Abstract
Objectives/Hypothesis
Compare the accuracy of wideband acoustic transfer functions (WATFs) measured in the ear canal at ambient pressure to methods currently recommended by clinical guidelines for predicting middle-ear effusion (MEE).
Study Design
Cross-sectional validating diagnostic study among young children with and without MEE to investigate the ability of WATFs to predict MEE.
Methods
WATF measures were obtained in a MEE group of 44 children (53 ears, mean age 1.9 years) scheduled for middle-ear ventilation tube placement and a normal age-matched control group of 44 children (59 ears, mean age 1.8 years) with normal pneumatic otoscopic findings and no history of ear disease or middle-ear surgery. An otolaryngologist judged whether MEE was present or absent and rated tympanic-membrane (TM) mobility via pneumatic otoscopy. A likelihood-ratio classifier reduced WATF data (absorbance, admittance magnitude and phase) from 0.25 to 8 kHz to a single predictor of MEE status. Absorbance was compared to pneumatic otoscopy classifications of tympanic membrane (TM) mobility.
Results
Absorbance was reduced in ears with MEE compared to ears from the control group. Absorbance and admittance magnitude were the best single WATF predictors of MEE, but a predictor combining absorbance, admittance magnitude and phase was the most accurate. Absorbance varied systematically with TM mobility based on data from pneumatic otoscopy.
Conclusions
Results showed that absorbance is sensitive to middle-ear stiffness and MEE, and WATF predictions of MEE in young children are as accurate as that reported for methods recommended by the clinical guidelines.
Keywords: Absorbance, admittance, clinical decision theory, effusion, myringotomy, pneumatic otoscopy, receiver operating characteristic curve, tympanometry, wideband acoustic transfer functions, Level of Evidence: 2c
INTRODUCTION
Otitis media is described as inflammation that can lead to accumulation of fluid in the middle ear.1 Clinical cases of otitis media increased from nearly 10 million in 1975 to 24.5 million in 1990, making it one of the most common diagnoses for children under the age of 15 years.2 Otitis media with effusion denotes the presence of middle-ear effusion (MEE) and affects roughly 2.2 million children annually with treatment costs upwards of $4 billion3. Up to 90% of children develop some form of otitis media with effusion before school age.4 MEE decreases tympanic membrane (TM) mobility and impedes the transfer of energy via the middle ear,5 resulting in hearing losses, commonly between 15 and 40 dB HL.6 A hearing loss associated with recurrent episodes of MEE during critical learning phases can lead to long-term consequences including deficits in language development,7-10 auditory processing,11 binaural hearing,12 speech perception,13-17 sound localization,18 cognitive ability,19 and academic success.20-22 The present study evaluates the accuracy of wideband acoustic transfer functions (WATFs) to predict MEE.
Given the incidence, cost, and consequences, it is essential to accurately diagnose MEE for its effective management. Myringotomy is the gold standard for identifying MEE, and, combined with applications of clinical decision theory,23,24 can be used to evaluate the performance of alternative techniques in predicting the presence of MEE.
For obvious reasons, myringotomy cannot be used with subjects for whom there are no indications of middle-ear dysfunction. Pneumatic otoscopy provides an alternative to surgical confirmation, especially in cases in which MEE are not suspected. Current clinical practice guidelines for treating MEE are based primarily on pneumatic otoscopy,25 which enables visual inspection of TM mobility in response to pressure changes in a hermetically sealed ear canal.26 Studies have evaluated the ability of pneumatic otoscopy to predict the presence of MEE as validated by surgical findings at myringotomy. For example, a meta-analysis27 showed that pneumatic otoscopy was the best of eight methods for diagnosing MEE in children (sensitivity and specificity of 94 and 80%, respectively). Separate studies report sensitivity values from 85 to 91%, and specificity from 58 to 89%.28-31 Although pneumatic otoscopy has potential in predicting MEE, interpretation of test results is characterized by highly variable outcomes26,30,32,33 either across otoscopists34,35 or across repeated tests by a single otoscopist.29
When the diagnosis of MEE via pneumatic otoscopy is in question, current clinical guidelines recommend either 226-Hz tympanometry or reflectometry as an adjunct to pneumatic otoscopy.25 Clinical 226-Hz tympanometry, which measures middle-ear admittance across a pressure change in the ear canal, is a reliable and objective measure characterized by good inter-tester agreement.36,37 Unlike 1000-Hz tympanometry, which is often used for children under 6 months of age, 226-Hz tympanometry is typically used for a clinical population beyond 6 months of age. When compared to myringotomy, 226-Hz tympanometry predicts MEE with sensitivity from 80 to 90%, and specificity from 74 to 100%.27,28,31,37,38 Such a range of values is likely the result of differences in the components of the tympanogram across studies that were used to make predictions of MEE. Toner and Mains30 report a single predictive value of 89% when using flat tympanograms to predict MEE. Of several tympanometric parameters (i.e., acoustic reflex, gradient, width, peak pressure, and admittance), width is reported to be the most accurate predictor of MEE with a sensitivity and specificity of 81 and 82%29, respectively. Other studies39,40 have reported good test performance for tympanometric predictors of MEE despite relying on otoscopic diagnosis instead of myringotomy for MEE verification. In reference to reflectometry, meta-analysis results indicate lower accuracy in predicting MEE relative to pneumatic otoscopy and tympanometry.27,40
A limitation of both pneumatic otoscopy and tympanometry is the need to obtain a hermetic seal in the ear canal so that pressure changes can be delivered across the TM. In ears with multiple pathologies, a TM with low-impedance as measured with 226-Hz tympanometry can mask more medial pathologies.41 Also, 226-Hz tympanometry assesses middle-ear status at a single frequency and is not suitable for detecting slight changes in middle-ear mechanics caused by otitis media.42
WATFs, which are not discussed in current clinical guidelines, were developed to measure middle-ear function across a broad frequency spectrum.43 WATFs, which includes absorbance (AB) and acoustic admittance, are measured in the ear canal and provide a spectral analysis and acoustic transfer function of the ear canal and middle ear.44 AB values range between 0, which occurs when all the incident energy is reflected back to the probe microphone, and 1, which occurs when all incident energy is absorbed by the ear canal and middle ear. Advantages of the WATF test include: (1) a short test time (typical duration of a few seconds), (2) a continuous broad frequency response between 0.25 and 8 kHz, (3) the option of obtaining pressurized or ambient responses, (4) an independence of probe placement within the ear canal,45 and (5) good test-retest reliability.46
WATFs have been investigated in various populations including normal adults,47-51 children and infants,46,47,52-55 healthy newborns,56-58 newborns in intensive care,59 and infants receiving a neonatal hearing screening.60,61 WATFs are sensitive to middle-ear disorders including children and adults with a conductive hearing impairments,62,67 otitis media in children with cleft palate,63 otitis media with effusion in children64 and adults,65 otosclerosis in adults,64-66 ossicular discontinuity in adults,65 and TM perforation in adults.64,65 Although case studies64,68 have described WATFs in ears with MEE, few have reported distributions of WATFs in ears of these children. Beers et al.54 studied WATFs at ambient pressure in children with normal middle-ear status and in children with MEE. They found that energy reflectance (ER; i.e., the ratio of reflected to incident energy) increased over a wider frequency range as middle-ear status progressed from mild negative pressure to MEE, and that the ER between 680 and 6000 Hz was higher in ears with MEE (i.e., lower AB) compared to ears with normal middle-ear status. The sensitivity and specificity of ER between 1200 to 2600 Hz for predicting MEE was 100% and 90%, respectively, and ER predictors at 1250 Hz were better than predictors based on 226-Hz static admittance, which suggests WATFs have potential in predicting MEE at least as well as 226-tympanometry.
The above summary suggests that there is a need to improve current methods of MEE diagnosis. The ideal test should be objective, quick, have good test-retest reliability, and be predictive of middle-ear status, both in ears with MEE and in normal ears. An ambient test might be preferable over pressurized methods for children experiencing ear pain. A prototype system that measures WATFs in the ear canal at 60 frequencies between 0.226 and 8 kHz in approximately 1 sec at ambient pressure60 has been described previously.45 The results from that study suggest that WATFs may result in more accurate diagnostic results compared to tests described in the current guidelines and in widespread clinical use. The primary goal of the present study is to evaluate WATFs in predicting MEE in children using myringotomy as the gold standard. Data are compared to results in age-matched children with normal middle-ear function and no history of ear disease or middle-ear surgery, in whom pneumatic otoscopy served as a substitute gold standard because myringotomies were not performed in these children. Test performance is assessed through the application of clinical decision theory, which provides a means of comparing the performance of an experimental measure (WATFs) to a gold standard (observations at surgery or pneumatic otoscopy). An additional goal is to assess the extent to which WATFs are related to qualitative estimates of tympanic-membrane mobility as determined via pneumatic otoscopy.
MATERIALS AND METHODS
Subjects
Following institutional review board approval, informed consent was obtained prior to data collection from the parents/legal guardians of all subjects. Data were collected from children between 0.5 and 7 years of age. Subjects with no history of ear disease or middle-ear surgery served as a control group. Normal middle-ear function was determined by an otolaryngologist via pneumatic otoscopy. The control group consisted of 59 ears from 44 subjects with a median age of 1.2 years and inter-quartile range (IQR) of 1.0 to 2.2 years. Details of the measurements in this group are provided below. Subjects in the MEE group were recruited from patients scheduled for myringotomy and tube placement, but had no history of myringotomies prior to the day of data collection. Subject data were included into the MEE group only if the physician conducting the surgery confirmed the presence of MEE at operation. Details of the status of the middle-ear at surgery were provided via a questionnaire completed by the surgeon. Pre-anesthetic sedation was delivered to each child in the MEE group according to a protocol specified by an anesthesiologist. WATF data from the MEE group were obtained in the patient holding room in the presence of the family following the administration of pre-anesthesia and within 1 hour of surgery. General anesthesia was then induced in the operating room just before surgery. The MEE group consisted of 53 ears from 44 subjects with a median age of 1.3 years and IQR of 1.1 to 2.1 years. This overall sample size of 112 test ears was concluded to be sufficient to measure test performance of WATFs in predicting MEE in a group of children relative to a baseline group with normal middle-ear function.
WATF Measurements and Analysis
A prototype system45 was used to acquire WATF responses. The prototype used a Windows-based computer with a CardDeluxe (Digital Audio Labs) internal soundcard in which custom software controlled stimulus generation and data acquisition. A prototype probe assembly (Interacoustics, Assens, Denmark) delivered a click signal of fixed voltage to the subjects’ ear via a single channel and had a miniature microphone to record the acoustic response. The probe assembly was fitted with a plastic tip appropriate for each subject’s ear-canal dimensions. The system was calibrated daily before data collection using a procedure that is explained elsewhere.45,60 The calibration procedure enabled the measurement of the WATFs and was completed in less than 1 minute. The measured WATFs in the ear-canal included absorbance (AB), admittance magnitude (YM), and admittance phase (YP). AB is defined as one minus the ER. ER is calculated as the ratio of reflected energy at the probe termination in the ear canal to incident acoustic energy supplied by the probe receiver. The admittance is the transfer function between the acoustic volume velocity to the acoustic pressure, with both variables measured at the tip of the ear-canal probe. Ear-canal measurements were based on the synchronous response to the presentation of 16 clicks and included tests for a leak-free probe insertion and artifact rejection to exclude excessively noisy trials. In the event of an insufficient seal, the operator was prompted to remove and reinsert the probe tip. The total test time per measurement was typically under 1 second once the probe was sealed in the ear canal.
Each WATF response (i.e., AB, YM, and YP) contained data at every 1/12th octave between 0.25 and 8 kHz (60 data points). The multivariate nature of the WATF response provides many inter-related values regarding middle-ear status. For this reason, a log-likelihood ratio classifier69 was calculated that combined the multivariate information across frequency into a single (univariate) predictor. See Appendix B in Sanford et al.60 for a more detailed description of the log-likelihood ratio calculation.
Pneumatic Otoscopy Measurements
Pneumatic otoscopy was completed by an otolaryngologist for ears considered for inclusion in the control group. Each ear was assigned a numeric value along a 5-point rating scale that classified the degree of TM movement to pressure changes in the ear canal. These classifications were defined as (1) Normal (N+), (2) Slightly stiff (SS), (3) Moderately stiff (MS), (4) Very stiff (VS), and (5) No movement (i.e., immobile; IM). Ratings corresponding to 1 and 2 (N+ and SS, respectively) were classified as having normal eardrum mobility and were included in the normal group. Data corresponding to ratings denoting reduced TM mobility (i.e., 3-5) were excluded from the main test-performance analyses; however, these data, along with the data corresponding to ratings of 1 and 2, were used to evaluate the relationship between TM mobility and AB. All pneumatic otoscopic evaluations were performed by the same otolaryngologist.
Measures of WATF Test Performance
Upon constructing the likelihood ratios for WATF predictors (i.e., AB, YM, YP), clinical decision theory23,24 was used to generate receiver operating characteristic (ROC) curves. Each ROC curve plots the sensitivity of a univariate predictor of MEE (such as admittance magnitude, YM) as a function of the false-alarm rate (i.e., 1 – specificity). One summary statistic of the ROC curve is the area under the curve (AUC), which was calculated using a non-parametric procedure. An alternative summary statistic curve is the point of symmetry (SYM) on the ROC curve where sensitivity and specificity are equal.60,70 AUC and SYM values each range between 0.5 and 1, with values of 1 representing perfect test performance and values of 0.5 representing chance performance. One advantage of the SYM value is that it represents a clinically relevant test criterion characterizing both sensitivity and specificity. For the current study, AUC and SYM values were calculated for each of the wideband measures of AB, YM, and YP. A combined predictor (CP), defined as the sum of the log-likelihood ratios of the three aforementioned predictors, was also evaluated. The CP value incorporates diagnostic information associated with all three wideband measures. The 95% confidence intervals (CIs) for both AUC and SYM values were calculated using a bootstrap procedure71 of the ROC curve based on 40,000 iterations.
RESULTS
Figure 1 shows the AB median and IQR from 0.25 to 8 kHz for the normal (solid line, light shading) and MEE (dotted line, heavy shading) groups. The intermediate shading represents the range over which the IQRs for each group overlap. The median for the MEE group is below the 25th percentile of the normal group at all frequencies while the median for the normal group is above the 75th percentile for the MEE group between approximately 0.6 and 3.3 kHz, and around 5 kHz. The least overlap of IQR between groups occurs for frequencies between 1.5 and 3 kHz. This is the frequency range over which AB is most sensitive to MEE status.
Figure 1.
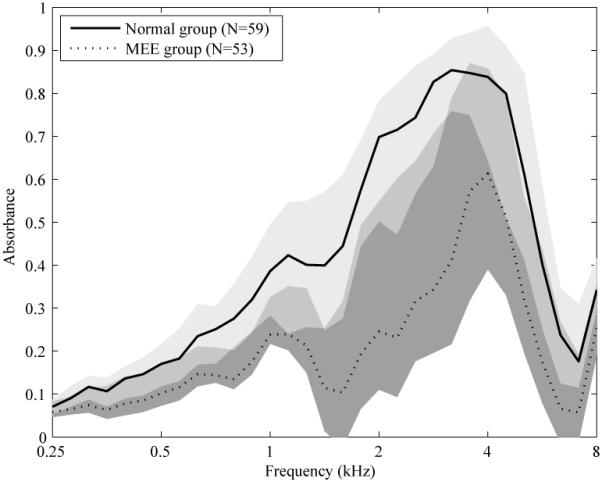
The solid and dotted lines represent the median absorbance for the normal and middle-ear effusion (MEE) group, respectively. The light gray fill represents the inter-quartile range (IQR) for the normal group, and the dark gray fill for the MEE group. The medium gray fill at each frequency represents the absorbance range over which the group interquartile ranges (IQRs) overlap.
Table 1 summarizes the accuracy of each WATF predictor of MEE along with its corresponding CI. The best overall predictor, as determined by the largest AUC, is CP followed by AB and YM, which are similar to each other. Although AB, YM, and CP have similar AUCs and CIs, CP has a larger SYM and narrower corresponding CI compared to both AB and YM. Specifically, the lower CI of SYM increases from 0.78 or 0.79 to 0.85, and the higher CI of SYM from 0.92 or 0.93 to 0.96 when using the CP predictor compared to using either the AB or YM predictor. While the CP predictor is preferred in terms of its larger AUC and SYM values, there were no significant differences between any of the WATF predictors listed in Table 1.
TABLE I.
The AUC, SYM and corresponding CIs for WATF predictors of MEE
| Predictor | AUC (95% CI) | SYM |
|---|---|---|
| AB | 0.93 (0.87-0.97) | 0.87 (0.78-0.92) |
| YM | 0.93 (0.87-0.97) | 0.87 (0.79-0.93) |
| YP | 0.90 (0.82-0.95) | 0.85 (0.77-0.91) |
| CP | 0.94 (0.88-0.98) | 0.90 (0.85-0.96) |
AB = absorbance; AUC = area under the receiver operating characteristic (ROC) curve; CI =
confidence intervals; CP = combined predictor; MEE = middle-ear effusion; SYM = point of symmetry on the ROC curve; WATF = wideband acoustic transfer function; YM = admittance magnitude; YP = admittance phase.
As described in the Introduction, AB ranges between 0 and 1 in a middle ear (in the reasonable limit that the cochlear generation of sound via otoacoustic emissions is neglected). The measured AB was less than 0 in some ears at some frequencies due to the presence of measurement error, but more often in the MEE group than in the control group because the median absorbance was smaller in the MEE group. The results reported in Table I used the AB data irrespective of whether the values ranged smaller than 0. This approach was selected as the most direct and practical assessment of test performance. An alternative and physically plausible approach in data analysis would be to set AB equal to 0 whenever the measured value was negative. The data were re-analyzed using this alternative, which resulted in the same SYM as that reported in Table I. The AUC was 0.94, which was slightly larger than the AUC of 0.93 in Table I. Nevertheless, this difference in AUC was small compared to the 95% CIs. Thus, methodological differences in how a predictor was constructed from the AB data had no significant effect on its ability to classify ears with MEE.
For purposes of visualizing the WATF responses measured in ears with varying mobility ratings in pneumatic otoscopy, only the AB was considered. In Figure 2, the median AB is plotted across frequency for each of the subgroups of ears with the same TM stiffness classification obtained via pneumatic otoscopy. The median AB for the MEE group is also plotted for comparison, which, assuming MEE reduces TM mobility, is hypothesized to be similar to AB in those ears rated as having increased stiffness (but for which no classification of MEE was available). The subgroups of TM mobility with the largest numbers of ears are those with N+ (N=36) and SS (N = 23) ratings, with both subgroups forming the normal group of responses in the clinical detection theory analysis. Overall, stiffer ears tended to have lower AB, with the most sensitive region (as defined by the largest differences in AB across subjective stiffness rating) between 0.8 and 2 kHz, which shows that absorbance decreases as TM stiffness increases. Regions beyond this range tend to have greater overlap across classifications. The AB for ears in the IM subgroup (i.e., scores of 5) is most similar across frequency to the AB of ears with MEE. That is, ears with MEE tend to be ears with an immobile eardrum.
Figure 2.
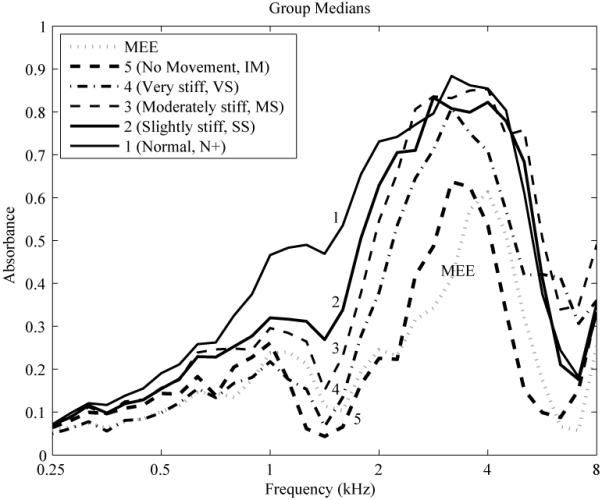
Median absorbance is plotted (black lines) across frequency for each of the subgroups of ears with the same TM stiffness classification (1 through 5) obtained via pneumatic otoscopy. Stiffness classifications are defined as follows: (1) Normal (N+), (2) Slightly stiff (SS), (3) Moderately stiff (MS), (4) Very stiff (VS), and (5) No movement (i.e., immobile; IM). The absorbance for the middle-ear effusion (MEE) group is plotted for comparison (gray dotted line).
To further investigate the relationship between AB and pneumatic otoscopy, AB responses were averaged over the frequency region most sensitive to changes in stiffness (i.e., 0.8 to 2 kHz) and plotted in Figure 3 for each TM stiffness classification group and the MEE group. The vertical notch on each side of each box represents the outcome from a nonparametric test for comparing each pair of medians for a significant difference at the 0.05 level. If notches are non-overlapping across TM stiffness classifications, medians are significantly different. The number of ears for each TM classification is listed along the x-axis at the top of the plot. As shown in Figure 3, the group classified as N+ has significantly higher AB than all other groups. The TMs classified as SS have significantly higher AB than the VS, IM, and MEE groups. Ears classified into the MS group have significantly higher AB than those in the IM group. Overall, the groups that are significantly different from one another include the N+ and SS groups compared to the VS and IM groups. The ears with TMs classified into the MS group represent a borderline group.
Figure 3.
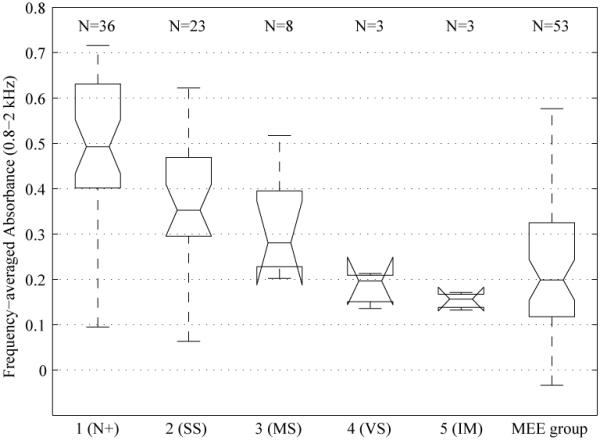
Absorbance (AB) responses averaged from 0.8 to 2 kHz are plotted as box and whiskers plots for each otoscopic subgroup and the middle-ear effusion (MEE) group. For each box, the center horizontal line represents the median AB, and the top and bottom horizontal lines represent the interquartile range (IQR) of AB. Each whisker length denotes the lesser of the full range of the data or 1.5 times the IQR. The notch is used with a nonparametric test to evaluate if two medians are significantly different at the 0.05 level. If notches are non-overlapping across tympanic membrane stiffness classifications, then the medians are significantly different. The notch for a box and whiskers plot can be wider than the IQR if the number of ears is small, such as is the case for the Moderately stiff, Very stiff, and No movement subgroups.
The interpretation of the results shown in Figures 2 and 3 is limited by the factor that only 8 ears are present in the MS otoscopic group and 3 ears are present in each of the VS and IM groups. The study of the relationship between AB in each of the five otoscopic groups is a secondary goal of the project. The relationship emerges from the approach of using pneumatic otoscopy to identify ears with normal middle-ear function (as comprised of the larger N+ and SS groups). As shown in Figures 2 and 3, the 14 test ears in the MS, VS and IM groups have reduced AB compared to ears in the N+ and SS groups, and the absorbance data for these groups (especially the VS and IM groups) were within the range of AB measured in the MEE group. The study of any residual differences amongst the MS, VS and IM groups would require additional testing with larger numbers of test ears. Notwithstanding this limitation, a general pattern is that eardrums judged to have reduced mobility via pneumatic otoscopy also have reduced absorbance.
DISCUSSION
The main purpose of this study was to evaluate WATFs in predicting MEE in young children by comparing WATFs in ears with surgically confirmed fluid to ears from age-matched peers with presumably normal middle-ear function and no history of middle-ear disease or surgery. In addition, this study assessed the extent to which AB is related to TM movement as determined via pneumatic otoscopy. Our results showed that AB was reduced in ears with MEE compared to ears without MEE, especially in the frequency range of 1.5 to 3 kHz, which suggests that the middle ear is less efficient at absorbing sound energy in the mid-frequencies when MEE is present. It should be noted that these “mid-frequencies” include test frequencies higher than those used in clinical tympanometry tests. In addition, although the variability of AB is reduced in the lower frequencies, the overlap between MEE and non-MEE groups is greater at lower frequencies than at higher frequencies. This is notable because clinical tympanometric evaluations often rely on measurements at 0.226 Hz, a frequency at which variance may be small, but its diagnostic information is also limited.
Tests to predict MEE that are presently recommended in accepted clinical guidelines include pneumatic otoscopy, and either 226-Hz tympanometry or reflectometry when the results of pneumatic otoscopy are questionable.25 Although meta-analysis has reported good sensitivity and specificity for pneumatic otoscopy in making predictions of MEE,27 it has produced variable inter- and intra-tester results.29,34,35 The performance of each WATF predictor is quantified in the present study by AUC and SYM values, which is not reported in the literature for pneumatic otoscopy. Nevertheless, it is possibly to estimate SYM from previous studies when the reported sensitivity and specificity values were similar. The present study reports a SYM value of 0.90 for the CP predictor (see Table I). The CP predictor would be preferred in clinical applications because it combines the diagnostic information from the AB, YP and YM predictors. The calculation of a combined predictor score could be automated with no meaningful increase in test time. The accuracy of pneumatic otoscopy for detecting MEE, based on surgical findings at myringotomy, was described by Toner and Mains30, who reported test sensitivity of 87% and specificity of 89%. These values translate to a SYM value between 0.87 and 0.89, which would lie within the CI of SYM for each of the WATF predictors in Table I (i.e., 0.85-0.96). This suggests that a diagnostic WATF test is at least as accurate as pneumatic otoscopy in detecting MEE, and, because it is objective, it is not affected by variability associated with visual examinations of the eardrum.
Toner and Mains30 reported sensitivity and specificity values of 81 and 82%, respectively, for 226-Hz tympanometry, which corresponds to a SYM value of approximately 0.81-0.82. Such values are less than the SYM values for all WATF variables reported in Table I, which range from 0.85 to 0.90. In addition, AUC values for WATF predictors in Table I (i.e., 0.90-0.94) were larger than values reported in other studies for 226-Hz tympanometry (i.e., 0.83-0.84)39,40 and reflectometry (i.e., 0.77).40 The tympanometry and reflectometry studies did not report a CI for any summary statistic of the ROC curve, so no conclusion is possible regarding the significance of the differences across studies in these tests and the WATF tests in the present study. Nevertheless, the SYM and AUC values for WATF predictors were, in every case, higher (i.e., more accurate) than the corresponding SYM and AUC values for 226-Hz tympanometry and acoustic reflectometry tests.
It should be noted that no 226-Hz tympanometry test was performed in the present study because such a test requires additional time and pressurization of the ear canal. A goal was to minimize any interference with the clinical protocol or discomfort that the child might experience, especially just before surgery. Another factor in the present study was to avoid any effects of pressurization on the amount of the MEE that was measured during surgery, which immediately followed the acquisition of the research data.
Beers et al.54 reported an AUC of 0.97 in detecting MEE in children of age 3-12 years relative to a normal control group of age 5-6 years. This AUC is larger than that found in the present study. One difference between the studies is that the children tested in the present study were younger with a mean age of 1.8-1.9 years. A more fundamental difference is that the presence of MEE in Beers et al. was defined on the basis of pneumatic otoscopy and video otomicroscopy for one half of the ears and on the basis of elevated air-conduction thresholds, flat tympanograms, and absent transient-evoked otoacoustic emissions for the other half of the ears. The presence of MEE in the present study was defined on the basis of surgical findings at myringotomy. These differences in methodology may have contributed to the observed difference in AUC values.
The main result of the present study suggests that WATF measurements are at least as effective, and possibly more effective, in predicting the presence of MEE in children aged 0.5 to 7 years, compared to methods currently recommended by clinical guidelines. The use of an ambient-pressure test, such as the WATF test in the present study, to predict the presence of MEE may be favored over tests such as pneumatic otoscopy and tympanometry that require pressurization in the ear canal, especially in a population of children experiencing ear-related discomfort. Nevertheless, the WATF test has the option of obtaining pressurized responses if desired.45 In addition, pneumatic otoscopy is characterized by variability in interpretation29,34,35 while tympanometry may not be suitable for detecting slight changes in middle-ear mechanics that result from MEE because it tests at only a single frequency.42 The WATF test overcomes these limitations by providing an objective assessment of middle-ear function across a broad frequency region (0.25 to 8 kHz) while also maintaining good test-retest reliability46,55 and a short test time.45 The WATF response is relatively insensitive to probe placement within the ear canal (so long as an adequate seal is achieved),45 which reduces variability associated with the use of multiple testers or individual testers across multiple test sessions.
Although associations between classifications, based on pneumatic otoscopy, and AB may be tempered by the small number of observations in some classification groups (i.e., MS, VS, and IM), the findings that AB decreases as TM stiffness increases, and that AB in ears classified as N+ and SS is significantly different from AB in ears classified as VS and IM, suggests a close association between pneumatic otoscopy and AB. This is further supported inasmuch as ears with an immobile TM have similar absorbance to ears with MEE (see Figure 3). While pneumatic otoscopy and WATF have similar accuracy in predicting MEE, the objective test outcome from a WATF response eliminates the need for subjective interpretation that is associated with the use of pneumatic otoscopy. The close relationship between AB and pneumatic otoscopy ratings suggest the potential value of AB in training programs for validating pneumatic otoscopy.
CONCLUSION
WATFs measured in the ear canal were accurate predictors of MEE in young children with the most important diagnostic information (i.e., the frequency range with the least overlap) occurring at frequencies between approximately 1.5 and 3 kHz. The best overall test performance was achieved when wideband acoustic absorbance and admittance predictors were combined across the entire frequency range (0.25-8 kHz), which resulted in correct classification of 90% of both normal and MEE ears. When TM stiffness was classified according to pneumatic otoscopy, the ear absorbed less energy as TM stiffness increased over the bandwidth of frequencies selected between 0.8 and 2 kHz. When compared to pneumatic otoscopy, the absorbance in ears with no TM mobility was similar to that in ears with MEE. WATFs are at least as effective in predicting the presence of MEE in children aged 0.5 to 7 years as those methods currently recommended by clinical guidelines.
ACKNOWLEDGEMENT
The authors thank the audiologists and surgical-medical staff of Boys Town National Research Hospital for their assistance in subject recruitment and help in data collection. The authors express special thanks to Yi-Wen Liu for software development and data analysis and Michelle Gortemaker for help in data collection.
This work was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD grant numbers DC006607, DC000013, DC004662).
Footnotes
Presented in part at the American Auditory Society Annual Meeting, Scottsdale, AZ, USA, March 8-10, 2011.
Financial Disclosure Information: Douglas H. Keefe is the President of Sonicom, Inc., which is a small business aiming to commercialize medical devices including devices that can be used for aural wideband acoustic transfer function testing. In support of this research, the NIDCD awarded a STTR grant R42 DC006607 to Sonicom, Inc. as Application Organization with BTNRH as Research Institution. No other authors have any conflict of interest to disclose.
Contributor Information
John C. Ellison, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA.
Michael Gorga, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA.
Edward Cohn, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA.
Denis Fitzpatrick, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA.
Chris A. Sanford, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA.
Douglas H. Keefe, Sonicom, Inc., 8105 Cedar Street, Omaha, NE 68124, USA.
REFERENCES
- 1.Gelfand SA. Essentials of Audiology. 2nd ed Thieme; New York: 2001. [Google Scholar]
- 2.Schappert SM. Office visits for otitis media: United States, 1975-90. Advance Data From Vital and Health Statistics of the Centers for Disease Control. 1992;214:1–18. [PubMed] [Google Scholar]
- 3.Shekelle P, Takata G, Chan LS, et al. Evidence Report/Technology Assessment No. 55 AHRQ Publication No. 03-E023) Agency for Healthcare Research and Quality; Rockville, MD: 2003. Diagnosis, Natural History, and Late Effects of Otitis Media With Effusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tos M. Epidemiology and natural history of secretory otitis. American Journal of Otology. 1984;5:459–462. [PubMed] [Google Scholar]
- 5.Williamson I. Otitis media with effusion. Clinical Evidence. 2002;7:469–476. [PubMed] [Google Scholar]
- 6.Bluestone CD, Klein JO. Otitis media in infants in children. 4th ed BC Decker; Hamilton, Ontario: 2007. [Google Scholar]
- 7.Wallace IF, Gravel JS, McCarton CM, Stapells DR, Bernstein RS, Ruben RJ. Otitis media, auditory sensitivity, and language outcomes at one year. Laryngoscope. 1988;98:64–70. doi: 10.1288/00005537-198801000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Friel-Patti S, Finitzo T. Language learning in a prospective study of otitis media with effusion in the first two years of life. J Speech Hear Res. 1990;33:188–194. doi: 10.1044/jshr.3301.188. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JE, Burchinal MR, Medley LP, et al. Otitis media, hearing sensitivity, and maternal responsiveness in relation to language during infancy. J Pediatr. 1995;126:481–489. doi: 10.1016/s0022-3476(95)70476-0. [DOI] [PubMed] [Google Scholar]
- 10.Creps C, Vernon-Feagans L. Infant daycare and otitis media: Multiple influences on children’s language development. J Appl Dev Psychol. 2000;21:357–378. [Google Scholar]
- 11.Menjuk P. Relationship of otitis media to speech and language development. In: Katz J, Stecker N, Henderson D, editors. Central Auditory Processing: A Transdisciplinary Approach. Mosby; St. Louis, MO: 1992. pp. 187–198. [Google Scholar]
- 12.Pillsbury HC, Grose JH, Hall JW., III Otitis media with effusion in children. Binaural hearing before and after corrective surgery. Arch Otolaryngol Head Neck Surg. 1991;117:718–723. doi: 10.1001/archotol.1991.01870190030008. [DOI] [PubMed] [Google Scholar]
- 13.Jerger S, Jerger J, Alford BR, Abrams S. Development of speech intelligibility in children with recurrent otitis media. Ear Hear. 1983;4:138–145. doi: 10.1097/00003446-198305000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Gravel JS, Wallace IF. Listening and language at 4 years of age: effects of early otitis media. J Speech Hear Res. 1992;35:588–595. doi: 10.1044/jshr.3503.588. [DOI] [PubMed] [Google Scholar]
- 15.Brown DP. Speech recognition in recurrent otitis media: results in a set of identical twins. J Am Acad Aud. 1994;5:1–6. [PubMed] [Google Scholar]
- 16.Schilder AG, Snik AF, Straatman H, van den Broek P. The effect of otitis media with effusion at preschool age on some aspects of auditory perception at school age. Ear Hear. 1994;15:224–231. doi: 10.1097/00003446-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld RM, Madell JR, McMahon A. In: Lim DJ, Bluestone CD, Casselbrant M, Klein JO, Ogra PL, editors. Auditory function in normal hearing children with middle ear effusion; Recent Advances in Otitis Media: Proceedings of the 6th International Symposium; Hamilton, ON, Canada: BC Decker. 1996.pp. 354–356. [Google Scholar]
- 18.Besing J, Koehnke J. A test of virtual auditory localization. Ear Hear. 1995;16:220–229. doi: 10.1097/00003446-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Feagans L, Sanyal M, Henderson F, Collier A, Applebaum M. Relationship of middle ear diseases in early childhood to later narrative and attention skills. J Pediatr Psychol. 1987;12:581–594. doi: 10.1093/jpepsy/12.4.581. [DOI] [PubMed] [Google Scholar]
- 20.Lous J. Silent reading and secretory otitis media in school children. Int J Pediatr Otorhinol. 1993;25:25–38. doi: 10.1016/0165-5876(93)90007-p. [DOI] [PubMed] [Google Scholar]
- 21.Peters SAF, Grievink EM, van Bon WHJ, Schilder AGM. The effects of early bilateral otitis media with effusion on educational attainment: a prospective cohort study. J Learn Disabil. 1994;27:111–121. doi: 10.1177/002221949402700206. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JE, Burchinal MR, Zeisel SA. Otitis media in early childhood in relation to children’s school-age language and academic skills. Pediatrics. 2002;110:696–706. doi: 10.1542/peds.110.4.696. [DOI] [PubMed] [Google Scholar]
- 23.Turner RG, Nielsen DW. Application of clinical decision analysis to audiological tests. Ear Hear. 1984;5:125–133. doi: 10.1097/00003446-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Family Physicians; American Academy of Otolaryngology-Head and Neck Surgery; American Academy of Pediatrics, Subcommittee on Otitis Media With Effusion Otitis media with effusion. Pediatrics. 2004;113:1412–1429. doi: 10.1542/peds.113.5.1412. [DOI] [PubMed] [Google Scholar]
- 26.Jones WS, Kaleida PH. How helpful is pneumatic otoscopy in improving diagnostic accuracy? Pediatrics. 2003;112:510–513. doi: 10.1542/peds.112.3.510. [DOI] [PubMed] [Google Scholar]
- 27.Takata GS, Chan LS, Morphew T, Mangione-Smith R, Morton SC, Shekelle P. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. Pediatrics. 2003;112:1379–1387. doi: 10.1542/peds.112.6.1379. [DOI] [PubMed] [Google Scholar]
- 28.Harris PK, Hutchinson KM, Moravec J. The use of tympanometry and pneumatic otoscopy for predicting middle ear disease. Am J Aud. 2005;14:3–13. doi: 10.1044/1059-0889(2005/002). [DOI] [PubMed] [Google Scholar]
- 29.Nozza RJ, Bluestone CD, Kardatzke D, Bachman R. Identification of middle ear effusion by aural acoustic admittance and otoscopy. Ear Hear. 1994;15:310–323. doi: 10.1097/00003446-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Toner JG, Mains B. Pneumatic otoscopy and tympanometry in the detection of middle ear effusion. Clinical Otolaryngology. 1990;15:121–123. doi: 10.1111/j.1365-2273.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 31.Finitzo T, Friel-Patti S, Chinn K, Brown O. Tympanometry and otoscopy prior to myringotomy: issues in diagnosis of otitis media. Int J Pediatr Otrhinolaryngol. 1992;24:1001–1110. doi: 10.1016/0165-5876(92)90136-d. [DOI] [PubMed] [Google Scholar]
- 32.Cantekin EI, Bluestone CD, Fria TJ, Stool SE, Beery QC, Sabo DL. Identification of otitis media with effusion in children. Annals Otol Rhinol Laryngol. 1980;89:190–195. doi: 10.1177/00034894800890s344. [DOI] [PubMed] [Google Scholar]
- 33.Gates GA, Avery CA, Cooper JC, Hearne EM, Holt GR. Predictive value of tympanometry in middle ear effusion. Annals Otol Rhinol Laryngol. 1986;95:46–56. doi: 10.1177/000348948609500110. [DOI] [PubMed] [Google Scholar]
- 34.Rogers DJ, Boseley ME, Adams MT, Makowski RL, Hohman MH. Prospective comparison of handheld pneumatic otoscopy, binocular microscopy, and tympanometry in identifying middle ear effusions in children. Int J Pediatr Otrhinolaryngol. 2010;74:1140–1143. doi: 10.1016/j.ijporl.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Karma PH, Penttila MA, Sipila MM, Kataja MJ. Otoscopic diagnosis of middle ear effusion in acute and non-acute otitis media. I. The value of different otoscopic findings. Int J Pediatr Otrhinolaryngol. 1989;17:37–49. doi: 10.1016/0165-5876(89)90292-9. [DOI] [PubMed] [Google Scholar]
- 36.van Balen FA, Aarts AM, De Melker RA. Tympanometry by general practitioners: reliable? Int J Pediatr Otorhinolaryngol. 1999;48:117–123. doi: 10.1016/s0165-5876(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 37.Palmu A, Puhakka H, Rahko T, Takala AK. Diagnostic value of tympanometry in infants in clinical practice. Int J Pediatr Otorhinolaryngol. 1999;49:207–213. doi: 10.1016/s0165-5876(99)00207-4. [DOI] [PubMed] [Google Scholar]
- 38.Watters GW, Jones JE, Freeland AP. The predictive value of typanometry in the diagnosis of middle ear effusion. Clinical Otolaryngol. 1997;22:343–345. doi: 10.1046/j.1365-2273.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith CG, Paradise JL, Sabo DL, et al. Tympanometric finding and the probability of middle-ear effusion in 3686 infants and young children. Pediatrics. 2006;118:1–13. doi: 10.1542/peds.2005-1879. [DOI] [PubMed] [Google Scholar]
- 40.Chianese J, Hoberman A, Paradise JL. Spectral gradient acoustic reflectometry compared with tympanometry in diagnosing middle ear effusion in children aged 6 to 24 months. Arch Pediatr Adolesc Med. 2007;161:884–888. doi: 10.1001/archpedi.161.9.884. [DOI] [PubMed] [Google Scholar]
- 41.Van Camp KJ, Margolis RH, Wilson RH, Creten WL, Shanks JE. Principles of Tympanometry. ASHA; Rockville, MD: 1986. ASHA Mongraphs, No. 24. [PubMed] [Google Scholar]
- 42.Vlachou SG, Tsakanikos M, Douniadakis D, Apostolopoulos N. The change in the acoustic admittance phase angle: a study in children suffering from acute otitis media. Scand Audiol. 2001;30:24–29. doi: 10.1080/010503901750069545. [DOI] [PubMed] [Google Scholar]
- 43.Keefe DH, Ling R, Bulen JC. Method to measure acoustic impedance and reflectance coefficient. J Acoust Soc Am. 1992;91:470–485. doi: 10.1121/1.402733. [DOI] [PubMed] [Google Scholar]
- 44.Keefe DH, Feeney MP. Principles of acoustic immittance and acoustic transfer functions. In: Katz J, Burkhard RF, Medwetsky L, Hood LJ, editors. Handbook of Clinical Audiology. Lippincott, Williams & Wilkins; Baltimore, MD: 2009. pp. 125–156. [Google Scholar]
- 45.Liu Y, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP, Keefe DH. Wideband absorbance tympanometry using pressure sweeps: system development and results on adults with normal hearing. J Acoust Soc Am. 2008;124:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunter LL, Tubaugh L, Jackson A, Propes S. Wideband middle ear power measurement in infants and children. J Am Acad Audiol. 2008;19:309–324. doi: 10.3766/jaaa.19.4.4. [DOI] [PubMed] [Google Scholar]
- 47.Keefe DH, Bulen JC, Arehart KH, Burns EM. Ear canal impedance and reflection coefficient in human infants and adults. J Acoust Soc Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- 48.Voss SE, Allen JB. Measurement of acoustic impedance and reflectance in the human ear canal. J Acoust Soc Am. 1994;95:372–384. doi: 10.1121/1.408329. [DOI] [PubMed] [Google Scholar]
- 49.Margolis RH, Saly GL, Keefe DH. Wideband reflectance tympanometry in normal adults. J Acoust Soc Am. 1999;106:265–280. doi: 10.1121/1.427055. [DOI] [PubMed] [Google Scholar]
- 50.Feeney MP, Sanford CA. Age effects in the human middle ear: wideband acoustical measures. J Acoust Soc Am. 2004;116:3546–3558. doi: 10.1121/1.1808221. [DOI] [PubMed] [Google Scholar]
- 51.Shahnaz N, Bork K. Wideband reflectance norms for Caucasian and Chinese young adults. Ear Hear. 2006;27:774–788. doi: 10.1097/01.aud.0000240568.00816.4a. [DOI] [PubMed] [Google Scholar]
- 52.Vander Werff KR, Prieve BA, Georgantas LM. Test-retest reliability of wideband reflectance measures in infants under screening and diagnostic test conditions. Ear Hear. 2007;28:669–681. doi: 10.1097/AUD.0b013e31812f71b1. [DOI] [PubMed] [Google Scholar]
- 53.Sanford CA, Feeney MP. Effects of maturation on tympanometric wideband acoustic transfer functions in human infants. J Acoust Soc Am. 2008;124:2106–2122. doi: 10.1121/1.2967864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beers AN, Shahnaz N, Westerberg BD, Kozak FK. Wideband reflectance in normal Caucasian and Chinese school-aged children and in children with otitis media with effusion. Ear Hear. 2010;31:221–233. doi: 10.1097/AUD.0b013e3181c00eae. [DOI] [PubMed] [Google Scholar]
- 55.Werner LA, Levi EC, Keefe DH. Ear-canal wideband acoustic transfer functions of adults and two- to nine-month-old infants. Ear Hear. 2010;31:587–598. doi: 10.1097/AUD.0b013e3181e0381d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keefe DH, Folsom RC, Gorga MP, Vohr BR, Bulen JC, Norton SJ. Identification of neonatal hearing impairment: ear canal measurements of acoustic admittance and reflectance in neonates. Ear Hear. 2000;21:443–461. doi: 10.1097/00003446-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Abdala C, Keefe DH, Oba SI. Distortion product otoacoustic emission suppression tuning and acoustic admittance in human infants: birth through 6 months. J Acoust Soc Am. 2007;121:3617–3627. doi: 10.1121/1.2734481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keefe DH, Abdala C. Theory of foreward and reverse middle ear transmission applied to otoacoustic emissions in infant and adult ears. J Acoust Soc Am. 2007;121:978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahnaz N. Wideband reflectance in neonatal intensive care units. J Am Acad Audiol. 2008;19:419–429. doi: 10.3766/jaaa.19.5.4. [DOI] [PubMed] [Google Scholar]
- 60.Sanford CA, Keefe DH, Liu Y, et al. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: test performance of wideband acoustic transfer functions and 1-kHz tympanometry. Ear Hear. 2009;30:635–652. doi: 10.1097/AUD.0b013e3181b61cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunter LL, Feeney MP, Lapsley Miller JA, Jeng PS, Bohning S. Wideband reflectance in newborns: normative regions and relationship to hearing-screening results. Ear Hear. 2010;31:599–610. doi: 10.1097/AUD.0b013e3181e40ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piskorski P, Keefe DH, Simmon JL, Gorga MP. Prediction of conductive hearing loss based on acoustic ear canal response using a multivariate clinical decision theory. J Acoust Soc Am. 1999;105:1749–1764. doi: 10.1121/1.426713. [DOI] [PubMed] [Google Scholar]
- 63.Hunter LL, Bagger-Sjöbäck D, Lundberg M. Wideband reflectance associated with otitis media in infants and children with cleft palate. Int J Audiol. 2008;47(suppl 1):S57–S61. doi: 10.1080/14992020802294057. [DOI] [PubMed] [Google Scholar]
- 64.Allen JB, Jeng PS, Levitt H. Evaluation of human middle ear function via an acoustic power assessment. J Rehabil Res Dev. 2005;42:63–78. doi: 10.1682/jrrd.2005.04.0064. [DOI] [PubMed] [Google Scholar]
- 65.Feeney MP, Grant IL, Marryott LP. Wideband energy reflectance measurements in adults with middle ear disorders. J Speech Lang Hear Res. 2003;46:901–911. doi: 10.1044/1092-4388(2003/070). [DOI] [PubMed] [Google Scholar]
- 66.Shahnaz N, Bork K, Polka L, Longridge N, Bell D, Westerberg BD. Energy reflectance and tympanometry in normal and otosclerotic ears. Ear Hear. 2009;30:219–233. doi: 10.1097/AUD.0b013e3181976a14. [DOI] [PubMed] [Google Scholar]
- 67.Keefe DH, Simmons JL. Energy transmittance predicts conductive hearing loss in older children and adults. J Acoust Soc Am. 2003;114:3217–3238. doi: 10.1121/1.1625931. [DOI] [PubMed] [Google Scholar]
- 68.Hunter LL, Margolis RH. Effects of tympanic membrane abnormalities on auditory function. J Am Acad Audiol. 1997;8:431–446. [PubMed] [Google Scholar]
- 69.Van Trees HL. Detection, Estimation, and Modulation Theory. Wiley Interscience; New York: 1967. [Google Scholar]
- 70.Pepe MS. The Statistical Evaluation of Medical Tests For Classification and Prediction. Oxford University Press; Oxford: 2003. [Google Scholar]
- 71.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–75. [Google Scholar]


