Introduction
Numerous clinical studies have evaluated the effects of beta blockade in cardiac, general, vascular and trauma surgery subpopulations with varying results. Lindenauer et al. (1) recently showed that the use of perioperative beta-blocker therapy is associated with a reduced risk of in-hospital death among high-risk patients undergoing major noncardiac surgery but was of no benefit in low-risk patients. This retrospective database analysis does not specify the type of beta blocker or the effectiveness of dosing but draws their conclusion on the basis of timing of beta blockade administration. The Metoprolol after Vascular Surgery trial was a prospective, double blind randomized control trial studying the effects of metoprolol (selective B1 blocker) on cardiac risk in patients undergoing abdominal aortic surgery or major lower extremity revascularization operations. Yang et al. (2) showed that metoprolol was not effective in reducing either 30 day or 6 month postoperative cardiac events in vascular patients. In a retrospective study of trauma patients, Arbabi et al. (3), found that the use of beta blockade before or after injury was associated with decreased mortality. This retrospective study in trauma patients does not specify the type of beta blockade used, however, they advocate the benefits of the drug being its cardioprotective effects, decreased myocardial oxygen demands, and decreased cerebral oxygen requirements in head injury (3). A decrease in heart rate (HR) has been shown to be cardioprotective after myocardial infarction and may also be beneficial after a severe traumatic injury and it associated systemic inflammatory response.
A significant increase in catecholamines secondary to the stress response to injury has been shown to be associated with bone marrow (BM) dysfunction (4). This BM dysfunction is prolonged and lasts for up to 14 days after injury and manifests clinically as increased susceptibility to infection and persistent anemia (5, 6). The need for multiple transfusions further contributes to the morbidity and mortality following their severe injuries (7, 8). The post-injury hypercatecholamine state has been shown to suppress BM function in a dose-dependent manner (9, 10). Previously we have demonstrated that the administration of non-selective beta blockade with propranolol, both before and after injury, restored BM function (11, 12). The BM protective effects of propranolol have been shown to be dose-dependent (12).
It remains unclear if the beneficial effects of non-selective beta blockade given after trauma are via an overall cardiovascular protective effect or an immunomodulatory effect, or both. Understanding the mechanism of beta blockade protection of BM is important to the development of effective treatment modalities in the clinical setting. Thus, the aim of this study is to more clearly define the role of the specific beta adrenergic receptors (B1, B2, B3) in BM protection following trauma and hemorrhagic shock (HS) and to determine if a cardiovascular effect is necessary for BM protection.
Methods
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 300-400g were housed at 25°C under barrier-sustained conditions with 12 hour light/dark cycles. Animal were provided ad lib access to water and chow (Teklad22/5 Rodent Diet W-8640; Harlan Teklad, Madison, WI). The animal facility environment and animals were maintained in accordance with the regulations detailed in the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care and Use Committee approved all animal protocols.
Reagents
Bovine serum albumin (BSA), and 2-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO). Methylcellulose was purchased from Stemcell Technologies (Vancouver, Canada). Fetal bovine serum (FBS), Iscove’s Modified Dulbecco’s Medium (IMDM), glutamine, penicillin/streptomycin, and trypan blue were obtained from Invitrogen (Carlsbad, CA). All cytokines rhEpo, rhIL-3, rhGM-CSF were purchased from R&D Systems (Minneapolis, MN). Sodium pentobarbital was purchased from Lundbeck Inc. (Deerfield, IL) and heparin was obtained from Hospira Inc. (Lakefront, IL). Atenolol (B1B), butoxamine (B2B), and SR59230A (B3B) were purchased from Sigma-Aldrich.
Experimental Groups
Animals were assigned to the following experimental groups. Control groups consisted of unmanipulated controls (UC) or animals treated with B1B alone that did not undergo any injury (B1B). B1B animals served as a control to assess the sustained cardiovascular effects of B1B on a non-injured animal and were given either a 5mg/kg or 10mg/kg daily dose prior to selecting a dose for the experimental groups. Experimental groups were defined as: lung contusion followed by hemorrhagic shock (LCHS), lung contusion followed by hemorrhagic shock and treatment with 5 mg/kg of either atenolol (LCHS/B1B), butoxamine (LCHS/B2B) or SR59230A (LCHS/B3B).
The immediate effects of injury and drug treatment were assessed by giving a single dose of selective beta blocker post-resuscitation after LCHS and sacrificing animals at 3 hours (3h). The sustained effects of injury and HS and beta blockade therapy were assessed by a daily dosing of selective beta blocker following LCHS and then sacrificing animals on 7 days (7d).
Physiologic Monitoring
Invasive mean arterial pressure (MAP) and heart rate (HR) were obtained via femoral arterial lines that were connected to a continuous blood pressure monitoring device (BP-2 Digital Blood Pressure Monitor; Columbus Instruments; Columbus, Ohio) before, during, and after hemorrhagic shock for up to 3 hours.
Non-invasive MAP and HR measurements were obtained using the CODA noninvasive tail-cuff acquisition system (Kent Scientific Corporation, Torrington, CT) at three time points (initial, day 1, day 7) to determine the effects of selective beta blockade during the study period.
Combined Lung Contusion and Hemorrhagic Shock Model
Experimental animals were anesthetized with IP injections of sodium pentobarbital (50 mg/kg). Unilateral lung contusion (LC) was inflicted using a blast wave percussive nail gun (Craftsman 968514 Stapler, Sears Brands Chicago, IL) applied to a 12mm metal plate adherent to the right axilla of the rat. This LC model has been shown to produce a clinically significant lung injury as demonstrated by histology and radiography (13). Using aseptic surgical technique, the right internal jugular vein and femoral artery were then cannulated with polyethylene (PE-50; Becton Dickinson and Co., Sparks, MD) and Silastic (Dow Corning Corp., Midland, MI) tubing, respectively. To prevent clotting, all tubing was flushed with 10 units/ml of heparinized saline. The femoral artery tubing was then connected to a continuous blood pressure monitoring device for measurement of mean arterial pressure (MAP) and heart rate (HR). Animals were bled to a MAP of 30-35mmHg for 45 minutes. Temperature was maintained at approximately 37 oC with the use of an electric heating pad under the surgical platform. Shed blood was re-infused at a rate of 1ml/min following the shock period. Selective beta-blockade was administered post resuscitation (when MAP > 80mmHg) via intraperitoneal (IP) injection.
Bone Marrow Cellularity
BM cells were obtained by removing the femoral epiphysis post-mortem and aspirating the bone marrow with an 18 gauge needle on a 5cc syringe filled with of 1mL IMDM supplemented with 10% FBS. A suspension was prepared by passing cells through a 40μm sterile nylon strainer to remove particulate matter. Total viable cell counts were then determined by 0.4% Trypan blue staining using a hemocytometer.
Bone Marrow Progenitor Cell Cultures
Colony-forming unit-granulocyte-, erythrocyte-, monocyte-, megakaryocyte (CFU-GEMM) were used to assess the effects of LCHS on earlier progenitor cells. To specifically explore the effects on the erythroid cell lines, burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) were assessed. The normal differentiation of these progenitor cells is as follows: CFU-GEMM →BFU-E→ CFU-E→ erythrocytes (14).
Based on the cellularity, a stock solution of BM mononuclear cells was prepared to yield a concentration of 1×106 cells/mL of IMDM. From this solution cultures were prepared in duplicate by removing 1.5×105 cells and plating them in IMDM containing 30% FBS, 2% BSA, 1% methylcellulose, rat growth factor, penicillin/streptomycin, 2×10−4 mol/L 2-mercaptoethanol, and glutamine. Plates were further supplemented with either, 1.3 U/mL rhEpo and 6U/mL rhIL-3 for BFU-E/CFU-E, or 3U/mL rhGM-CSF for CFU-GEMM. Cultures were incubated at 37°C in 5% CO2. CFU-E colonies were counted at day 7, BFU-E colonies at day 14, and CFU-GEMM colonies at day 17 by an observer blinded to the origin of the samples.
Hemoglobin Measurements
Hemoglobin (Hb) was measured in all 7d animals. Peripheral blood was obtained by via cardiac puncture using a 10-mL heparinized syringe. Three hundred microliters aliquots were then sampled and analyzed within 10 minutes of collection using a Coulter Hmx Hematology Analyzer (Beckman Coulter; Brea, CA). Samples were run in duplicate.
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparison post-test with GraphPad Prism (Version 4.0, San Diego, CA). Results were considered significant if *p <0.05 vs. UC or **p <0.05 vs. LCHS.
Results
Control Testing
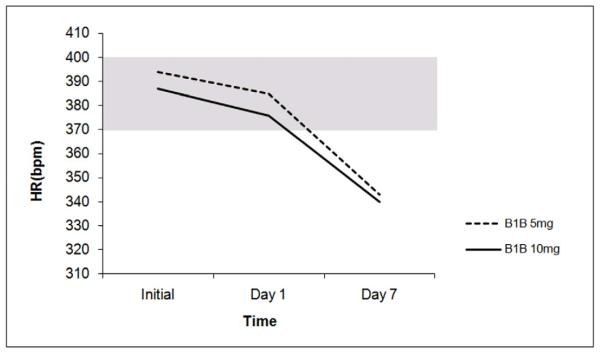
The average normal UC HR rate in a sedated animal measured by invasive monitoring was approximately 344±27 beats per minute (bpm). The average UC HR range in an awake animal measured by non-invasive means was 370-400 bpm. In the non-injury model, to determine effective cardiac protection dosing of the B1B, dose response testing was performed. There was a decrease in HR over the course of 7 days by 12% with B1B at both 5mg (394±19 to 343±15) and 10mg (387±21 to 340±18) (Figure 1).
Figure 1. Heart rate in non-injury model.
After seven days of treatment with B1B at either 5mg or 10mg/kg/day, a non-injured animal had almost a 15% decrease in HR. N=4-10/animals per group. (UC=unmanipulated control; B1B=animals given daily doses of beta 1 blockade). Shaded area represents normal UC heart range.
In a non-injury model, animals treated with B1B alone for seven days at either 5mg or 10mg had no effect on BM cellularity as compared to UC (239±15 and 211±4 vs. 225±7). Similarly, there was no change in growth of BM CFU-GEMM, BFU-E and CFU-E with the use of B1B at either 5mg or 10mg as compared to UC (37± 1, 67± 1, 76± 1 and 34±1, 66±1, 74±1 respectively vs. 35±1, 66±2, and 75±1). In the non-injured animals treated with B1B alone at both 5 and 10mg, Hb values were equivalent to UC levels (14.0±0.9 and 14.1±0.5 vs. 13.8±0.8).
Physiologic Monitoring
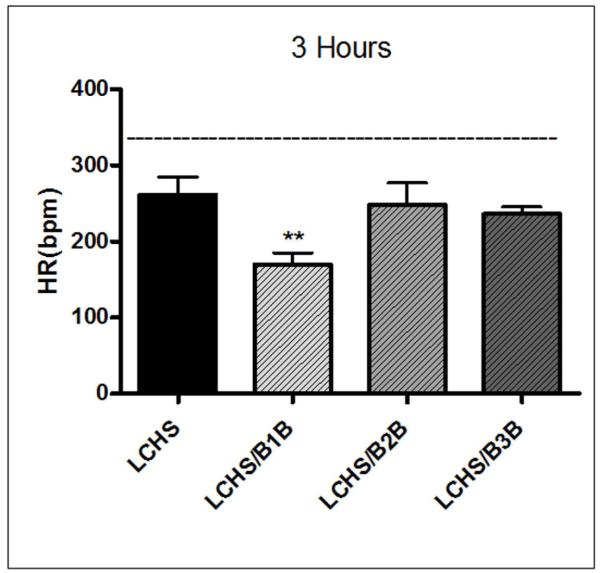
Three hours following LCHS, the HR is much lower as compared to UC (261±46 vs. 344±27). Immediate treatment with a B1B after LCHS caused a further significant decrease in heart rate at 3h compared to LCHS alone (170±38** vs. 261±46, **p<0.05)(Figure 2). There is no statistically significant decrease in HR when either B2B or B3B was given 3h after LCHS (Figure 2).
Figure 2. Heart rate three hours after injury and shock.
B1B was the only beta-adrenergic antagonist to cause a statistically significant decrease in HR when given after LCHS. N=4-6/animals per group. (UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation). **p<0.05 vs. LCHS. Dashed line represents UC levels.
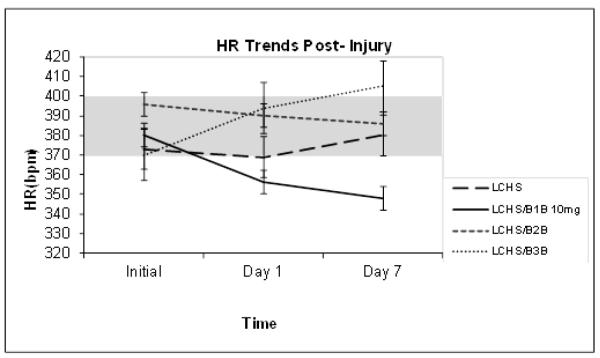
In an LCHS injury model, over 7 days there was no change in HR in LCHS animals (372±17 to 378±15) or those treated with B2B(396±9 to 386±11) or B3B(370±26 to 405±17) after LCHS. However, though not statistically significant, there was a clear 10% decrease in HR in LCHS animals given 10mg/kg/day of B1B (379±10 to 348±13) (Figure 3). The decrease in HR in LCHS animals given 5mg/kg/day was less than 10% (data not shown), therefore, 10 mg/kg/day was used as the treatment dose for LCHS/B1B group.
Figure 3. Heart rate over seven days following injury and shock.
LCHS alone, LCHS/B2B, and LCHS/B3B animals had no overall change in HR compared to the normal UC range. However, LCHS animals receiving 7 days of B1B had a 10% decrease in HR. N=4-10/animals per group. p >0.05 for LCHS vs. each selective BB counterpart. (UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation). Shaded area represents normal UC heart range.
The average normal UC MAP in a sedated animal measured by invasive monitoring was approximately 90±7mm Hg. The average UC MAP in an awake animal measured by non-invasive means was 98±8mm Hg. There were no statistically significant differences noted in mean arterial pressure (MAP) for any control or experimental group following LCHS at either three hours or seven days (data not shown).
Short Term Effects of Selective Beta Blockade
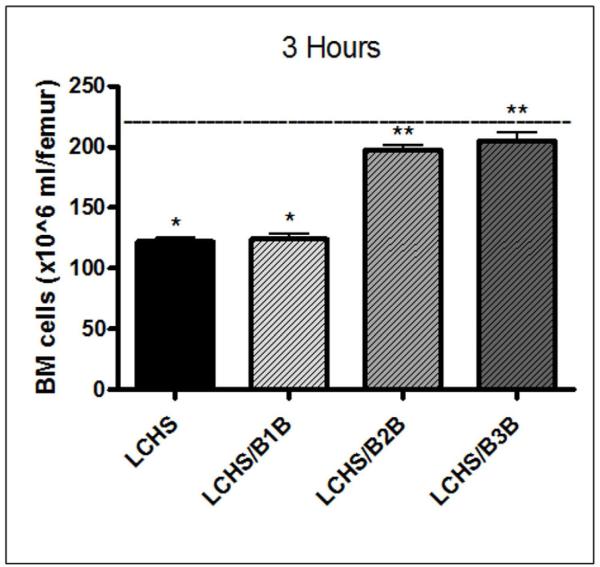
Within 3 hours of injury, LCHS resulted in a statistically significant decrease in overall BM cellularity of 45% from UC levels (122±6* vs. 220±7, *p<0.05) (Figure 4). Treatment with a B2B after LCHS prevented the decrease in BM cellularity and restored BM cellularity to within 10% of UC values (198±9 vs. 220±7) (Figure 4). Similarly, treatment with a B3B after LCHS restored cellularity to within 7% of UC values. Treatment with a B1B after LCHS had no effect on BM cellularity compared to LCHS alone (124±10 vs. 122±6) (Figure 4).
Figure 4. BM cellularity three hours after injury and shock.
The cellularity after LCHS alone or treatment with B1B is significantly decreased from UC levels. Treatment with B2B or B3B after LCHS restores BM cellularity to UC levels. N=4-6/animals per group. (BM=bone marrow; UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation). * p<0.05 vs. UC, **p<0.05 vs. LCHS. Dashed line represents UC levels.
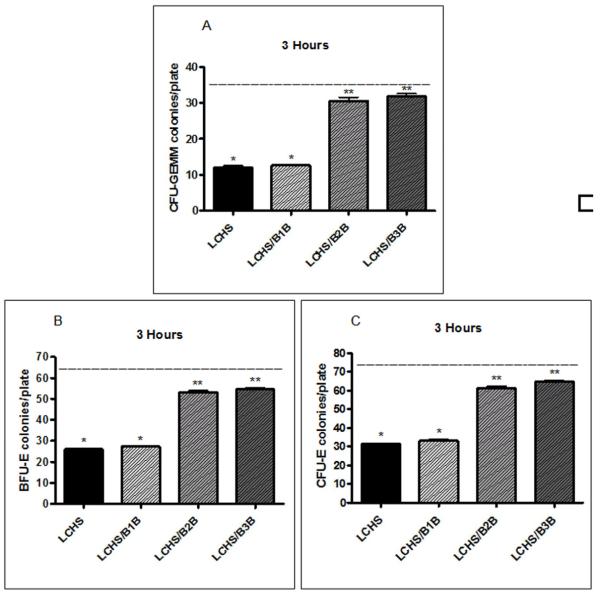
LCHS caused nearly a 60% decrease in all BM HPC colony growth, including CFU-GEMM, BFU-E, and CFU-E, compared to UC at 3h (12±1*,26±1*,31±1* vs. 36±1,65±1, 73±1 *p<0.05)(Figure 5A-C). Both B2B and B3B given after LCHS prevented all HPC colony growth suppression as compared to LCHS alone (Figure 5A-C). The use of B2B recovered BM HPC growth to within 15-18% of UC levels for CFU-GEMM, BFU-E, and CFU-E (31±2, 53±2, 61±3 vs. 36±1, 65±1, and 73±1). Similarly, treatment with a B3B after LCHS restored colony growth to within 11-15% of UC levels for the CFU-GEMM, BFU-E, and CFU-E (32±2, 55±1, 65±2 vs. 36±1, 65±1, and 73±1). Treatment with a B1B after LCHS had did not prevent HPC colony growth suppression (Figure 5A-C).
Figure 5A-C. BM HPC colony growth three hours after injury and shock.
BM HPC colony growth after LCHS alone or LCHS/B1B treatment is significantly decreased versus control in CFU-GEMM(Figure 2A), BFU-E(Figure 2B), and CFU-E(Figure 2C). After LCHS, treatment with B2B or B3B prevents BM HPC growth suppression in all three cell lines. N=4-6/animals per group. (BM=bone marrow; HPC=hematopoietic progenitor cells; UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation). * p<0.05 vs. UC, **p<0.05 vs. LCHS. Dashed line represents UC levels.
Long Term Effects of Selective Beta Blockade
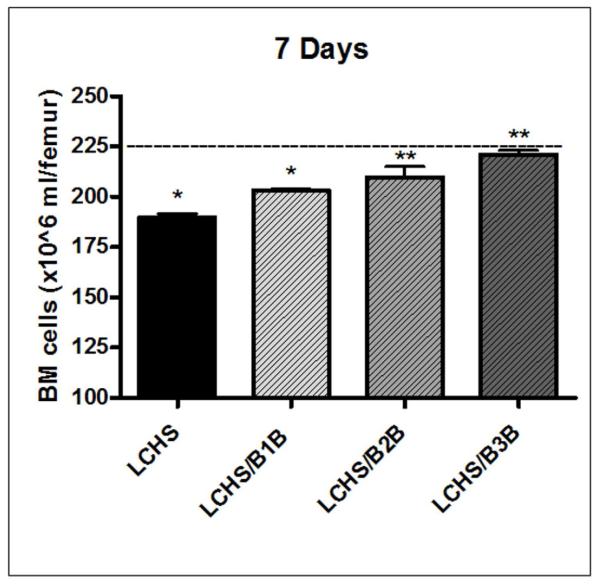
After 7 days, LCHS BM cellularity had improved compared to the 3h time point, but remained significantly lower than UC levels(190±3* vs. 225±7, *p<0.05)(Figure 6). Both B2B and B3B restored BM cellularity to UC levels at 7d (210±10 and 221±4 vs. 225±7). The cellularity of both LCHS/B2B and LCHS/B3B was significantly greater than LCHS alone at 7d (Figure 6). After 7 days of treatment, LCHS animals given B1B at 10mg still had significantly less BM cellularity as compared to UC values(203±1.6* vs. 225±7, *p<0.05).
Figure 6. BM cellularity seven days after LCHS.
After 7 days, LCHS BM cellularity was still significantly decreased when compared to UC levels. Treatment with B2B or B3B after LCHS showed a significant improvement in cellularity. Treatment with B1B after LCHS had no effect on BM cellularity. N=4-10/animals/group. (BM=bone marrow; UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation). * p<0.05 vs. UC, **p<0.05 vs. LCHS. Dashed line represents UC levels.
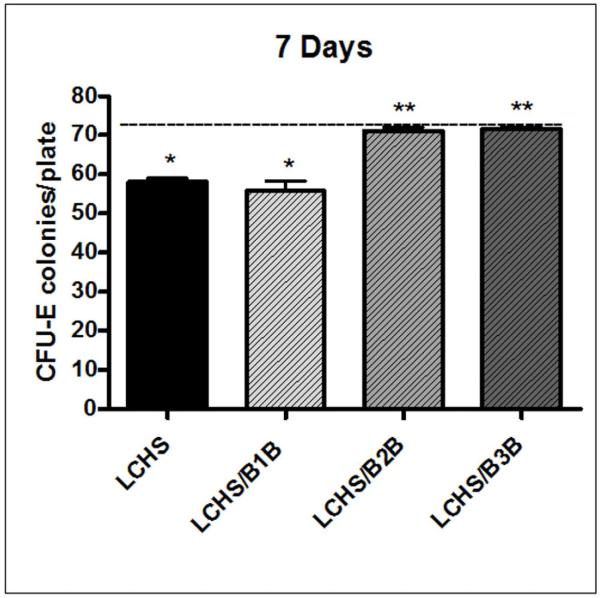
After 7 days, LCHS BM HPC growth for CFU-GEMM, BFU-E, and CFU-E remained significantly less than UC (28±2*, 50±3*, 58±1* vs. 35±1, 66±2, 75±2*p<0.05) (Figure 7). Treatment with either a B2B or a B3B for 7 days after LCHS improved CFU-E colony growth and prevented persistent BM HPC growth suppression as compared to LCHS alone (Figure 7). Similar trends were seen for the growth of CFU-GEMM and BFU-E, representative data from CFU-E is shown in figure 7. The administration of 7 days of B1B after LCHS had no effect on CFU-E colony growth and BM HPC colony growth remained suppressed (Figure 7).
Figure 7. CFU-E colony growth seven days after injury and shock.
After 7 days, LCHS still suppresses CFU-E colony growth as compared to UC. Treatment with B1B after LCHS had no effect on colony growth, while treatment with B2B or B3B significantly improved colony growth. N=4-10/animals per group. (UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation). * p<0.05 vs. UC, **p<0.05 vs. LCHS. Dashed line represents UC levels.
The Effect of Selective Beta Blockade on Hemoglobin
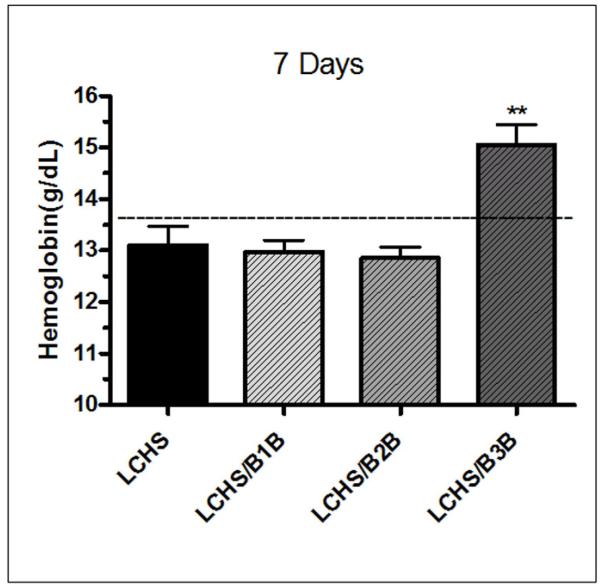
Hemoglobin (Hb) was used as an additional marker of hematopoietic function in the seven day animals. LCHS alone decreased Hb levels approximately 1 gram from UC (13.1±0.9 vs. 13.8±0.8). Both B1B and B2B following LCHS did not change Hb levels and they were similar to LCHS (13.0±0.5, 12.9±0.4 vs. 13.1±0.9). The animals receiving B3B following LCHS had a significant increase in Hb as compared to LCHS (15.1±0.8** vs. 13.1±0.9, **p<0.05) (Figure 8).
Figure 8. Hemoglobin seven days after injury and shock.
One week after LCHS, average Hb levels are lower than UC levels. Treatment with B1B or B2B after LCHS did not impact Hb level. However, there was a statistically significant 1 g/dL improvement in Hb in LCHS animals given B3B. N=4-10/animals per group. (Hb=hemoglobin; UC=unmanipulated control; LCHS=lung contusion followed by hemorrhagic shock; LCHS/B1B=LCHS followed by beta 1 blockade after resuscitation, LCHS/B2B=LCHS followed by beta 2 blockade after resuscitation, LCHS/B3B=LCHS followed by beta 3 blockade after resuscitation)**p<0.05 vs. LCHS Dashed line represents UC levels.
Discussion
The results of this study demonstrate a protection of the BM when B2B or B3B is given after trauma and HS, and that this protection is early but also maintained over time with daily use of either a B2B or B3B. The use of B1B after LCHS produced a 10% decrease in HR but it was ineffective in protecting the BM either early or late. Thus despite a cardiovascular effect, B1B does not offer any BM protection. These findings suggest the beta blockade protection of the BM is not via a cardiovascular effect but rather via an immunomodulatory effect involving the B2 and B3 receptors.
Our model examines the effects of severe tissue injury (LC), followed by systemic injury (HS) and their combined effects on BM function. This combined model causes a significant immediate BM suppression as measured by BM cellularity and BM HPC colony growth. The suppression of BM cellularity and BM HPC colony growth after LCHS has been demonstrated previously and is more severe than either LC or HS alone (15, 16). Seven days following injury, BM cellularity and BM HPC colony growth improve slightly but remain suppressed as compared to controls. In this study, Hb was used as an additional marker of BM function and was shown to be decreased 1 g/dL from control values. These findings are consistent with previous studies and suppression of Hb was demonstrated seven days after LCHS previously (16).
The use of B2B or B3B alleviates the BM suppression caused by LCHS. The use of either drug improves BM cellularity and BM HPC colony growth within hours after injury or over time with continued administration. Previously, Beiermeister et al. (17), showed a similar BM protection when B2B or B3B was given prior to injury in a LC model. With daily pretreatment of B2B or B3B before LC, animals had improvement in BM HPC colony growth (17). This study differs from ours in that it is single tissue injury model with no shock and that the drugs were administered before injury. In contrast, our study is the first to examine the effects of selective BB when given after trauma and HS. The current model is more clinically relevant as it examines both severe local tissue injury and systemic injury and it leads to a more severe suppression of BM HPC growth which is a more practical comparison to the trauma population.
Hemoglobin was chosen as a marker of BM function to demonstrate that with preserved BM function there is protection of hematopoietic function in peripheral blood. While both B2B and B3B had similar protective effects on both BM cellularity and BM HPC growth, their effects on Hb were different. B2B after LCHS had no effect on Hb level, whereas B3B significantly increased Hb by 1g/dL as compared to LCHS alone. This seems to suggest that while both B2 and B3 receptors play a role in BM regulation, they may do so in different ways. Recent work by Mendez-Ferrer et al. (18) shows that these two receptors may be regulating the BM by distinct mechanisms. The B3 receptor may downregulate Chemokine CXC ligand 2 (CXCL2), while stimulation of B2 receptors induces clock gene expression in stromal cells. Their study also demonstrated that under certain circumstances there may be cooperation of B2 and B3 receptors and a double deficiency of B2 and B3 receptors significantly decreased mobilization of HPCs to the peripheral blood (18). The B2 receptors are known to be located in the heart, vascular smooth muscle, bronchial smooth muscle, intestinal tract, liver, pancreas, and skeletal muscle and the B3 receptors are found primarily on adipocytes and also intestinal smooth muscle (19). However, recent studies have demonstrated the presence of both B2 and B3 receptors in the bone and bone marrow environment. Osteoblasts have been found to express B2 receptors (20). B3 receptors are found on stromal cells and regulate the circadian release of HPCs to the bloodstream (21). It appears that it is the cooperation of the B2 and B3 receptors that provides BM protection following LCHS.
While both B2B and B3B offered BM protection, the use of B1B after LCHS had no effect on BM function. Measures of BM cellularity, BM HPC growth, and Hb in LCHS animals given B1B were similar to LCHS alone across all time points. This lack of effect on BM function was shown previously when B1B was given pre-injury in a LC alone model (17). No recent studies have demonstrated the presence of B1 receptors in the BM or any BM protective effect provided by treatment with B1B. The B1 receptors are found mainly in cardiac and renal tissue and have a well-known cardioprotective effect when dosed appropriately. Neither B2B nor B3B produced any observable cardiovascular effect. In contrast, the use of B1B did have a clear effect on HR. Though cardiomyocytes express both B1 and B2 receptors, B1 is the predominant subtype, accounting for 75-80% of B receptors in cardiac muscle (22). Hakuno et al. (23) demonstrated in vitro that BM derived cardiomyocytes had an increased spontaneous beating rate when stimulated by isoproteronal, which was substantially decreased by 71% when a selective B1 blocker was given, whereas there was only a 21% reduction in beating rate when a selective B2 blocker was given. B1B did produce a 15% decrease in HR over seven days in non-injured animals as opposed to only a 10% decrease in HR when given following LCHS at the same dose. This suggests that after LCHS, there are higher circulating catecholamines that trigger both BM colony growth suppression and egress of HPCs from the BM. This finding is supported by studies showing that norepinephrine causes a dose dependent suppression of BM HPC growth (10). Beta blockade works by competitive inhibition of the beta adrenergic receptor (24). Thus in the presence of such extreme levels of NE, seen after severe injury and shock, a higher dose of B1B is needed to produce the same change in HR in a non-injured animal with less circulating catecholamines. In addition, beta adrenergic receptors are down regulated by the persistent stimulation from elevated catecholamines. This is the mechanism seen clinically in chronic congestive heart failure, where the density of cardiac beta-receptors is downregulated in response to the chronically elevated plasma catecholamine levels (25). Despite dose and injury related effects of B1B there is no impact on BM function. Thus, cardiac protection after trauma while it may be beneficial due to the inflammatory state is not directly related to BM protection.
The results of this study may have some broader implications for the use of propranolol, a non-selective beta blocker, in the clinical setting. A nonselective beta blocker would be associated with a decreased mortality rate in the trauma population due to the cardiovascular protection and/or decreased oxygen demands in these patients (3). As nosocomial infections, persistent anemia, increased transfusions all contribute to worse outcomes in the critically injured trauma population, the non-selective beta blocker could also provide BM and immune protection. Using HR to titrate a non-selective beta blocker for a positive non-cardiac effect has been done successfully in the burn population. Herndon et al. (27), have titrated propranolol for a goal HR decrease of 20% in order to decrease hypermetabolism and reverse muscle-protein catabolism in pediatric burn patients.
Recent work in our lab shows that non-selective BB after LCHS is both time and dose-dependent and that the effective therapeutic window is three hours from the time of injury in rats (12). This is similarly supported by the work of Krzyzaniak et al. (28) who demonstrated vagal nerve stimulation following thermal injury in rats must be performed within ninety minutes of injury to provide protection of the gut mucosal barrier. Though the BM protective effect of beta blockade only requires the use of B2B or B3B, the use of these drugs selectively may not be practical without any visible clinical parameters to assess efficacy and appropriate dosing of the medications. The endpoints of hemoglobin or infection rates are not evident until days after injury. However, by using a non-selective beta blocker, the B1B effect on HR could be used as a guide for effective dosing, while also providing BM protection with B2B and B3B. Also, since it appears that B2B and B3B offer BM protection in different ways, administering a non-selective BB to get the protective effect of both seems prudent. This is especially true if the cooperation of B2 and B3 receptors enhances mobilization of HPCs from the BM (21). Therefore, use of a non-selective BB after shock and injury may be more effective than the use of selective BB.
In summary, this study demonstrates that the cardiovascular effect produced by B1B does not provide any protection against BM dysfunction. The use of B2B and B3B following injury and HS provide BM protection and prevent BM HPC growth suppression and decreased BM cellularity. B2B and B3B likely protect the BM through different mechanisms because only B3B provided 1 g/dL increase in hemoglobin seven days after injury and HS. Therefore, the use of non-selective beta blockade following severe traumatic injury needs to be further studied to determine its potential therapeutic effects.
Acknowledgments
This research was supported by the National Institutes of Health grant K08 NIH GM078304 and the Clowes American College of Surgeons/American Association for the Surgery of Trauma Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–61. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–90. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Arbabi S, Campion EM, Hemmila MR, Barker M, Dimo M, Ahrns KS, et al. Beta-blocker use is associated with improved outcomes in adult trauma patients. J Trauma. 2007;62:56–61. doi: 10.1097/TA.0b013e31802d972b. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect. 2004;5:385–93. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 5.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, et al. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–53. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu JC, Livingston DH, Hauser CJ, Deitch EA, Rameshwar P. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Ann Surg. 2001;234:224–32. doi: 10.1097/00000658-200108000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 8.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–9. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 10.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, et al. Dose-response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold. J Surg Res. 2010;163:e85–90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhassan IO, Hannoush EJ, Sifri ZC, Jones E, Alzate WD, Rameshwar P, et al. Beta-blockade prevents hematopoietic progenitor cell suppression after hemorrhagic shock. Surg Infect. 2011;12:273–278. doi: 10.1089/sur.2010.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baranski GM, Cook KM, Sifri ZC, Alzate WD, Livingston DH, Mohr AM. Beta blockade following injury: A critical link between heart rate and immunomodulation. Surg Infect. 2011;12:S53–54. doi: 10.4172/2329-8820.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badami CD, Livingston DH, Sifri ZC, Caputo FJ, Bonilla L, Mohr AM, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 14.Papayannopoulou T, Migliaccio AR, Abkowitz JL, D’Andrea AD. Chapter 25. Biology of erythropoiesis, erythroid differentiation, and maturation. In: Hoffman R, Benz E, Shattil S, Furie B, et al., editors. Hematology: Basic Principles and Practice. 5th ed Churchill Livingstone Elsevier; Philadelphia: 2009. [Google Scholar]
- 15.Baranski GM, Offin MD, Sifri ZC, Elhassan IO, Hannoush EJ, Alzate WD, et al. β-blockade protection of bone marrow following trauma: the role of G-CSF. J Surg Res. 2011;170:325–331. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr AM, ElHassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, et al. Does beta blockade postinjury prevent bone marrow suppression? J Trauma. 2011;70:1043–1049. doi: 10.1097/TA.0b013e3182169326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beiermeister KA, Keck BM, Sifri ZC, ElHassan IO, Hannoush EJ, Alzate WD, et al. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 receptors after injury. J Trauma. 2010;69:338–343. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Méndez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–144. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald PA. Chapter 11. Adrenal medulla and paraganglia. In: Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology. 9th ed McGraw-Hill; New York: 2011. [Google Scholar]
- 20.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin Regulates Bone Formation via the Sympathetic Nervous System. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 21.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 22.Rockman HA, Koch WJ, Lefkowitz RJ. Cardiac function in genetically engineered mice with altered adrenergic receptor signaling. Am J Physiol. 1997;272:H1553–H1559. doi: 10.1152/ajpheart.1997.272.4.H1553. [DOI] [PubMed] [Google Scholar]
- 23.Hakuno D, Fukuda K, Makino S, Konishi F, Tomita Y, Manabe T, et al. Bone marrow-derived regenerated cardiomyocytes (CMG Cells) express functional adrenergic and muscarinic receptors. Circulation. 2002;105:380–386. doi: 10.1161/hc0302.102593. [DOI] [PubMed] [Google Scholar]
- 24.Robertson D, Biaggioni I. Chapter 10. Adrenoceptor antagonist drugs. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic & Clinical Pharmacology. 11ed McGraw-Hill; China: 2009. [Google Scholar]
- 25.Bristow M, Ginsburg R, Umans V, et al. Beta 1- and beta 2-adrenergic receptor subpopulations in non-failing and failing human ventricular myo-cardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor downregulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Brunton LL, Chabner BA, Knollmann BC. Chapter 12: Adrenergic agonists and antagonists. In: Brunton LL, Blumenthal DK, Murri N, Hilal-Dandan R, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12e McGraw-Hill; China: 2011. [Google Scholar]
- 27.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 28.Krzyzaniak M, Peterson C, Loomis W, Hageny AM, Wolf P, Reys L, et al. Postinjury vagal nerve stimulation protects against intestinal epithelial barrier breakdown. J Trauma. 2011;70:1168–1175. doi: 10.1097/TA.0b013e318216f754. [DOI] [PMC free article] [PubMed] [Google Scholar]