SUMMARY
The incidence of chronic allergic dermatitis is rapidly increasing. Regulatory control of this disease has not been adequately explored. Here we report that mast cell derived interleukin-2 (IL-2) contributes to the suppression of chronic allergic dermatitis. Mice deficient in IL-2 production, or deficient in mast cells (KitW-sh/W-sh), showed exacerbated dermatitis upon repeated oxazolone challenge when compared to their wild-type counterparts. Adoptive transfer of wild type, but not Il2−/−, mast cells into KitW-sh/W-sh mice dampened the inflammatory response. During the course of disease mast cell expansion occurred at the site of inflammation and also in the spleen, where production of IL-2 by mast cells was markedly enhanced. In the absence of mast cell IL-2 production, the ratio of activated to regulatory T cells at the site of inflammation was increased. Thus, MC-derived IL-2 contributes to the maintenance of suppression in chronic allergic skin inflammation.
INTRODUCTION
Eczematous dermatitis is a chronic inflammatory skin disease, associated with cutaneous hyper-reactivity, which usually occurs in individuals with an allergic background (also referred to as atopic dermatitis; AD). One of its most striking features is the rising incidence during the past two decades, currently approaching 15-30% of children at some point during childhood (Bieber, 2008; Schultz Larsen et al., 1996). Both genetic and environmental factors contribute to the development of AD (Bieber, 2008; Leung et al., 2007), however, the underlying mechanisms by which such factors contribute to the pathogenesis of dermatitis are poorly defined.
Animal models have been valuable in studying the mechanisms underlying the development and severity of dermatitis. Current models can be divided into three groups consisting of spontaneous dermatitis, genetically engineered mice, and hapten-induced inflammation (Jin et al., 2009). In this latter category, haptens such as oxazolone (Matsumoto et al., 2004) and dinitrofluorobenzene (DNFB) (Phanuphak et al., 1974) are used to induce skin disease in a convenient and reproducible manner. Oxazolone induces a T-cell dependent allergic contact hypersensitivity with mast cell (MC) involvement (Evans et al., 1971; Pritchard and Micklem, 1972) but in the course of repeated challenges, it evolves into a chronic allergic inflammatory response similar to human AD (Matsumoto et al., 2004). In hairless mice (hr/hr) chronic treatment with oxazolone results in dermatitis that demonstrates barrier abnormalities, a T helper 2 cell (Th2 cell) predominant inflammation, MC and eosinophil rich infilitrates as well as elevated IL-4 and IgE (Man et al., 2008), all hallmarks of human AD. However, the mechanistic roles played by some of these features in the chronicity of disease are unexplored.
MCs can enhance inflammation not only in allergic diseases (Kalesnikoff and Galli, 2008) but also in autoimmune models such as experimental autoimmune encephalomyelitis (Secor et al., 2000). Alternatively, they can act as suppressors as demonstrated in their contribution to skin allograft tolerance (Lu et al., 2006). MCs can also exert stimulatory or inhibitory effects on both effector and regulatory T cells (Tregs) (Galli et al., 2008; Gri et al., 2008; Hershko and Rivera, 2010; Mekori and Metcalfe, 1999). The potential mediators of MC - T cell communication are numerous and include secreted cytokines, chemokines, leukotrienes, histamine as well as varied surface molecules (Bachelet and Levi-Schaffer, 2007; Hershko and Rivera, 2010; Nakae et al., 2006). The putative anatomical sites where the interaction between these two cell types takes place are also varied and may include such tissues as the skin (Lu et al., 2006), draining and remote lymph nodes (LN) (Gri et al., 2008), spleen as well as other sites which harbor these cells.
The expanding prevalence of AD and the considerable gaps in understanding its etiology and the regulation of its severity prompted us to investigate the strategic players that govern disease intensity. Here we report that MCs are protective in late stages of oxazolone-induced chronic allergic dermatitis. Protection occurs by a previously unrecognized regulatory mechanism in which MC-derived IL-2 is required to maintain the appropriate ratio of activated T cells to regulatory T cells (Tregs) at the site of inflammation during the chronic phase of disease.
RESULTS
IL-2 controls the severity of chronic allergic dermatitis
Induction of oxazolone-induced chronic allergic dermatitis in mice recapitulates many of the characteristics of human AD (Leung et al., 2004), which prompted us to use this model as a tool for investigating regulatory processes.
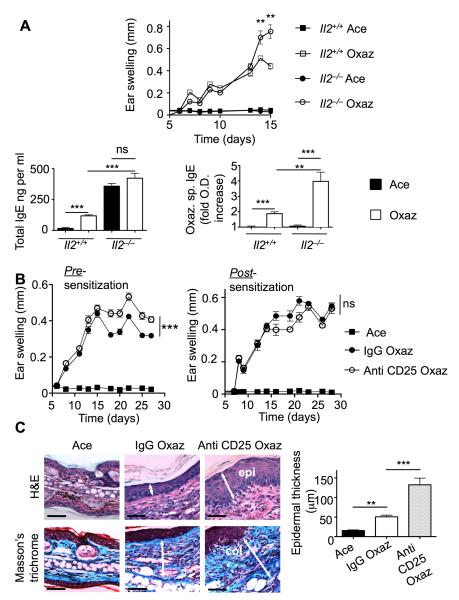
IL-2 is a key cytokine for Treg function (Malek and Bayer, 2004), which has been implicated in the control of allergic disease, particularly asthma (Boudousquie et al., 2009; Curotto de Lafaille et al., 2008; Wilson et al., 2008). Il2−/− mice were used as an initial tool to investigate the regulatory features in control of chronic allergic dermatitis. On a C57BL/6 background, these animals exhibit a 40% reduction in Tregs (Zheng et al., 2007), and have a profound impairment in Treg function (Malek and Bayer, 2004). In contrast to Foxp3sf mice, the Il2−/− mice have a longer life span, do not suffer from spontaneous dermatitis, and are thus a preferable model for hapten-induced disease. When exposed to oxazolone, severe ear swelling was noted in Il2−/− mice (Figure 1A, top panel). Hyper-responsiveness was accompanied by elevated total IgE titers and over-production of oxazolone-specific IgE (Figure 1A, bottom panels). Given the known spontaneous manifestation of inflammation in these genetically altered mice (Sadlack et al., 1995), we confirmed the role of IL-2 through administration of a CD25 monoclonal antibody (Kohm et al., 2006). This antibody binds to the alpha chain of the IL-2 receptor (IL-2R), which is constitutively expressed on Tregs, and causes transient inactivation (Kohm et al., 2006). Injection of anti-CD25 prior to sensitization with oxazolone resulted in enhanced inflammation at a late stage of the disease relative to the control injection of isotype identical IgG. This was manifested as a sustained increase in ear thickness (Figure 1B, left panel), at a range previously reported as physiologically significant (Grimbaldeston et al., 2007), as well as expansion of dermatitis to the adjacent skin (not shown). When antibodies were administered one week after sensitization, only a minimal effect on disease course was observed (Figure 1B, right panel). Examination of tissue sections disclosed that oxazolone-induced epidermal hyperplasia and collagen deposition are considerably enhanced following anti-CD25 administration (Figure 1C). Collectively, these experiments indicate that IL-2 is vital to the control of oxazolone-induced dermatitis.
Figure 1. IL-2 is important in suppression of oxazolone-induced chronic dermatitis.
(A) Ear swelling during induction of disease in 8-10 week old Il2−/− and Il2+/+ mice (n = 4 mice/group) (top). Total IgE levels (bottom left) and oxazolone specific (Oxaz sp) IgE (bottom right) as measured in the serum by ELISA. (B) Disease course in WT C57BL/6 mice that were injected with anti-CD25 pre-sensitization on day −1 (n = 5 mice/group), or post-sensitization on day 7 (n = 4). Data is reported beginning at the day of challenge where detectable ear swelling might be expected. Each experiment was performed twice. (C) Sections of ear tissue (left panel) stained as indicated and quantitation of epidermal thickening (right panel). epi., epidermis; col, collagen. Statistical analysis was by two-way ANOVA (B) or by a two-tailed student’s t test (A and C right panel). Results are means + SEM. **p<0.01; ***p<0.001. See also Figure S1.
MCs suppress oxazolone-induced dermatitis
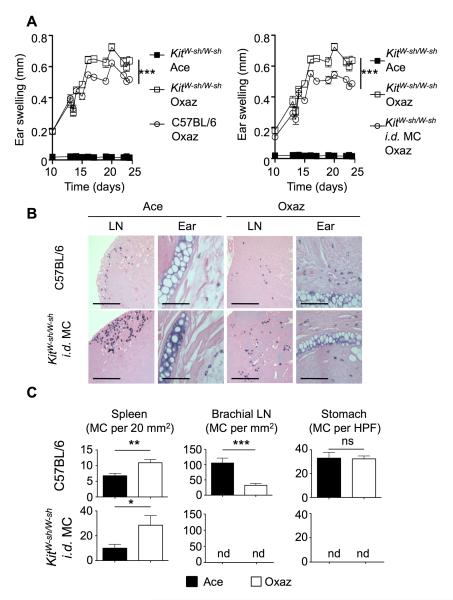
We explored the role of MCs in the oxazolone-induced dermatitis using MC-deficient KitW-sh/W-sh mice (Grimbaldeston et al., 2005). After 5 challenges with oxazolone, ear swelling was markedly increased in KitW-sh/W-sh mice, compared to their WT counterparts (Figure 2A, left panel). Adoptive transfer of WT bone marrow-derived MCs into the ear-skin of KitW-sh/W-sh mice resulted in reduced ear swelling (Figure 2A, right panel), demonstrating that MCs dampened inflammation. Similarly, disease suppression was observed following the administration of IL-2-anti-IL-2 complexes, a manipulation known to increase the bioavailability of IL-2 and to promote Treg expansion (Kamimura et al., 2006; Wilson et al., 2008) (Figure S1 available online). Histological analysis of tissues from KitW-sh/W-sh mice revealed that, following intradermal (i.d.) reconstitution with MCs, engraftment occurs in the skin of the ear as well as in the cervical draining LN and spleen (Figures 2B and 2C). No MCs were found in LNs or spleens of unreconstituted KitW-sh/W-sh mice, however, an occasional MC was found in ear skin, in mice with dermatitis (data not shown). Following reconstitution, the distribution of MCs was similar to that of WT mice, albeit more MCs were found in the LNs of KitW-sh/W-sh relative to WT mice while the latter mice had more MCs in the ear (Figure S2). In both types of mice, induction of dermatitis caused an accumulation of MCs in the (ear) skin and an apparent redistribution of MCs from the subcapsular sinus to the LN cortex (Figure 2B). In reconstituted KitW-sh/W-sh mice, no MCs were found in tissues far from the site of inflammation, like a distal (brachial) LN or in the stomach (Figure 2C). However, MCs were observed in the spleens of unchallenged WT mice and i.d. reconstituted KitW-sh/W-sh mice, and for both, their numbers increased following oxazolone treatment. This accumulation was intriguing due to the fact that the spleen did not show dynamic changes in total cell numbers or even in CD4+ T cells, with the exception of a modest but distinct increase in Tregs (Figure S2). Because MCs were introduced only into the ears of KitW-sh/W-sh mice, this finding argues that MCs migrate from the skin to the draining LN and then to the spleen but not to the brachial LN or the stomach. This view was further supported by i.d. injection of small numbers of GFP-expressing BMMCs in the ear-skin of KitW-sh/W-sh mice as these MCs were found to migrate to the draining LNs and the spleen with concomitant disease suppression (Figure S3).
Figure 2. MCs suppress dermatitis and expand in the spleen.
(A) Comparison of ear swelling between KitW-sh/W-sh (n = 7 mice/group) and C57BL/6 (WT) mice (n = 6) (left) or KitW-sh/W-sh i.d. injected with C57BL/6-derived MCs (n = 5) (right). Graphs shown in (A) are from the same experiments but presented individually in comparison to WT for clarity. Two independent experiments were performed. Statistical analysis was by two-way ANOVA. (B) Toluidine blue-staining for MC in the ear skin and draining cervical lymph node (LN). Tissues were harvested from WT and KitW-sh/W-sh mice i.d. injected with WT MCs following full treatment with oxazolone. Scale bars represent 200 μm. (C) Quantitation of MC numbers in tissues distal to the inflammatory site. All tissues were collected following completion of chronic dermatitis induction. (HPF=high power field, X 200). Statistical significance was determined by a two-tailed student’s t test. Results (A and C) are means + SEM. *p<0.05; **p<0.01; ***p<0.001. See also Figure S2.
Dermatitis is enhanced by the spleen and dampened by splenic MCs
The observation of increased MC recruitment to the spleen (Figure 2C), upon oxazolone challenge of WT or i.d.-reconstituted KitW-sh/W-sh mice, led us to explore the relevance of the spleen and of MCs within it to disease regulation. Mice skewed towards Th1 (C57BL/6) or Th2 (Balb/c) cell responses were assessed for accumulation of MCs in the spleen upon oxazolone challenge. As shown in Figure S4A, C57BL/6 or Balb/c mice developed a similar response to oxazolone challenge, although Balb/c mice had a more rapid onset. Both strains of mice showed marked changes in MC numbers in the ear-skin and the draining LN following oxazolone challenge (Figures S4B and S4C). Although the absolute numbers of spleen resident MCs differed considerably in the two strains (Figure S4D), oxazolone challenge increased their numbers by approximately 3-5 fold in both strains (Figure S4E). This shows that oxazolone-induced dermatitis followed a similar pattern in both C57BL/6 and Balb/c mice and caused an increase of MCs in the spleen of both strains.
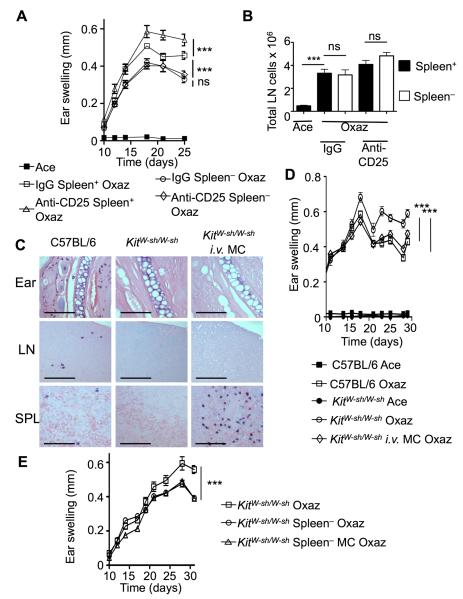
To explore the role of the spleen in the course of disease, wild type (C57BL/6) mice were splenectomized and challenged with oxazolone. These mice developed a mild allergic dermatitis relative to their WT controls (Figure 3A), and were resistant to pre-sensitization injection of anti-CD25 (Figure 3A). These findings demonstrated that the spleen enhances the inflammatory response, and that the increased inflammation that results from blocking the IL-2 receptor also requires the spleen. Conversely, splenectomy did not affect the response of draining LNs (Figure 3B), and oxazolone-specific IgE production (Figure S5A) upon oxazolone challenge and administration of anti-CD25. Likewise, CD4+ T cell and Treg proliferation in the LN were not affected by the absence of a spleen (Figure S5B). To directly address if the suppression mediated by MCs was independent of their localization to the skin, we adoptively transferred MCs into KitW-sh/W-sh mice by intravenous (i.v.) injection. As previously described (Grimbaldeston et al., 2005), the i.v. introduction of MCs failed to reconstitute the ear pinna and these cells were not recruited to the ear after oxazolone challenge (Figure 3C), whereas the spleen of i.v. injected mice showed large numbers of MCs relative to their WT counterparts (Figure 3C). When challenged, KitW-sh/W-sh mice i.v. reconstituted with MCs showed a marked reduction in dermatitis relative to MC-deficient KitW-sh/W-sh mice (Figure 3D), as well as milder epidermal hyperplasia (Figure S6). Furthermore, splenectomized KitW-sh/W-sh mice mounted an attenuated response to oxazolone, with no additional alleviation after MC reconstitution (Figure 3E). In this experiment, however, we introduced MCs i.d. to ensure engraftment, since i.v. injected cells showed a strong predilection for the spleen and its removal altered reproducible engraftment. Regardless, the findings demonstrate that the spleen contributes to the inflammation induced by oxazolone challenge and that the presence of MCs in the spleen is associated with suppression of chronic allergic dermatitis.
Figure 3. The spleen exacerbates dermatitis and facilitates anti-CD25 enhancement or MC suppression of disease.
(A) Splenectomized (Spleen−) C57BL/6 (WT) mice (n = 5) were treated with either control IgG or anti-CD25 and with oxazolone to induce dermatitis. Ear swelling was compared to non-splenectomized (Spleen+) littermates. (B) Draining cervical LNs were harvested for evaluation of total cell numbers. The LNs were extracted from normal and splenectomized mice shown in (A) after full treatment with acetone or oxazolone. (C) MCs were introduced through the tail-vein prior to induction of oxazolone-dermatitis, and tissues from the inflamed ear, draining lymph nodes (LN) and spleens (SPL) were stained with toluidine blue. Scale bars represent 100 μm. (D) Disease course in WT, KitW-sh/W-sh or KitW-sh/W-sh following i.v. injection of MCs (n = 5). Two independent experiments were conducted. (E) Comparison of ear swelling between KitW-sh/W-sh mice and KitW-sh/W-sh following splenectomy or splenectomy and i.d. MC reconstitution (n = 5). Statistical analysis was done by a two-way ANOVA test (A, B, D and E) or by a two-tailed student’s t test (B lower panels). Results are means + SEM. ***p<0.001. See also Figures S3 and S5.
IL-2R blockade enhances IgE-dependent accumulation of splenic MCs
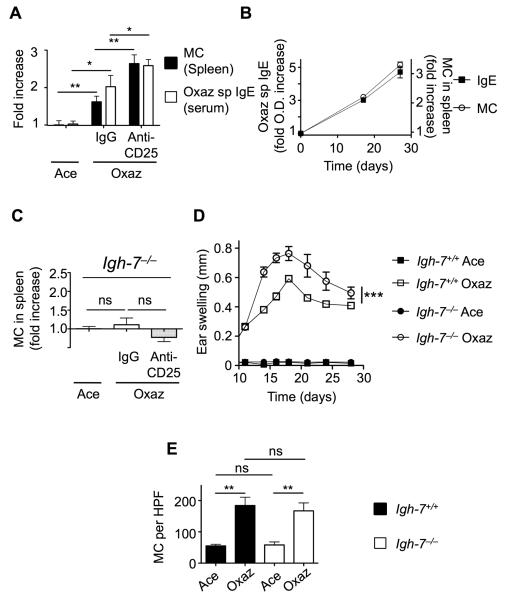
Having found that pre-sensitization treatment with anti-CD25 inhibits regulation of disease (Figure 1B), we examined the impact of this manipulation on the expansion of MCs in the spleen. Consistent with our previous result (Figure 2C), MC numbers in the spleen increased with oxazolone treatment and were further elevated when IL-2R was inactivated by anti-CD25 treatment (Figure 4A). Oxazolone-specific IgE also increased in a similar manner. Analysis of oxazolone-specific IgE in the spleen prior to, midcourse, and during the full course of disease revealed a time-dependent increase that mirrored the increase of MCs in the spleen (Figure 4B). To assess if IgE was important for the increased numbers of MCs in the spleen, we challenged the IgE-deficient (Igh-7−/− ) mice with oxazolone in the presence of active or inactive (anti-CD25-treated) IL-2R. As shown in Figure 4C, accumulation of MCs in the spleen was absent in challenged Igh-7−/− mice and the administration of anti-CD25 did not substantially change MC numbers demonstrating that IgE production was required for MC accumulation in the spleen. Moreover, oxazolone challenge of Igh-7−/− mice resulted in more severe eczematous dermatitis than seen in Igh-7+/+ mice (Figure 4D). However, unlike the spleen, MC accumulation in the ear was equal in both Igh-7+/+ and Igh-7−/− (Figure 4E) suggesting that MC numbers in the ears are not associated with disease suppression. Collectively, the findings show that the IgE-dependent recruitment of MCs to the spleen is associated with disease suppression.
Figure 4. MC recruitment and disease suppression is IgE dependent.
(A) Comparison between MC recruitment to the spleen (n = 6) and oxazolone specific (Oxaz sp) IgE levels in the serum (n = 4) of C57BL/6 mice treated with oxazolone. Dermatitis was induced following pre-sensitization treatment of mice with either control IgG or anti-CD25. (B) Kinetics of IgE and MC accumulation in the spleens of Balb/c mice, as measured on days 0, 17 and 27 of oxazolone dermatitis (n = 4). (C) MC numbers in the spleens of control (acetone) or oxazolone-challenged IgE deficient (Igh-7−/−) in the absence (IgG only) or in the presence of anti-CD25 (n = 5). (D) Disease course in Igh-7−/− mice, as compared to C57BL/6 wild type controls (Igh-7 +/+) (n = 5). (E) Quantitation of MCs in the ears of the mice in (D). Three independent experiments were performed. Statistical analysis was by a two-tailed student’s t test (A, C and E) or by two-way ANOVA (D). Results are means + SEM. *p<0.05; **p<0.01; ***p<0.001. See also Figure S4.
Chronic oxazolone-challenged KitW-sh/W-sh mice are hypersensitive to IL-2R blockade
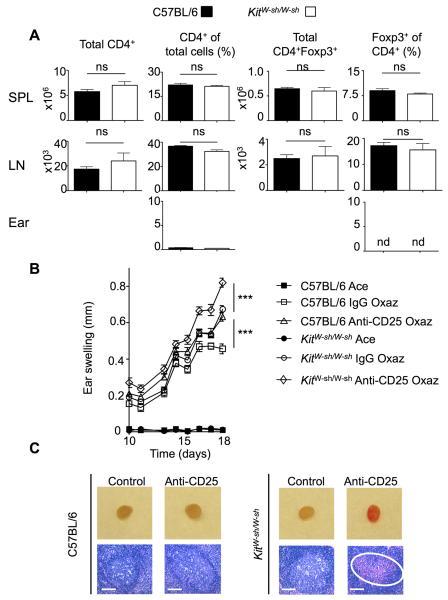
Given the importance of IL-2 in regulating the severity of the dermatitis, we investigated if IL-2 function might be altered in KitW-sh/W-sh mice. Tregs are highly dependent on IL-2 and analysis of cells from the spleen, LN and ear of unchallenged KitW-sh/W-sh mice revealed no significant difference in the total CD4+ cells or in the proportion of CD4+Foxp3+ Tregs when compared to unchallenged WT control mice (Figure 5A). Few CD4+ cells were found in the ears of unchallenged control and KitW-sh/W-sh mice and Tregs were not detectable in the ear (Figure 5A). This observation indicated that Treg homeostasis in naïve animals is independent of MCs implying no defect in IL-2 homeostasis. However, this did not rule out the possibility that under conditions of repeated oxazolone challenges MCs are required to maintain adequate IL-2 production. In order to assess the extent of IL-2 function in the absence of MCs, KitW-sh/W-sh and control WT mice were injected with anti-CD25 and subsequently challenged with oxazolone. As shown in Figure 5B, WT mice treated with anti-CD25 showed a strikingly similar profile of disease intensity to MC-deficient KitW-sh/W-sh mice. However, when KitW-sh/W-sh mice were treated with anti-CD25 and were subsequently challenged with oxazolone an exaggerated disease was observed when compared to the KitW-sh/W-sh mice injected with control IgG or control WT mice injected with anti-CD25. Occasionally, the cervical draining LNs in this group of mice became hemorrhagic and necrotic (Figure 5C) reflecting the physiological severity of the inflammatory response. Thus, although MCs do not appear to be required for Treg homeostasis in naïve animals, at the chronic stage of dermatitis, MCs appear to support IL-2-mediated Treg responses. To assess this possibility, we quantitated the numbers of Foxp3+ cells in the ear skin of oxazolone challenged KitW-sh/W-sh mice or MC-reconstituted KitW-sh/W-sh mice. Whereas no Foxp3+ cells were observed in the ear skin of unchallenged KitW-sh/W-sh mice (Figure 5A), oxazolone challenge led to recruitment of Foxp3+ cells in the ear skin and reconstitution of KitW-sh/W-sh mice with MCs increased the numbers of Tregs at this site (Figure S7A). These findings argue that MCs contribute to Treg-mediated suppression of chronic allergic dermatitis at a late stage of disease.
Figure 5. MCs cooperate with Tregs in disease suppression.
(A) Flow cytometric analysis of total cell populations extracted from tissues and stained for CD4 and Foxp3 (SPL=spleen, LN=cervical lymph node). Statistical analysis was by a two-tailed student’s t test. ns, not significant; nd – not detectable. (B) Ear swelling in C57BL/6 and KitW-sh/W-sh treated with oxazolone following pre-sensitization injection with either control IgG or anti-CD25 (n = 5). Data is presented as mean + SEM; statistical significance was determined by a two-way ANOVA, ***p<0.01. (C) Representative draining lymph nodes from mice with oxazolone dermatitis and corresponding H&E stained sections. The circle indicates hemorrhage within the lymph node. Scale bars represent 50 μm. See also Figures S5 and S7.
MC IL-2 production supports Treg-mediated disease suppression
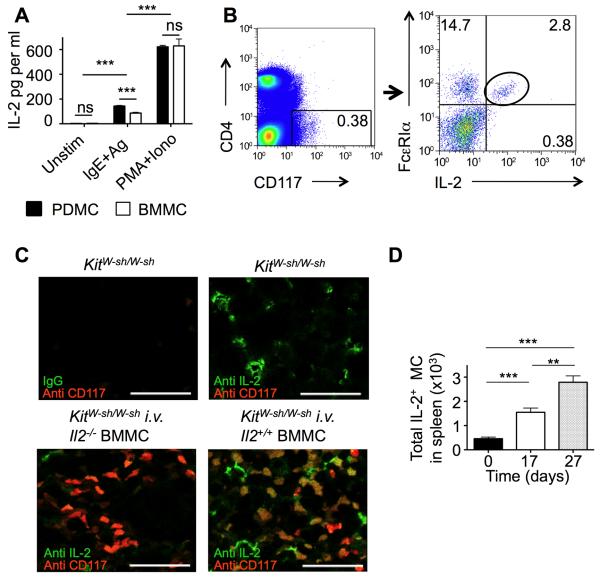
Given that MCs were necessary to promote disease suppression and caused increased Treg numbers at the site of inflammation, we examined the hypothesis that MCs could mediate suppression by providing IL-2 to control the balance between Tregs and activated T cells in the context of oxazolone-induced chronic dermatitis. FcεRI stimulation of MCs induced significant levels (50-100 pg/ml) of IL-2 secretion in both BMMCs and peritoneal-derived cultured MCs (PDMCs) (Figure 6A). PMA + ionomycin stimulation was also a potent inducer of MC IL-2 secretion demonstrating that MCs have the capacity to produce copious amounts of IL-2. To confirm these findings in vivo, we analyzed expression of IL-2 in splenic MCs from mice with dermatitis by flow cytometry (Figure 6B). Importantly, immunofluorescent staining (Figure 6C) of spleen sections from i.v. reconstituted KitW-sh/W-sh mice, using either Il2+/+ or Il2−/− MCs, formally demonstrated the in situ production of IL-2 by MCs in the spleen (Figure 6C). Further quantitative analysis revealed that the total numbers of splenic MCs producing IL-2 was markedly increased during the course of disease (Figure 6D). In contrast, the numbers of splenic basophils (CD117−FcεRI+CD49b+) decreased upon oxazolone challenge (data not shown), ruling out these cells as a confounding factor in the accumulation of MCs in the spleen.
Figure 6. Oxazolone dermatitis increases MC-IL-2 production in vivo.
(A) Quantitation of IL-2 production by in vitro stimulation of peritoneal- and bone-marrow-derived MC (PDMC and BMMC, respectively) after FcεRI or PMA + ionomycin stimulation. IL-2 was measured by ELISA. (B) Flow cytometric detection of IL-2+ MCs in the spleen of Balb/c mice with oxazolone dermatitis. This strain was preferred for cytometry over C57BL/6 due to the higher numbers of splenic MCs. (C) Immunofluorescent detection of IL-2 production in KitW-sh/W-sh mice reconstituted (i.v.) with BMMCs from Il2−/− and Il2+/+ mice. Double staining was done with anti-IL-2 (green), and anti-CD117 (Kit) (red), as indicated. Bars represent 30 μm. (D) Total numbers of IL-2+ MCs was determined in spleens harvested from Balb/c mice on days 0, 17 and 27 of oxazolone-induced dermatitis (n =4 Data are means + SEM and significance was determined by a two-tailed student’s t test (A) or by two-way ANOVA (D); **p<0.01; ***p<0.001. See also Figure S6.
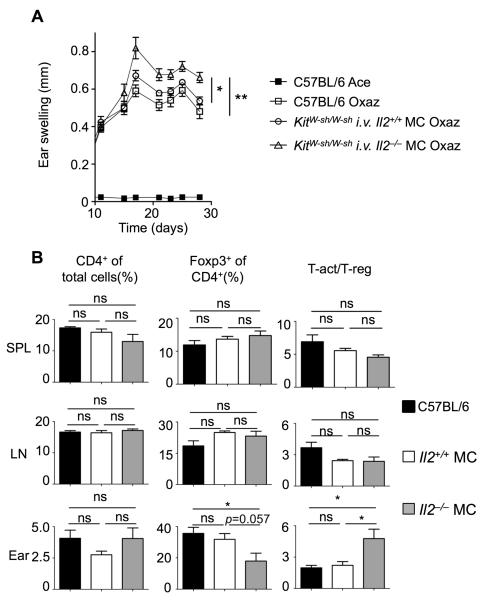
To directly assess the role of splenic MC IL-2 production in controlling the severity of disease KitW-sh/W-sh mice, reconstituted i.v. with MCs from Il2−/− or from WT littermates, were challenged with oxazolone. In contrast to the mice carrying WT MCs, the loss of IL-2 production by MCs resulted in an increased severity of disease (Figure 7A). We hypothesized that this may be due to a compromised balance of activated T cells to Tregs relative to the general CD4+ response. Analysis of cells from tissues of mice with dermatitis disclosed no difference in CD4+, CD4+ Foxp3+, and the activated- to regulatory-T cell ratio (Tact:Treg), in the spleen and lymph node, for WT mice or KitW-sh/W-sh mice carrying IL-2 producing or deficient MCs (Figure 7B). In mice with Il2−/− MCs there was also no difference in the proportion of CD4+ T cells in the ear. Nonetheless, a decrease in the percent of CD4+Foxp3+ cells relative to total CD4+ cells was observed with a significant (p<0.05) increase in the ratio between activated (CD4+CD5+CD69+) T cells and Tregs (Figure 7B). Taken together, these data indicate that, in the absence of MC IL-2, Treg expansion is sluggish compared to T cell activation and CD4+ T cell proliferation, thus creating an imbalance between the regulatory and effector responses. Histological analysis confirmed comparable engraftment in the spleens of the recipients of Il2+/+ and Il2−/− MCs (Figure S7B). These findings demonstrate a previously unappreciated role for MCs, and their IL-2 producing ability, in controlling the appropriate ratio of activated T cells:Tregs at the site of inflammation during chronic allergic dermatitis.
Figure 7. Splenic MC IL-2 production controls the ratio of activated T cells:Tregs and is needed for late stage suppression of dermatitis.
(A) Ear swelling in C57BL/6 mice or KitW-sh/W-sh mice reconstituted with Il2+/+ or Il2−/− MCs via an i.v. injection (n = 5). (B) FACS analysis of total cell populations from tissues of KitW-sh/W-sh mice following i.v. injection with either Il2+/+ or Il2–/− MCs and induction of oxazolone dermatitis (day 30); Activated T cells (T-act.)=CD4+CD5+CD69+, regulatory T cells (Treg)= CD4+Foxp3+. Data are means + SEM and significance was determined by two-way ANOVA (A) and a two-tailed student’s t test (B) ; *p<0.05; **p<0.01. See also Figure S7.
Discussion
There is increasing evidence of the importance of MC - T cell crosstalk in regulating the immune response. MC effector activity is suppressed by Tregs (Gri et al., 2008) and Tregs can also use MCs as mediators of immune suppression (Lu et al., 2006). MCs have also been implicated in promoting CD4+ and CD8+ T cell expansion (Piconese et al., 2009; Stelekati et al., 2009). Herein, we describe a regulatory mechanism in which secretion of IL-2 by MCs suppresses chronic allergic dermatitis by enhancing the proportion of Tregs at the site of inflammation and decreasing the fraction of activated T cells at this site.
It has been reported that MCs can either promote (Biedermann et al., 2000; Bryce et al., 2005; Dudeck et al., 2011; Suto et al., 2006) or dampen contact hypersensitivity (Depinay et al., 2006; Grimbaldeston et al., 2007; Hart et al., 1998), the latter being mediated primarily through MC production of IL-10. Dudeck and coworkers recently reported (Dudeck et al., 2011) that MCs are key promoters of contact hypersensitivity and not suppressors of this response. They induced contact hypersensitivity in a manner similar to that reported in the Grimbaldeston study (Grimbaldeston et al., 2007), using a mouse model (Mcpt5-Cre+iDTR+) in which connective tissue type MCs are selectively depleted. Their findings showed that the absence of connective tissue type MCs impaired migration of DCs to the lymph nodes and caused a marked reduction in contact hypersensitivity. They proposed that previous studies in mast cell deficient mice (KitW-sh/W-sh or KitW/W-v) showing MC protective effects were flawed due to potential immunological abnormalities in these mice. While it is clear that KitW-sh/W-sh or KitW/W-v mice have various abnormalities (Grimbaldeston et al., 2005; Wolters et al., 2005), such overt dismissal for the role of MCs as suppressors should be cautioned by the fact that the particular disease model, mouse strain or genetics, route of sensitization and challenge, and strength of stimulus can all influence the immune response. In agreement with the Dudeck study, we (data not shown) and others (Norman et al., 2008; Suto et al., 2006) have seen that MCs can promote acute contact hypersensitivity in KitW-sh/W-sh or KitW/W-v mice in models where strength of the stimulus may be modest. Interestingly, Norman and colleagues (Norman et al., 2008) showed that induction of contact hypersensitivity by a weak versus strong stimulus was a determinant of whether MCs induce or suppress disease, respectively. In our study, we utilize a model that differs from that used by Grimabaldeston and Dudeck in that repeated challenges of oxazolone on the ear were done for a period of approximately 28 days. This chronic model shows many of the characteristics of human AD (Leung et al., 2004) including the presence of both Th1 and Th2 cell cytokines. Our finding of MC-mediated suppression is consistent with the results of Norman et al. (Norman et al., 2008) where a strong stimulus induced both Th1 and Th2 cell cytokines and resulted in a protective role for MCs. In addition, our findings also show that MCs can act to cause suppression from tissues (spleen) distal to the site of inflammation. Thus one cannot exclude that the presence of mucosal MCs, which are seemingly not depleted in the diptheria toxin-induced Mcpt5-Cre+iDTR+ mice, could also influence observed responses.
Our deduction that the spleen represents a site involved in MC regulatory function is based on several findings. Splenectomized mice had a diminished inflammatory response and anti-CD25 treatment of these mice failed to exacerbate disease. This demonstrated the contribution of the spleen in oxazolone-induced inflammation and showed that the effect of anti-CD25 was dependent on the spleen. The effect of anti-CD25 treatment was independent of MCs since oxazolone-induced dermatitis was markedly exacerbated in the MC-deficient KitW-sh/W-sh mice. Intravenous MC reconstitution of KitW-sh/W-sh mice, which causes engraftment of the spleen, restored regulation of disease despite no engraftment of MCs in ear pinna. It is important to note that i.v. reconstitution of KitW-sh/W-sh mice with MCs engrafts several other sites (Grimbaldeston et al., 2005; Wolters et al., 2005), including LNs (a key site of MC - T cell interactions (Gri et al., 2008; Piconese et al., 2009)), and thus these sites may be contributory. Migration of MCs to the spleen was dependent on IgE accumulation in the spleen, and mirrored both the increased production of IL-2 by MCs in the spleen and suppression of disease. The precise mechanism underlying the increase of MCs in the spleen has yet to be elucidated. It does not appear to involve FcεRI, as mice deficient in FcεRIβ (and thus unable to express FcεRI) showed increased numbers of MCs in the spleen following oxaxzolone challenge (data not shown). However, antibody blockade of CD23 inhibited the increase of splenic MCs (data not shown), suggesting the possible involvement of this low affinity IgE receptor. IgE engagement of CD23 on monocytes (which infiltrate the spleen upon oxazolone challenge (Sugiura et al., 2010)) has been shown to induce secretion of CCL3 (Ezeamuzie et al., 2009), a potent chemoattractant for MCs. Collectively, these findings demonstrate a connection between the spleen and the skin in modulating chronic allergic dermatitis, and the putative trafficking of Tregs between the two sites warrants further investigation. It should be noted that the spleen has also been shown to be essential for the induction of antigen-specific allergic diarrhea due to mobilization of primed splenic CD4+ T cells to the large intestine (Kurashima et al., 2007). Moreover, migration of MCs to the spleen is a well described phenomena, as acute models of DNFB (Wang et al., 1998) and peptidoglycan (Matsui and Nishikawa, 2006) dermatitis, and a model of helminth infection (Friend et al., 2000) showed increased numbers of MCs in the spleen. MC-mediated regulation of disease severity at a location distal to the site of inflammation was also shown in a mouse model of experimental autoimmune encephalomyelitis, where MCs exacerbated disease following adoptive transfer without repopulating the inflammatory site, i.e. the central nervous system (Tanzola et al., 2003). Thus, we propose that MC migration to the spleen may be important beyond allergic skin disease.
Localization of Treg - MC interactions to a site such as the spleen may provide an explanation to the observation that exogenous administration of IL-2 in post-sensitized mice did not modify disease course, whereas MC-derived IL-2 alleviates chronic disease. We hypothesize that a systemic bolus of IL-2, while disease is ongoing, may stimulate both regulatory and effector T cells that express the IL-2R thus failing to alter the course or severity of disease. Conversely, a systemic bolus prior to disease would target those cells constitutively expressing the IL-2R, which are primarily Tregs (Malek and Bayer, 2004), and cause amplification of their activity thus suppressing the disease. The production of IL-2 by MCs at a distal lymphoid organ may serve a similar role in compartmentalizing the effect of IL-2 during the disease course. Our data do not define the numbers of MCs required in order to maintain normal regulation in dermatitis. In fact, comparable degrees of regulation were obtained in C57BL/6 as in KitW-sh/W-sh mice following i.d. or i.v. injection of the latter with MCs. Although in C57BL/6 mice MC numbers in the spleen (per 20mm2) were in the range of 5-10, i.d. reconstituted KitW-sh/W-sh showed four to five fold greater numbers and for i.v. reconstituted KitW-sh/W-sh several hundreds (Grimbaldeston et al., 2005)(and data not shown). Thus, the absolute numbers of MCs migrating to the spleen does not appear to affect the onset or extent of the suppressive regulatory effect on disease. We also theorize that in the early phase of dermatitis (or throughout disease course in the case of KitW-sh/W-sh mice) IL-2 is provided by cells other than MCs. This is supported by the finding of a small population of splenic CD4+ T cells (approximately 0.8%) that stain positive for IL-2 in naïve WT mice (data not shown). However, unlike MCs, this population of T cells did not expand during disease course. In addition, we detected IL-2 producing cells in the spleens of non MC-reconstituted KitW-sh/W-sh mice with dermatitis. We cannot formally exclude that some of the observed responses in KitW-sh/W-sh mice might result from other described abnormalities in these mice beyond the MC compartment (Wolters et al., 2005). Nonetheless, one must conclude from the MC reconstitution of KitW-sh/W-sh mice that IL-2 expression in MCs is vital for late stage disease suppression, but it is not the rate-limiting factor in initiating regulatory control.
Whereas our results indicate that Tregs and MCs exert comparable effects in suppressing the response to repeated oxazolone challenge, the overall impact on immune homeostasis differs greatly. This is exemplified by comparing the severe phenotype resulting from Treg deficiency in Foxp3sf mice (Brunkow et al., 2001) and the mild phenotype associated with the absence of MCs in KitW-sh/W-sh mice (Grimbaldeston et al., 2005). Whereas Foxp3sf mice have a life span of 16-25 days, KitW-sh/W-sh have a nearly normal life span. Foxp3sf mice can develop a spontaneous and severe eczematous skin disease (which made them unsuitable for our studies) whereas KitW-sh/W-sh mice may show mild skin lesions in their late life. In fact, the data argue that Tregs do not require MCs to suppress disease at early stages (prior to the fourth or fifth challenge) rather their role appears to be in providing help to Tregs once disease has reached almost maximal severity. This view is supported by the findings that inactivation of Tregs increases the numbers of splenic MCs in the spleen and that consequent IL-2 production supports Treg numbers at the site of inflammation.
In summary, we propose that MC - Treg cell communication during the course of oxazolone-induced chronic allergic dermatitis is vital to disease regulation. Our data argues that the spleen plays a role in the inflammatory process and serves as a site where MCs are recruited as second line regulators in an IgE-dependent manner. By producing IL-2, MCs serve to maintain the Treg:Teff cell ratio at the site of inflammation, which is manifested by control of disease severity. Thus, the findings show that MCs play an overall protective role in late stages of chronic allergic dermatitis and suggest that failure of MC function in this disease leads to increased severity.
Experimental Procedures
Mice
For the majority of experiments, littermate and aged matched (8-16 week or as indicated) WT mice of C57BL/6 background were used along KitW-sh/W-sh, Il2−/−, and Igh-7−/− mice. WT Balb/c mice were used as indicated. All mice were purchased from Taconic Farms and the Jackson Laboratories. Splenectomized C57BL/6 mice and normal controls were purchased from the Jackson Laboratories. Mice were maintained and used in accordance with National Institutes of Health (NIH) guidelines and National Institute of Arthritis and Musculoskeletal and Skin Diseases-approved animal study proposal A007-03-01.
Oxazolone induced dermatitis
Mice were sensitized on day 0 by application of 15 μl 1% oxazolone (Aldrich) in acetone on both aspects of both ears (total of 60 μl per challenge per mouse). From day 7, mice were challenged in the same way with 0.5% oxazolone, three times per week for up to a total of ten challenges. Under conditions where disease was considerably exaggerated the protocol was shortened. In control mice, treatment was performed with acetone only. Ear thickness was measured and recorded daily using a dial thickness gauge (Mitutoyo).
In vivo administration of cytokines and antibodies
Anti-mouse CD25 (clone B6.1), anti-mouse IL-2 and rat IgG1 were purchased from BD Bioscience and IL-2 from PeproTec, Inc. Anti-CD25 or IgG1 isotype control were injected intraperitoneally (i.p.) at doses of 300 μg per injection, either on day −1 (pre-sensitization) or day 6 (post-sensitization). Immune complexes were prepared by incubating 1 mg anti-IL-2 with 2 μg IL-2 at 4 0C, overnight with rotation. This solution was given i.p. in two separate doses either on days −2 and −1 (pre-sensitization) or on days 6 and 7 (post-sensitization).
Histological staining
Tissue samples were collected at the end of each experiment and placed in formalin solution, 10% neutral buffered (Sigma-Aldrich). Embedding, sectioning, H&E and toluidine blue staining were done by American Histolabs. Immunohistochemical analysis for IL-2/CD117(Kit) was done on formalin-fixed paraffin-embedded tissues. Antigen retrieval was performed by a microwave oven in either Tris-EDTA buffer (pH 9) or citrate-based antigen unmasking solution (Vector Laboratories). Binding of an anti-Kit (Abcam) was detected with a Alexa Fluor 568 conjugated secondary antibody. Detection of IL-2 was with Alexa Fluor 488-conjugated IL-2 antibody (Santa Cruz, 5ug/mL) or for control a similarly conjugated normal IgG. Stained sections were mounted using an anti-fade medium (Vector Laboratories) and analyzed by confocal laser scanning microscopy (LSM 510 Meta, Carl Zeiss, Germany).
Cytokine and IgE titers
Tissues were homogenized in 700 μl PBS supplemented with complete protease inhibitor cocktail tablets, 1 tablet/50 ml PBS (Roche Diagnostics). Following 10 min centrifugation at 14,000 x g (4 0C) the supernatant was collected and kept at −80 0C. Total protein concentration was measured with Bio-Rad DC Protein Assay (Bio-Rad Laboartories) and cytokines titers were detected in 1:5 diluted homogenate samples using ExcelArray Mouse Inflammation Array I (Pierce). Total IgE titers in tissue homogenates and in sera were determined using mouse IgE ELISA quantitation kit (Bethyl Laboratories, Inc). Oxazolone-specific IgE was measured in a semi-quantitative ELISA. For coating, 2% oxazolone was prepared in ethanol, and added to 2% bovine serum albumin (BSA)(MP Biomedicals) at a ratio of 3:50. The oxazolone/BSA mixture was diluted 1:10 in carbonate buffer and used for coating overnight at 4 0C. Blocking was performed with 10% fetal bovine serum (FBS) (Gibco) in PBS for 3 h at room temperature. Sera was diluted 1:10 in buffer prior to use. Buffers, plates, antibodies and HRP substrate were from Bethyl Laboratories, Inc. For in vitro IL-2 detection, MCs were suspended at 5 × 106 cells per ml. Activation was induced with anti-dintrophenyl specific IgE and DNP-HSA (Ag) or with 40 nM PMA (phorbol 12-myristate 13-acetate; Sigma) and 400 nM ionomycin (Calbiochem) for 6 hours at 37°C. The supernatant was analyzed with Mouse IL-2 ELISA (eBioscience).
Generation of MCs and reconstitution of mice
Bone marrow-derived cultured MCs were generated as previously described (Gonzalez-Espinosa et al., 2003). Differentiation of MCs was confirmed by FACS analysis using c-Kit and FcεRI as markers. Cells were used when cultures were more than 95% Kit+FcεRI+. MCs were injected at a concentration of 2× 106 or 107 cells (as indicated) in 200 μl RPMI either to the ear skin or into the tail vein of 6-8 week old male KitW-sh/W-sh recipients. Engraftment was allowed for a minimum of 6 or 10 weeks depending on the method of reconstitution (i.d. or i.v., respectively). Subsequently, mice were subjected to oxazolone sensitization and challenge, after which reconstitution in all mice was confirmed by toluidine blue staining of tissue sections.
Flow cytometric analysis
For determination of cells in the skin, ears were removed from mice and split into dorsal and ventral halves. The skin was placed in 2 ml digestion buffer containing 0.1 mg/ml DNase I and 0.2 mg/ml Liberase TL (Roche Diagnostics) diluted in 1% FBS in RPMI (Gibco). Following a 1 h incubation at 37 0C, ears were placed into a 70 μm cell srainer (BD Biosciences), and mashed through the mesh. The strainers were washed with 2 ml of 1% FBS, 2 mM EDTA in PBS (Gibco). Detection of cell surface markers and intracellular staining were performed as previously described (Charles et al., 2009). Flow cytometry acquisitions were done with a FACSCalibur (BD Biosciences) and Cell Quest software. Analysis was with FlowJo software (Treestar, Inc.). For IL-2 intracellular staining, cells were fixed with 3.7% paraformaldehyde solution in PBS, permeabilized in PBS, 0.1% BSA, 0.1% saponin, and stained in the same buffer with fluorescently-tagged IL-2 antibody. Foxp3 expression in CD4+ T cells was determined by Foxp3 staining (eBioscience).
Statistical Analysis
For comparisons between two populations, two-tailed Student’s t-tests were performed. Disease course was analyzed using a two-way ANOVA test. p values less than 0.05 were considered statistically significant and the following designation used: *p<0.05; **p<0.01; ***p<0.001. Statistical analysis was performed with Prism software (GraphPad Software, Inc.).
Supplementary Material
Highlights.
Mast cells are suppressive in the late stages of chronic allergic dermatitis.
Mast cell derived IL-2 is required for this suppression.
Mast cell IL-2 controls the ratio of activated to regulatory T cells at the site of inflammation.
Acknowledgements
This work was supported by the intramural research program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health. A.Y.H. was also supported by the American Physician Fellowship and by the Legacy Heritage Fund – Israel Science Foundation. We also thank the Laboratory Animal Care and Use Section, the Flow Cytometry Unit, and the Light Imaging Section of NIAMS for support of this work. The authors have no conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachelet I, Levi-Schaffer F. Mast cells as effector cells: a co-stimulating question. Trends Immunol. 2007;28:360–365. doi: 10.1016/j.it.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. N. Engl. J. Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hultner L, Rocken M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudousquie C, Pellaton C, Barbier N, Spertini F. CD4+CD25+ T cell depletion impairs tolerance induction in a murine model of asthma. Clin. Exp. Allergy. 2009;39:1415–1426. doi: 10.1111/j.1365-2222.2009.03314.x. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE on mast cells. Novartis Found. Symp. 2005;271:15–24. discussion 24-38, 95-19. [PubMed] [Google Scholar]
- Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O’Shea JJ, Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, Peschke K, Vohringer D, Waskow C, Krieg T, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Evans DP, Hossack M, Thomson DS. Inhibition of contact sensitivity in the mouse by topical application of corticosteroids. Br. J. Pharmacol. 1971;43:403–408. [PMC free article] [PubMed] [Google Scholar]
- Ezeamuzie CI, Al-Attiyah R, Shihab PK, Al-Radwan R. Low-affinity IgE receptor (FcepsilonRII)-mediated activation of human monocytes by both monomeric IgE and IgE/anti-IgE immune complex. Int. Immunopharmacol. 2009;9:1110–1114. doi: 10.1016/j.intimp.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Friend DS, Gurish MF, Austen KF, Hunt J, Stevens RL. Senescent jejunal mast cells and eosinophils in the mouse preferentially translocate to the spleen and draining lymph node, respectively, during the recovery phase of helminth infection. J. Immunol. 2000;165:344–352. doi: 10.4049/jimmunol.165.1.344. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Espinosa C, Odom S, Olivera A, Hobson JP, Martinez ME, Oliveira-Dos-Santos A, Barra L, Spiegel S, Penninger JM, Rivera J. Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J. Exp. Med. 2003;197:1453–1465. doi: 10.1084/jem.20021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko AY, Rivera J. Mast cell and T cell communication; amplification and control of adaptive immunity. Immunol. Lett. 2010;128:98–104. doi: 10.1016/j.imlet.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J. Invest. Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat. Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- Kurashima Y, Kunisawa J, Higuchi M, Gohda M, Ishikawa I, Takayama N, Shimizu M, Kiyono H. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J. Immunol. 2007;179:1577–1585. doi: 10.4049/jimmunol.179.3.1577. [DOI] [PubMed] [Google Scholar]
- Leung AK, Hon KL, Robson WL. Atopic dermatitis. Adv. Pediatr. 2007;54:241–273. doi: 10.1016/j.yapd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J. Clin. Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, Leung DY, Holleran W, Uchida Y, Elias PM. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Invest. Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Nishikawa A. Percutaneous application of peptidoglycan from Staphylococcus aureus induces mast cell development in mouse spleen. Int. Arch. Allergy Immunol. 2006;139:271–278. doi: 10.1159/000091598. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizukoshi K, Oyobikawa M, Ohshima H, Tagami H. Establishment of an atopic dermatitis-like skin model in a hairless mouse by repeated elicitation of contact hypersensitivity that enables to conduct functional analyses of the stratum corneum with various non-invasive biophysical instruments. Skin Res. Technol. 2004;10:122–129. doi: 10.1111/j.1600-0846.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J. Allergy Clin. Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- Norman MU, Hwang J, Hulliger S, Bonder CS, Yamanouchi J, Santamaria P, Kubes P. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am. J. Pathol. 2008;172:1638–1649. doi: 10.2353/ajpath.2008.070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanuphak P, Moorhead JW, Claman HN. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J. Immunol. 1974;112:115–123. [PubMed] [Google Scholar]
- Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- Pritchard H, Micklem HS. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin. Exp. Immunol. 1972;10:151–161. [PMC free article] [PubMed] [Google Scholar]
- Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- Schultz Larsen F, Diepgen T, Svensson A. The occurrence of atopic dermatitis in north Europe: an international questionnaire study. J. Am. Acad. Dermatol. 1996;34:760–764. doi: 10.1016/s0190-9622(96)90009-2. [DOI] [PubMed] [Google Scholar]
- Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelekati E, Bahri R, D’Orlando O, Orinska Z, Mittrucker HW, Langenhaun R, Glatzel M, Bollinger A, Paus R, Bulfone-Paus S. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Fujimiya M, Ebise H, Miyahira Y, Kato I, Sugiura Y, Kimura T, Uehara M, Sato H, Sugiura H. Immunosuppressive effect of prolactin-induced protein: a new insight into its local and systemic role in chronic allergic contact dermatitis. Br. J. Dermatol. 2010;162:1286–1293. doi: 10.1111/j.1365-2133.2010.09756.x. [DOI] [PubMed] [Google Scholar]
- Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J. Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J. Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Pesce JT, Ramalingam TR, Thompson RW, Cheever A, Wynn TA. Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J. Immunol. 2008;181:6942–6954. doi: 10.4049/jimmunol.181.10.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin. Exp. Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Sharma R, Gaskin F, Fu SM, Ju ST. A novel role of IL-2 in organ-specific autoimmune inflammation beyond regulatory T cell checkpoint: both IL-2 knockout and Fas mutation prolong lifespan of Scurfy mice but by different mechanisms. J. Immunol. 2007;179:8035–8041. doi: 10.4049/jimmunol.179.12.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.