Abstract
Background
Little is known about the effects of use of a cane on balance during perturbed gait or whether people with Parkinson disease (PD) benefit from using a cane.
Objectives
The purpose of this study was to evaluate the effects of cane use on postural recovery from a slip due to repeated surface perturbations in individuals with PD compared with age- and sex-matched individuals who were healthy.
Design
This was a prospective study with 2 groups of participants.
Methods
Fourteen individuals with PD (PD group) and 11 individuals without PD (control group) walked across a platform that translated 15 cm rightward at 30 cm/s during the single-limb support phase of the right foot. Data from 15 trials in 2 conditions (ie, with and without an instrumented cane in the right hand) were collected in random order. Outcome measures included lateral displacement of body center of mass (COM) due to the slip and compensatory step width and length after the perturbation.
Results
Cane use improved postural recovery from the first untrained slip, characterized by smaller lateral COM displacement, in the PD group but not in the control group. The beneficial effect of cane use, however, occurred only during the first perturbation, and those individuals in the PD group who demonstrated the largest COM displacement without a cane benefited the most from use of a cane. Both PD and control groups gradually decreased lateral COM displacement across slip exposures, but a slower learning rate was evident in the PD group participants, who required 6, rather than 3, trials for adapting balance recovery.
Limitations
Future studies are needed to examine the long-term effects of repeated slip training in people with PD.
Conclusions
Use of a cane improved postural recovery from an unpracticed slip in individuals with PD. Balance in people with PD can be improved by training with repeated exposures to perturbations.
Failure to recover from a trip or slip during gait is one of the most common reasons for falls in people with Parkinson disease (PD).1,2 Previous studies demonstrated normal latencies, but reduced magnitudes, of postural responses in reaction to forward or backward slips induced by surface perturbations in people with PD while maintaining standing posture.3–5 Other studies suggested that people with PD may be even more unstable in the lateral direction than in the forward/backward direction.6,7 Studies have shown that lateral slips in people with PD result in even smaller feet-in-place responses and delayed initiation of stepping responses, resulting in more falls, compared with age-matched control participants.4,8
Canes are commonly prescribed for people with PD to improve mobility and to help maintain balance. It has been suggested that a cane may prevent or reduce falls in people with PD.9 However, until now, this notion has not been investigated in people with PD, and the effects of cane use on postural recovery from a slip in other neurological populations are controversial. A cane can reduce postural sway during unperturbed stance in people who are visually impaired10,11 and in patients with stroke.12,13 In the study by Ashton-Miller et al,14 when the support surface was moving, a cane reduced loss of balance during single-legged stance in patients with peripheral neuropathy. In contrast, results from studies of young individuals who were healthy suggested that the use of a cane could increase the risk of falling induced by lateral movement of the support surface by interfering with lateral compensatory stepping or grasping.15,16 However, these studies assessed the effect of cane use on postural recovery during stance, so they may not represent the true effect of using a cane to recover equilibrium during walking.
Repeated exposure to slip perturbations during walking has been advocated as an intervention strategy to prevent slip-related falls.17 Adaptation to repeated slip perturbations is considered a component of motor skill learning that occurs within seconds to minutes over a period of trial-and-error practice when exposed to a novel condition.18 Assessment of capability for motor adaptation is useful for assessing rehabilitation potential in people with brain damage.19 Previous studies have shown that PD affects the ability to quickly change postural response strategy when the initial conditions change.20,21 The role of the basal ganglia in motor learning, however, may be task dependent.22,23 Whether short-term postural adaptation also would be impaired by PD is unclear. At present, only a few studies have addressed motor adaptation to balance tasks in people with PD, and the results are inconclusive.24–30
This study compared the effect of cane use on postural recovery from a slip during walking in people with PD and age-matched control participants. We also assessed how people learned to minimize disequilibrium by practicing repeated slips during walking. Slip perturbation in the direction that triggered the fall away from the cane was selected, as it was the most disturbing direction to balance.14 We hypothesized that use of a cane would improve postural recovery from a slip during walking in people with PD. We also predicted that people with PD would demonstrate slower postural adaptation to repeated exposure to slips than people without PD.
Method
Participants
In this prospective study, 14 individuals with idiopathic PD (PD group) and 11 age- and sex-matched individuals who were healthy (control group) participated (Tab. 1). All participants were recruited from the Portland, Oregon, area, and data collection was performed from July 2010 to January 2011. Participants in both groups could walk independently for 10 m without using a walking aid. The Activities-specific Balance Confidence (ABC) scale31 and the Edinburg Handedness Inventory32 were administered to all participants. Semmes-Weinstein monofilaments were used to assess somatosensation on the index finger of the right hand and the plantar surface of the feet. The vibration sensation was examined on the great toe and the index finger by using a 128-Hz tuning fork. None of the participants in the control group demonstrated impaired somatosensation, whereas 5 participants with PD showed a decrease in somatosensation (>4.31-g monofilaments33) and 8 showed a decrease in vibration sensation of the finger and feet.
Table 1.
Participant Characteristicsa
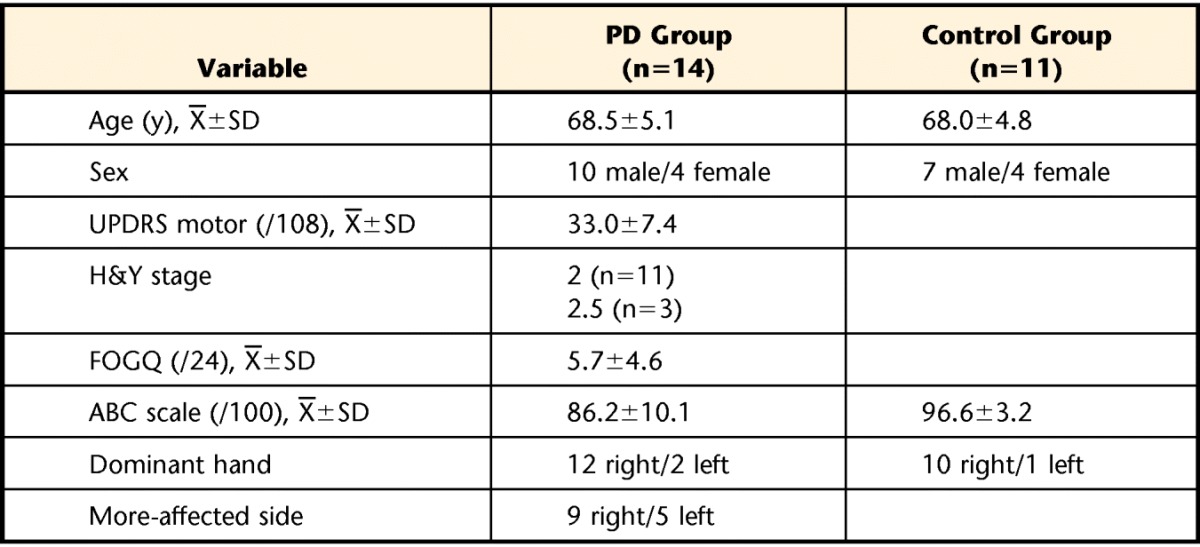
PD=Parkinson disease, UPDRS motor=motor subscale the Unified Parkinson's Disease Rating Scale, H&Y stage=Hoehn and Yahr stage, FOGQ=Freezing of Gait Questionnaire, ABC scale=Activities-specific Balance Confidence scale.
In addition, the motor subscale (part III) of the Unified Parkinson's Disease Rating Scale, the Hoehn and Yahr scale, and the Freezing of Gait Questionnaire34 were administered to participants with PD during the “on” stage by a physical therapist. Recruited participants had mild to moderate PD with minimal gait problems (Tab. 1). All participants with PD took their usual medications within 2 hours of testing (“on” stage), and none reported a wearing off of their medication or exhibited freezing during data collection. All participants provided written informed consent.
Tasks and Procedure
Two conditions of walking (with perturbation and no perturbation) and 2 cane conditions (with cane and no cane) were examined in this study. The cane was customized with a wireless force sensor (MLP-100, Transducer Techniques Inc, Temecula, California) to detect the vertical force exerted through its length. This cane was adjusted so that the top of the cane was at the level of each participant's greater trochanter,35 and all participants held the cane in the right hand. Participants were instructed how to use the cane “to touch the ground without much weight” in the same phase as the left foot (ie, the cane was put on the ground when the left foot was in contact with the ground). Prior to data collection, a 10-minute practice session was given to familiarize the participants with using a cane during walking. They then were asked to walk 10 m, including steps onto a hydraulically driven, servo-controlled, movable force platform embedded in the floor. Twenty trials of unperturbed walking, 10 each for the cane and no-cane conditions, were captured at the beginning of data collection to provide the baseline gait information. A 10-minute rest was given to each participant at the end of unperturbed walking to prevent fatigue.
After the unperturbed walking trials, participants were informed that the next set of trials were all perturbation trials in which the floor would move to mimic slips, but the participants were unaware of the direction or the timing of perturbation and they were not allowed to practice the perturbation. The slip perturbations involved a 15-cm rightward movement of the force platform at 30 cm/s, triggered by 40-N loading of the right foot on the forceplate. The starting location of walking was manipulated so that the participant's right foot would naturally be the first to step on the forceplate. Posttest analysis revealed that the perturbation was triggered at the single-limb support phase of the right foot as the left foot was lifted off the ground prior to the surface translation (control group: X̅=21.9 milliseconds; PD group: X̅=4.7 milliseconds). Similarly, in the cane condition, the perturbation was triggered when the cane in the right hand was lifted off the ground.
The participants were instructed to walk straight ahead and, when the perturbation occurred, to try to recover their balance and continue walking. To prevent falls without restricting motion, participants wore a safety harness (NeuroCom, a division of Natus, Clackamas, Oregon) tethered to a sliding hook on an overhead rail. Thirty trials of slip perturbations, 15 each for the cane and no-cane conditions, were randomly assigned in blocks of 10 to each participant. The first trial of perturbation was controlled so that an equal numbers of participants started in the cane and no-cane conditions. All participants were able to perform 30 trials of perturbed walking without requiring additional rest, and no one fell during the surface perturbation.
Data Collection and Analysis
A Motion Analysis System (Motion Analysis Corporation, Santa Rosa, California) with 8 cameras was used to capture 3-dimensional spatial coordinate information about body segment displacements, the movement of the platform, and the movement of the force sensor cane. Reflective markers were placed bilaterally on the following anatomical landmarks: temporal bone, lateral mandibular joint, acromion, olecranon, radial styloid process, greater trochanter, lateral femoral condyle, lateral malleolus, heel, and fifth metatarsophalangeal joint. In addition, 1 marker was placed on the back corner of the platform, and 2 markers were placed on the top and bottom of the cane shaft. Kinematic data were sampled at 60 Hz and low-pass filtered using a second-order, dual-pass Butterworth filter with a cutoff frequency of 6 Hz. The Matlab software program (MathWorks Inc, Natick, Massachuetts) was used to perform subsequent data analysis. Body COM position was calculated from segmental COM position and the anthropometric data36 and was adjusted to the position of the force platform. The velocity of the body COM was calculated from the derivative of body COM position in the anteroposterior direction. The average COM velocity in each trial was used to identify gait speed during walking.
As the rightward perturbation resulted in large postural displacements to the left, lateral displacement of the body COM was the primary outcome for effectiveness of postural responses and postural adaptation to repeated slips (Fig. 1). The initial peak lateral displacement of the COM after the perturbation was used to measure postural recovery from the slip (PRS), after subtracting the normal lateral COM displacement during gait and the passive effect of the translation. Peak-to-peak lateral body COM displacements during unperturbed walking were averaged across trials to represent gait lateral COM in the no-cane and cane conditions. To determine the PRS, the maximum lateral displacement of the platform (15 cm) and the individual's gait lateral COM were subtracted from the peak lateral COM displacement. Benefit of the cane was defined as the difference between PRS at the first trial in the no-cane and cane conditions (PRSno_cane − PRScane), such that a more positive value indicated more benefit of the cane on improving postural recovery.
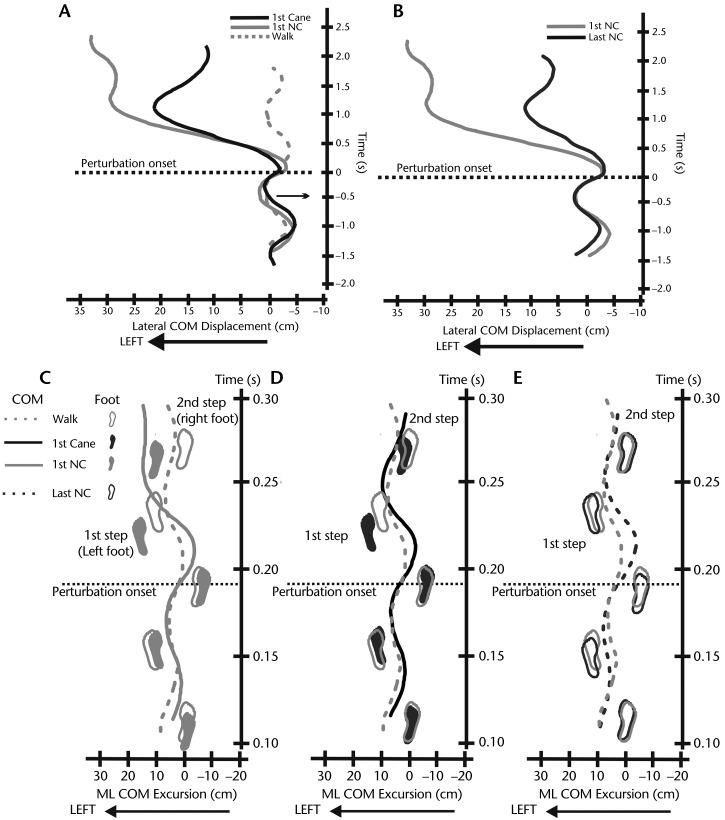
Figure 1.
Characteristics of lateral center of mass (COM) displacements and mediolateral (ML) COM excursions. A. Lateral COM displacement (with positive values indicating leftward displacements from normal unperturbed gait) in a representative individual with Parkinson disease (PD) during an unperturbed walk and during a slip perturbation with a cane and with no cane (NC) to illustrate the typical effect of cane use on reducing lateral COM displacement in the first trial. B. Lateral COM displacement in the same individual with PD during the first and last trials of a slip perturbation with NC to demonstrate postural adaptation to repeated slip exposures. Perturbation onset is aligned with time 0, so the positive values indicate the time post-perturbation. C. ML COM excursion and foot placements in the same individual with PD during an unperturbed walk compared with the first slip perturbation with NC. D. ML COM excursion and foot placements during an unperturbed walk compared with the first slip perturbation with a cane. E. ML COM excursion and foot placements during an unperturbed walk compared with the last slip perturbation with NC. The first step and second step indicate the foot placements as a result of a slip perturbation. Note the deviation of the foot placement from the plane of progression occurred after the first slip perturbation with NC (Fig. 1C).
To determine the rate of postural adaptation, we identified the number of trials, starting at the first trial, that were taken until a person could maintain stable postural responses. Stable postural responses were calculated from the 95% confidence interval (CI) of maximum lateral COM displacement across the 8th through 15th trials of the perturbed no-cane condition. The 8th trial has been shown to be fully habituated, with minimal further changes occurring in the subsequent trials.30 The first trial that reached within 95% CI of maximum lateral COM displacement during perturbed gait was identified as the rate of postural adaptation.
Postural responses due to slip perturbations also involved a change in gait spatiotemporal parameters. Step width and step length were determined from the distance between the right and left heel markers in the lateral and anteroposterior directions, respectively. The average step width from individuals during unperturbed gait was subtracted from step width in each perturbed trial to calculate step width adjustments due to slip (SWS). Similar calculations were performed (ie, step length during perturbed trial minus average step length during unperturbed walking) to determine step length adjustments due to slip (SLS).
The step response time was used to indicate how fast the participant put his or her foot down after the platform perturbation onset and was selected at the first, lowest position of the heel marker. Thus, the first step response time corresponded to placement of the left foot on the ground, and the second step response time corresponded to placement of the right foot on the ground. The cane response time was used to determine how fast the cane was put on the ground after the platform perturbation onset and was selected based on the first, lowest position of the bottom cane marker. The lateral base of support (BOS) after platform perturbation was calculated from the distance between the first and second heel markers or between the first heel marker and the cane marker, whichever touched the ground first.
The longitudinal force exerted on the cane was captured at a sampling rate of 7 Hz by the force sensor cane and sent wirelessly via Bluetooth to the computer. Cane force was calculated by the Matlab software based on our calibration curve. The peak longitudinal cane force was averaged separately in 2 conditions: unperturbed walking and the first ground contact after perturbation. The data from the Motion Analysis System and the cane were synchronized to characterize the temporal component of cane use.
Sample size calculation was performed using G*power 3.37 Based on a similar study of people with PD,38 we calculated that a sample of at least 9 people in each group would be required to achieve 90% power (using the mean anteroposterior COM difference of 5 cm [SD=3.5] between the PD and control groups). Other statistical analysis was performed using Statistica software 7 (StatSoft Inc, Tulsa, Oklahoma). A 2-way mixed analysis of variance (ANOVA) was used to compare the baseline gait characteristics (ie, gait lateral COM, step width, step length, and gait speed) between the 2 groups and the 2 cane conditions. Effect of cane use and postural adaptation from each group was determined using a 2-way repeated-measures ANOVA (2 cane conditions × 15 trials). Comparison of the cane benefit was performed on the PRS during the first trial of cane and no-cane conditions using a 2-way mixed ANOVA (2 groups × 2 cane conditions). An independent t test was used to compare the amount of force exerted on the cane between the PD and control groups. The main and interaction effects were set at a 2-sided .05 significance level. The Tukey test was used to find post hoc differences when there was a significant main or interaction effect.
Results
Effect of Cane Use on Unperturbed Gait
Cane use did not change gait characteristics. Gait characteristics for both groups, including gait lateral COM displacement, step width, step length, and gait speed, were not significantly different whether using a cane or not using a cane (Tab. 2). These gait characteristics also were not different between groups, with the exception of step length, which was significantly shorter in the PD group (F1,23=9.5, P=.01). Both groups maintained their gait speed prior to surface perturbations similar to gait speed in trials without a perturbation (Tab. 2). Cane force was not different between groups either during perturbed walking (PD group: X̅=20.5 N, SD=8.6; control group: X̅=26.7 N, SD=16.1) or during unperturbed walking (PD group: X̅=18.4 N, SD=13.7; control group: X̅=23.8 N, SD=16.2).
Table 2.
Baseline Gait Characteristicsa
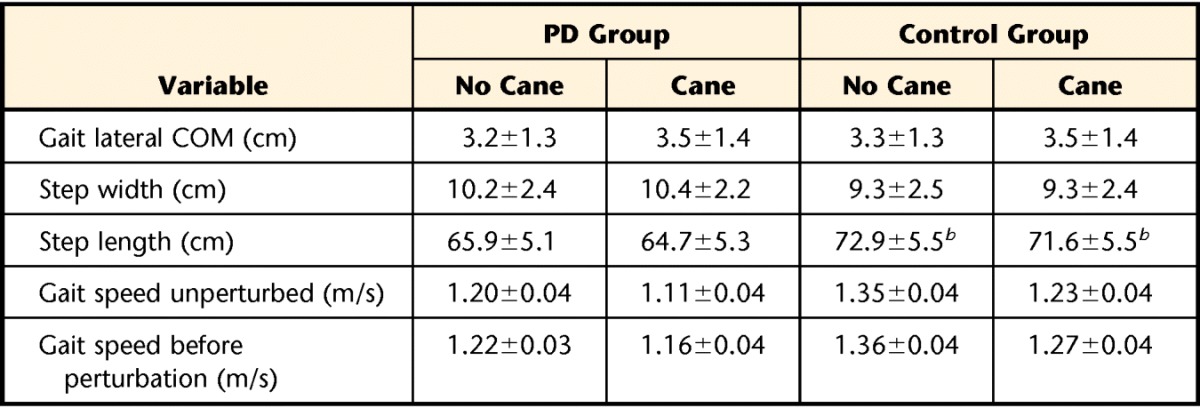
Values are presented as mean±standard deviation. PD=Parkinson disease, COM=center of mass.
Significant difference in step length, but not in gait speed, between PD and control groups at P<.05.
Effect of Cane Use and Postural Adaptation During Perturbed Gait
Figure 1A illustrates an example of leftward displacement of the body COM due to the rightward surface perturbations with and without a cane compared with body COM lateral motion during unperturbed gait. Use of a cane and practice both reduced the lateral displacement of the body COM in response to slips while walking (Figs. 1A and 1B). Widening of the base of foot support in the first step after the first perturbation also was observed (Figs. 1C and 1D). The foot placements were adjusted upon repeated slip exposure so that they were similar to the steps during unperturbed gait (Fig. 1E).
The lateral COM displacements across 15 trials of perturbation during the 2 cane conditions were 4 to 7 times larger than during unperturbed gait in the PD group (Fig. 2A) and the control group (Fig. 2B). Maximum lateral COM displacement was significantly larger for the PD group than that for the control group during the 1st trial, but not significantly different during the 15th trial. A 2-way repeated-measures ANOVA (15 trials × 2 cane conditions) revealed a significant trial effect, but no cane or interaction effects, and these statistical results were similar in the PD group (F14,364=9.3, P=.00) and in the control group (F14,280=4.1, P=.00). Post hoc analysis for the trial effect showed that the COM displacement to the perturbation was significantly larger during the 1st trial than during all of the following trials in the PD group, and the 2nd trial differed significantly from the 6th trial through the 15th trial. In contrast, the COM displacement in the control group at the 1st trial did not differ from that at the 2nd trial, but both were significantly larger than displacement in the rest of the trials. The 95% CI of lateral COM displacement across all perturbed trials was 11.8 to 13.0 cm in the PD group and 11.5 to 13.4 cm in the control group. The PD group showed a slower rate of postural adaptation compared with the control group. The 6th trial of the PD group and the 3rd trial of the control group were the first trials that reached the 95% CI of lateral COM displacement during perturbed stance.
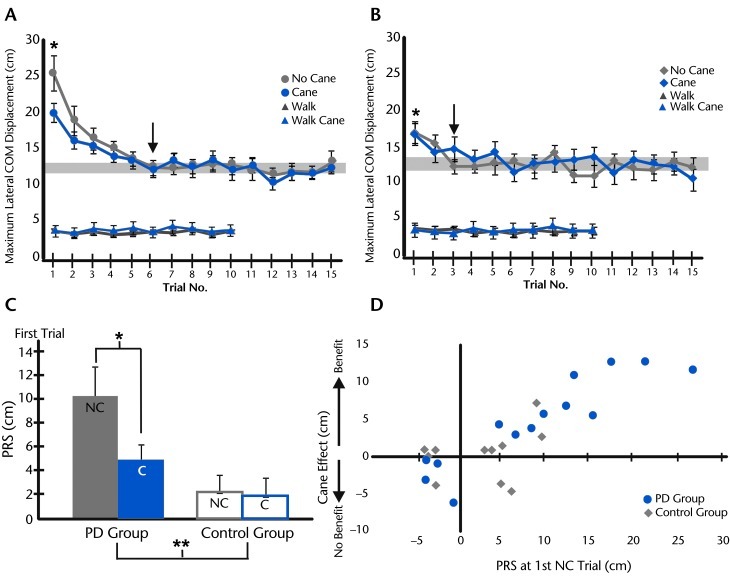
Figure 2.
Postural responses to slips across trials in (A) participants with Parkinson disease (PD) and (B) control group. Group average of maximum lateral center of mass (COM) displacement (with standard error of the mean) across 10 trials of unperturbed walking with cane use (walk cane) and without cane use (walk) compared with group average lateral COM displacement across 15 trials of perturbed gait with and without cane use. The gray zone represents 95% confidence interval of lateral COM displacement in the 8th to 15th trials, with the arrow indicating the first trial that reached the gray zone. The asterisk indicates a significant difference of the COM displacement in the first trial from the other trials at P<.05. C. Group average postural response from the slip (PRS) (with standard error of the mean) during the first trial of perturbed gait with cane use (C) and without cane use (NC) in the PD and control groups. The asterisk indicates difference between NC and C trials at P<.05 in the PD group. The double asterisk indicates difference in PRS between the PD and control groups at P<.05. D. Plot of relationship between PRS calculated during the first NC trial and the cane effect (PRSno_cane − PRScane) in the PD and control groups. The positive value of cane effect indicates the cane benefit. Six participants in the PD group showed larger postural instability during the PRS than any participant in the control group.
The PD group, but not the control group, significantly benefited from cane use in the first perturbation trial (see video clip). Figure 2C compares the PRS from peak COM displacement during first trial of the cane and no-cane conditions in both groups of participants. A 2-way ANOVA (2 groups × 2 cane conditions) demonstrated significant cane (F1,23=6.1, P=.02) and interaction (F1,23=4.8, P=.03) effects, suggesting that the use of a cane reduced PRS significantly in the PD group but not in the control group. However, individual participants' data showed that not every participant with PD benefited from use of a cane; 4 out of 14 participants with PD did not benefit from use of a cane. The relationship between the benefit of using a cane and the PRS in the first trial of the no-cane condition (Fig. 2D) showed that participants who had the largest PRS (ie, the largest postural displacement) benefited the most from use of a cane.
Spatiotemporal Gait Parameters After Perturbation
Step response times after a perturbation did not differ whether using a cane or not using a cane (Figs. 3A and 3B). In addition, the first and second step response times were not different between groups. However, step response times significantly increased across repetitions (P=.00), showing that participants in both groups put their foot on the ground more slowly in the later trials (Fig. 3A). Also, no group or cane effects were found for the second step time (Fig. 3B). Only the trial effect was significant, showing that the second step time became longer across trials (PD group: F14,364=5.5, P=.00; control group: F14,280=7.4, P=.00).
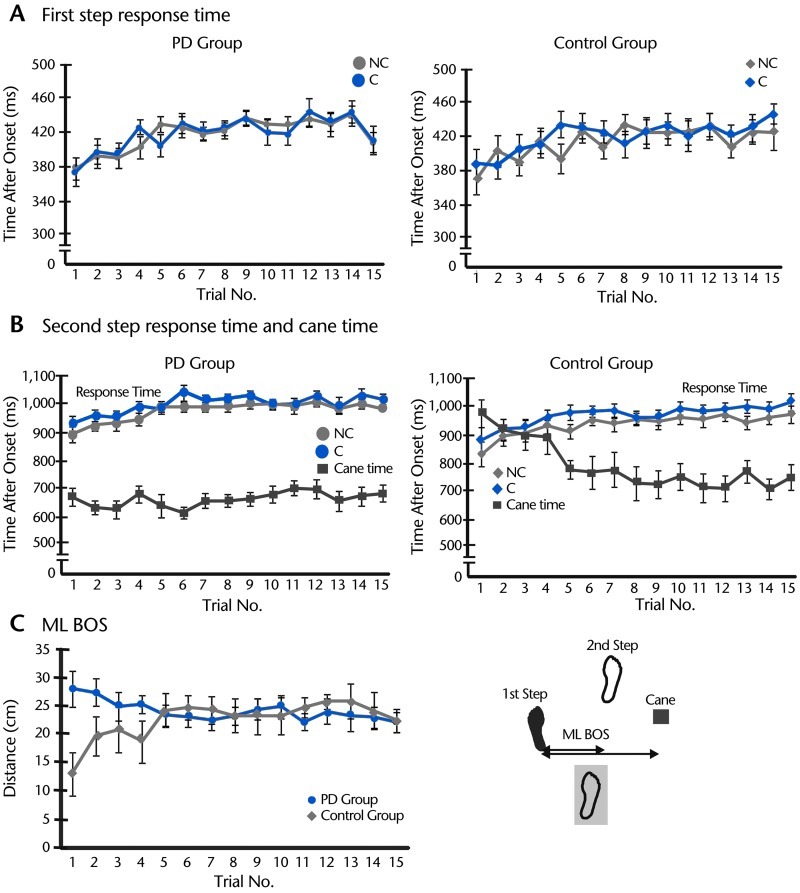
Figure 3.
Step response time, cane time, and lateral base of support (BOS). A. Group average of first step response time (with standard error) during perturbed walking with a cane (C) and without a cane (NC) across 15 trials in participants with Parkinson disease (PD) and controls. B. Group average of second step response time during perturbed walking in C and NC conditions across 15 trials in the PD and control groups. Group average of cane time (with standard error) also is demonstrated in the same graphs. C. Group average of mediolateral (ML) BOS when using a cane across 15 trials of perturbed walking in the PD and control groups. The measurement of ML BOS is shown in the right panel as ML distance from the first step to the second step or to the cane, whichever was put down on the ground first.
The participants in the PD group put their canes on the ground faster after a perturbation compared with those in the control group (Fig. 3B). However, the participants in the control group gradually adjusted the way they used the cane so that after the fifth trial, they put the cane down on the ground as fast as the participants in the PD group. Figures 3A and 3B also show that the participants in the PD group put the first recovering (left) foot on the ground first, followed by the cane and then the right foot. In contrast, during the first trial of platform perturbation, the participants in the control group put their left foot on the ground first, followed by the right foot and then the cane. A 2-way mixed ANOVA (2 groups × 15 trials) revealed the significant group (F1,23=10.08, P=.00), trial (F14,322=4.83, P=.00), and interaction (F14,322=7.02, P=.00) effects for the cane time.
The sequence of touching the ground by either foot or cane creates a different BOS. Figure 3C shows that the lateral BOS in the participants with PD was widest during the first 2 trials, whereas the BOS in the control group was narrower during their first four trials than in later trials.
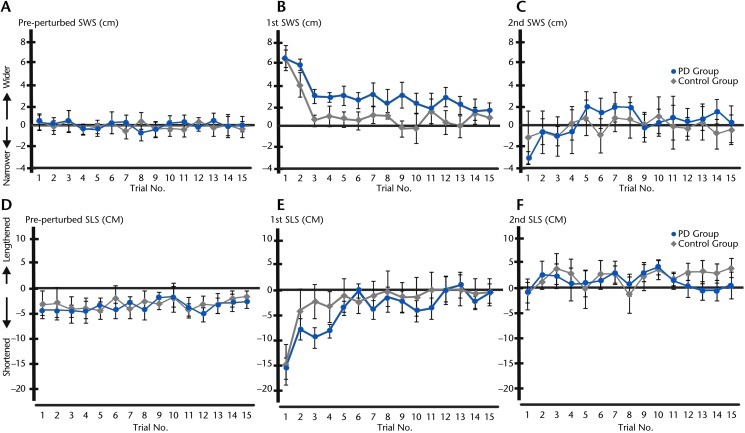
Step width and step length adjustments due to slip are presented in Figure 4. The SWS of the pre-perturbed step did not differ from the step width during unperturbed walking (Fig. 4A), but both groups shortened their pre-perturbed step slightly (Fig. 4D). A 2-way repeated-measures ANOVA (15 trials × 2 cane conditions) showed no differences between cane conditions but a significant trial effect for 1st SWS (F14,322=7.47, P=.00) and 1st SLS (F14,322=8.58, P=.00) due to slip in the 1st trial. Post hoc analysis revealed that 1st SWS during the 1st trial was wider than during the 3rd through 15th trials (Fig. 4B) and was not different from unperturbed step width by the 3rd trial in the control group and the 14th trial in the PD group. In contrast, the 1st SLS in the 1st trial was significantly shorter than in the rest of the trials and became similar to unperturbed step length by the 3rd trial in the control group and the 6th trial in the PD group (Fig. 4E). As the SLS was much larger than the SWS, these results suggest that the faster step response time in the 1st trial was related to the shorter step length and the longer step response times in later trials were associated with longer step lengths. This finding, however, is not applicable to the second step response time because there were no significant adjustments in 2nd SWS and 2nd SLS (Figs. 4C and 4F).
Figure 4.
Step width adjustment (SWS) and step length adjustment (SLS) due to the slip across 15 trials of slip perturbation with no cane in participants with Parkinson disease (PD) and controls. A. Group average of pre-perturbed SWS in the PD and control groups. B. Group average of first SWS in the PD and control groups. C. Group average of second SWS in the PD and control groups. D. Group average of pre-perturbed SLS in the PD and control groups. E. Group average of first SLS in the PD and control groups. F. Group average of second SLS in the PD and control groups.
Discussion
Benefit of Cane Use for Balance During Walking
The present study is the first to investigate the benefits of using a cane on balance control in response to a slip while walking. We compared the benefits of using a cane in people with PD and elderly control participants. Our results demonstrated that use of a cane improved postural recovery from the first, novel slip in individuals with PD, but not in the healthy control participants, who showed less COM displacement to the slip, with or without a cane. With the average cane force of 21 N (2% body weight), the reduction of lateral COM displacement in people with PD is not likely from mechanical support to the body. Earlier studies showed that individuals poststroke required a cane force of 7% to 25% of body weight to provide mechanical support to the body during walking.39,40
When holding the cane, the central nervous system receives augmented somatosensory cues from the hand and arm that provide spatial orientation information to control balance.10,41 Previous studies have shown postural stability in stance improves even when the cane force is insufficient for mechanical support (ie, <1 N in people who were healthy10 and in people with peripheral neuropathy,42,43 <4 N in people with stroke44,45). In the cane condition, participants with PD anticipated the cane contact with the ground prior to the first recovering step (left foot) and, hence, expanded their lateral BOS during left single-leg support. Our analysis confirmed that the BOS was significantly larger during the first trial for the PD group than for the control group. This increased BOS can allow a larger COM displacement to be tolerated without loss of stability.46 Another potential benefit of using a cane could be explained by the contribution of stabilizing forces at the hand holding the cane, such that a small force through the cane could create sufficient corrective moment around the hand to compensate for the loss of balance.14–16 Using calculations similar to those of Bateni and Maki,47 the average cane force of 21 N located 28 cm from the left foot created a moment of 5.88 N·m acting to oppose the rotational moment of the body. However, the body COM was perturbed away from the side using the cane, so the cane was not directly used to mechanically stop the fall of the body COM due to the perturbation.
Another finding in this study was that the cane benefit was associated with the amount of instability measured during the first novel slip, such that individuals who showed greater instability benefited the most from the use of a cane. This result could explain why the cane benefited people with PD more than the control participants and why the benefit of using a cane was less during subsequent trials when stability improved after repeated exposure to perturbations. These findings supported the notion that a cane is helpful only in people whose stability is compromised by inadequate postural responses.
In the present study, our participants with PD showed greater postural instability during the first slip compared with their age- and sex-matched controls. This result is in contrast to the findings of a previous study in our laboratory that examined the ability to stop walking during unexpected, forward surface slips, which indicated that PD did not impair the ability to integrate a balance-correcting response into gait termination.38 The disagreement in the results may be due to differences in perturbation direction, such that people with PD are more prone to lateral, than forward, perturbations. Some studies of postural responses to lateral slips in a standing position demonstrated that people with PD had longer step latency and shorter step length in response to lateral slips.8,48 However, we did not find differences in step response time, SLS, or SWS between people with PD and controls, suggesting that postural requirements may be higher during standing than during walking.
Postural Adaptation in People With PD
In this study, we found that participants with PD preserved postural adaptation capability, but the rate of adaptation was slower than that of their age- and sex-matched controls. Our finding that PD does not impair the ability to learn an automatic balance task agrees with the findings of previous studies demonstrating postural task learning in people with PD practicing a limits of stability balance task26 and a balance perturbation by pulling a weight27 and in response to surface rotation during stance.24,30 In those studies, people with PD improved their postural responses at the end of the acquisition phase, and the effect of learning was sustained from 1 week to 2 months after the training.26 Upon repeated exposure to surface perturbations, the central nervous system builds or updates the internal representations of the potential threats to stability to improve feedforward control and reduce reliance on slower, feedback corrective mechanisms for successful recovery.49 Changes in feedforward control with practice, prior to an encounter with a perturbation, lead to improvement of dynamic stability prior to the onset of a perturbation, which reduces the need for a postural response following the perturbation.50 In our study, the role of gradual improvement via feedforward control was demonstrated by the gradual reduction of lateral COM displacement after repeated perturbations, together with the adjustment of step width until it was close to those of unperturbed, walking steps. The reduction of COM displacement with practice is likely due to optimized postural responses.38,51,52 Reviews of previous motor learning studies reveal the slow learning rate of people with PD,29,53,54 but our study is the first to show that this finding is also true for a postural response task. In our study, however, the PD group required more trials to adapt to the same level as the control group, perhaps because their early trials resulted in such large disequilibrium and large error to correct.
Limitations and Clinical Implications
The benefit of using a cane for postural recovery during unpracticed slips during gait was confirmed in this study. Most of the slips commonly occurring in daily life that could lead to falls in people with PD are unpracticed (ie, those that have not been practiced). Therefore, our results suggest that a cane could be an effective and inexpensive aid for a majority of people with PD to prevent a fall due to a slip, especially when they need to navigate in unfamiliar environments. The limitation of this study is that postural perturbations generated by the hydraulic platform may not fully represent slip perturbations that occur in the real world. Thus, the benefits of cane use also should be validated outside a laboratory during daily walking.
In the present study, the cane benefit was unlikely to be associated with handedness, as the participants who benefited from using a cane included those with both right-hand and left-hand dominance. Although traditionally the cane is suggested to be held in the less-affected hand,55 our participants with PD showed benefit from use of a cane regardless whether they held it in the more-affected or less-affected hand. This finding may have been due to the fact that the cane was not used for biomechanical support to resist the perturbation, but rather to increase the lateral BOS.
Consistent with other studies,56–58 we found that postural adaptation to repeated exposure to perturbations is possible in people with PD, and this finding is encouraging for therapeutic interventions. The discovery that the postural adaptation rate was slower in people with PD than in elderly controls could guide clinicians to arrange more practice trials for training postural control in people with PD. This study, however, focused on postural adaptation during the acquisition phase of learning, so it is not known how long this learning would last. Future studies are needed to examine the long-term effects of repeated slip training in people with PD.
The Bottom Line
What do we already know about this topic?
Canes are commonly prescribed to people with Parkinson disease (PD) to improve mobility and balance. It has been suggested that a cane may help people with PD prevent or reduce falls by helping them recover from a slip. However, the effects of cane use on postural recovery from a slip in other neurological populations, such as people with visual impairments or people with stroke, are controversial.
What new information does this study offer?
This study investigated whether people with PD benefit from a cane. Results show that people with PD who have impaired balance benefit from a cane when encountering slips during walking. The use of the cane reduces body sway druing unpracticed slips. Balance in PD also can be improved by training with repeated slip exposures.
If you're a patient, what might these findings mean to you?
Use of a cane when walking may be helpful in recovering from a slip if you have PD. Balance training that includes practice recovering from slips can help improve balance, and more practice appears to be needed in people with PD than in healthy older people.
Supplementary Material
Footnotes
Dr Boonsinsukh, Dr Carlson-Kuhta, and Dr Horak provided concept/idea/research design, writing, and project management. Dr Boonsinsukh and Dr Saengsirisuwan provided data collection. Dr Boonsinsukh provided data analysis. Dr Carlson-Kuhta and Dr Horak provided participants. Dr Horak provided fund procurement, facilities/equipment, and institutional liaisons. Dr Saengsirisuwan and Dr Horak provided consultation (including review of manuscript before submission). The authors thank Edward King and Martina Mancini for technical assistance. They appreciate the help from Dr Misha Pavel for use of the instrumented cane.
This study was approved by the Institutional Ethical Review Board of Oregon Health & Science University.
Funding support for this study was provided by National Institutes of Health grants F05AG032213 and R37AG006457.
References
- 1. Ashburn A, Stack E, Ballinger C, et al. The circumstances of falls among people with Parkinson's disease and the use of Falls Diaries to facilitate reporting. Disabil Rehabil. 2008;30:1205–1212 [DOI] [PubMed] [Google Scholar]
- 2. Mak MK, Pang MY. Parkinsonian single fallers versus recurrent fallers: different fall characteristics and clinical features. J Neurol. 2010;257:1543–1551 [DOI] [PubMed] [Google Scholar]
- 3. Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson's disease. J Neurophysiol. 2004;91:489–501 [DOI] [PubMed] [Google Scholar]
- 4. Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson's disease. Exp Neurol. 2005;193:504–521 [DOI] [PubMed] [Google Scholar]
- 5. Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396 [DOI] [PubMed] [Google Scholar]
- 6. Mitchell SL, Collins JJ, De Luca CJ, et al. Open-loop and closed-loop postural control mechanisms in Parkinson's disease: increased mediolateral activity during quiet standing. Neurosci Lett. 1995;197:133–136 [DOI] [PubMed] [Google Scholar]
- 7. van Wegen EE, van Emmerik RE, Wagenaar RC, Ellis T. Stability boundaries and lateral postural control in Parkinson's disease. Motor Control. 2001;5:254–269 [DOI] [PubMed] [Google Scholar]
- 8. King LA, Horak FB. Lateral stepping for postural correction in Parkinson's disease. Arch Phys Med Rehabil. 2008;89:492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Constantinescu R, Leonard C, Deeley C, Kurlan R. Assistive devices for gait in Parkinson's disease. Parkinsonism Relat Disord. 2007;13:133–138 [DOI] [PubMed] [Google Scholar]
- 10. Jeka JJ, Easton RD, Bentzen BL, Lackner JR. Haptic cues for orientation and postural control in sighted and blind individuals. Percept Psychophys. 1996;58:409–423 [DOI] [PubMed] [Google Scholar]
- 11. Maeda A, Nakamura K, Otomo A, et al. Body support effect on standing balance in the visually impaired elderly. Arch Phys Med Rehabil. 1998;79:994–997 [DOI] [PubMed] [Google Scholar]
- 12. Maeda A, Nakamura K, Higuchi S, et al. Postural sway during cane use by patients with stroke. Am J Phys Med Rehabil. 2001;80:903–908 [DOI] [PubMed] [Google Scholar]
- 13. Milczarek JJ, Kirby RL, Harrison ER, MacLeod DA. Standard and four-footed canes: their effect on the standing balance of patients with hemiparesis. Arch Phys Med Rehabil. 1993;74:281–285 [PubMed] [Google Scholar]
- 14. Ashton-Miller JA, Yeh MW, Richardson JK, Galloway T. A cane reduces loss of balance in patients with peripheral neuropathy: results from a challenging unipedal balance test. Arch Phys Med Rehabil. 1996;77:446–452 [DOI] [PubMed] [Google Scholar]
- 15. Bateni H, Heung E, Zettel J, et al. Can use of walkers or canes impede lateral compensatory stepping movements? Gait Posture. 2004;20:74–83 [DOI] [PubMed] [Google Scholar]
- 16. Bateni H, Zecevic A, McIlroy WE, Maki BE. Resolving conflicts in task demands during balance recovery: does holding an object inhibit compensatory grasping? Exp Brain Res. 2004;157:49–58 [DOI] [PubMed] [Google Scholar]
- 17. Pai YC, Bhatt T, Wang E, et al. Inoculation against falls: rapid adaptation by young and older adults to slips during daily activities. Arch Phys Med Rehabil. 2010;91:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin TA, Keating JG, Goodkin HP, et al. Throwing while looking through prisms; II: specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(pt 4):1199–1211 [DOI] [PubMed] [Google Scholar]
- 19. Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther. 2010;90:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chong RK, Horak FB, Woollacott MH. Parkinson's disease impairs the ability to change set quickly. J Neurol Sci. 2000;175:57–70 [DOI] [PubMed] [Google Scholar]
- 21. Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111:46–58 [DOI] [PubMed] [Google Scholar]
- 22. Puttemans V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci. 2005;25:4270–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–483 [DOI] [PubMed] [Google Scholar]
- 24. Allum JH, Tang KS, Carpenter MG, et al. Review of first trial responses in balance control: influence of vestibular loss and Parkinson's disease. Hum Mov Sci. 2011;30:279–295 [DOI] [PubMed] [Google Scholar]
- 25. Dietz V, Michel J. Locomotion in Parkinson's disease: neuronal coupling of upper and lower limbs. Brain. 2008;131(pt 12):3421–3431 [DOI] [PubMed] [Google Scholar]
- 26. Jessop RT, Horowicz C, Dibble LE. Motor learning and Parkinson disease: refinement of movement velocity and endpoint excursion in a limits of stability balance task. Neurorehabil Neural Repair. 2006;20:459–467 [DOI] [PubMed] [Google Scholar]
- 27. Jobges M, Heuschkel G, Pretzel C, et al. Repetitive training of compensatory steps: a therapeutic approach for postural instability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:1682–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S, Horak FB, Carlson-Kuhta P, Park S. Postural feedback scaling deficits in Parkinson's disease. J Neurophysiol. 2009;102:2910–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michel J, Benninger D, Dietz V, van Hedel HJ. Obstacle stepping in patients with Parkinson's disease: complexity does influence performance. J Neurol. 2009;256:457–463 [DOI] [PubMed] [Google Scholar]
- 30. Visser JE, Oude Nijhuis LB, Janssen L, et al. Dynamic posturography in Parkinson's disease: diagnostic utility of the “first trial effect.” Neuroscience. 2010;168:387–394 [DOI] [PubMed] [Google Scholar]
- 31. Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50:M28–M34 [DOI] [PubMed] [Google Scholar]
- 32. Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 33. Kamei N, Yamane K, Nakanishi S, et al. Effectiveness of Semmes-Weinstein monofilament examination for diabetic peripheral neuropathy screening. J Diabetes Complications. 2005;19:47–53 [DOI] [PubMed] [Google Scholar]
- 34. Giladi N, Shabtai H, Simon ES, et al. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–170 [DOI] [PubMed] [Google Scholar]
- 35. Tyson SF. The support taken through walking aids during hemiplegic gait. Clin Rehabil. 1998;12:395–401 [DOI] [PubMed] [Google Scholar]
- 36. Winter DA, Patla AE, Frank JS. Assessment of balance control in humans. Med Prog Technol. 1990;16:31–51 [PubMed] [Google Scholar]
- 37. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160 [DOI] [PubMed] [Google Scholar]
- 38. Oates AR, Frank JS, Patla AE, et al. Control of dynamic stability during gait termination on a slippery surface in Parkinson's disease. Mov Disord. 2008;23:1977–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen CL, Chen HC, Wong MK, et al. Temporal stride and force analysis of cane-assisted gait in people with hemiplegic stroke. Arch Phys Med Rehabil. 2001;82:43–48 [DOI] [PubMed] [Google Scholar]
- 40. Dickstein R, Abulaffio N, Pillar T. Vertical force loaded on walking canes in hemiparetic patients. Gait Posture. 1993;1:113–118 [Google Scholar]
- 41. Jeka JJ, Lackner JR. The role of haptic cues from rough and slippery surfaces in human postural control. Exp Brain Res. 1995;103:267–276 [DOI] [PubMed] [Google Scholar]
- 42. Dickstein R, Peterka RJ, Horak FB. Effects of light fingertip touch on postural responses in subjects with diabetic neuropathy. J Neurol Neurosurg Psychiatry. 2003;74:620–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dickstein R, Shupert CL, Horak FB. Fingertip touch improves postural stability in patients with peripheral neuropathy [erratum in: Gait Posture. 2003;17:189–192]. Gait Posture. 2001;14:238–247 [DOI] [PubMed] [Google Scholar]
- 44. Boonsinsukh R, Panichareon L, Phansuwan-Pujito P. Light touch cue through a cane improves pelvic stability during walking in stroke. Arch Phys Med Rehabil. 2009;90:919–926 [DOI] [PubMed] [Google Scholar]
- 45. Boonsinsukh R, Panichareon L, Saengsirisuwan V, Phansuwan-Pujito P. Clinical identification for the use of light touch cues with a cane in gait rehabilitation post stroke. Top Stroke Rehabil. 2011;18(suppl 1):633–642 [DOI] [PubMed] [Google Scholar]
- 46. Tagawa Y, Shiba N, Matsuo S, Yamashita T. Analysis of human abnormal walking using a multi-body model: joint models for abnormal walking and walking aids to reduce compensatory action. J Biomech. 2000;33:1405–1414 [DOI] [PubMed] [Google Scholar]
- 47. Bateni H, Maki BE. Assistive devices for balance and mobility: benefits, demands, and adverse consequences. Arch Phys Med Rehabil. 2005;86:134–145 [DOI] [PubMed] [Google Scholar]
- 48. Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson's disease. Neuroscience. 2006;141:999–1009 [DOI] [PubMed] [Google Scholar]
- 49. Horak FB. Adaptation of automatic postural responses. In: Bloedel JR, Ebner TJ, Wise SP.Acquisition of Motor Behavior in Vertebrates. Cambridge, MA: MIT Press; 1996:57–85 [Google Scholar]
- 50. Pai YC, Wening JD, Runtz EF, et al. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol. 2003;90:755–762 [DOI] [PubMed] [Google Scholar]
- 51. Van Ooteghem K, Frank JS, Allard F, et al. Compensatory postural adaptations during continuous, variable amplitude perturbations reveal generalized rather than sequence-specific learning. Exp Brain Res. 2008;187:603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Ooteghem K, Frank JS, Allard F, Horak FB. Aging does not affect generalized postural motor learning in response to variable amplitude oscillations of the support surface. Exp Brain Res. 2010;204:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nieuwboer A, Rochester L, Muncks L, Swinnen SP. Motor learning in Parkinson's disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15(suppl 3):S53–S58 [DOI] [PubMed] [Google Scholar]
- 54. Smiley-Oyen AL, Lowry KA, Emerson QR. Learning and retention of movement sequences in Parkinson's disease. Mov Disord. 2006;21:1078–1087 [DOI] [PubMed] [Google Scholar]
- 55. Dean E, Ross J. Relationships among cane fitting, function, and falls [erratum in: Phys Ther. 1993;73:712]. Phys Ther. 1993;73:494–500 [DOI] [PubMed] [Google Scholar]
- 56. Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640 [DOI] [PubMed] [Google Scholar]
- 57. Keus SH, Munneke M, Nijkrake MJ, et al. Physical therapy in Parkinson's disease: evolution and future challenges. Mov Disord. 2009;24:1–14 [DOI] [PubMed] [Google Scholar]
- 58. Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson's disease. Neurorehabil Neural Repair. 2010;24:826–834 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.