Abstract
Evidence suggests that lateral frontal cortex is hierarchically organized such that rostral frontal regions support more abstract representations than caudal regions. A recent fMRI study of language processing proposes that striatum may exhibit an analogous organization. We consider this hypothetical correspondence at both the cognitive and anatomical levels.
The prefrontal cortex (PFC) is crucial for cognitive control and goal-directed behavior [1]. In recent years, growing evidence has suggested that lateral PFC is organized hierarchically along its rostro-caudal axis, such that rostral regions support more abstract control processing [2–5]. A new study of syntactic processing by Mestres-Missé et al. [6] raises the possibility that a similar organization exists in the caudate nucleus (CN) – an area heavily interconnected with frontal cortex. This observation raises two questions: 1) Do the forms of abstraction previously associated with lateral frontal organization correspond to the progression of complexity in sentence processing in the CN, and 2) Is the similarity in rostro-caudal functional organization between frontal cortex and CN reflective of a common anatomical circuit?
To manipulate the degree of complexity in language processing, Mestres-Missé et al. asked subjects to make judgments about the grammaticality of sentences. Three kinds of sentences were used: ambiguous (e.g., the subject of the sentence could not be determined until the verb), ungrammatical (e.g., the subject was unambiguous but did not agree with the verb), and unambiguous (e.g., the subject was unambiguous and agreed with the verb). Ambiguous and ungrammatical sentences both elicit conflict and so trigger a greater demand for cognitive control than unambiguous, grammatical sentences. Both ambiguous and ungrammatical sentences produced activation in caudal PFC and caudal and dorsal CN relative to unambiguous sentences.
Importantly, however, ambiguous sentences can undergo further controlled processing to resolve conflict, whereas ungrammatical sentences cannot. Contrasting ambiguous with ungrammatical sentences resulted in activation in more rostral and ventral CN, potentially reflective of this additional processing. Thus, rostral/ventral CN was distinguished from caudal/dorsal CN as a function of the amount of syntactic processing required. Mestres-Missé et al. relate this rostro-caudal distinction in CN to the gradient of cognitive control function observed along the rostro-caudal axis of lateral PFC.
The putative relationship between the rostro-caudal distinction in CN observed by Mestres-Missé et al. and previous work is intriguing, and could fit with a general frontal organizing principle. However, caution is merited in drawing strong conclusions about the direct relationship between the two sets of findings. First, though observed repeatedly, functional distinctions along the rostro-caudal axis of frontal cortex have been described differently across studies, and there remains considerable debate about what factors drive this apparent functional topography [2]. Thus, it would be challenging to determine what precise aspect of the ambiguous sentence manipulation maps onto prior distinctions in lateral frontal cortex. For example, the source of the rostral activation differences for ambiguous sentences could involve the need for additional nested processing steps, as suggested by the authors, but also the demand to consider multiple alternative sentence resolutions, the demand to maintain an unresolved noun phrase in working memory, or other factors that might co-vary with sentence complexity and/or overall difficulty. Second, though the locations of the activation in lateral frontal cortex in Mestres-Missé et al. do exhibit a rostro-caudal difference, their precise correspondence to previously identified regions cannot be precisely assessed without having included these prior manipulations in the experiment.
Thus, beyond the general observation that increases in task complexity across these distinct task domains resulted in more rostral frontal cortex activation, further research will be required to directly relate the two lines of research at the level of cognitive mechanism. Nevertheless, if such a relationship were established, it would speak both to the domain generality of the cognitive control processes involved, as well as the nature of the control mechanisms deployed during sentence processing.
Quite apart from how these lines of work correspond in cognitive terms, an exciting implication of the Mestres-Missé et al. study is that the rostro-caudal organization of frontal cortex may be mirrored in striatum. Interestingly, a similar parallel fronto-striatal functional architecture has recently been hypothesized to support hierarchical cognitive control, specifically during hierarchical rule learning [7]. In order to simulate human learning data, a biologically-plausible computational model simulated interacting corticostriatal circuits organized hierarchically. Within each circuit, the striatum learns to select frontal actions based on their value. Importantly, the circuits are nested such that information maintained in higher-order (“rostral”) PFC layers influences lower-order, (“caudal”) fronto-striatal loops. This nested gating architecture allows the system to learn rapidly in environments with hierarchical structure, and importantly, it predicts a parallel functional organization between frontal cortex and striatum.
Consistent with this prediction, a companion fMRI study [8] found that focal regions in the CN correlated with learning signals (i.e., reward prediction error) at a specific level of abstraction. Moreover, these CN foci were closest anatomically to the frontal cortical region that was sensitive to the same level of abstraction. However, this result did not provide evidence of multiple foci in CN that were selective for different levels of abstraction. The Mestres-Missé et al. provides the first observation of such an effect.
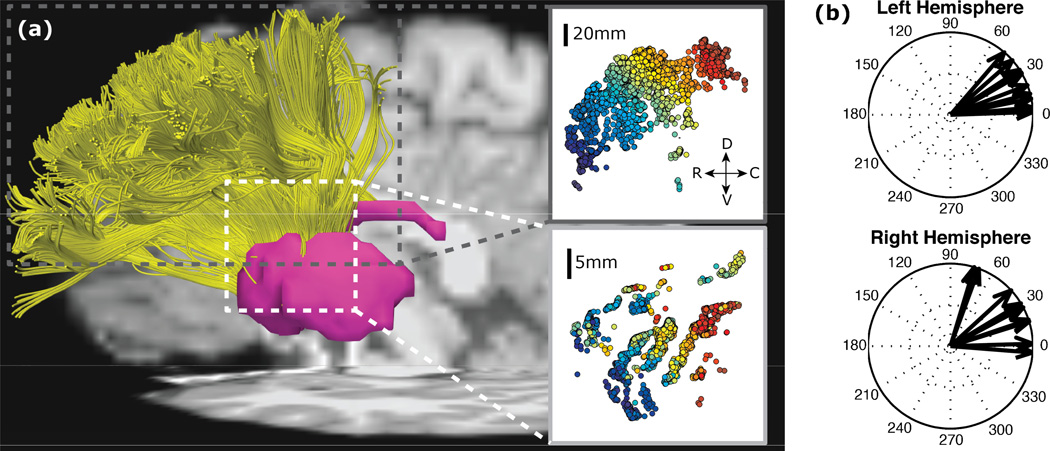
A question raised by both the learning study and Mestres-Missé et al. concerns whether the anatomical connections between lateral frontal cortex and striatum could support a parallel topographic relationship. First, a parallel fronto-striatal organization is broadly consistent with evidence in animals and humans, showing macro-level loops between frontal cortex and striatum that array rostro-caudally [9]. Second, recent evidence using high angular resolution diffusion tractography has provided evidence that the micro-organization of fronto-striatal connections is arrayed rostro-caudally within the macrolevel dorsolateral PFC (“associative”) loop [10]. Specifically, tracts seeded from rostral to caudal middle frontal gyrus exhibit a systematic shift of termination positions in the CN that was progressively caudal and dorsal (Fig 1). This finding aligns with that of Mestres-Missé et al., who observed a dorsal and caudal shift from higher to lower levels of processing.
Figure 1.
Fiber tracts between dorsolateral PFC and striatum from human diffusion tractography. From [10]. (a) Fibers in an example subject that start in the dorsolateral PFC (top inset) and terminate in the striatum (bottom inset) as seen in the sagittal plane (D, dorsal; V, ventral; R, rostral; C, caudal). Color gradient shows start from more rostral (blue) to caudal (red) along the frontal gyrus. (b) Vectors show shifts in fiber position in the striatum as cortical start position goes rostral to caudal in the sagittal plane for ten subjects.
To conclude, there is emerging evidence that the functional organization of PFC, and possibly other regions of the brain, may be constrained by the way they connect with the striatum. More specifically, motivated by Mestres-Missé et al. and others, an emerging hypothesis is that the widely observed rostro-caudal gradient in lateral frontal cortex may be at least partly an emergent property of the nature of fronto-striatal dynamics.
Acknowledgements
Supported by the National Institutes of Health (5T32MH019118-21, T.M.D.; 5R01NS065046, D.B.). We wish to thank L. Kertz for helpful discussions during the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 4.Christoff K, et al. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- 5.Koechlin E, et al. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 6.Mestres-Missé A et al. An anterior-posterior gradient of cognitive control within dorsomedial striatum. Neuroimage. doi: 10.1016/j.neuroimage.2012.05.021. (in press) [DOI] [PubMed] [Google Scholar]
- 7.Frank MJ, Badre D. Mechanisms of hierarchical reinforcement learning in corticostriatal circuits 1: computational analysis. Cereb Cortex. 2012;22:509–526. doi: 10.1093/cercor/bhr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badre D, Frank MJ. Mechanisms of hierarchical reinforcement learning in cortico-striatal circuits 2: evidence from FMRI. Cereb Cortex. 2012;22:527–536. doi: 10.1093/cercor/bhr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draganski B, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstynen TD, et al. Microstructural organizational patterns in the human corticostriatal system. J Neurophysiol. 2012;107:2984–2995. doi: 10.1152/jn.00995.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]