Abstract
Background
Prenatal allergen exposure has been linked to both induction and protection of allergic sensitization in offspring. We hypothesized that prenatal exposure of mice (F0) to Aspergillus fumigatus (A. fumigatus) would be associated with decreased immunoglobulin (Ig) E and airway eosinophilia and alterations in CpG methylation of T-helper genes in third-generation mice (F2).
Methods
Female BALB/c mice were sensitized to A. fumigatus (62.5, 125, 1250 μg, or saline) and re-exposed to the same dose on days 7 and 14 (early) or days 12 and 17 (late) gestation. Grandoffspring were treated with A. fumigatus (62.5 μg) at 9 weeks. IgE, IgG1, and IgG2a levels and cell counts from bronchoalveolar lavage fluid were determined. Lung DNA was pyrosequenced at multiple sites in the interferon (IFN)-γ and interleukin (IL)-4 promoters.
Results
Grandoffspring of mothers dosed with 1250 μg early during pregnancy developed increased airway eosinophilia (P < 0.05). Grandoffspring of mothers dosed late in pregnancy developed lower IgE (P < 0.05) and airway eosinophilia (P < 0.05). Grandoffspring of mothers dosed early had lower methylation at IL-4 CpG−408 and CpG−393 compared to late dosed mice (P < 0.005 across all doses). Few correlations were found between methylation levels and airway eosinophilia and IgE.
Conclusion
Prenatal exposure to A. fumigatus late during pregnancy, but not early, was associated with lower IgE and airway eosinophilia in grandoffspring. Prenatal exposure to A. fumigatus was associated with changes in CpG methylation in the IFN-γ and IL-4 promoters that did not correlate consistently with indicators of allergic sensitization.
Keywords: allergy, Aspergillus fumigatus, DNA methylation, prenatal exposure
Asthma in parents is associated with increased risk of asthma in children, with maternal asthma more strongly associated with risk compared to paternal (1). This pattern suggests that maternal exposures, particularly during gestation, may alter developmental programming, leading to molecular, physiologic, and metabolic adaptations in the offspring (1, 2). For example, prenatal allergen exposure may ‘prime’ T-helper (Th) cells toward a more proallergic Th2 phenotype (3). Previous studies of prenatal exposures on the development of allergy have been mixed, with exposures linked to both increased (4) and decreased (5) allergy in the offspring, likely due to differing effects of dose, timing of exposure during gestation, and other methodological variations.
An emerging paradigm is that epigenetic regulation of genes important to Th gene transcription and cytokine production may be responsible for the development of allergy (6). Cell systems have shown that site-specific CpG methylation of Th gene promoters can steer differentiation toward the Th2 phenotype (7, 8). In particular, the CpG−53 of the interferon (IFN)-γ promoter was shown to undergo rapid methylation during Th2 polarization that blocked ATF2-c/Jun and CREB transcription factor binding (7). More limited in vivo studies have been conducted. In one study, our group showed that combined exposure to diesel exhaust particles and allergen in mice altered methylation patterns at CpG sites in the IFN-γ and IL-4 promoters that correlated with changes in immunoglobulin (Ig) E production (9).
Epigenetic regulation also may explain partially the heritability of asthma (2). A gestational diet high in folic acid, a methyl donor for methylation reactions, induced allergic airway inflammation in offspring mice (F1) in association with hypermethylation of RUNX3, a T-cell regulator (10). Differences in levels of global DNA methylation among dendritic cells, and in their antigen-presenting activity, derived from offspring mice also were associated with the presence or absence of an asthma-like phenotype in the mother (11). Epidemiologic studies additionally have demonstrated associations between prenatal exposures, including tobacco smoke and polycyclic aromatic hydrocarbons (PAH), with differential methylation patterns in several asthma candidate genes and symptoms in children (12, 13). Also, prenatal smoking, determined using retrospective questionnaire, was associated with greater asthma risk in the grandchildren (14). Others have suggested that the timing of exposure during gestation may be important, with folic acid supplementation either during the first trimester or during late pregnancy being associated with asthma-related symptoms in young children in separate studies (15, 16).
We hypothesized that allergen exposure during gestation may alter the allergic phenotype through multiple generations of mice and that such changes would be associated with DNA methylation of Th genes. We also hypothesized that these associations may vary depending on the timing of prenatal exposure. Given our previous report that prenatal Aspergillus fumigatus (A. fumigatus) administration combined with diesel exhaust particles was associated with reduced IgE in sensitized offspring (F1) (17), here we exposed female mice (F0) to multiple doses of A. fumigatus during the early or late period of gestation and determined the effects on IgE, airway eosinophilia, and Th gene IFN-γ and IL-4 promoter methylation in the lungs of their grandoffspring (F2).
Methods
Aspergillus fumigatus sensitization
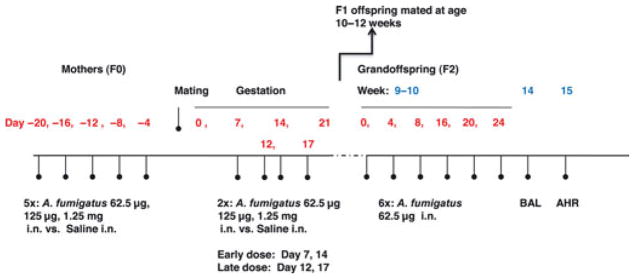
BALB/c female mice were exposed to A. fumigatus (62.5 μg, 125 μg, or 1.25 mg) intranasally in 50 μl of saline, or saline alone, five times 4 days apart, beginning 20 days prior to mating. Pregnant mice were retreated with same dose of A. fumigatus or saline on days 7 and 14 (early gestation) or days 12 and 17 (late gestation) after mating (Fig. 1). At 10– 12 weeks of age, offspring (F1) were mated with littermates. Their offspring (F2, grandoffspring) at 9 weeks of age were treated with six doses of A. fumigatus (62.5 μg in 50 μl), each dose 4 days apart, and IgE, IgG1, and IgG2 levels were measured.
Figure 1.
Experimental protocol. AHR, Airway hyper-reactivity; BAL, Bronchoalveolar lavage; i.n, intranasal; 5×: five doses of Aspergillus fumigatus; 6×: six doses of A. fumigatus.
Bronchoalveolar lavage (BAL), airway cellular analysis, and airway hyper-reactivity
Mice were killed at median age of 12.5 weeks, and BAL was performed. One hundred cells were counted for each sample from 10 randomly chosen viewing fields, and eosinophils, lymphocytes, macrophages, and neutrophils were quantified by a blinded reader. At median age of 15.5 weeks, mice were anesthetized and intubated with a 20-g catheter inserted directly into the trachea, placed on a flexiVent ventilator, and airway resistance was determined.
DNA methylation
DNA was extracted from lung tissue using the Wizard SV Genomic DNA Purification System (Promega, Madison, WI, USA). Purified DNA (400 ng) underwent bisulfite modification using the EZ DNA Methylation-Gold kit (Zymo, Irvine, CA, USA). Bisulfite-converted DNA (20 ng) was used in PCR with primers that spanned two regions of the IFN-γ promoter (CpG−205 and CpG−190; CpG−53 and CpG−45, relative to transcription start site) and one region of the IL-4 promoter (CpG−408, CpG−393) (9) (Table 1). PCR products were pyrosequenced, and methylation levels at each CpG site were determined using PyroMark 24 run software (Qiagen) as described (9).
Table 1.
Primers used for PCR amplification and pyrosequencing experiments
| IFN-γ | |
| PCR forward | TGGTGTGAAGTAAAAGTGTTTTTAGA |
| PCR reverse | biotin-TACACCTCTCTAACTTCCAATTTT |
| Pyrosequencing 1 | GAATGGTATAGGTGGGTA |
| Pyrosequencing 2 | AAAAAAAATTTGTGAAAATA |
| IL-4 | |
| PCR forward | GTTTTTAAGGGGTTTTTATAGTAGGAAGT |
| PCR reverse | biotin-AATTACCACTAACTCTCCTCCTACA |
| Pyrosequencing | AGATTTTTTTGATATTATTTTGTTT |
Nonparametric statistical analyses were performed. These details, as well as additional methods, are provided in the online supplement.
Results
IgE in adult female mice and grandoffspring
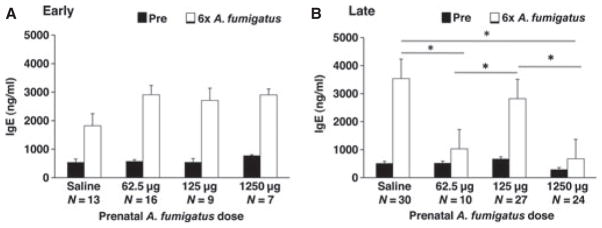
Adult female mice treated with A. fumigatus developed dosedependent increases in total IgE levels compared to mice treated with saline (P < 0.0001 Kruskal–Wallis, Fig. S1). Grandoffspring (F2) of mothers (F0) who received A. fumigatus 1250 μg developed lower levels of IgE following sensitization with A. fumigatus 62.5 μg compared to grandoffspring of mothers who were treated with saline or A. fumigatus 125 μg during pregnancy (P < 0.05, Dunn–Nemenyi). When the grandoffspring were stratified according to the timing of prenatal dose (early vs late pregnancy), grandoffspring of mice who were treated with A. fumigatus 62.5 or 1250 μg later in pregnancy, but not early, developed reduced IgE levels following sensitization to A. fumigatus compared to grandoffspring of mice that were treated with saline or 125 μg (Fig. 2A,B, P < 0.05, Dunn–Nemenyi). Significant reductions in IgE levels of grandoffspring also were found when comparing the levels after the fifth dose of A. fumigatus in the grandoffspring when compared to saline (data not shown, Kruskal–Wallis, P < 0.0001).
Figure 2.
Mean IgE levels in grandoffspring (F2) following sensitization of female mice (F0) to Aspergillus fumigatus. IgE data are presented as mean ± standard error. (A) Early dosed: No changes in IgE were detected across treatment groups. (B) Late dosed: IgE was reduced in grandoffspring (F2) of mothers treated with 62.5 and 1250 μg of A. fumigatus, compared to saline and 125 μg. *P < 0.05.
Eosinophilic inflammation in the grandoffspring
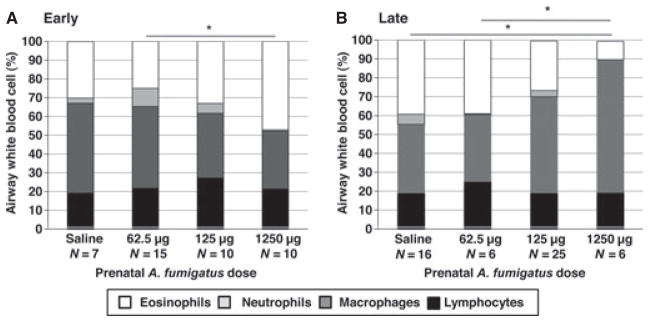
Grandoffspring of mice who were challenged with the highest A. fumigatus dose (1250 μg) during early gestation developed greater levels of airway eosinophilia following sensitization with A. fumigatus compared to grandoffspring of mice who were challenged with A. fumigatus 62.5 μg (Dunn–Nemenyi P < 0.05, Fig. 3A). Grandoffspring of mice who received A. fumigatus 1250 μg dosed late during pregnancy developed lower levels of airway eosinophilia following sensitization with A. fumigatus compared to grandoffspring of mice who were treated during pregnancy with saline or A. fumigatus 62.5 μg (Dunn–Nemenyi P < 0.05, Fig. 3B).
Figure 3.
BAL counts in grandoffspring (F2) following sensitization of female mice (F0) to Aspergillus fumigatus. One hundred cells in total were counted for each sample from 10 randomly chosen viewing fields, and eosinophils, lymphocytes, macrophages, and neutrophils were quantified by a blinded reader. BAL data are presented as percents of total cell counts. (A) Early dosed: Percent eosinophils in bronchoalveolar lavage fluid was increased in grandoffspring (F2) of mice treated with 1250 μg of A. fumigatus (47.1 ± 3.9%), compared to 62.5 μg (25.0 ± 6.7%). (B) Late dosed: Percent eosinophils in bronchoalveolar lavage fluid was decreased in grandoffspring (F2) of mice treated with 1250 μg of A. fumigatus (9.7 ± 4.8%), compared to saline (39.3 ± 3.7%) and 62.5 μg (39.0 ± 4.6%). *P < 0.05.
Lung DNA methylation at the IFN-γ and IL-4 promoters
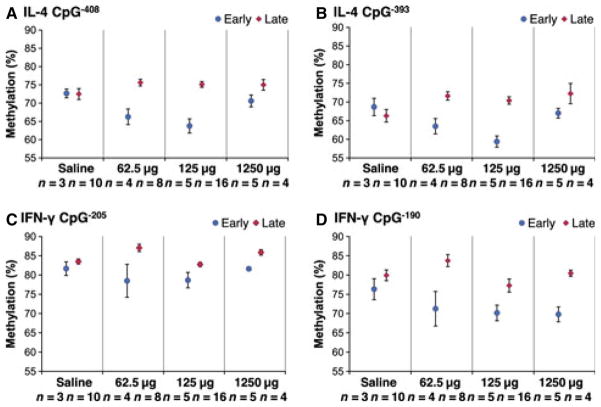
Levels of methylation at CpG−408 and CpG−393 in the IL-4 promoter were lower among grandoffspring of mice dosed early in all experimental groups compared to those dosed later (Kruskal–Wallis, P < 0.0005, Fig. 4A,B). Following stratification by timing of exposure, significant differences in methylation levels at CpG−393 and CpG−408 across A. fumigatus doses were detected only among mice prenatally dosed early (Kruskal–Wallis, P = 0.03 and P = 0.029, Fig. 4A,B). Levels of methylation at CpG−205 and CpG−190 in the IFN-γ promoter also were lower among grandoffspring of mice dosed early in all experimental groups compared to those dosed later (Kruskal–Wallis, P < 0.0005, Fig. 4C,D). DNA methylation in IFN-γ CpG−53 or CpG−45 did not differ by timing (Kruskal–Wallis, P = 0.81 and P = 0.76, Fig. S3) or across treatment groups. Following stratification by timing of prenatal exposure, significant differences across experimental doses were not detected in the early dosed group.
Figure 4.
CpG methylation at the IL-4 and IFN-γ promoters in grandoffspring (F2) following sensitization of female mice (F0) to Aspergillus fumigatus. Methylation data are presented as mean ± standard error. (A) IL-4 CpG−408: Methylation in grandoffspring (F2) of mice dosed early with A. fumigatus was lower than in grandoffspring of mice dosed late (P < 0.0005). (B) IL-4 CpG−393: Methylation in grandoffspring (F2) of mice dosed early with A. fumigatus was lower than in grandoffspring of mice dosed late (P < 0.0005). (C) IFN-γ CpG−205: Methylation in grandoffspring (F2) of mice dosed early with A. fumigatus was lower than in grandoffspring of mice dosed late (P < 0.0005). (D) IFN-γ CpG−190: Methylation in grandoffspring (F2) of mice dosed early with A. fumigatus was lower than in grandoffspring of mice dosed late (P < 0.0005).
Additional results are provided in the Supporting Information.
Discussion
Maternal exposure during pregnancy (F0) to A. fumigatus was associated with reductions in IgE and airway eosinophilia in grandoffspring (F2). Upon stratification by timing of prenatal allergen exposure, the reductions in IgE and airway eosinophilia were only observed in the late dosed offspring. Conversely, early gestational exposure to allergen produced an increase in airway eosinophilia. Grandoffspring of mice following early prenatal exposure to allergen had lower lung DNA methylation levels at multiple CpG sites at both the IL-4 and IFN-γ promoters compared to offspring of late dosed mice. Grandoffspring of mice dosed early in pregnancy showed evidence of allergen dose-dependent differences in CpG−393 and CpG−408 DNA methylation at the IL-4 promoter. Only a few borderline and significant correlations were found in IL-4 and IFN-γ methylation with indicators of allergic sensitization (i.e., IgE, airway eosinophilia) in the F2 offspring. Collectively, these results indicate that maternal prenatal exposure to A. fumigatus dosed later in pregnancy may affect the allergic airway responses across multiple generations. Epigenetic changes in IL-4 and IFNγ occur in grandoffspring mice (F2) following prenatal exposure to allergen, but those measured here do not appear responsible for the changes in IgE and airway eosinophilia.
Previously, we found that sensitization to A. fumigatus in combination with prenatal exposure to diesel exhaust particles led to a reduction in IgE and airway eosinophilia in the F1 offspring (17). While the current study did not test F1 mice, the two studies together suggest that the effects of prenatal administration of A. fumigatus on IgE may extend through the third generation under certain conditions.
After fertilization and before implantation, methylation marks are re-established de novo via the actions of DNA methyltransferases (18). Early gestational exposure to A. fumigatus potentially could have downregulated these methyltransferases, lowering methylation levels among the early dosed groups. However, if this mechanism was biologically important here, one may have predicted that associations between methylation levels and IgE in the grandoffspring would have been observed if the exposure occurred early during pregnancy instead of later. Instead, these observations may be more consistent with evidence that methylation changes may occur throughout gestation (19). Alternately, because the reductions in IgE were most pronounced among mice prenatally exposed later in gestation, one could speculate that prenatal allergen exposure induced Th cytokine shifts among F0 mice that were transferred to F1 mice across the placenta or through breast milk (20), and possibly induced their own epigenetic marks that subsequently were inherited by the F2 mice.
Only a few studies to date have examined the influence of timing of environmental exposures within gestation on allergic phenotypes in offspring. In one study, relatively high-dose ovalbumin (OVA) administration in BALB/c mice early in gestation was associated with lower OVA-IgE levels compared to OVA exposure late in gestation (21). In a human cohort, increased exposure to PAHs and fine particulate matter (PM2.5) in the second month of gestation was associated with lower cord IgE levels and relative increases in CD3+ and CD4+ lymphocytes. Increased exposure to PAH and PM2.5 in mid- to late pregnancy was associated with opposite findings (22, 23), suggesting that the timing of exposure during gestation may have a distinct effect on B-cell and T-cell-related outcomes relevant to allergic sensitization.
Another novel aspect of this study is the focus on lung DNA methylation of Th genes in the grandoffspring that varied by both timing of prenatal allergen exposure and dose. The strongest results suggest that methylation levels of both cytokine genes were lower among mice whose prenatal exposures were earlier in gestation. The absence of correlations between gene methylation and IgE indicates that methylation of these CpG sites does not appear to regulate directly the IgE response in these mice. Instead, the observed differences in methylation following prenatal exposure to A. fumigatus suggest that epigenetic regulation occurred in association with environmental exposures two generations ago. The biological significance of these epigenetic changes is uncertain. One could speculate that the effect of prenatal allergen exposure on allergic immune responses measured here in F2 mice was a result of significant epigenetic changes in unmeasured genes like Foxp3, a gene susceptible to both DNA methylation and histone modification and implicated in the regulation of allergy (24).
The relative importance of methylation of individual CpG sites in the IFN-γ and IL-4 promoters, in relation to gene expression, is not established (25). For example, although significant changes in methylation levels at CpG−205 in IFN-γ were observed with allergen treatment, it is uncertain whether these led to sustained effects on IFN-γ gene expression, particularly when methylation at CpG−53 and CpG−45 was unchanged. Moreover, the balance of Th1 and Th2 cytokines is thought to contribute to the outcome of the immune response (26). Increases in the methylation of both the IFN-γ and IL-4 promoters may fail to induce relative shifts in cytokine production. Also, the absolute changes in methylation level required to affect the cytokine gene transcription are unknown. The levels of methylation of the IFN-γ promoter measured in this study were much higher than those reported in previous studies of peripheral CD4+ cells in mice (9). These observations may reflect differences in gene methylation in lung vs CD4+ cells (27).
Methylation levels at IFN-γ CpG−205 and CpG−190 were associated negatively with airway eosinophilia. Because increased IFN-γ expression is generally associated with Th1 responses and reduced eosinophilia (28), this finding appears to be counterintuitive. However, IFN-γ production can stimulate eosinophil activation and survival in some models (29, 30).
These results also add to a limited number of studies that suggest the timing of prenatal exposures may modify epigenetic outcomes. For example, the study of the Dutch Hunger Winter in 1944–1945 found that famine was associated with changes in methylation in several genes implicated in metabolic disease and growth that were modified by timing of famine during pregnancy (31). A study of baboons demonstrated that the extent of global methylation in offspring kidneys varied by timing of prenatal dietary restriction (32).
Limitations of this study warrant discussion. The observations may be specific to BALB/c mice. The use of whole lung tissue for DNA extraction, which included a mixture of cell types, may have impeded our ability to detect significant methylation changes among the treatment groups. The effect of inbreeding the F1 generation, rather than mating F1 females with ‘fresh’ male mice, is also unknown. We did not measure IFN-γ and IL-4 cytokine levels and thus were unable to correlate the promoter methylation with cytokine production. The lack of F0 and F1 measures prevented the analysis of A. fumigatus exposure on allergy and CpG methylation in these generations. Additionally, because we did not continue the experiment through the F3 generation, we cannot conclude that these findings reflect transgenerational inheritance (18). Some experiments, including the airway hyper-reactivity experiments and measures of eosinophilia, could have been underpowered. Finally, the observed batch effect for IgE may stem from assay variability. However, IgE levels were compared primarily across all A. fumigatus doses within each timing group, not between timing groups, diminishing to some extent the impact of assay variability.
In summary, these findings suggest that A. fumigatus exposure during the prenatal period affects allergic immune responses in grandoffspring. Timing of prenatal allergen exposure determined the allergic response in grandoffspring, with exposures late in pregnancy diminishing the systemic and airway allergic response. Exposures early in pregnancy induced greater airway eosinophilia. While changes in DNA methylation were observed in grandoffspring in association with prenatal allergen exposure, they do not appear responsible for the observed changes in IgE or airway eosinophilia.
Supplementary Material
Supplementary Figure 1. IgE following sensitization of female mice to A. fumigatus
Supplementary Figure 2. Mean IgG1 levels in grandoffspring (F2) following sensitization of female mice (F0) to A. fumigatus
Supplementary Figure 3. CpG methylation at the IFN-γ promoter in grandoffspring (F2) following sensitization of female mice (F0) to A. fumigatus
Acknowledgments
This work was supported by P30ES009089, R21ES013063, TL1RR024158, P01ES09600, and EPA RD-83214101. All authors of this manuscript meet the conditions of authorship set up by The International Committee of Medical Journal Editors (ICMJE).
Footnotes
Conflict of interest
We confirm that no authors have any conflicts of interest to disclose.
References
- 1.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS ONE. 2010;5:e10134. doi: 10.1371/journal.pone.0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008;177:567–573. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devereux G, Seaton A, Barker RN. In utero priming of allergen-specific helper T cells. Clin Exp Allergy. 2001;31:1686–1695. doi: 10.1046/j.1365-2222.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 4.Peters JL, Suglia SF, Platts-Mills TAE, Hosen J, Gold DR, Wright RJ. Relationships among prenatal aeroallergen exposure and maternal and cord blood IgE: project ACCESS. J Allergy Clin Immunol. 2009;123:1041–1046. doi: 10.1016/j.jaci.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirsten TB, Soares de Oliveira BP, Ligeiro de Oliveira AP, Kieling K, Tavares de Lima W, Palermo-Neto J, et al. Single early prenatal lipopolysaccharide exposure prevents subsequent airway inflammation response in an experimental model of asthma. Life Sci. 2011;89:15–19. doi: 10.1016/j.lfs.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. 2010;65:7–15. doi: 10.1111/j.1398-9995.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones B, Chen J. Inhibition of IFN-gamma transcription by site-specific methylation during T helper cell development. EMBO J. 2006;25:2443–2452. doi: 10.1038/sj.emboj.7601148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamper CJ, Agoston AT, Nelson WG, Powell JD. Identification of DNA methyltransferase 3a as a T cell receptor-induced regulator of Th1 and Th2 differentiation. J Immunol. 2009;183:2267–2276. doi: 10.4049/jimmunol.0802960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, et al. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008;102:76–81. doi: 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am J Respir Cell Mol Biol. 2011;44:285–292. doi: 10.1165/rcmb.2009-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breton CV, Byun H-M, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS ONE. 2009;4:16. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 15.Ha° berg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–184. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–1493. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Zhu H, Quan C, Grunig G, Ballaney M, Jin X, et al. Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice. Allergy Asthma Clin Immunol. 2010;6:7. doi: 10.1186/1710-1492-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozharny Y, Lambertini L, Ma Y, Ferrara L, Litton CG, Diplas A, et al. Genomic loss of imprinting in first-trimester human placenta. Am J Obstet Gynecol. 2010;202:391.e391–391.e398. doi: 10.1016/j.ajog.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, et al. Allergen- independent maternal transmission of asthma susceptibility. J Immunol. 2003;170:1683–1689. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 21.Fusaro AE, de Brito CA, Taniguchi EF, Muniz BP, Victor JR, Orii NM, et al. Balance between early life tolerance and sensitization in allergy: dependence on the timing and intensity of prenatal and postnatal allergen exposure of the mother. Immunology. 2009;128(1 Suppl):e541–e550. doi: 10.1111/j.1365-2567.2008.03028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herr CEW, Ghosh R, Dostal M, Skokanova V, Ashwood P, Lipsett M, et al. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatr Allergy Immunol. 2011;22(1-Part-I):75–84. doi: 10.1111/j.1399-3038.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- 23.Herr CE, Dostal M, Ghosh R, Ashwood P, Lipsett M, Pinkerton KE, et al. Air pollution exposure during critical time periods in gestation and alterations in cord blood lymphocyte distribution: a cohort of livebirths. Environ Health. 2010;9:46. doi: 10.1186/1476-069X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852. e810. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Lee CG, Sahoo A, Im SH. Epigenetic regulation of cytokine gene expression in T lymphocytes. Yonsei Med J. 2009;50:322–330. doi: 10.3349/ymj.2009.50.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 27.Falek PR, Ben-Sasson SZ, Ariel M. Correlation between DNA methylation and murine IFN-gamma and IL-4 expression. Cytokine. 2000;12:198–206. doi: 10.1006/cyto.1999.0528. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigeninduced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4 + T cells. J Exp Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishihara C, Ochiai K, Kagami M, Takashahi H, Matsuyama G, Yoshida S, et al. Human peripheral eosinophils express functional interferon-gamma receptors (IFN-gammaR) Clin Exp Immunol. 1997;110:524–529. doi: 10.1046/j.1365-2249.1997.4511469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim CK, Choi J, Callaway Z, Iijima K, Volcheck G, Kita H. Increases in airway eosinophilia and a th1 cytokine during the chronic asymptomatic phase of asthma. Respir Med. 2010;104:1436–1443. doi: 10.1016/j.rmed.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unterberger A, Szyf M, Nathanielsz PW, Cox LA. Organ and gestational age effects of maternal nutrient restriction on global methylation in fetal baboons. J Med Primatol. 2009;38:219–227. doi: 10.1111/j.1600-0684.2008.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. IgE following sensitization of female mice to A. fumigatus
Supplementary Figure 2. Mean IgG1 levels in grandoffspring (F2) following sensitization of female mice (F0) to A. fumigatus
Supplementary Figure 3. CpG methylation at the IFN-γ promoter in grandoffspring (F2) following sensitization of female mice (F0) to A. fumigatus