Abstract
Declarative memory is known to depend on the medial temporal lobe memory system. Recently, there has been renewed focus on the relationship between the basal ganglia and declarative memory, including the involvement of striatum. However, the contribution of striatum to declarative memory retrieval remains unknown. Here, we review neuroimaging and neuropsychological evidence for the involvement of the striatum in declarative memory retrieval. From this review, we propose that, along with the prefrontal cortex (PFC), the striatum primarily supports cognitive control of memory retrieval. We conclude by proposing three hypotheses for the specific role of striatum in retrieval: (1) Striatum modulates the re-encoding of retrieved items in accord with their expected utility (adaptive encoding), (2) striatum selectively admits information into working memory that is expected to increase the likelihood of successful retrieval (adaptive gating), and (3) striatum enacts adjustments in cognitive control based on the outcome of retrieval (reinforcement learning).
Introduction
Declarative memory retrieval refers to the conscious recovery of previously stored experiences, facts, and concepts that are verifiable through verbal report (Tulving, 1972). It has long been known that the medial temporal lobe (MTL) system is necessary for the formation, consolidation, and retrieval of declarative memories (Cohen, Eichenbaum, & Poldrack, 1997; Squire, 1992). By contrast, other types of long-term memory, such as skill learning or classical conditioning do not appear to require the MTL memory system (Corkin, 1968; Knowlton, Squire, & Gluck, 1994; Cohen, Eichenbaum, & Poldrack, 1997). Rather, these forms of “non-declarative” memory are strongly associated with the reward driven mechanisms of the basal ganglia (Packard, Hirsh, & White, 1989; Knowlton, Mangels, & Squire, 1996; Cohen, Eichenbaum, & Poldrack, 1997; Shohamy et al., 2004). However, mounting evidence from both neuroimaging and neuropsychological studies of declarative memory have renewed focus on interactions between the declarative and non-declarative systems, and have highlighted the potential role of the basal ganglia, including striatum (Figure 1A), in declarative memory performance both at encoding and retrieval (Shohamy & Adcock, 2010; Cohn et al., 2010; Han et al, 2010; Long et al., 2010; Poldrack & Foerde, 2008; Moustafa & Gluck, 2011).
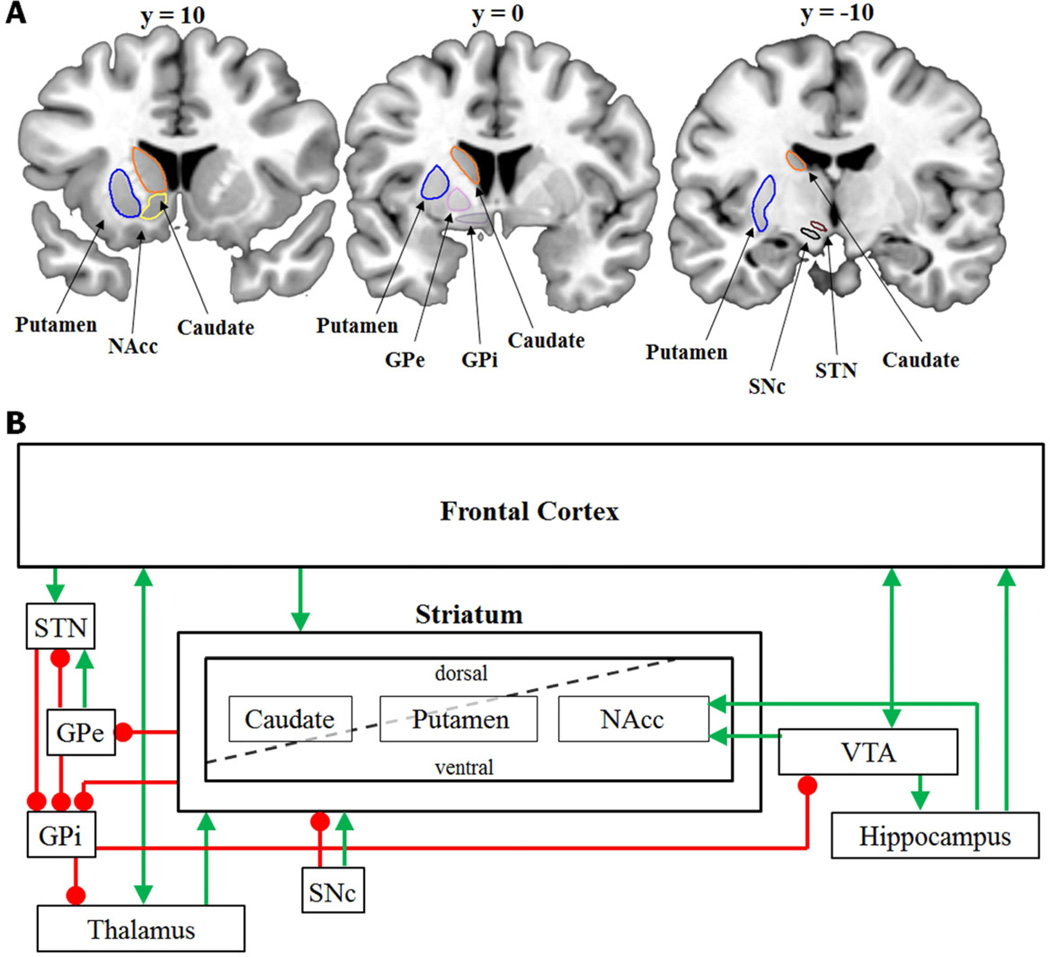
Figure 1.
(A) Basic anatomy of the basal ganglia including striatum (caudate, putamen, and nucleus accumbens [NAcc]); GPe: external segment of the globus pallidus; GPi: internal segment of the globus pallidus; SNc: substantia nigra pars compacta; and STN: subthalamic nucleus. (B) Schematic of frontal-striatal circuitry and the VTA-hippocampus loop. Projections from frontal cortex through the striatum (caudate, putamen, and nucleus accumbens), subthalamic nucleus (STN), globus pallidus, and thalamus drive adaptive gating of working memory representations. The VTA-hippocampal loop includes direct projections from ventral tegmental area (VTA) to hippocampus, and a circuit through the nucleus accumbens (NAcc) and globus pallidus from the hippocampus to VTA. The striatum is frequently characterized by a dorsal/ventral division (e.g. Bornstein & Daw, 2011), with different subregions of caudate and putamen existing on both sides of the divide. Green arrows indicate excitatory connections; red circles indicate inhibitory connections. GPe: external segment of the globus pallidus; GPi: internal segment of the globus pallidus; SNc: substantia nigra pars compacta.
Outside of the long-term memory domain, there has been growing recognition of a broader role for striatal-frontal interactions beyond basic motor control. Specifically, recent years have seen a growth in our understanding of the mechanisms by which striatum supports higher cognitive functions like working memory, decision making, categorization, and cognitive control (Graybiel & Mink, 2009; Doll & Frank, 2009; Cools, 2011; Seger and Miller, 2010; Landau et al., 2009; Stelzel et al., 2010; Lewis et al., 2004; Badre and Frank, 2012; Badre et al. 2012). However, to date, we still have a limited understanding of the role of these striatal mechanisms in declarative memory retrieval.
Here, we review evidence for the involvement of the striatum in declarative memory retrieval. First, based on evidence from neuroimaging and neuropsychological studies of declarative memory, we argue that, along with the prefrontal cortex (PFC), the striatum supports the cognitive control of memory retrieval. Then, leveraging models of reinforcement learning and cognitive control theory outside of the memory domain, we propose a set of novel hypotheses regarding the potential mechanistic role of the striatum in declarative memory as a basis for future research.
Striatal responses to item recognition
An adaptive function of the declarative memory system is the ability to discriminate items and contexts with which an animal has prior experience versus those that are novel. The ability to recognize previously encountered items is known to require MTL structures, including perirhinal, parahippocampal, and hippocampal cortex (Squire, 1992; Schacter & Wagner, 1999; Eichenbaum, Yonelinas, & Ranganath, 2007; Squire & Wixted, 2011). Nevertheless, the wider view afforded by functional neuroimaging studies has provided initial evidence for striatal involvement during item discrimination; though this system has rarely been a focus of these experiments.
In the item recognition paradigm, participants first encode a series of items, usually words or pictures, and are then shown a mix of items that they had seen previously during encoding along with new items that have not been seen before. For each item, the participant judges whether the item has been seen previously (old) or not (new). Thus, contrasting trials on which participants correctly judged an old item as “old” (hits) against trials on which a participant correctly judged a new item as “new” (correct rejections [CR]) probes the neural correlates of “retrieval success”.
Since the earliest event-related fMRI studies of the item-recognition task (i.e., Buckner et al., 1998; Donaldson, Peterson, Ollinger, & Buckner, 2001; Rombouts et al., 2001), retrieval success has yielded striatal activation. Moreover, this effect has been replicated across multiple variants of encoding tasks and stimulus materials. Spaniol and colleagues (2009) analyzed 81 fMRI studies of episodic memory, a subset of which included contrasts of encoding success (subsequent hits greater than misses) and/or retrieval success (hits greater than CR). A quantitative meta-analytic procedure indicated that retrieval success consistently activated striatum across studies, including both dorsal striatum in the left caudate and ventral striatum in regions of caudate, putamen, and nucleus accumbens (also see Kim, 2011). Figure 2 shows this effect in an updated recoding and reanalysis of these data conducted for this review. Moreover, a contrast between retrieval success and encoding success showed that the ventral caudate was more reliably associated with retrieval success than encoding success across studies. Importantly, retrieval success in striatum is not dependent on an actual prior experience with an item. Rather, striatum shows greater activation for false alarms (new items incorrectly judged as old) than CR or misses (Abe et al, 2008). Thus, like most regions showing retrieval success effects (Wagner et al., 2005), striatal activation tracks the perception of an item as being old during a recognition memory task, rather than it having been previously encountered on the study list. Thus, striatal retrieval success effects cannot be trivially explained based on a prior association with positive reinforcement formed at encoding.
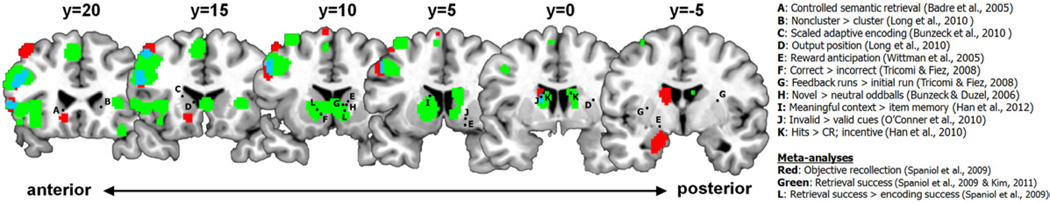
Figure 2.
Comparison of activations from meta-analyses and neuroimaging data regarding basal ganglia involvement in novelty detection, reward processing, and declarative memory. Points labeled A through K are the basal ganglia foci reported in the respective studies. The red and green activations are from our recoding and reanalysis of the relevant activations from the Spaniol et al. (2009) and Kim (2011) meta-analyses in GingerALE Version 2.1.1 (brainmap.org) in Talaraich space; their overlap is marked in blue. Points labeled L are the foci from the meta-analysis reported by Spaniol et al. (2009). See the main text and individual references for more information about individual contrasts. CR: correct rejections.
Generally consistent with the neuroimaging data, deficits in patients with Parkinson’s disease (PD) – a disease arising from degeneration of cells in the substantia nigra that are a primary source of dopaminergic input into the striatum (Figure 1B) – indicate that the basal ganglia are broadly necessary for normal levels of recognition memory performance. In particular, though not suffering from the profound amnesias accompanying MTL damage, PD patients do demonstrate deficits in recognition memory relative to controls in studies with sufficient power (Whittington, Podd, & Kan, 2000).
Accounting for these recognition deficits in PD has proven difficult and multifaceted. Across studies, deficits have been evident sometimes in recollection (Barnes, Boubert, Harris, Lee, & David, 2003; Edelstyn, Mayes, Condon, Tunnicliffe, & Ellis, 2007; Drag, Bieliauskas, Kasniak, Bohnen, & Glisky, 2009; Edelstyn, Shepherd, Mayes, Serhman, & Ellis, 2010) and sometimes in familiarity (Davidson, Anaki, Saint-Cyr, Chow, & Moscovitch, 2006; Weiermann, Stephan, Kaelin-Lang, & Meier, 2010). Moreover, there seems ample evidence that at least a portion of memory deficits observed in PD arise from a failure to engage in effective encoding strategies (Knoke, Taylor, & Saint-Cyr, 1998; Vingerhoets, Vermeule, & Santens, 2005). However, a recent study has provided convincing evidence for a recollection deficit in PD when encoding strategy was controlled. Cohn et al. (2010) had PD patients and age-matched controls study word-pairs under shallow and deep encoding conditions, and estimated familiarity and recollection using the process-dissociation procedure (Yonelinas, Regher, & Jacoby, 1995). Relative to shallow encoding, deep encoding normalized the patients’ familiarity relative to the controls, demonstrating the efficacy of the deep encoding manipulation. However, whereas recollection improved for controls when items were deeply encoded, patients showed no improvement in recollection for deeply encoded items. As also noted by the authors, this retrieval deficit could be interpreted as a failure of the “executive” component of retrieval, such that patients did not take strategic advantage of the elaborative encoding strategy. We will return to the question of executive (i.e. cognitive control) deficits below. However, regardless of the specific source of the deficit, the evidence for a component of impaired retrieval in PD from this and prior work seems compelling.
It should be noted, however, that PD is not a selective striatal disorder, making it difficult to assign deficits to striatum specifically, as opposed to frontal disruption or dysfunctional dynamics within the broader basal ganglia system. However, recognition deficits in PD indicate that the nigra-striatal dopamine system is broadly necessary for retrieval. Moreover, declarative memory deficits have been demonstrated in a variety of disease conditions involving the nigra-striatal dopamine system such as Huntington’s Disease, which is more localizable to striatum, and schizophrenia (e.g., Hodges et al., 1990; van Oostrom et al., 2003; Solomon et al., 2007). Thus, when considered together with the neuroimaging data that localizes retrieval effects within the striatum, the evidence begins to converge on a necessary role for these structures during retrieval. However, as will be discussed below, this role likely relates to the way that memory retrieval is modulated by retrieval goals, as opposed to being a source of the mnemonic signal itself.
Striatal responses to item novelty
The apparent sensitivity of striatum to perceived oldness is, perhaps, surprising in light of the established association of the broader mesolimbic/nigra-striatal dopamine system with the opposite property, namely item novelty. Physiological recording studies in the rodent (Schultz, 1998; Horvitz et al., 1997; Horvitz, 2000) have observed activation to stimulus novelty of cells in the ventral tegmental area (VTA) and substantia nigra (SN). Importantly, novelty responses in these cells are modulated by salience and goal-relevance of the novel stimulus and are separable experimentally from the established responses of these cells to expected reward (e.g., Horvitz, 2000). Similar effects of item novelty SN/VTA have also been observed in human fMRI studies (Bunzeck & Düzel, 2006) and are again separable from reward-related activation.
Novelty responses in the SN/VTA are hypothesized to arise via inputs from the hippocampus (Lisman & Grace, 2005), which computes the novelty of encountered items. Novelty responses in VTA neurons, in turn, are hypothesized to project back to hippocampus where they may enhance encoding of the novel item through dopaminergic modulation of hippocampal long-term potentiation (LTP). This hippocampal-VTA loop can serve the adaptive function of selectively enhancing encoding for novel items that are behaviorally relevant for the animal (Lisman & Grace, 2005; Shohamy & Adcock, 2010). Evidence from human fMRI studies has been consistent with this hypothesis, demonstrating that novelty at encoding elicits responses in VTA/SN that are associated with beneficial effects on subsequent memory (Wittmann et al., 2007; Schott et al., 2004; Krebs et al., 2009).
Importantly, as noted above, VTA/SN cells also provide dopaminergic input into the striatum (Figure 1B) where the information they convey about expected reward and other behaviorally relevant features of an input, like novelty, can influence learning, action selection, and decision-making. For example, when harvesting reward in a stochastic environment, strategically directing exploratory behavior to novel items has the potential to glean the most new information about that environment (Kakade & Dayan, 2002; Daw et al., 2006; Frank et al., 2009; Badre et al., 2012). Indeed, striatal novelty responses have been specifically associated with novelty-driven choices during economic decisions (Guitart-Masip et al., 2010; Wittmann et al., 2008; Krebs et al., 2009). Moreover, many studies citing SN/VTA activation in response to novelty, also report responses to novel greater than familiar items in the striatum (e.g. Bunzeck & Duzel, 2006; Guitart-Masip et al., 2010). Notably, these activations fall in close proximity to those associated with retrieval success (Figure 2).
Thus, considered together with retrieval success effects, the evidence for novelty responses in the striatum argues against obligatory coding of item oldness in striatum as a consequence of episodic retrieval. Rather, striatal responses to episodic memory signals are likely modulated depending on the adaptive significance of “oldness” or “newness” to the animal’s current actions and desired outcomes.
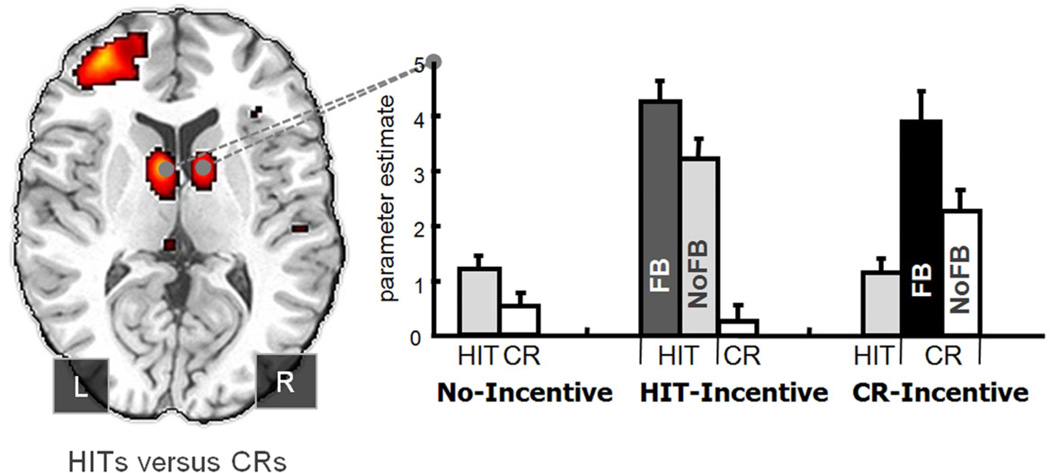
Two recent findings provide support for this hypothesis. Bunzeck et al. (2010) showed that responses in the striatum are scaled adaptively based on expectations of the relative novelty and oldness of items in the environment. Han et al. (2010) more directly manipulated the goal-relevance of item novelty versus oldness and revealed a similar dynamic flexibility in striatal responses. Specifically, retrieval goals were manipulated by associating either “old” or “new” responses with potential monetary reward. When “old” responses were incentivized, participants earned money for correct old responses (hits) and lost money for incorrect old responses (false alarm) and neither gained nor lost money for “new” responses (and vice versa when “new” was incentivized). Activity in the caudate tracked the incentivized response independent of whether the item was studied or novel (Figure 3). It should be noted that this pattern was seen regardless of whether or not participants received explicit feedback after their response, suggesting that striatal activity can be driven by satisfaction of internal goals and incentives.
Figure 3.
Striatal activation modulated by incentive in Han et al. (2010). Activation in caudate tracking the incentivized response - either hits or correct rejections (CR) – regardless of whether or not explicit feedback (FB) was present, suggesting that striatal activity can be driven by incentives and goal-relevant responses. (Figure adapted from Han et al., 2010; Will ask for permission to reprint
Of course, a key question is whether these results can be reconciled with retrieval success effects, when there is no overt incentive to locate old versus new items. First, as is evident in Figure 2, the subregion of caudate that demonstrated these dynamic effects matched closely that observed across studies of retrieval success. Second, in a condition where neither response was incentivized, Han and colleagues found greater activity for hits compared to correct rejections, consistent with previous work. Similarly, striatal activity was seen for hits even when new responses were incentivized. Thus, all else being equal, participants subjectively valued “old” responses more than “new” responses when performing recognition memory tasks.
In summary, the evidence from studies of retrieval success and novelty detection indicate that striatum plays a role in the basic ability to behave according to the oldness or novelty of an item. Though in light of the qualitative differences in the severity of memory deficits accompanying striatal versus MTL dysfunction, it is unlikely that striatum is the source of memory signals conveying oldness versus novelty, which is the province of the MTL memory system. Accordingly, as with perceptual and other inputs to the striatal system, MTL signals coding item novelty or oldness will elicit striatal responses dependent on the value of this information for current behavioral goals.
Importantly, however, goals need not be restricted to outcomes achieved through overt behavior. Rather, the process of retrieval itself can be conducted with the expectation of a particular information retrieval outcome. For example, when trying to remember a recent conversation with a good friend, we might try thinking of our friend’s face as a cue. We adopt this strategy with the implicit expectation that it will yield an outcome that meets our goal, namely remembering our previous conversation. To distinguish this type of outcome from an exogenous reward or behavioral goal, we will refer to this type of desired information retrieval outcome as a retrieval goal. In what follows, we will argue that the striatum is particularly important for declarative memory when cognitive control is required to achieve a retrieval goal.
Striatum and the cognitive control of memory
The ability to internally modulate ongoing processing based on goals, expectations, and strategies is generally referred to as cognitive control. As introduced above, in the context of memory, cognitive control mechanisms are important for guiding and monitoring retrieval in order to achieve a particular retrieval goal. Cognitive control of memory has an established dependence on frontal lobe function, evident in the unique memory impairments of frontal lobe patients. In contrast to the profound amnesia seen in patients with damage to the hippocampus and associated MTL areas (Scoville & Milner, 1957; Squire, 1986, 1992), patients with damage to PFC demonstrate deficits in memory tasks that involve strategic control of memory, goal-directed manipulation of mnemonic information, or overcoming interference (Moscovitch, 1992; Stuss et al., 1994; Wheeler et al., 1995; Aly et al., 2011; Thompson-Schill et al., 1998). Neuroimaging studies have contributed specificity, highlighting different frontal systems in support of separate control processes that contribute to these demanding retrieval tasks (e.g. Badre et al., 2005; Badre & Wagner, 2007; Buckner, 1996; Buckner et al., 1998; Poldrack et al., 1999; Anderson et al., 2004; Kuhl et al., 2007; Yonelinas, Otten, Shaw, & Rugg, 2005; Anderson et al., 2008; Gallo, McDonough, & Scimeca, 2010; Long, Oztekin, & Badre, 2010). Importantly, similar lines of neuroimaging and neuropsychological evidence also implicate the striatum in the cognitive control of declarative memory retrieval.
Cognitive control of episodic retrieval
Within the episodic retrieval domain, source memory tasks place explicit demands on cognitive control. In a source memory experiment, participants are required to verify a specific detail from a prior encoding event, such as indicating what type of task was performed with the item. In these tasks, the retrieval goal is explicit and highly specific, and so retrieval must be directed to successful recovery of only the task-relevant “source” detail to exclusion of other competing details. Thus, source memory decisions involve greater demands on cognitive control mechanisms than do simple item recognition decisions.
Contrasts between source and item recognition memory consistently locate activation in a network of frontal and parietal regions that include the striatum. In their meta-analysis, Spaniol et al. (2009) reported consistent source memory effects (i.e., “objective recollection”) in left dorsal caudate, overlapping with the left dorsal striatal focus observed for retrieval success (Figure 2). In our reanalysis and recoding of these data, we found that the effects in caudate were evident both for studies contrasting correct source versus correct item decisions and those contrasting correct versus incorrect source decisions. Thus, the preferential effects of source memory in caudate were neither simply due to performing the more difficult source task nor merely to successful retrieval, irrespective of whether it was goal directed or not.
Importantly, the association of striatum with source memory relative to item decisions is not necessarily reflective of the contribution of recollection versus familiarity in these two types of tasks. Studies that have distinguished between spontaneous recollection versus familiarity during item recognition (such as is assessed by using the remember/know procedure) have not consistently located activation in the striatum when participants merely experienced recollection relative to familiarity. Direct contrast of source retrieval versus recollection during item recognition indicated that left caudate was more consistently observed across studies of source memory (Spaniol et al., 2009). Thus, the need for cognitive control, as opposed to the mere occurrence of recollection, modulates striatal activation during episodic memory retrieval.
PD patients also demonstrate consistent deficits in cognitive control of memory. In general, PD patients show greater deficits in less structured retrieval contexts, such as free recall paradigms, relative to recognition memory paradigms (Taylor, Saint-Cyr, & Lang, 1990; Dubois, Boller, Pillon, & Agid, 1991; Zgaljardic et al., 2003). Though likely partially arising from ineffective encoding (Knoke, Taylor, & Saint-Cyr, 1998; Vingerhoets, Vermeule, & Santens, 2005), their deficits on these tasks could also be traced to a failure to employ effective retrieval strategies. For example, studies using the California Verbal Learning Test (Delis, et al., 1987) have shown that PD patients show decreased semantic clustering at retrieval relative to controls (van Oostrom et al., 2003; Bronnick et al., 2011). Thus, deficits in recall among PD patients may partially be traced to a failure to effectively employ strategic control processes at retrieval.
Cognitive control during memory retrieval is also important to overcome interference, such as that arising through automatic retrieval of irrelevant information. PD patients show difficulty in overcoming such memory interference (Helkala et al., 1989; Rouleau et al., 2001; but see Sagar et al., 1991). Again, though likely partly due to encoding, these effects may also be attributable to retrieval deficits. For example, Crescentini and colleagues (2011) employed a part-list cuing paradigm designed to induce interference via external retrieval cues. PD patients and healthy age-matched controls studied separate word lists under shallow and deep encoding. Following shallow encoding, both groups showed decreased retrieval in the interference condition relative to a non-interference control. In contrast, following deep encoding, control participants showed equivalent performance in the interference and control condition, while the patients still showed impaired retrieval in the interference condition. Thus, akin to the result from Cohn et al. (2010) in recognition memory, this part-list cueing effect could be interpreted as a failure to effectively take advantage of a good encoding strategy at retrieval; in this case, in order to overcome interference.
Cognitive Control of Semantic Retrieval
Striatal involvement in the cognitive control of declarative memory retrieval generalizes beyond MTL-dependent episodic memory to include semantic memory retrieval. Semantic memory refers to knowledge of facts, concepts, and word meanings that are independent of a specific encoding context and that may be stored in a distributed neocortical representation outside of the medial temporal lobe (Tulving, 1972; McClelland & Rogers, 2003). As with episodic retrieval, access to semantic knowledge can be bottom up and cue driven or it can be goal directed, requiring cognitive control (Badre & Wagner, 2007). Unlike episodic memory, however, it is easier to isolate observed effects, such as in patients, as arising during retrieval rather than encoding. Here again, the evidence generally suggests that the striatum is important for control of semantic memory retrieval.
Badre et al. (2005) investigated the neural systems supporting the cognitive control of semantic memory retrieval. This study focused on the contribution of left ventrolateral PFC (VLPFC) to different forms of cognitive control of memory retrieval. In a reanalysis conducted for this review, a manipulation of control semantic retrieval located activation in the left dorsal caudate (Figure 2). Perhaps consistent with this finding, a recent study from Han et al. (2012) found that VLPFC was preferentially engaged during a demanding retrieval task (source memory vs. item memory), but only for semantically meaningful items, suggesting that VLPFC was engaged in semantic elaboration to enhance retrieval. The caudate showed a qualitatively identical pattern of activation. Thus, as with the Badre et al., (2005) result noted above, activation in caudate is observed under the same conditions requiring cognitive control of semantic memory that engaged VLPFC.
Consistent with the imaging data, at least one study has located interference-induced deficits in semantic retrieval in PD patients. Compared to age-matched controls, PD patients showed an impaired ability to produce a semantically related verb when presented with a noun (Crescentini et al., 2008). The deficit was greatest in a condition where there was no strongly associated response for the presented stimulus, and instead many weakly associated target verbs.
Hence, as with episodic retrieval, the striatum likely interacts with the PFC to play a causal role in the goal-directed retrieval and selection of semantic information from memory. Importantly, this suggests fronto-striatal cricuits may play a similar role in the cognitive control of both episodic and semantic retrieval. However, future research will need to test whether this common function in semantic versus episodic memory is instantiated the same or separable fronto-striatal circuits.
Hypotheses for the role of striatum in declarative memory retrieval
From the preceding review, it seems evident that the striatum plays a necessary role in optimal declarative retrieval performance, particularly under conditions requiring the cognitive control of memory. In this way, the contribution of striatum appears to mirror that of the frontal cortex during declarative memory tasks (Stuss et al., 1994; Wheeler et al., 1995; Aly et al., 2011; Thompson-Schill et al., 1998). However, research on the neural mechanisms of cognitive control and reinforcement learning, outside of the context of memory, has suggested that striatum and frontal cortex have distinct but complementary roles (Braver & Cohen, 2000; Cools, Clark, & Robbins, 2004; O’Reilly & Frank, 2006; McNab & Klingberg, 2008; Cools, 2011; Badre & Frank, 2012). In particular, whereas lateral PFC supports cognitive control by sustaining task-relevant information in working memory (i.e., Miller and Cohen, 2001), the striatum plays a key role in flexibly updating and selecting from among candidate frontal motor or cognitive representations based on their utility or adaptive value for current goals (Mink 1996; Gurney et al. 2001; Brown et al. 2004; Cools, 2011; Frank and Badre, 2012). In this way, fronto-striatal circuits allow for separable maintenance and updating (Hochreiter and Schmidhuber 1997), with striatum playing a key role in mapping acquired value/utility to action selection.
Drawing on this basic cognitive control and reinforcement learning literature, we propose three hypotheses for striatal mechanisms during declarative memory retrieval: (1) Striatum modulates the re-encoding of retrieved items in accord with their expected utility (i.e., adaptive encoding), (2) striatum selectively admits information into working memory that is expected to increase the likelihood of successful retrieval (i.e., adaptive gating), and (3) striatum enacts adjustments in cognitive control based on the outcome of retrieval (i.e., reinforcement learning). These hypotheses are not intended as an exhaustive list nor are they mutually exclusive. However, each accounts for a portion of the extant data on striatal involvement in declarative memory (see Table 1) and has some limited evidence in its support.
Table 1.
Evidence regarding striatum in declarative memory retrieval
| Effect | Example Reference |
|---|---|
| Neuroimaging | |
| · Retrieval success (hits > CR) during item recognition | Spaniol et al., 2009 |
| · Perceived retrieval success (FA > CR) | Abe et al., 2008 |
| · Retrieval success > encoding success | Spaniol et al., 2009 |
| · Novelty responses during novelty detection | Bunzeck et al., 2006 |
| · Adaptive scaling based on behavioral significance/incentive value of familiarity/novelty | Bunzeck et al., 2010 |
| · Incentive value of old or new memory response | Han et al., 2010 |
| · Objective recollection (source > item recognition) | Spaniol et al., 2009 |
| · Retrieval of weak semantic associates | Badre et al., 2005 |
| · Clustered > non-clustered items during free recall | Long et al., 2010 |
| · Increases with output position during free recall | Long et al., 2010 |
| · Violation of expectations during retrieval | O’Conner et al., 2010 |
| Neuropsychology | |
| · Recognition memory deficits | Whittington, Podd, & Kan, 2000 |
| · Recollection deficits | Edelstyn, Shepherd, Mayes, Serhman, & Ellis, 2010 |
| · Familiarity deficits | Weiermann, Stephan, Kaelin-Lang, & Meier, 2010 |
| · Impaired recollection in deep LoP encoding condition | Cohn et al., 2010 |
| · Impaired familiarity in shallow LoP encoding condition | Cohn et al., 2010 |
| · Decreased semantic clustering | Van Oostrom, et al., 2003 |
| · Difficulty overcoming retrieval interference | Crescentini et al., 2011 |
| · Deficits in verb production based on semantic-relatedness | Crescentini et al., 2008 |
Hypothesis 1: Retrieval as adaptive encoding
Striatal activation during declarative memory retrieval may serve to modulate re-encoding of previously encoded items as a function of their behavioral relevance and their likelihood of future utility. The goal of memory retrieval may be expressed as the recovery of items that have an expected utility for an agent exceeding the costs associated with retrieval itself (Anderson & Milson, 1989). From this perspective, it is important for the availability of items in memory to be prioritized by their expected utility, particularly in a given task context. Among the various cues to utility for a given item is its history of prior use: items that were retrieved in a particular context previously are more likely than others to be useful in that context again. So, retrieval itself is an important cue to the utility of an item. Indeed, analyses of retrieval that leverage prior use statistics – both in human declarative memory and in other analogous information retrieval contexts like library book withdrawals or Google search queries – account for a wide range of retrieval phenomena (Burrell, 1980; Anderson & Milson, 1989; Brin & Page, 1998; Griffiths, Steyvers, and Firl, 2007). Thus, it is adaptive to have a means of prioritizing memories based on context-dependent prior utility.
Striatal dopamine signals elicited by retrieval could provide one mechanism by which memories are strengthened in accord with their context-dependent utility. It is well established that classical midbrain dopamine structures, such as the SN and VTA, along with medial prefrontal cortex, and ventral and dorsal striatum respond as a function of expected utility (e.g. Knutson et al., 2001a, 2001b, 2005). Projections from the midbrain to hippocampus can support modulation of hippocampal encoding by cells in these regions. For instance, dopamine can modulate synaptic change via long-term potentiation (LTP) within hippocampus, such as by decreasing LTP thresholds within CA1 fields (Li et al., 2003; Jay, 2003; Lemon & Manahan-Vaughan, 2006). Thus, the nigra-striatal dopamine system is generally well suited for coordinating dopaminergic modulation of hippocampal encoding while processing items associated with high expected utility (Shohamy & Adcock, 2010).
Recent evidence directly supports the hypothesis that the nigra-striatal dopamine system modulates hippocampal encoding as a function of the expected utility of an item (reviewed in Shohamy & Adcock, 2010), albeit not during retrieval itself. As already discussed, the hippocampal-VTA loop is thought to enhance memory for novel items in an adaptive fashion (Schott et al., 2004; Wittmann et al, 2007; Krebs et al., 2009). Moreover, two recent studies have provided evidence for dopaminergic modulation at encoding in accord with anticipated reward statistics. Wittmann et al. (2005) demonstrated that cues predicting subsequent reward lead to greater activation in ventral striatum and midbrain relative to pictures that did not predict reward. Moreover, activation in these striatal and midbrain regions was predictive of subsequent memory at the longer test delay for the rewarded but not the neutral pictures. By contrast, hippocampus showed subsequent memory effects for both the rewarded and neutral items and did not differentiate the two. Adcock and colleagues (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006) more directly incentivized retrieval itself, by providing participants a cue indicating that remembering an upcoming picture during a later recognition test would be worth either high or low reward. Again, regions of VTA and ventral striatum (nucleus accumbens) showed greater activation to high-reward cues. Moreover, correlation between these regions and hippocampus was positively correlated with enhanced subsequent memory. Thus, these results demonstrate that the basal ganglia can modulate hippocampal encoding to enhance memory based on an estimate of future, as opposed to immediate, utility.
Though theorizing has primarily focused on initial encoding, a similar adaptive encoding account could be extended to nigra-striatal involvement during retrieval, as well. As noted above, the successful retrieval of an item from memory is itself evidence that this item holds some utility in the current context. Thus, it is generally adaptive to increase the likelihood of future retrieval of that item, given an analogous context (also see Roediger & Butler, 2011). Hence, to the extent that cells in SN/VTA fire at retrieval – whether as an obligatory marker of retrieval success or reflective of the expected utility of the retrieved information for the current context – dopamine projections to hippocampus could enhance re-encoding and so prioritize items in memory as a function of their retrieval history. Importantly, descending inputs from ventral striatum to VTA (Figure 1B; Lisman and Grace, 2005) could provide modulatory input related to the adaptive value of retrieved information for current goals, providing greater contextual specificity to re-encoding.
Adaptive encoding can provide a reasonable account of a portion, though not all, of the evidence regarding striatal involvement at retrieval. Certainly, retrieval success and novelty effects in striatum, as observed with fMRI, could reflect encoding modulation in accord with the current utility of an item. Indeed, even differences between source retrieval and item familiarity/general recollection could relate to the degree of match between retrieved information and a maintained goal. However, the evidence of retrieval deficits in patient groups with basal ganglia dysfunction (e.g., Cohn et al., 2010) argues that striatum also plays a role in retrieval itself, rather than exclusively influencing future retrieval attempts. With this in mind, we will now consider two hypotheses that propose a role for striatum in on-going retrieval.
Hypothesis 2: Adaptive gating of working memory to control retrieval
Striatum may modulate retrieval itself in accord with the expected utility of retrieval success in the current context. As noted above, if one takes the goal of memory retrieval to be recovering those items with high expected utility given the context, then cognitive control of memory is a means by which the priority of items in memory can be modified on-line to increase the likelihood of retrieving relevant information and minimize the influence of irrelevant information. In its specifics, this objective can be reached by a number of means, and indeed, there are likely several control mechanisms that operate complementarily at different stages of retrieval. For example, attention might be directed to cues in the environment that increase the likelihood of successful retrieval. Likewise, a cue might be maintained in working memory or semantically elaborated to allow it to influence retrieval. Following retrieval, monitoring of retrieved information and selection of information that matches decision criteria or behavioral goals can ensure accuracy and precision. Cognitive neuroscience research on the cognitive control of retrieval has provided a share of evidence regarding how PFC is organized to support these functions (e.g. Shimamura, 1995; Rugg, Henson, & Robb, 2003; Dobbins & Wagner, 2005; Badre & Wagner, 2007; Öztekin & Badre, 2011; Gallo, McDonough, & Scimeca, 2010).
Importantly, however, all of these cognitive control mechanisms share a common demand to maintain a goal or relevant contextual feature in working memory in order to provide a top-down bias on current processing (Desimone & Duncan, 1995; Miller & Cohen, 2001). PFC is widely thought to support this working memory maintenance function. Also critical for working memory is a “gate” that will let goal relevant contextual information into working memory and will keep irrelevant information out (Hazy et al., 2006; Braver & Cohen, 2000). The striatum may act as this working memory gate (O’Reilly & Frank, 2006).
As one example of how gating of working memory could influence retrieval, consider that certain cues are more likely to yield retrieval of goal-relevant information than others. Hence, maintaining those particular cues (and not others) in working memory – such as by sustaining a distributed pattern of neural activity in the PFC – provides a top-down input to the MTL system that will bias retrieval toward associates of that particular cue. At least two capacities are critical for this mechanism to operate: (1) Cues must be identified that are of potentially high expected value in the retrieval context, where here expected value is directly related to the likelihood of retrieving task relevant information, and (2) high value cues should be selectively allowed into working memory while inhibiting irrelevant or misleading cues.
As noted above, the striatum has been implicated in this type of adaptive gating of PFC to support working memory and cognitive control over action (McNabb & Klingberg, 2008; Landau et al., 2009; Cools, 2011). In computational models of working memory (e.g., O’Reilly & Frank, 2006), neural networks simulate parallel cortico-striatal loops that are responsible for working memory gating, determining which representations are maintained in recurrent “PFC” layers. Based on dopaminergic learning signals, striatum learns to gate representations into PFC that lead to better outcomes (i.e., have high utility given the context) and suppress those leading to less rewarding outcomes. Once learned, gating proceeds upon encounter with a contextual input associated with high utility. Gating itself can be accomplished through fronto-striatal-thalamic loops (Alexander, DeLong, & Strick, 1986) that modulate maintenance activity in PFC. Relative to the learning or evaluative component of this system that may be more associated with ventral striatum, this gating function may be differentially carried out by the dorsal striatum (O’Doherty et al., 2004; Tricomi, Delgado, & Fiez, 2004; Cohen & Frank, 2009).
This network architecture is generally supported by various lines of behavioral, pharmacological, and patient work (Cools, Ivry, & D’Esposito, 2006; Dahlin, Neely, Larsson, Backman, & Nyberg, 2008; Frank & Fossella, 2011; Badre and Frank, 2012), and computational models using this fronto-striatal “gating” network architecture have been applied to tasks involving working memory, task-switching, and contingent action selection (e.g., O’Reilly & Frank, 2006; Moustafa, Sherman, & Frank, 2008; Frank & Badre, 2012). Thus, extended to memory retrieval, cues or retrieval strategies that previous experience has associated with high expected value for retrieval could be gated into or excluded from working memory by these same fronto-striatal circuits.
The adaptive gating hypothesis is generally consistent with the evidence regarding the contribution of striatum to declarative retrieval, though limited direct evidence exists. Certainly, retrieval success effects and novelty responses could reflect an encounter with a cue or deploying a strategy that is relevant to a decision about oldness/novelty. Moreover, gating demands will increase in any retrieval context requiring more cognitive control; as contextual elements, goals, retrieval strategies, and interim products of retrieval are updated and maintained in working memory. Hence, evidence of greater striatal activation that accompanies PFC activation for source relative to item retrieval, during controlled semantic retrieval, or with increased output position during free recall (Long, Öztekin, and Badre, 2010) is broadly consistent with the gating hypothesis. Also, potentially consistent with this interpretation, one multi-modal imaging study using fMRI and SPECT reported a correlation of increased D2 receptor binding in striatum with greater left VLPFC activation during proactive interference resolution (Nyberg et al., 2009).
Retrieval deficits in patients under conditions requiring greater control could likewise be traced to ineffective working memory gating. For example, as already discussed, the recollective deficit observed in PD patients following deep encoding (Cohn et al., 2010) could reflect a failure to take advantage of an effective encoding strategy, perhaps because of a failure to gate adaptive cues or retrieval strategies into working memory that were afforded by the deep encoding task.
Thus, across neuroimaging and neuropsychological studies, the gating hypothesis is broadly consistent with striatal involvement in cognitive control of memory retrieval. However, none of the studies cited provide direct evidence for this interpretation over others. Directed future research will be required to test this hypothesis and to dissociate striatal updating/selection from PFC maintenance during memory retrieval.
Hypothesis 3: Reinforcement learning and adaptation of cognitive control at retrieval
Just as striatum may mark the expected value associated with anticipated retrieval in a particular context, it may also be important for adapting cognitive control based on deviations from expectations about retrieval outcome. As introduced in the preceding discussion, striatum must acquire expectations about the value of particular retrieval strategies and control representations in order to support a gating function. Likewise, when these strategies prove to be ineffective or become obsolete, the system must revise its expectations or even shift to new strategies. In the reinforcement learning literature, the deviation of an outcome from an expectation is referred to as a reward prediction error (RPE; Schultz, Dayan, & Montague, 1998; Sutton & Barto, 1998; O’Doherty et al., 2004). In order to learn the relationship between a context, a course of action, and a particular outcome, a positive RPE reinforces a particular behavior and makes it more likely to be chosen in an analogous context in the future. Conversely, a negative RPE punishes a particular course of action and makes it less likely to be chosen. Thus, over the course of learning, behavior incrementally converges on statistically optimal behavioral strategies given the context. In the striatum, which representations to gate into working memory and which to suppress may be learned through modulation of synaptic plasticity by dopaminergic RPE signals computed in the midbrain. For example, these signals may modulate the activity of separate populations of “Go” and “NoGo” neurons that express D1 and D2 dopamine receptors respectively (Shen et al., 2008; O’Reilly and Frank, 2006).
Applied to the cognitive control of memory, RPE could hypothetically operate in a similar manner, reinforcing or punishing selection/maintenance of a particular retrieval strategy given the context. Becker and Lim (2003) proposed a model of semantic clustering in free recall that provides an example of how RPE might drive adjustments in control of memory (also see Gorski and Laird, 2011). This model sought to simulate semantic clustering strategies during recall. Clustering was implemented by maintaining a semantic context in “PFC” working memory units where it influenced serial retrieval by the MTL/hippocampus. After each item was retrieved, it was assessed for its familiarity. Items associated with too much or too little familiarity were judged as errors (i.e., repetitions or intrusions, respectively). Either of these errors produced a negative RPE that punished the maintenance of a particular semantic context (i.e., retrieval strategy) in PFC. When enough such errors accumulated, the category maintained in PFC shifted.
This model simulates classical semantic clustering, as well as reductions in recall due to a “frontal” challenge, namely dividing attention (Moscovitch, 1994). Importantly, the model highlights that recall itself can be affected not only by demands on maintaining a strategy but also detecting when a strategy has become obsolete and a shift is in order. Consistent with this insight, frontal patients have been shown to use fewer numbers of semantic categories for clustering than controls, even when controlling for deficits in the degree to which they retrieve semantically related items consecutively (Jetter et al., 1986; Hildebrandt et al., 1998). Hence, this model illustrates that RPE could be an important signal used by the brain to adjust memory retrieval strategies.
Within the declarative memory domain, there is some behavioral evidence that participants adjust their retrieval strategies based on feedback about outcomes. Han & Dobbins (2009) manipulated explicit feedback to differentially reinforce “old” responses in a recognition memory task and found that participants become more or less likely to endorse memory probes as “old”. This shift in behavior occurred gradually over the course of learning and persisted even in blocks after the feedback was removed. This suggests that participants had adaptively adjusted a latent criterion threshold that they use to evaluate retrieved mnemonic evidence and/or to choose their response. Theoretically, these adjustments could arise from an RPE, as in a mismatch between the expectations of participants regarding the outcome of their report (old/new) and the feedback they received. Such an RPE could be computed in the striatum.
Considerable evidence has already linked the basal ganglia in general and striatum in particular to incremental adjustments in behavior in accord with RPE (though see Berridge, 2007). Classically, patients with basal ganglia disorders, like PD patients, show deficits in tasks, like the weather prediction task, in which links between a state, action, and outcome must be learned based on reinforcement (Knowlton, Squire, & Gluck, 1996; Gluck et al., 2002; Poldrack et al., 2001). Similarly, evidence from reinforcement learning tasks that estimate learning rates in individual participants and model RPE based on a participant’s specific sequence of responses and rewards has repeatedly shown that activation in ventral striatum tracks trial-to-trial changes in RPE (O’Doherty et al., 2004, 2007; Glascher, Daw, Dayan, & O’Doherty, 2010; Daw et al., 2011; Badre & Frank, 2012). There is also some evidence that this type of reinforcement learning may influence learning of working memory gating functions by dorsal striatum (Frank & O’Reilly, 2006; Moustafa et al., 2008; Badre and Frank, 2012). Thus, RPE may play a similar role in memory control and either reinforce memory control strategies or drive changes in them in accord with the deviation from expected retrieval outcomes.
As with the gating hypothesis, the reinforcement learning hypothesis is broadly consistent with evidence linking striatum to cognitive control. Retrieval success effects could reflect the positive RPE associated with the success of a retrieval strategy (i.e., achieving a goal; e.g., Han et al., 2010). Likewise, evidence linking striatum to retrieval tasks that place greater demands on cognitive control could reflect adjustments in control as retrieval unfolds.
More directly, there is also some limited evidence that striatal activation can vary as a function of deviations from expectation during memory retrieval. Tricomi and Fiez (2008) reported a paired-associate learning task, in which participants first learned the associations by randomly choosing between two answer choices and then receiving feedback on their accuracy. On subsequent memory trials, participants made their decisions based on their memory of the correct response from earlier trials, again receiving feedback on their performance. Caudate activation was evident on the memory trials but not the initial learning trials, suggesting that the caudate was selectively engaged when participants are expecting the feedback to provide information about the accuracy of their memory decisions. O’Connor and colleagues (2010) examined the interaction between expectations and retrieval success effects by manipulating participants’ expectations of upcoming oldness in a recognition memory test. Participants saw a valid or invalid anticipatory cue (“likely old” or “likely new”) before each recognition judgment. The caudate was active not only in the “retrieval success” contrast, but also in a contrast comparing invalid cue trials vs. valid cue trials, suggesting that the caudate activity may serve as a marker of the violation of memory expectations.
To summarize, then, there is evidence that people can take advantage of feedback to adjust their memory retrieval strategies; a process that could reasonably be assumed to rely on some form of RPE. And, there is evidence that striatal activation tracks deviations from expectation during retrieval tasks and so is potentially modulated by RPE. These observations motivate the hypothesis that RPE signals in striatum support experience-driven adjustments in cognitive control strategies during retrieval. However, it remains to be demonstrated that these RPE signals are the source of behavioral adjustments in memory control.
Conclusion
There has been a growing recognition of the role of striatum across cognition, extending beyond basic motor control and being implicated in domains such as action selection, working memory, reinforcement learning, and cognitive control. Indeed, the results reviewed here suggest that striatum interacts with other brain regions, such as prefrontal cortex and hippocampus, in declarative memory retrieval. In particular, the extant neuroimaging and neuropsychological literature implicate striatum in oldness and novelty detection, goal-relevant decision processes in recognition memory, and the cognitive control of episodic and semantic memory (Table 1).
Considering these observations, it is evident that striatum plays a critical role in optimal memory retrieval, but the specific mechanistic contributions of striatum are underspecified. Drawing on existing theories of striatal function, we have proposed three possible ways in which striatum might contribute to declarative memory retrieval, namely through (1) adaptive encoding at retrieval, (2) adaptive gating of working memory to control retrieval, and (3) reinforcement learning of retrieval strategies. These hypotheses are likely not mutually exclusive. Indeed, it seems likely that all three may characterize a component of what striatum and/or the broader basal ganglia system is supporting during retrieval. Moreover, there may be differences within striatum regarding how these hypothesized functions are supported. For example, the difference between adaptive gating and reinforcement learning/evaluation of memory control strategies – a kind of actor-critic architecture for memory control (e.g., Bornstein & Daw, 2012; Botvinick et al., 2009; Niv, 2009; Holroyd and Yeung, 2012) – could be supported by dorsal versus ventral striatum respectively.
It is also important to clarify that the hypothetical contributions of striatum to declarative retrieval performance proposed here need not exclusively support those cognitive control processes that lead to improved retrieval itself. Cognitive control can affect the accuracy and precision of retrieval, as illustrated by the examples provided above. However, cognitive control may also adjust decision criteria and response selection policy during memory tasks in order to gain positive task outcomes, independently of the underlying retrieval outcomes. In other words, cognitive control may also bias reports during memory tasks as opposed to affecting discrimination, per se (Lauwereyns et al., 2002; Maddox and Bohil, 2005). And indeed, certain manipulations such as those that incentivize particular reports (old versus new, for example; Han and Dobbins, 2009; Han et al., 2010) are likely examples of adaptation occurring at this decision stage, as opposed to affecting retrieval or discrimination directly. Nevertheless, whether cognitive control mechanisms are directed toward achieving a particular retrieval goal, such as recovering a particular type of information from memory or maximizing positive outcomes by biasing reports, striatum may play a similar role in utility-driven updating and selection of working memory representations to influence performance.
Finally, it is important to note that though we have drawn an analogy between striatal function during declarative memory tasks and existing models of striatum developed outside of the memory domain, mapping value to memory signals and processes – which is at the base of all three hypotheses – is different in important ways from typical reinforcement learning tasks that map value to a stimulus. In particular, declarative memory representations are abstract and multidimensional and are shaped by the retrieval process itself. Thus, items or contexts with different features may elicit similar memory signals and conversely items with highly overlapping features may be treated differently depending on the nature of the memory signal being computed. Thus, in the context of memory, striatal function should not be conceptualized as mapping value to stimuli. Rather, one must consider the problem of assigning value to levels and types of mnemonic representations. Similarly, valuation itself within the memory domain is somewhat different than in traditional contexts. For example, value could be based on the match of a latent memory state to expectations, the degree of effort minimization that follows from successful retrieval, and/or the variability in retrieval outcome (akin to outcome variance in reinforcement learning; e.g., Niv et al., 2012). Hence, moving forward, it is crucial to study the contribution of striatum to declarative memory in the context of memory retrieval itself, rather than by analogy with other domains. Future directed investigations will be required to provide a more concrete view of the mechanistic role of striatum in declarative memory retrieval.
Acknowledgements
The present work was supported by a grant from the National Institute of Neurological Disease and Stroke (5R01NS065046) and a fellowship from the Alfred P. Sloan foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Okuda J, Suzuki M, Sasaki H, Matsuda T, Mori E, Tsukada M, Fujii T. Neural correlates of true memory, false memory, and deception. Cerebral Cortex. 2008;18:2811–2819. doi: 10.1093/cercor/bhn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Aly M, Yonelinas A, Kishiyama MM, Knight RT. Damage to lateral prefrontal cortex impairs familiarity but not recollection. Behavioural Brain Research. 2011;225:297–304. doi: 10.1016/j.bbr.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Milson R. Human Memory: An Adaptive Perspective. Psychological Review. 1989;96:703–719. [Google Scholar]
- Barnes J, Boubert L, Harris J, Lee A, Davis AS. Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia. 2003;34:565–574. doi: 10.1016/s0028-3932(02)00182-3. [DOI] [PubMed] [Google Scholar]
- Badre D, Doll BB, Long NM, Frank MJ. Rostrolateral prefrontal cortex and individual differences in uncertainty-driven exploration. Neuron. 2012;72:595–607. doi: 10.1016/j.neuron.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Frank MJ. Mechanisms of hierarchical reinforcement learning in corticostrial circuits 2: Evidence from fMRI. Cerebral Cortex. 2012;22:537–536. doi: 10.1093/cercor/bhr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler R, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Becker S, Lim J. A computational model of prefrontal control in free recall: strategic memory use in the California Verbal Learning Task. Journal of Cognitive Neuroscience. 2003;15:821–832. doi: 10.1162/089892903322370744. [DOI] [PubMed] [Google Scholar]
- Berridge K. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bornstein AM, Daw ND. Multiplicity of control in the basal ganglia: computational roles of striatal subregions. Current Opinion in Neurobiology. 2011;21:374–380. doi: 10.1016/j.conb.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Niv Y, Barto AC. Hierarchically organized behavior and its neural foundations: A reinforcement learning perspective. Cognition. 2009;113:262–280. doi: 10.1016/j.cognition.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: the role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance. Cambridge, MA: MIT Press; 2000. pp. 713–737. [Google Scholar]
- Brin S, Page L. The Anatomy of a Large-Scale Hypertextual Web Search Engine; Seventh International World-Wide Web Conference; (WWW 1998), April 14–18 1998; Brisbane, Australia. 1998. [Google Scholar]
- Bronnick K, Alves G, Aarsland D, Tysnes O, Larsen JP. Verbal memory in drug-naïve, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25:114–124. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- Brown J, Bullock D, Grossberg S. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Networks. 2004;17:471–510. doi: 10.1016/j.neunet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Beyond HERA: Contributions of specific prefrontal brain areas to long-term memory retrieval. Psychonomic Bulletin & Review. 1996;3:149–158. doi: 10.3758/BF03212413. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner A, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI: I. Retrieval effort versus retrieval success. NeuroImage. 1998;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Dayan P, Dolan RJ, Duzel E. A common mechanism for adaptive scaling of reward and novelty. Human Brain Mapping. 2010;31:1380–1394. doi: 10.1002/hbm.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell Q. A simple stochastic model for library loans. Journal of Documentation. 1980;36:115–132. [Google Scholar]
- Crescentini C, Marin D, Del Missier F, Biasutti E, Shallice T. Interference from retrieval cues in Parkinson’s disease. Neuropsychology. 2011 Jul 4;25:720–733. doi: 10.1037/a0024674. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Mondolo F, Biasutti E, Shallice T. Supervisory and routine processes in noun and verb generation in nondemented patients with Parkinson’s disease. Neuropsychologia. 2008;46:434–447. doi: 10.1016/j.neuropsychologia.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory, and choice. Behavioural Brain Research. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H, Poldrack RA. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Cohn M, Moscovitch M, Davidson PSR. Double dissociation between familiarity and recollection in Parkinson’s disease as a function of encoding tasks. Neuropsychologia. 2010;48:4142–4147. doi: 10.1016/j.neuropsychologia.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic control of the striatum for high-level cognition. Current Opinion in Neurobiology. 2011;21:402–407. doi: 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. The Journal of Neuroscience. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D’Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. Journal of Cognitive Neuroscience. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6:255–265. [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;13:2008. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. Exploring the recognition memory deficit in Parkinson’s disease: Estimates of recollection versus familiarity. Brain. 2006;129:1768–1779. doi: 10.1093/brain/awl115. [DOI] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP. California Verbal Learning Test. New York: Psychological Corporation; 1987. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Doll BB, Frank MJ. The basal ganglia in reward and decision making: computational models and empirical studies. In: Dreher JC, editor. Handbook of Reward and Decision Making. Academic Press; 2009. pp. 399–425. [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA, Kaszniak AW, Bohnen NI, Glisky EL. Source memory and frontal functioning in Parkinson’s disease. Journal of the International Neuropsychological Society. 2009;15:399–406. doi: 10.1017/S1355617709090572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Boiler F, Pillon B, Agid Y. Cognitive deficits in Parkinson's disease. In: Boiler F, Grafman J, editors. Handbook of neuropsychology. Vol. 5. Amsterdam: Elsevier; 1991. pp. 195–240. [Google Scholar]
- Edelstyn NM, Mayes AR, Condon L, Tunnicliffe M, Ellis SJ. Recognition, recollection, familiarity and executive function in medicated patients with moderate Parkinson’s disease. Journal of Neuropsychology. 2007;1:131–147. doi: 10.1348/174866407x182565. [DOI] [PubMed] [Google Scholar]
- Edelstyn NM, Shepherd TA, Mayes AR, Sherman SM, Ellis SJ. Effect of disease severity and dopaminergic medication on recollection and familiarity in patients with idiopathic nondementing Parkinson’s. Neuropsychologia. 2010;48:1367–1375. doi: 10.1016/j.neuropsychologia.2009.12.039. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:1223–1252. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Badre D. Mechanisms of hierarchical reinforcement learning in corticostrial circuits 1: Computational analysis. Cerebral Cortex. 2012;22:1–18. doi: 10.1093/cercor/bhr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nature Neuroscience. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Fossella JA. Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology. 2011;36:133–152. doi: 10.1038/npp.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in human cognition: Psychopharmacological studies with cabergoline and haloperidol. Behavioral Neuroscience. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Gallo DA, McDonough IM, Scimeca J. Dissociating source memory decisions in the prefrontal cortex: fMRI of diagnostic and disqualifying monitoring. Journal of Cognitive Neuroscience. 2010;22:955–969. doi: 10.1162/jocn.2009.21263. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: Dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–595. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the “weather prediction” task?: Individual variability in strategies for probabilistic category learning. Memory & Learning. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski NA, Laird JE. Learning to use episodic memory. Cognitive Systems Research. 2011;12:144–153. [Google Scholar]
- Graybiel AM, Mink JW. The Basal Ganglia and Cognition. In: Gazzaniga MS, editor. The Cognitive Neurosciences. MIT Press; 2009. pp. 565–585. [Google Scholar]
- Griffiths TL, Steyvers M, Firl A. Google and the mind: Predicting fluency with PageRank. Psychological Science. 2007;18:1069–1076. doi: 10.1111/j.1467-9280.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Bunzeck N, Stephan KE, Dolan RJ, Duzel E. Contextual novelty changes reward representations in the striatum. Journal of Neuroscience. 2010;30:1721–1726. doi: 10.1523/JNEUROSCI.5331-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. II. Analysis and simulation of behaviour. Biological Cybernetics. 2001;84:411–424. doi: 10.1007/PL00007985. [DOI] [PubMed] [Google Scholar]
- Han S, Dobbins IG. Regulating recognition decisions through incremental reinforcement learning. Psychonomic Bulletin & Review. 2009;16:469–474. doi: 10.3758/PBR.16.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Huettel SA, Raposo A, Adcock RA, Dobbins IG. Functional significance of striatal responses during episodic decisions: Recovery or goal attainment? Journal of Neuroscience. 2010;30:4767–4775. doi: 10.1523/JNEUROSCI.3077-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, O’Connor AR, Eslick AN, Dobbins IG. The role of left ventrolateral prefrontal cortex during episodic decisions: Semantic elaboration or resolution of episodic interference? Journal of Cognitive Neuroscience. 2012;24:223–234. doi: 10.1162/jocn_a_00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Banishing the homunculus: Making working memory work. Neuroscience. 2006;139:105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Helkala E-L, Laulumaa V, Soininen H, Riekkinen PJ. Different error pattern of episodic and semantic memory in Alzheimer’s disease and Parkinson’s disease with dementia. Neuropsychologia. 1989;27:1241–1248. doi: 10.1016/0028-3932(89)90036-5. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Brand A, Sachsenheimer W. Profiles of patients with left prefrontal and left temporal lobe lesions after cerebrovascular infarcations on California Verbal Learning Test-like indices. Journal of Clinical and Exp Neuropsychology. 1998;20:673–683. doi: 10.1076/jcen.20.5.673.1119. [DOI] [PubMed] [Google Scholar]
- Hochreiter S, Schmidhuber J. Long short term memory. Neural Computation. 1997;9:1735–1780. doi: 10.1162/neco.1997.9.8.1735. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Differential impairment of semantic and episodic memory in Alzheimer’s and Huntington’s diseases: a controlled prospective study. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:1089–1095. doi: 10.1136/jnnp.53.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulated cortex. Trends in Cognitive Sciences. 2012;16:121–127. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Research. 1997;759:251–258. doi: 10.1016/s0006-8993(97)00265-5. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progress in Neurobiology. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jetter W, Poser U, Freeman RB, Markowitsch JH. A verbal long term memory deficit in frontal lobe damaged patients. Cortex. 1986;22:229–242. doi: 10.1016/s0010-9452(86)80047-8. [DOI] [PubMed] [Google Scholar]
- Kakade S, Dayan P. Dopamine: generalization and bonuses. Neural Networks. 2002;15:549–559. doi: 10.1016/s0893-6080(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: An Activation Likelihood Estimation Meta-Analysis. Human Brain Mapping. 2011 doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoke D, Taylor AE, Saint-Cyr JA. The Differential Effects of Cueing on Recall in Parkinson’s Disease and Normal Subjects. Brain and Cognition. 1998;38:261–274. doi: 10.1006/brcg.1998.1042. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Schutze H, Duzel E. The novelty exploration bonus and its attentional modulation. Neuropsychologia. 2009;47:2272–2281. doi: 10.1016/j.neuropsychologia.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nature Neuroscience. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cerebral Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. Journal of Neuroscience. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. European Journal of Neuroscience. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nature Neuroscience. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Long NM, Oztekin I, Badre D. Separable prefrontal cortex contributions to free recall. Journal of Neuroscience. 2010;30:10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox WT, Bohil CJ. Optimal classifier feedback improves cost-benefit but not base-rate decision criterion learning in perceptual categorization. Memory & Cognition. 2005;33(2):303–319. doi: 10.3758/bf03195319. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nature Reviews Neuroscience. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: a component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:1984–1994. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Cognitive resources and dual-task interference effects at retrieval in normal people: the role of the frontal lobes in medial temporal cortex. Neuropsychology. 1994;8:524–534. [Google Scholar]
- Moustafa AA, Gluck MA. Computational cognitive models of prefrontal-striatal-hippocampal interactions in Parkinson's disease and schizophrenia. Neural Networks. 2011;24:575–591. doi: 10.1016/j.neunet.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attional shifting in Parkinsonism. Neuropsychologia. 2008;46:3144–3156. doi: 10.1016/j.neuropsychologia.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Niv Y. Reinforcement learning in the brain. The Journal of Mathematical Psychology. 2009;53:139–154. [Google Scholar]
- Niv Y, Edlund JA, Dayan P, O’Doherty JP. Neural prediction errors reveal a risk-sensitive reinforcement-learning process in the human brain. The Journal of Neuroscience. 2012;32(2):551–562. doi: 10.1523/JNEUROSCI.5498-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Andersson M, Forsgren L, Jakobsson-Mo S, Larsson A, Marklund P, Nilsson L, Riklund K, Backman L. Striatal dopamine D2 binding is related to frontal BOLD response during updating of long-term memory representations. NeuroImage. 2009;46:1194–1199. doi: 10.1016/j.neuroimage.2009.03.035. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? Journal of Neuroscience. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental condition. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Hampton A, Kim H. Model-based fMRI and its application to reward learning and decision making. Annals of the New York Academy of Sciences. 2007;11104:35–53. doi: 10.1196/annals.1390.022. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: A computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Öztekin I, Badre D. Distributed patterns of brain activity that lead to forgetting. Frontiers in Human Neuroscience. 2011;5:1–8. doi: 10.3389/fnhum.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Hirsch R, White M. Differential Effects of Fornix and Caudate Radial Maze Tasks : Evidence for Multiple Nucleus Lesions on Two Memory Systems. The Journal of Neuroscience. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Foerde K. Category learning and the memory systems debate. Neuroscience and Biobehavioral Reviews. 2008;32:197–205. doi: 10.1016/j.neubiorev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Butler AC. The critical role of retrieval practice in long-term retention. Trends in Cognitive Sciences. 2011;15:20–27. doi: 10.1016/j.tics.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Witter MP, Machielsen WC, Scheltens P. Anteriormedial temporal lobe activation during attempted retrieval of encoded visuospatial scenes: An event-related fMRI study. NeuroImage. 2001;14:67–76. doi: 10.1006/nimg.2001.0799. [DOI] [PubMed] [Google Scholar]
- Rouleau I, Imbault H, Laframboise M, Bedard MA. Pattern of intrusions in verbal recall: comparison of Alzheimer’s disease, Parkinson’s disease, and frontal lobe dementia. Brain and Cognition. 2001;46:244–249. doi: 10.1016/s0278-2626(01)80076-2. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA, Robb WGK. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Sagar HJ, Sullivan EV, Cooper JC, Jordan NJ. Normal release from proactive interference in untreated patients with Parkinson’s disease. Neuropsychologia. 1991;29:1033–1044. doi: 10.1016/0028-3932(91)90075-j. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schott BH, Sellner DB, Lauer C-J, Habib R, Frey JU, Guderian S, Heinze H-J, et al. Activation of midbrain structures by associative novelty and the formation of explicit memory in humans. Learning & Memory. 2004;11:383–387. doi: 10.1101/lm.75004. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampus lesions. Journal of Neurology, Neurosurgery, and Neuropsychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Miller EK. Category learning in the brain. Annual Review of Neuroscience. 2010;33:203–219. doi: 10.1146/annurev.neuro.051508.135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Gazzaniga MS. The cognitive neurosciences. Cambridge, MA: MIT Press; 1995. Memory and frontal lobe function; pp. 803–813. [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Sciences. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Solomon AC, Stout JC, Johnson SA, Langbehn DR, Aylward EH, Bradnt J, et al. Verbal episodic memory declines prior to diagnosis in Huntington’s disease. Neuropsychologia. 2007;45:1767–1776. doi: 10.1016/j.neuropsychologia.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annual Review of Neuroscience. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on egenetic differences in D2 receptor density. Journal of Neuroscience. 2010;30:14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo CL, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Memory and learning in early Parkinson’s disease: Evidence for a “Frontal Lobe Syndrome”. Brain and Cognition. 1990;13:211–232. doi: 10.1016/0278-2626(90)90051-o. [DOI] [PubMed] [Google Scholar]
- Thomson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Fiez JA. Feedback signals in the caudate reflect goal achievement on a declarative memory task. NeuroImage. 2008;41:1154–1167. doi: 10.1016/j.neuroimage.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 382–403. [Google Scholar]
- Vingerhoets G, Vermeule E, Santens P. Impaired intentional content learning but spared incidental retention of contextual information in non-demented patients with Parkinson’s disease. Neuropsychologia. 2005;43:675–681. doi: 10.1016/j.neuropsychologia.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Von Zerssen G, Mecklinger A, Opitz B, von Cramon DY. Conscious recollection and illusory recognition: an event-related fMRI study. European Journal of Neuroscience. 2001;13:2148–2156. doi: 10.1046/j.0953-816x.2001.01589.x. [DOI] [PubMed] [Google Scholar]
- Van Oostrom I, Dollfus S, Brazo P, Abadie P, Halbecq I, Thery S, Marie RM. Verbal learning and memory in schizophrenic and Parkinson’s disease patients. Psychiatry Research. 2003;117:25–34. doi: 10.1016/s0165-1781(02)00302-5. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Science. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weiermann B, Stephan MA, Kaelin-Lang A, Meier B. Is there a recognition memory deficit in Parkinson’s disease? Evidence from estimates of recollection and familiarity. International Journal of Neuroscience. 2010;120:211–216. doi: 10.3109/00207450903506510. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. Journal of the International Neuropsychological Society. 1995;1:525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Podd J, Kan MM. Recognition memory impairment in Parkinson’s disease: Power and Meta-Analyses. Neuropsychology. 2000;14:233–246. doi: 10.1037//0894-4105.14.2.233. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Duzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. NeuroImage. 2007;38:194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Daw ND, Seymour B, Dolan RJ. Striatal activity underlies novelty-based choice in humans. Neuron. 2008;58:967–973. doi: 10.1016/j.neuron.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus- dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Regehr G, Jacoby LL. Incorporating response bias in a dual-process theory of memory. Journal of Memory and Language. 1995;34(6):821–835. [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS. A review of the cognitive and behavioral sequelae of Parkinson’s disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]