minutes awake after sleep onset increased significantly in post-chemotherapy intervals, but no other changes were significant. The percentage of time patients spent sleeping during the day increased linearly after chemotherapy infusions. Daytime activity increased, then decreased significantly in post-infusion intervals. Nighttime activity showed linear and quadratic changes after infusions, spiking on the first night, then decreasing on the second night and remaining relatively stable thereafter. The dichotomy index displayed linear and quadratic increases in post-chemotherapy intervals, with increases significantly more pronounced following the first chemotherapy infusion.
Baseline Clinical Predictors of Symptom Change
Time since surgery significantly predicted daytime activity (r =.49, p<.01) and percent of time spent sleeping during the day (r =−.31, p<.01) post-chemotherapy, such that patients who had surgery more recently had less daytime activity and slept more during the day after infusions. Cancer recurrence predicted time in bed at night post-chemotherapy (r =−.26, p <.05) such that patients who had recurred spent less time in bed at night after infusions. Patients who received only a single chemotherapeutic agent (i.e., carboplatin or cisplatin) displayed a lower dichotomy index after chemotherapy (r=−.23, p<.04), or less disrupted sleep/activity patterns. Patients prescribed antidepressants at baseline displayed decreases in depression over time before infusions (r=−.28, p<.01). There were no significant relationships between sleep medication prescription at baseline and changes in symptoms over time (ps>.15). There were also no significant relationships between cancer type (i.e., ovarian vs. other) and changes in symptoms over time (ps>.06).
Correlations Between Concurrent Symptom Changes
Spearman correlations between concurrent symptom changes were calculated separately for pre-chemotherapy intervals (see Table 2) and post-chemotherapy intervals (see Table 3). Across pre-chemotherapy intervals, increases in fatigue were associated with increases in depression, while increases in depression were associated with decreases in daytime activity and increases in sleep during the day. Increases in daytime activity were associated with decreases in daytime sleep but not with nighttime sleep variables. Changes in nighttime sleep variables were also correlated with one another. Across post-chemotherapy intervals, increases in fatigue were associated with increases in depression, decreases in daytime activity, increases in time awake after sleep onset, and increases in sleep/activity disruption. Increases in depression were associated with increases in sleep/activity disruption but not individual measures of sleep and activity. Decreases in daytime activity were associated with increases in daytime sleep, decreases in nighttime activity, increases in sleep efficiency, and increases in sleep/activity disruption. Changes in nighttime sleep variables were also correlated with one another (all ps<.05).
Table 2.
Correlations between concurrent symptom changes during pre-chemotherapy intervals
| Average daily fatigue |
Average daily depression |
Time in bed at night |
Sleep efficiency |
WASO | Percent daytime sleep |
Daytime activity |
Nighttime activity |
Dichotomy index (I/O) |
|
|---|---|---|---|---|---|---|---|---|---|
| Average daily fatigue | 1.00 | ||||||||
| Average daily depression | .44** | 1.00 | |||||||
| Time in bed at night | .03 | .03 | 1.00 | ||||||
| Sleep efficiency | −.05 | −.05 | −.05 | 1.00 | |||||
| WASO | −.05 | .09 | .28** | −.52** | 1.00 | ||||
| Percent daytime sleep | .07 | .29** | .10 | −.09 | .07 | 1.00 | |||
| Daytime activity | −.14 | −.23* | −.07 | .04 | .01 | −.81** | 1.00 | ||
| Nighttime activity | .01 | .11 | .20 | −.48** | .55** | −.06 | .09 | 1.00 | |
| Dichotomy index (I/O) | .06 | .19 | .21 | −.36** | .46** | .22 | −.16 | .73** | 1.00 |
WASO minutes awake after sleep onset
p <.05,
p<.01
Table 3.
Correlations between concurrent symptom change during post-chemotherapy intervals
| Average daily fatigue |
Average daily depression |
Time in bed at night |
Sleep efficiency |
WASO | Percent daytime sleep |
Daytime activity |
Nighttime activity |
Dichotomy index (I/O) |
|
|---|---|---|---|---|---|---|---|---|---|
| Average daily fatigue | 1.00 | ||||||||
| Average daily depression | .54** | 1.00 | |||||||
| Time in bed at night | .10 | .08 | 1.00 | ||||||
| Sleep efficiency | .07 | −.03 | −.04 | 1.00 | |||||
| WASO | .26* | .18 | .41** | −.51** | 1.00 | ||||
| Percent daytime sleep | .25* | .17 | .15 | .11 | .15 | 1.00 | |||
| Daytime activity | −.28** | −.15 | −.20 | −.24* | −.06 | −.70** | 1.00 | ||
| Nighttime activity | .12 | .11 | −.10 | −.38** | .31** | −.29* | .29** | 1.00 | |
| Dichotomy index (I/O) | .30** | .23* | .11 | −.39** | .47** | .50** | −.34** | .47** | 1.00 |
WASO minutes awake after sleep onset
p<.05,
p<.01
Discussion
The current study examined daily variation in fatigue, depression, and objective sleep and activity in the week before and the week after gynecologic cancer patients’ first three chemotherapy infusions. The study provides evidence regarding daily symptom trajectories during chemotherapy, clinical predictors of symptom change, intraday variation in fatigue and depression, and relationships between concurrent symptom changes over time. Data indicate that symptoms display complex associations with one another. These findings have important implications for patient education and future research.
While previous research has investigated changes in fatigue, depression, sleep, and activity assessed periodically in patients undergoing chemotherapy, the present study is one of the first to examine daily and intraday variation in symptoms during this time. Similar to previous research [21–23, 25], the current study documented a roller coaster pattern in which symptoms were highest in the week following each infusion and lowest in the week prior to the next infusion. This pattern was evident for fatigue and disruptions in sleep and activity but not depression. Previous data from our research group [25] suggest that fatigue during chemotherapy becomes progressively worse over time, while other data indicate that peaks in fatigue remain relatively constant [11]. The current study found nonsignificant interactions between chemotherapy cycle and post-chemotherapy symptom change, indicating that post-infusion symptoms peaked at comparable levels across infusions. The exceptions to this pattern were fatigue, depression, and the dichotomy index which indicated that the worst symptoms occurred after the first chemotherapy cycle. The current study also found nonsignificant interactions between chemotherapy cycle and pre-chemotherapy symptom change, indicating that pre-infusion symptom levels were comparable across infusions. Thus, the study did not find evidence for a cumulative effect of chemotherapy on symptoms. Nevertheless, the present study examined only the first three infusions. Perhaps a cumulative effect of chemotherapy on symptoms would have been evident if the study had examined additional infusions.
The current study contributes significantly to previous literature examining the roller coaster pattern by pinpointing when symptom peaks occur after infusions. Nighttime sleep disruption was the first symptom to show a peak, at night 1, likely due to steroids received during the infusion. Sleep disruptions decreased at night 2, typically increased again at night 3, and remained relatively stable thereafter. Daytime sleep increased steadily after infusions, peaking on day 5, while daytime activity steadily decreased after infusions. Fatigue was high on infusion days, decreasing the day after, then increasing again at day 2 and peaking at day 5. These data are consistent with a previous study suggesting that sleep disturbances following chemotherapy infusions peak before fatigue during chemotherapy [23]. The same pattern was evident for depression. In contrast, most symptoms remained relatively stable in the weeks prior to infusions. The exception was duration of rest periods, which declined significantly during pre-chemotherapy intervals. These findings are exciting because they suggest a temporal sequence of symptom peaks, in which sleep disruption peaks first after chemotherapy infusions, followed by fatigue and depression. This temporal sequence is consistent with the view that sleep disruption may contribute to fatigue and depression, rather than vice versa. This temporal sequence is supported by data from a study in posttreatment breast cancer survivors with insomnia which found that sleep disturbance predicted next-day fatigue and depression, but that daytime fatigue and depression did not predict subsequent sleep disturbance [46]. Not surprisingly, in the current study, patients who recently had surgery and who received multiple chemotherapeutic agents showed higher symptom peaks after infusions.
The current study also contributes significantly to previous literature by examining intraday variation in fatigue and depression during chemotherapy. Results indicate that fatigue increases over the course of the day, while depression does not change. Our data are consistent with findings of Badr and colleagues [33] indicating that fatigue increased over the course of the day in ovarian cancer patients during chemotherapy. The intraday patterns observed in the current study did not change across pre-chemotherapy or post-chemotherapy intervals, nor were there cumulative effects of infusion number on within-day changes in fatigue and depression.
The current study was among the first to examine relationships among trajectories of fatigue, depression, and objectively measured sleep and activity. Although it has long been recognized that these symptoms co-occur during chemotherapy [6, 14, 22, 23, 26–28, 33], most studies to date have examined relationships among symptoms at a single time point rather than relationships among symptom change. An exception is a study by Roscoe and colleagues [31], which examined correlations among change in fatigue, depression, and sleep/activity patterns in breast cancer patients at two time points: the second and fourth chemotherapy infusions. The current study examined change in daily symptoms across 36 days of assessment during chemotherapy. As hypothesized, significant relationships were found among symptom trajectories. Similar to the Roscoe study, we found that increases in fatigue were correlated with increases in depression. This relationship was significant in both pre- and post-chemotherapy weeks in the current study. Pre-chemotherapy, the current study also found that increases in depression were significantly associated with decreases in daytime activity and increases in daytime sleep. Post-chemotherapy, the current study found that increases in fatigue were significantly associated with decreases in daytime activity and increases in daytime sleep. Increases in fatigue were also significantly associated with increases in minutes awake after sleep onset and increases in the dichotomy index, an overall indicator of disruptions sleep/ activity patterns. These findings extend prior research by suggesting that not only are fatigue, depression, sleep, and activity correlated during chemotherapy, but that daily changes in these symptoms are also correlated.
Taken together, findings from the current study have important implications for patient education. Gynecologic patients who request information regarding symptoms during chemotherapy could be advised that symptoms are highest in the week after an infusion and fatigue is highest late in the day [33]. Patients can expect disrupted nighttime sleep immediately after an infusion, while fatigue and depression will tend to peak 5 days after infusion [23, 24]. In addition, they can be informed that depression is typically highest at the first chemotherapy infusion and that daytime activity will decline after the first infusion while daytime sleep increases. Patients can also be told to expect recovery to baseline (i.e., pre-chemotherapy) symptom levels prior to the next infusion [22, 25], at least for the first three chemotherapy infusions.
The study is characterized by several strengths, including daily and intraday assessments of symptoms, use of actigraphy to objectively measure sleep and activity, and statistical analyses that model the complexity inherent in longitudinal daily data. In addition, the study focused on symptoms in gynecologic cancer patients, a group that has been studied infrequently despite high levels of fatigue, depression, and disruptions in sleep and activity. Study limitations should also be noted. Symptoms were assessed only during the week before and the week after each infusion and not over the entire chemotherapy cycle. This was done to avoid participant burden. Consequently, however, symptom change in the non-assessment intervals is not known. Subjective sleep quality and physical activity were not assessed on a daily basis in the current study; therefore, their relationships with daily fatigue, depression, and objective sleep and activity are not known. Paper diaries of fatigue and depression were used, as opposed to electronic momentary assessment. Although actigraphs were configured to beep to remind participants to complete diaries on time, we cannot be sure that participants did not backfill diary entries. The current sample was relatively small and homogenous in terms of race and ethnicity. As a result, it is not clear whether results are generalizable to the larger population of gynecologic cancer patients undergoing chemotherapy. It is also not clear whether results generalize to individuals with other types of cancer or who are undergoing other treatments. The current sample displayed heterogeneity in disease characteristics, encompassing patients with various types of gynecologic cancer, patients with new diagnoses or recurrent disease, varying lengths of time since surgery, and patients receiving various chemotherapy agents. While we examined baseline clinical predictors of symptom change, it should be noted that some predictors (e.g., recurrence, receipt of a single chemotherapeutic agent) occurred infrequently in our sample. As a result, caution is required regarding the generalizability of results. Examination of predictors of symptom trajectory during chemotherapy for gynecologic cancer is important and should be the subject of continued research.
Future research should also investigate biological mechanisms underlying symptom change. Several studies have found associations between biological markers and fatigue, depression, sleep, and activity [42, 43, 47]. Neuroendocrine and immune systems are theorized to play an important role in symptoms of cancer and its treatment [48] and thus should be the subject of further scrutiny both in vivo and in vitro. While the current study represents one step towards a better understanding of symptoms during chemotherapy, there is still a great deal more work to do to help cancer patients achieve the best possible quality of life.
Acknowledgments
This study was supported by the National Cancer Institute grant number R03-126775. Dr. Jim is supported in part by the National Cancer Institute grant number K07-138499.
Footnotes
Conflict of Interest Statement The authors have no conflicts of interest to disclose.
The equation used is as follows: y =b1Xinfusion number+b2Xprechemo+b3Xprechemo2+b4Xinfusion number Xprechemo+b5Xinfusion number Xprechemo2+b6Xpostchemo+b7Xpostchemo2+b8Xinfusion number Xpostchemo+b9Xinfusion number Xpostchemo2+c.
Contributor Information
Heather S. L. Jim, Moffitt Cancer Center, 12902 Magnolia Drive MRC-PSY, Tampa, FL 33612, USA
Brent Small, University of South Florida, Tampa, FL, USA
Leigh Anne Faul, Georgetown University, Washington DC, USA
Jamie Franzen, University of South Florida, Tampa, FL, USA.
Sachin Apte, Moffitt Cancer Center, 12902 Magnolia Drive MRC-PSY, Tampa, FL 33612, USA
Paul B. Jacobsen, Moffitt Cancer Center, 12902 Magnolia Drive MRC-PSY, Tampa, FL 33612, USA
References
- 1.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 2.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. European Journal of Cancer. 2002;38:27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 3.Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. Journal of Clinical Oncology. 2005;23:6613–6622. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros M, Oshima CT, Forones NM. Depression and anxiety in colorectal cancer patients. J Gastrointest Cancer. 2010;41:179–184. doi: 10.1007/s12029-010-9132-5. [DOI] [PubMed] [Google Scholar]
- 5.Iconomou G, Iconomou AV, Argyriou AA, et al. Emotional distress in cancer patients at the beginning of chemotherapy and its relation to quality of life. J BUON. 2008;13:217–222. [PubMed] [Google Scholar]
- 6.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27:5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 8.Beesley VL, Price MA, Butow PN, et al. Physical activity in women with ovarian cancer and its association with decreased distress and improved quality of life. Psychooncology. 2011 doi: 10.1002/pon.1834. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Stephenson LE, Bebb DG, Reimer RA, Culos-Reed SN. Physical activity and diet behaviour in colorectal cancer patients receiving chemotherapy: Associations with quality of life. BMC Gastroenterol. 2009;9:60. doi: 10.1186/1471-230X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck SL, Berger AM, Barsevick AM, et al. Sleep quality after initial chemotherapy for breast cancer. Support Care Cancer. 2010;18:679–689. doi: 10.1007/s00520-009-0662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs Forum. 2010;37:E359–369. doi: 10.1188/10.ONF.E359-E369. [DOI] [PubMed] [Google Scholar]
- 12.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18(1):105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 13.Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 14.Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Scholarly Inquiry for Nursing Practice. 2000;14:275–290. discussion 291–278. [PubMed] [Google Scholar]
- 15.Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiology International. 2002;19:313–323. doi: 10.1081/cbi-120002606. [DOI] [PubMed] [Google Scholar]
- 16.Munir F, Yarker J, McDermott H. Employment and the common cancers: Correlates of work ability during or following cancer treatment. Occup Med (Lond) 2009;59:381–389. doi: 10.1093/occmed/kqp088. [DOI] [PubMed] [Google Scholar]
- 17.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 18.Tiedtke C, de Rijk A, Dierckx de Casterle B, Christiaens MR, Donceel P. Experiences and concerns about ‘returning to work’ for women breast cancer survivors: A literature review. Psychooncology. 2010;19:677–683. doi: 10.1002/pon.1633. [DOI] [PubMed] [Google Scholar]
- 19.Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J Pain Symptom Manage. 2007;34:40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Poulson MJ. Not just tired. J Clin Oncol. 2001;19:4180–4181. doi: 10.1200/JCO.2001.19.21.4180. [DOI] [PubMed] [Google Scholar]
- 21.Savard J, Liu L, Natarajan L, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32:1155–1160. doi: 10.1093/sleep/32.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. 2009;18:187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: A pilot study. Oncology Nursing Forum. 2000;27:1443–1448. [PubMed] [Google Scholar]
- 24.de Jong N, Kester AD, Schouten HC, Abu-Saad HH, Courtens AM. Course of fatigue between two cycles of adjuvant chemotherapy in breast cancer patients. Cancer Nurs. 2006;29:467–477. doi: 10.1097/00002820-200611000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen PB, Hann DM, Azzarello LM, et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. Journal of Pain and Symptom Management. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 26.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncology Nursing Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 28.Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: Evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23:127–135. doi: 10.1016/j.soncn.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Stevinson C, Steed H, Faught W, et al. Physical activity in ovarian cancer survivors: Associations with fatigue, sleep, and psychosocial functioning. Int J Gynecol Cancer. 2009;19:73–78. doi: 10.1111/IGC.0b013e31819902ec. [DOI] [PubMed] [Google Scholar]
- 30.Servaes P, Verhagen CA, Bleijenberg G. Relations between fatigue, neuropsychological functioning, and physical activity after treatment for breast carcinoma: Daily self-report and objective behavior. Cancer. 2002;95:2017–2026. doi: 10.1002/cncr.10891. [DOI] [PubMed] [Google Scholar]
- 31.Roscoe JA, Morrow GR, Hickok JT, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Supportive Care in Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 32.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badr H, Basen-Engquist K, Taylor CL Carmack, De Moor C. Mood states associated with transitory physical symptoms among breast and ovarian cancer survivors. J Behav Med. 2006;29:461–475. doi: 10.1007/s10865-006-9052-9. [DOI] [PubMed] [Google Scholar]
- 34.Holzner B, Kemmler G, Meraner V, et al. Fatigue in ovarian carcinoma patients: A neglected issue? Cancer. 2003;97:1564–1572. doi: 10.1002/cncr.11253. [DOI] [PubMed] [Google Scholar]
- 35.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecologic Oncology. 2000;78:302–308. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 36.Fox SW, Lyon D. Symptom clusters and quality of life in survivors of ovarian cancer. Cancer Nurs. 2007;30:354–361. doi: 10.1097/01.NCC.0000290809.61206.ef. [DOI] [PubMed] [Google Scholar]
- 37.Morgan RJ, Alvarez RD, Armstrong DK, et al. [Retrieved Sept. 23, 2009];NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer. from http://www.nccn.org/professionals/physician_gls/PDF/ovarian.pdf.
- 38.Webster JB, Kripke DF, Messin S, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Sleep. 1982;5:389–399. doi: 10.1093/sleep/5.4.389. [DOI] [PubMed] [Google Scholar]
- 39.Patterson SM, Krantz DS, Montgomery LC, et al. Automated physical activity monitoring: Validation and comparison with physiological and self-report measures. Psychophysiology. 1993;30:296–305. doi: 10.1111/j.1469-8986.1993.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto A, Hara Y, Findley TW, Yonemoto K. A useful method for measuring daily physical activity by a three-direction monitor. Scandanavian Journal of Rehabilitation Medicine. 1997;29:37–42. [PubMed] [Google Scholar]
- 41.Berger AM, Wielgus KK, Young-McCaughan S, et al. Methodological challenges when using actigraphy in research. Journal of Pain and Symptom Management. 2008;36:191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clinical Cancer Research. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 43.Mormont MC, Langouet AM, Claustrat B, et al. Marker rhythms of circadian system function: A study of patients with metastatic colorectal cancer and good performance status. Chronobiology International. 2002;19:141–155. doi: 10.1081/cbi-120002593. [DOI] [PubMed] [Google Scholar]
- 44.Wainer H. Piecewise regression: A simplified procedure. British Journal of Mathematical and Statistical Psychology. 1971;24:83–92. doi: 10.1111/j.2044-8317.1971.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 45.McGee VE, Carleton WT. Piecewise regression. Journal of the American Statistical Association. 1970;65:1109–1124. [Google Scholar]
- 46.Rumble ME, Keefe FJ, Edinger JD, et al. Contribution of cancer symptoms, dysfunctional sleep related thoughts, and sleep inhibitory behaviors to the insomnia process in breast cancer survivors: A daily process analysis. Sleep. 2010;33:1501–1509. doi: 10.1093/sleep/33.11.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne J, Piper B, Rabinowitz I, Zimmerman B. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: A pilot study. Oncol Nurs Forum. 2006;33:775–783. doi: 10.1188/06.ONF.775-783. [DOI] [PubMed] [Google Scholar]
- 48.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]