Abstract
Background
In this research inactivity was simulated by immobilizing the forearm region in a plaster cast. Changes in skeletal muscle oxidative function were measured using near-infrared spectroscopy (NIRS), and the preventative effect of the training protocol on deterioration of skeletal muscle and the clinical utility of NIRS were examined.
Methods
Fourteen healthy adult men underwent immobilization of the forearm of the non-dominant arm by plaster cast for 21 days. Eight healthy adult subjects were designated as the immobilization group (IMM) and six were designated as the immobilization + training group (IMM+TRN). Grip strength, forearm circumference and dynamic handgrip exercise endurance were measured before and after the 21-day immobilization period. Using NIRS, changes in oxidative function of skeletal muscles were also evaluated. Muscle oxygen consumption recovery was recorded after the completion of 60 seconds of 40% maximum voluntary contraction (MVC) dynamic handgrip exercise 1 repetition per 4 seconds and the recovery time constant (TcVO2mus) was calculated.
Results
TcVO2mus for the IMM was 59.7 ± 5.5 seconds (average ± standard error) before immobilization and lengthened significantly to 70.4 ± 5.4 seconds after immobilization (p < 0.05). For the IMM+TRN, TcVO2mus was 78.3 ± 6.2 seconds before immobilization and training and shortened significantly to 63.1 ± 5.6 seconds after immobilization and training (p < 0.05).
Conclusions
The training program used in this experiment was effective in preventing declines in muscle oxidative function and endurance due to immobilization. The experimental results suggest that non-invasive monitoring of skeletal muscle function by NIRS would be possible in a clinical setting.
Keywords: Near infrared spectroscopy, Immobilization, Muscle O2 consumption, Endurance training
Background
Long periods of inactivity, brought about by hospitalization or broken limbs, influence the body in a variety of ways. Among the effects related to the musculoskeletal system, decline in bone mineral content and skeletal muscle atrophy have been observed [1,2]. The continuous assessment of these changes is thought to be useful in many ways, including the evaluation of courses of treatment. Conventional invasive methods [3,4] are a great burden to patients and, as they are not repeatable measurements, their use in the clinical setting is difficult. Thus, a convenient and non-invasive method to monitor oxidative metabolism function in muscle is desirable.
Magnetic resonance spectroscopy (MRS) is one method currently in use to directly measure local oxidative metabolism in skeletal muscle. The recovery time constant (Tc) for phosphocreatine (PCr), as measured by MRS, is known to be clearly correlated with the data of mitochondrial enzyme activity in skeletal muscle gained from the conventional biopsy techniques [5]. MRS is thus widely used as the gold standard in non-invasive measurement of muscle oxidative function. However, as MRS is costly and cumbersome and requires much time for maintenance, it is not a simple measurement process. As another method for measuring oxygen dyanamics, beginning with the research of Millikan [6] in 1937 and continuing with Jobsis [7] and Chance [8], visible light in the long wavelength region and near-infrared (NIR) light have been used successfully to measure oxygenation in the human brain as well as in the human skeletal muscle. Furthermore, oxygen dynamics in localized regions of skeletal muscle during exercise and recovery has also been made clear by the use of NIR light [9-11]. NIR spectroscopy (NIRS) is comparatively low in cost, portable and lightweight, thus well positioned to be practically useful in the clinical setting.
In previous research using NIRS to evaluate muscle oxidative function in muscle, the decrease in muscle oxygenation level at the onset of exercise and the time to recovery of muscle oxygenation level (Tr) have been used as evaluation indices [9,11,12]. In other previous research also using the Tr index, it has been found that patients with bone fractures immobilized by plaster cast displayed a significant lengthening of Tr [13].
In our previous research, a correlation was found between the TcPCr measured by MRS and the recovery time constant for muscle oxygen consumption (TcVO2mus) following the completion of handgrip exercise as measured by NIRS [14]. This result shows that through the use of NIRS the convenient and non-invasive evaluation of muscle oxidative function is possible. In the current research, we used TcVO2mus at the completion of exercise as an index to examine the possibility of convenient measurement of muscle oxidative function in subjects whose forearms where immobilized by plaster cast in a simulation of inactivity. Additionally, the possibility of using NIRS to evaluate the results of a training protocol to prevent decline in skeletal muscle function was also examined using the TcVO2mus index. We also measured Tr, grip strength, exercise endurance time and forearm circumference, as in our past research, and made comparative investigations.
Methods
Subjects
The experiment was administered after undergoing a review by the National Space Development Agency of Japan (NASDA) ethics committee and receiving informed consent from 14 healthy adult men. Subjects were 23 ± 2.6 years of age (average ± standard deviation), weighed 70.7 ± 10.6 kg and were 176.0 ± 5.0 cm in height. For all subjects, the forearm of the non-dominant arm from the fingers to the point two-thirds up the upper arm was immobilized in a natural position by plaster cast for 21 days. Each subject was then placed into one of two groups randomly, one group to undergo immobilization only (IMM, 8 subjects) and one group to undergo immobilization + training (IMM+TRN, 6 subjects). Measurements were made for both IMM and IMM+TRN before and after the 21-day immobilization period, and change due to the immobilization of skeletal muscle and the effect of training during the immobilization period were examined. For the IMM group, the same measurements were made for both the immobilized non-dominant arm and the free dominant arm, with the dominant arm acting as control (CON) for changes due to immobilization.
NIRS device
In order to measure multiple subjects simultaneously, two NIRS were used in this research (OMRON, HEO 200 and Shimadzu, OM-100A). The NIRS used to measure VO2mus and Tr, respectively, following the completion of exercise is a device able to measure the oxygenation in biological tissue in vivo by utilizing the absorption characteristics of oxygenated and deoxygenated hemoglobin (Hb) and myoglobin (Mb). The NIRS device projects light from the near-infrared wavelength region, the region between visible and infrared light, into the body.
The device is composed of probe and main computer body, and the probe is equipped with emitter and detectors for near-infrared light of 760 nm and 840 nm wavelengths. The emitted light passes through the skin and, while scattering, reaches tissue where a portion is absorbed by Hb and Mb and then returns to the detectors. With the 803 nm wavelength region – the region of equal absorption for oxygenated and de-oxygenated Hb – as the isosbestic point, deoxygenated Hb and Mb cause an increase in absorption in the shorter wavelengths, while oxygenated Hb and Mb cause an increase in absorption in the longer wavelengths. It thus follows that light from the emitter of 760 nm in wavelength is more easily taken up by de-oxygenated Hb and Mb, while light of 840 nm in wavelength is more easily taken up by oxygenated Hb and Mb. The amount of light that then returns to the detectors can be measured and in this way it is possible to measure approximately the oxygenation and de-oxygenation states of Hb and Mb based on the Beer-Lambert Law [11].
The mean penetration depth of NIRS in living tissue is approximately one-half of the distance between the emitter and detector as verified directly and by Monte Carlo simulation [12,15,16]. The distance between the emitter and the detector is conventionally set from 30–40 mm [17]; for this research the distance was set at 30 mm, and the penetration depth was approximately 15 mm from the skin surface. VO2mus was measured using the OMRON HEO 200; Tr was measured using the Shimadzu OM-100A.
Forearm Circumference Measurement
Forearm circumference was measured at the point of greatest circumference in the region of the forearm. The site of the initial measurement was marked, and care was taken to measure at the same site following immobilization.
Maximum Voluntary Contraction (MVC) Measurement
MVC was measured early in the morning before exercise was performed. Three measurements were made, and the largest was used as MVC value.
NIRS Measurement
Subjects were seated, and after measuring MVC, the NIRS probe was placed over the forearm finger flexor muscles and the sphygmomanometer cuff was placed around the upper arm. With the subject in a resting condition, measurements were thus begun.
VO2mus Measurement
VO2mus measurement was carried out in the following fashion: one minute of rest, one minute of cuff occlusion at 300 mmHg, 5 minutes of recovery after occlusion, one minute of dynamic hand grip exercise by grip ergometer at 40%MVC 1 repetition per 4 seconds as measured before immobilization. Brief arterial occlusions were repeated every 10 seconds at post-exercise recovery.
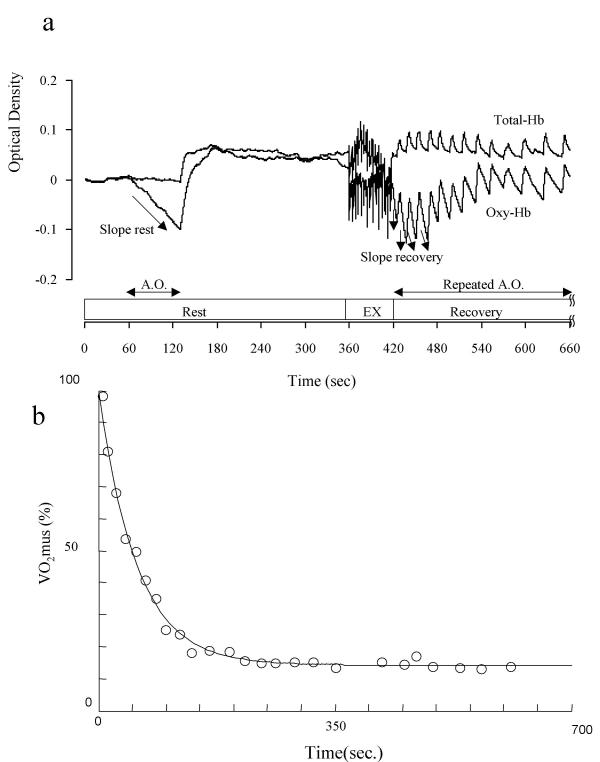
TcVO2mus was obtained from the repetitive brief arterial occlusions following the completion of exercise. The occlusion of arterial blood flow was terminated soon after total amount of Hb reached an almost uniform, constant level (fig. 1a). It has been shown in previous research that the percentage of de-oxygenated Hb/Mb at the time of arterial occlusion is a direct index of VO2mus [18], and this value of VO2mus is shown as a fraction of the resting value. TcVO2mus was calculated from the VO2mus value following the completion of exercise according to the formula shown below and fit to a mono-exponential curve:
Figure 1.
a. Schematic representation of VO2 mus and typical changes in muscle oxygenated Hb/Mb. Schematic representation of VO2mus and typical changes in muscle oxygenated Hb/Mb at rest, during exercise, and recovery. VO2mus was calculated from the rate of the decline of the oxygenated Hb/Mb during arterial occlusion at rest (Slope rest) and recovery period (Slope recovery). b. Typical kinetics of VO2 mus recovery after exercise. Typical kinetics of VO2mus recovery after exercise. Time constant for this subject was 55.8 s (pre) → 54.7 s (post).
y = a [(1-exp (i-kt) )]
For this equation, y represents the relative value of VO2mus during arterial occlusion in the rest period following exercise, a represents the total amount of change in VO2mus from the value at the completion of exercise to the value at recovery, k is a rate constant (1/k = Tc), and t is time. An example of the result of the TcVO2mus analysis is shown in Fig. 1b.
Tr Measurement
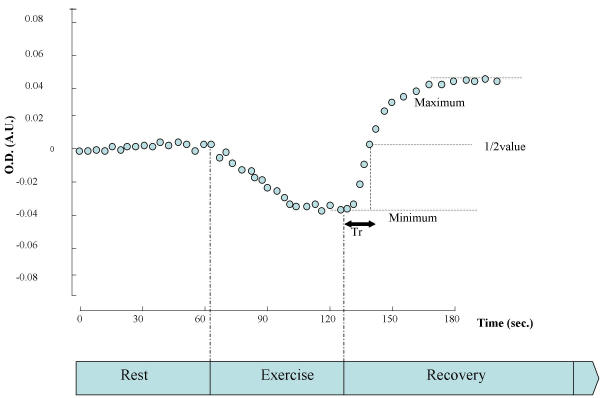
Tr was obtained from the full time necessary for muscle oxygenation to recover from the level following the completion of the dynamic handgrip exercise – 1 minute of rest followed by 1 minute of 40%MVC at 1 repetition per 4 seconds – to the peak value at recovery. This time was taken as a full scale, and Tr was measured as one-half of the full scale (fig. 2).
Figure 2.
Method to determine muscle oxygenation level and Tr. Method to determine muscle oxygenation level and Tr, the time required to reach the value halfway between the muscle oxygenation level immediately after exercise and that at peak hyperemia during recovery. The m-O2 value during exercise was defined as the minimum relative oxygenation level during exercise.
Grip Exercise Endurance Measurement
Grip exercise endurance was measured after the completion of NIRS measurement and a sufficient period of rest. Following skeletal muscle recovery, dynamic hand grip exercise was carried out at 30%MVC, 1 repetition per second, until the point of exhaustion. Two intensities were used: 30% of premaximum grip strength (absolute load) and 30% of the day-maximum grip strength (relative load). The time to exhaustion was recorded as the endurance measurement. It was not possible to measure grip exercise endurance for two subjects of the IMM group due to matters of personal convenience.
Endurance Training
Endurance training was carried out for the subjects of the IMM+TRN twice a week. The training consisted of dynamic handgrip exercise at 30%MVC, 1 repetition per second, until exhaustion. This grip exercise training method was the same as the one utilized in previous research that reported an average 39% improvement in muscle oxidative function [19].
Data Analysis
Paired t-test was used in the comparison of pre- and post-immobilization values of grip exercise endurance time, forearm circumference, grip strength, Tr and TcVO2mus for each of the IMM and IMM+TRN. Standard of significance was established as an uncertainty of less than 5% (p < 0.05) and an uncertainty of less than 1% (p < 0.01).
Results
The results of the measurements for the CON, the IMM and the IMM+TRN before and after immobilization are as shown in Table 1. For all measurements made before immobilization, there was no significant difference observed among CON, IMM and TRN. No significant differences were found in variables after the 21 day period in the CON. In the IMM, TcVO2mus following immobilization was found to be significantly longer (from 59.7 ± 5.8 to 70.4 ± 5.8 second, p < 0.01) than before immobilization. For the IMM+TRN, TcVO2mus was significantly shorter (from 78.3 ± 6.2 to 63.1 ± 5.6 second, p < 0.05) following the immobilization period. No significant difference was observed in Tr for either the IMM or IMM+TRN. No significant difference was found in forearm circumference for the IMM. However, a significant difference was found for the IMM+TRN. This significant change was an average of -3 mm, while the non-significant change for the IMM was an average of -2 mm.
Table 1.
Results of measurements of Control group, IMM group and IMM+TRN group before and after immobilization. Data are expressed as means ± S.E. Statistically significant differences from pre: *P < 0.05, **P < 0.01
| TcVo2mus (sec.) | Tr (sec.) | |||
| pre | post | pre | post | |
| CON | 51.6 ± 4.7 | 56.8 ± 10.5 | 10.5 ± 3.2 | 7.7 ± 1.8 |
| IMM | 59.7 ± 5.8 | 70.4 ± 5.8** | 9.8 ± 3.0 | 9.9 ± 3.2 |
| IMM+TRN | 78.3 ± 6.2 | 63.1 ± 5.6* | 8.0 ± 1.4 | 7.2 ± 1.3 |
| grip strength (kg) | arm circumferences (cm) | |||
| pre | post | pre | post | |
| CON | 44.3 ± 2.0 | 44.7 ± 2.7 | 25.8 ± 0.5 | 25.7 ± 0.4 |
| IMM | 40.6 ± 2.1 | 34.3 ± 2.3** | 25.0 ± 0.3 | 24.8 ± 0.3 |
| IMM+TRN | 44.9 ± 2.4 | 37.5 ± 2.7** | 26.3 ± 0.7 | 26.0 ± 0.6* |
| duration of grip exercise <absolute load> (sec.) | ||||
| pre | post | |||
| CON | 52.2 ± 2.3 | 49.2 ± 2.7 | ||
| IMM | 50.3 ± 2.0 | 40.5 ± 2.3 | P = 0.05 | |
| IMM+TRN | 46.3 ± 3.8 | 47.8 ± 4.8 | ||
| duration of grip exercise <relative load> (sec.) | ||||
| pre | post | |||
| CON | 52.3 ± 5.1 | 49.1 ± 5.9 | ||
| IMM | 50.3 ± 4.9 | 47.5 ± 11.3 | ||
| IMM+TRN | 46.3 ± 9.2 | 56.3 ± 5.2* | ||
At the absolute load, a trend of decrease in endurance time (from 50.3 ± 2.0 to 40.5 ± 2.3 second, p = 0.05) was found for the IMM. For the IMM+TRN, the decrease in time was checked. At the relative load, no significant difference was found for the IMM, but a significant increase in endurance time (from 46.3 ± 9.2 to 56.3 ± 5.2 second, p < 0.05) was found for the IMM+TRN.
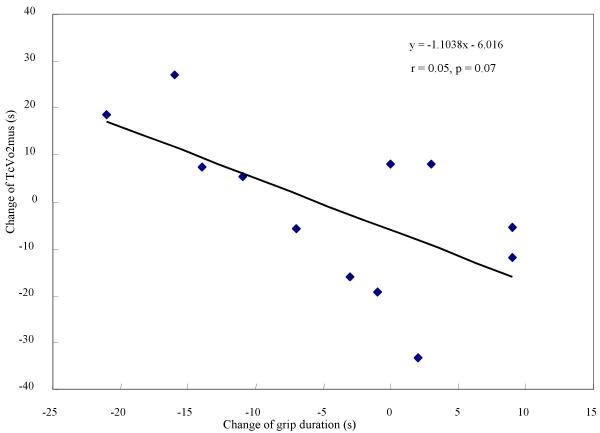
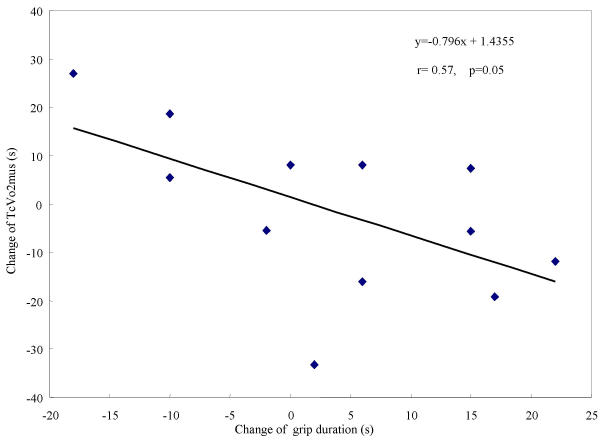
No correlation was found between TcVO2mus and grip strength, between Tr and grip strength, or between Tr and grip exercise endurance time. However, a negative correlation trend (for absolute load, r = 0.55; p = 0.07, and for relative load, r = 0.57; p = 0.05.) was found between the change of TcVO2mus and the change of grip exercise endurance duration (Figure 3, 4).
Figure 3.
The relationship between the changes of TcVO2 mus and grip exercise duration time (absolute load). Representation of the relationship between changes over the course of immobilization in TcVO2mus and grip exercise duration time (absolute load). A correlative trend (r = 0.55, p = 0.07) was observed between the two.
Figure 4.
The relationship between the changes of TcVO2 mus and grip exercise duration time (relative load). Representation of the relationship between changes over the course of immobilization in TcVO2mus and grip exercise duration time (relative load). A correlative trend (r = 0.57, p = 0.05) was observed between the two.
Discussion
In this research, an average decrease of 18% in TcVO2mus of forearm skeletal muscle was confirmed by the use of NIRS in subjects after a 21-day upper limb cast immobilization to simulate inactivity. Furthermore, a brief training program has been shown to prevent a decline in oxidative function due to cast immobilization. TcVO2mus, as stated previously, has been proved in previous research to be correlated with TcPCr as measured by MRS [14], and it has been shown by the current research that TcVO2mus would be a useful index for evaluating muscle oxidative function in a clinical setting.
Upper limbs have less muscle mass in comparison to lower limbs, and as upper limbs are not weight-bearing, atrophy due to immobilization is difficult to observe. Due to this difficulty, while there is previous research on the immobilization of lower limbs [20,21] and IMM+TRN of the lower limbs [22-24], there are in comparison fewer such reports on upper limbs. Therefore, obtaining results like those stated above can be considered most significant. A decrease in grip strength following immobilization was observed for both IMM and IMM+TRN. Muscle oxidative function was found to have decreased in IMM following immobilization. For the IMM+TRN, a trend of increasing muscle oxidative function was observed after immobilization. However, when compared to changes in skeletal muscle function, changes in muscle morphology as supposed from the circumference measurement were smaller, and in the case of the IMM, there was no significant change. Among reasons for this result, first it should be considered that the immobilization region was an upper limb, a region of comparatively less muscle mass. Additionally, it has been reported in previous research that while muscle cross-section area decreased by 1.9% following cast immobilization of the forearm, muscle power decreased in greater amounts, 29.3% for extension and 32.5% for flexion [25]. So it should be considered that the significant change of muscle oxidative function in this current research, in the absence of observed muscle morphology, may be caused by change in the nervous system, for example change in structures of nerve endings and neuromuscular junctions [26]. Furthermore, in research that placed lower limbs in a non-load bearing state, it has been reported that qualitative changes such as changes in muscle fiber type were more remarkable than quantitative changes in muscle fiber size and number [20]. From this result it can be thought that qualitative changes may precede quantitative changes in inactive skeletal muscle.
The general influence of inactivity due to immobilization on the structure and function of skeletal muscle can be found, as stated above, in change in muscle morphology (atrophy) or in change (decrease) in muscle function. It is held that changes in skeletal muscle morphology and function in conjunction with inactivity are more pronounced in continuous tension, slow-twitch muscle fibers (type I), like those responsible for maintaining posture, than in comparatively high strength, intermittently and repetitively contracting fast-twitch fibers (type II) [27]. It has been also reported that, independent of muscle fiber type, large size muscle fibers are more easily influenced by inactivity [26]. In previous research utilizing muscle biopsy, decrease in activity of oxidative enzymes [28-34], change in composition of muscle fiber type [35,36] and the accompanying damage to protein synthesis [37] have all been found due to cast immobilization. These changes are possible factors of influence on muscle oxidative function. Skeletal muscle energy metabolism is also affected by inactivity, giving rise to increased levels of glycolytic enzyme activity. Mitochondria, responsible for a large role in the synthesis of ATP, the energy source for muscle contraction, are known to suffer decreases in enzyme activity and mitochondria volume per cell due to inactivity [38]. This in turn leads to a decline in muscle endurance and causes fatigue to occur more easily. It is thought that the decline in muscle function due to immobilization in this research occurred by this mechanism.
Tr, as a measure to evaluate muscle oxidative function, was measured before and after immobilization in this research and the values were compared. No significant change was found for either the IMM or the IMM+TRN, a departure from the result reported in previous research [13] discussed above. Tr reflects the recovery of oxygen supply and consumption following the completion of exercise and is known to be related to the recovery of PCr [10]. It has been reported that recovery of muscle oxygenation level following exercise in lower leg muscles of peripheral vascular disease (PVD) patients is lengthened by a decrease in oxygen supply due to inhibition of blood flow [39-41]. Thus, Tr is considered a reflection of blood flow and a useful index not restricted in application only to the evaluation of oxidative function in skeletal muscle. Furthermore, Tr values in this research (7–12 seconds) are quite fast in comparison to PCr recovery, which should be similar to the Tcmus values (60 seconds). This supports the idea that Tr reflects oxygen delivery rather than oxidative metabolism. Blood flow was not measured in detail in the current research. However, we suppose that the lack of change of Tr in this research suggests that no changes in blood flow or oxygen delivery were seen with immobilization.
Moreover, it has also been reported that there is no correlation between oxidative function in skeletal muscle and Tr measured locally following exercise [42]. As in the current research, in the case that influence is anticipated on both intra-muscle oxygen supply and oxygen consumption due to decline in skeletal muscle oxidative function brought about by immobilization, it is difficult to interpret Tr as an index. Thus, although Tr measurement does not require arterial occlusion and is suited to clinical use, its use as an index in this research requires further study. It is unclear how much pH decreased due to the protocol in this research, but previous research has demonstrated that Tr, unlike Pcr recovery, is not influenced by muscle PH [10]. Thus, PH changes are not a reason to discount the Tr measurement.
Concerning the difference of oxidative capacity between dominant and non-dominant arms, previous research has demonstrated that muscle oxidative capacity is higher in the dominant arm than in the non-dominant arm [43]. In this research, there was no significant difference found between TcVO2mus measurements of non dominant and dominant arms. However, a trend not quite reaching the level of significant difference was found in the shortening of TcVO2mus for the dominant arm.
Past research has reported on a program of 30%MVC, 1 repetition per second, grip exercise carried out five sessions per week for six weeks and the correlation found between Pi/PCr and grip exercise endurance time [19]. In this study we found a close correlation between the change in endurance performance and the change in TcVO2mus for both absolute workload and relative workload. We confirmed that grip exercise performance at 30% MVC, 1 repetition per second is associated with oxidative function. In the current research, exercise training was performed at the same intensity and same cadence as in the previous research, but the frequency of training sessions was changed to twice a week to reduce the burden on the subjects. As a result, while previous research reported an average change of +39% in the absolute load grip exercise endurance time following training, in this research an average change of +3% (absolute load) was found for the IMM+TRN. An average change in grip exercise endurance time of -19.5% (absolute load) was found for the IMM. For relative load, no significant difference was observed in the IMM; an average change of +21.5% in grip exercise endurance time was observed for the IMM+TRN. Thus, the preventative effect of the training program on the decrease in grip exercise endurance time due to immobilization was recognized. While the training method carried out in the previous research was found to have a training effect on endurance-type exercise such as grip exercise endurance time, there was no such training effect found to increase MVC. In this research as well, it was not possible to check the decline in grip strength caused by immobilization through the training program. It is thought that training at 70% MVC or greater is required to stop decline in MVC.
As this research was carried out with healthy adult men as subjects, it is recognized that consideration of healthy strength and training of areas unaffected by bone fracture is necessary in regard to patients with real broken bones. However, it is possible to view the preventative effect of training, as described in this research, on the decline in endurance-type skeletal muscle function in long-term bed hospitalization with hope. In this research, the exercise protocol was only a protocol for bringing about an increase in the oxidative function of the forearm, but in the future, using NIRS, we will reconsider a training protocol that includes not just upper limbs but lower limbs as well, a training protocol that can maintain not only oxidative function but also MVC.
The number of subjects for the current research was a limitation and increasing the number of subjects is a concern worthy of future investigation. In addition, in future research it is likely that the scale of changes in skeletal muscle function can be further clarified through the extension of the immobilization period or through performing measurements several days or weeks following the end of the cast immobilization.
Conclusion
In this research, we were able to use NIRS to monitor the decrease in skeletal muscle function due to immobilization and the effect of training on the decrease both non-invasively and conveniently. MRS, held as the gold-standard in the measurement of muscle metabolism, is restricted in use due to large size and high cost. As used in this research, NIRS is compact and easy to use, even portable. It is thought to be useful for the non-invasive monitoring of changes in muscle function due to the cast immobilization of broken bones and long-term bed hospitalization, among other uses.
Authors' contributions
Mayuko Motobe, M.D., performed selection and medical checks of the experiment subjects as well as the measurement and evaluation of NIRS data. Takafumi Hamaoka, M.D., Ph.D., served as the general administrator for the experiment. Norio Murase, M.D., Ph.D., and Takuya Osada, M.D., Ph.D., performed selection and medical checks of the experiment subjects. Yuko Kurosawa, Ph.D., performed measurement and evaluation of NIRS data. Toshiyuki Homma, Chihoko Ueda, Takeshi Nagasawa and Shiro Ichimura also performed measurement and evaluation of NIRS data. Aya Kitahara, M.D., performed the cast immobilization process. Toshihito Katsumura, M.D., Ph.D., and Akinori Hoshika, M.D., Ph.D., examined the training method.
Acknowledgments
Acknowledgements
I would like to thank Professor Teruichi Shimomitsu of Tokyo Medical University, Department of Public Health and Preventive Medicine, for his supervision throughout the research and help in revising the report. I would also like to thank the graduate students and researchers of the same department for their great help in research and composition of the report, especially Eric Sell for his assistance in preparing the English version.
Contributor Information
Mayuko Motobe, Email: snowman-209@nifty.com.
Norio Murase, Email: murase@tokyo-med.ac.jp.
Takuya Osada, Email: DENTACMAC@aol.com.
Toshiyuki Homma, Email: t07hma@aol.com.
Chihoko Ueda, Email: chobo@tokyo-med.ac.jp.
Takeshi Nagasawa, Email: nagasawa@tokyo-med.ac.jp.
Aya Kitahara, Email: Ayakitahar@aol.com.
Shiro Ichimura, Email: shiro@kitty.ch.
Yuko Kurosawa, Email: kuro@tokyo-med.ac.jp.
Toshihito Katsumura, Email: katsu@tokyo-med.ac.jp.
Akinori Hoshika, Email: jsppn@tokyo-med.ac.jp.
Takafumi Hamaoka, Email: kyp02504@nifty.ne.jp.
References
- Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand. 2001;172:269–278. doi: 10.1046/j.1365-201x.2001.00869.x. [DOI] [PubMed] [Google Scholar]
- Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days bed rest on muscle morphology. J Gravit Physiol. 1997;4:S10–14. [PubMed] [Google Scholar]
- Gayeski TEJ, Honig CR. Direct measurements of intracellular O2 gradients. Role of convection and myoglobin. Adv Med Biol. 1983;159:613–621. doi: 10.1007/978-1-4684-7790-0_54. [DOI] [PubMed] [Google Scholar]
- Henricksson J, Reitman LS. Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome exidase activites and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand. 1977;99:91–97. doi: 10.1111/j.1748-1716.1977.tb10356.x. [DOI] [PubMed] [Google Scholar]
- McCully KK, Fielding A, Evans WJ, Leigh JS, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75:813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- Millikan GA. Experiments on muscle hemoglobin in vivo; the instantaneous measurement of muscle metabolism. Proc Roy Soc. 1937;123:218–241. [Google Scholar]
- Jobsis FF. Noninvasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Chance B, Nioka S, Kent J, McCully K, Fountain M, Greenfeld R, Holtom G. Time resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Anal Biochem. 1988;174:698–707. doi: 10.1016/0003-2697(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Albani C, Chance B, Iwane H. A new method for the evaluation of muscle aerobic capacity in relation to physical activity measured by near-infrared spectroscopy. Med Sport Sci. 1992;37:421–429. [Google Scholar]
- McCully K, Iotti S, Kendrick K, Wang Z, Posner J, Leigh J, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol. 1994;77:5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- Chance B, Dait TM, Chang C, Hamaoka T, Hagerman F. Recovery from exercise induced desaturation in the quadriceps muscle of elite competitive runners. Am J Physiol. 1992;262:766–775. doi: 10.1152/ajpcell.1992.262.3.C766. [DOI] [PubMed] [Google Scholar]
- Chance B, Maris M, Sorge J, Zhang MZ. A phase modulation system for dual wavelength difference spectroscopy of hemoglobin deoxygenation in tissues. Proc Soc Photo Optical Instrum Engr. 1990;1204:481–491. [Google Scholar]
- Nishio S, Iwane H, Hamaoka T, Shimomitsu T, Katsumura T, Ito K, Murase N, Fujinami J. Metabolism in local atrophied skeletal muscle after immobilization monitored by near infrared spectroscopy. Med Sci Sports Exerc. 1994;26:S98. [Google Scholar]
- Nagasawa T, Hamaoka T, Sako T, Murakami M, Kime R, Homma T, Ueda C, Ichimura C, Katsumura T. A practical indicator of muscle oxidative capacity determined by recovery of muscle O2 consumption using NIR spectroscopy. Eur J Sports Sci. 2003;3 [Google Scholar]
- Delpy DT. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33:1422–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- Patterson MS, Chance B, Wilson BC. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. J Appl Optics. 1989;28:2331–2336. doi: 10.1364/AO.28.002331. [DOI] [PubMed] [Google Scholar]
- Hampson NB, Piantadosi CA. Near infrared monitoring of human skeletal muscle oxygenation during forearm ischemia. J Appl Physiol. 1988;64:2449–2457. doi: 10.1152/jappl.1988.64.6.2449. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B. Non-invasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol. 1996;81:1410–1417. doi: 10.1152/jappl.1996.81.3.1410. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Katsumura T, Murase N, Kurosawa Y, Shimomitsu T, Kuwamori M, Kagaya A, Chance B. Exercise induced improvement in muscle oxidative function in young female measured by 31 phosphorus magnetic resonance spectroscopy (31 P-MRS) Jpn J Appl Physiol. 1998;28:1–9. [Google Scholar]
- Hather B, Adams G, Tesch A, Dudley G. Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol. 1992;72:1493–1498. doi: 10.1152/jappl.1992.72.4.1493. [DOI] [PubMed] [Google Scholar]
- Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand. 1996;157:63–70. doi: 10.1046/j.1365-201X.1996.476217000.x. [DOI] [PubMed] [Google Scholar]
- Schulze K, Gallagher P, Trappe S. Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc. 2002;34:303–313. doi: 10.1097/00005768-200202000-00019. [DOI] [PubMed] [Google Scholar]
- Akima H, Kubo K, Kanehisa Y, Suzuki Y, Gunji A, Fukunaga T. Leg-press resistance training during 20 days of 6 degrees head-down-tilt bed rest prevents muscle deconditioning. Eur J Appl Physiol. 2000;82:30–38. doi: 10.1007/s004210050648. [DOI] [PubMed] [Google Scholar]
- Bamman M, Hunter G, Stevens B, Guilliams M, Greenisen M. Resistance exercise prevents plantar flexor deconditioning during bed rest. Med Sci Sports Exerc. 1997;29:1462–1468. doi: 10.1097/00005768-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Miles M, Clarkson P, Bean M, Ambach K, Mulroy J, Vincent K. Muscle function at the wrist following 9d of immobilization and suspension. Med Sci Sports Exerc. 1994;26:615–623. [PubMed] [Google Scholar]
- Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: Microgravity and skeletal muscle. J Appl Phsiol. 2000;89:823–829. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- Desplanches D, Mayet H. Skeletal muscle adaptation in rats flown on Cosmos 1667. J Appl Physiol. 1990;68:48–52. doi: 10.1152/jappl.1990.68.1.48. [DOI] [PubMed] [Google Scholar]
- Edstrom L. Selective atrophy of red muscle fivers in the quadriceps in long-standing knee-joint dysfunction injuries to the anterior cruciate ligament. J Neurol Sci. 1970;11:551–558. doi: 10.1016/0022-510X(70)90105-X. [DOI] [PubMed] [Google Scholar]
- Haggmark T, Jannson E, Eriksson E. Fiber type area and metabolic potential of the thigh muscle in man after surgery and immobilization. Int J Sports Med. 1981;2:12–17. doi: 10.1055/s-2008-1034577. [DOI] [PubMed] [Google Scholar]
- Jansson E, Sylven C, Arvidsson I, Eriksson E. Increase in myoglobin content and decrease in oxdative enzyme activities by leg muscle immobilization in man. Acta Physiol Scand. 1988;132:515–517. doi: 10.1111/j.1748-1716.1988.tb08358.x. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ, Davies CTMS, Edwards RHT, Maunger C, Young A. Functional and structural changes after disuse of human muscles. Clin Sci Molecular Med. 1977;52:337–342. doi: 10.1042/cs0520337. [DOI] [PubMed] [Google Scholar]
- Fell RD, Steffen JM, Musacchia XJ. Effect of hypokinesia-hypodynamia on rat muscle oxidative capacity and glucose uptake. Am J Physiol. 1985;249:308–312. doi: 10.1152/ajpregu.1985.249.3.R308. [DOI] [PubMed] [Google Scholar]
- Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimbs suspension in rat muscle. J Appl Physiol. 1987;63:558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- Simard C, Lacaille M, Vallieres J. Enzymatic adaptations to suspension hypokynesia in skeltal muscle of young and old rats. Mech Ageing Dev. 1985;33:1–9. doi: 10.1016/0047-6374(85)90104-6. [DOI] [PubMed] [Google Scholar]
- McDougall JD, Elder GCB, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibers. Eur J Appl Physiol. 1980;43:25–34. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- Haggmark T, Eriksson E, Jansson E. Muscle fiber type in human skeletal muscle after injuries and immobilization. Orthopedics. 1986;9:181–185. doi: 10.3928/0147-7447-19860201-08. [DOI] [PubMed] [Google Scholar]
- Booth FW, Seider MJ. Early changes in skeletal muscle in protein synthesis after limb immobilization in rats. J Appl Physiol. 1979;47:974–977. doi: 10.1152/jappl.1979.47.5.974. [DOI] [PubMed] [Google Scholar]
- Matoba H, Gollnick PD. Response of skeletal muscle to training. Sport Med. 1984;1:240–251. doi: 10.2165/00007256-198401030-00006. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Shigematsu H, Yasuhara H, Muto T. An objective assessment of intermittent claudication by near infrared spectroscopy. Eur J Vasc Surg. 1994;8:294–296. doi: 10.1016/s0950-821x(05)80144-6. [DOI] [PubMed] [Google Scholar]
- McCully KK, Landsberg L, Suarez M, Hoffman M, Posner JD. Identification of peripheral vascular disease in elderly subjects using optical spectroscopy. J Gerontol. pp. B159–B165. [DOI] [PubMed]
- McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol. pp. B128–134. [DOI] [PubMed]
- Hamaoka T, Mizuno M, Katsumura T, Osada T, Shimomitsu T, Quistorff B. Correlation between indicators determined by near infrared spectroscopy and muscle fiber types in humans. Jpn J Appl Physiol. 1998;28:243–248. [Google Scholar]
- Minotti JR, Johnson EC, Hudson TL, Sibbitt RR, Wise LE, Fukushima E, Icenogle MV. Forearm metabolic asymmetry detected by 31P-NMR during submaximal exercise. J Appl Physiol. 1989;67:324–329. doi: 10.1152/jappl.1989.67.1.324. [DOI] [PubMed] [Google Scholar]