Abstract
OBJECITIVE
To assess whether incidental screening resulting from imaging conducted for other purposes has resulted in earlier detection or better outcomes in patients with adrenocortical carcinoma (ACC).
MATERIALS AND METHODS
We used the National Cancer Database (NCDB) to assemble a cohort diagnosed with ACC from 1985 to 2007. Trends in the distribution of grouped tumor sizes were assessed with the Cochran Armitage Chi-square test. Relative 5-year survival rates were calculated for cases diagnosed through 2002.
RESULTS
Median survival for the full cohort (n=4,275) was 24 months. Localized ACC accounted for 43.9% of cases. No stage migration over time was noted. No statistical trends were noted in changes of tumor size over the years in patients who underwent surgery for localized disease (p=0.32). Furthermore, no improvement in 5-year survival over the time period was observed (p>0.1).
CONCLUSIONS
In this cohort of ACC patients – the largest reported to date – fewer than half presented with localized disease (43.9%). No shift toward lower stage nor smaller tumor size over a 22 year period was noted, despite the advent of abdominal imaging and its resulting “incidental screening” of the adrenal gland. These data are in contrast to the well-documented stage and size migration for tumors of the kidney – a neighboring retroperitoneal organ. Furthermore, no improvement in survival was noted. As such, better risk-stratifying patients with adrenal incidentalomas, while improving treatment efficacy for those with proven ACC is an essential clinical and epidemiological task.
Keywords: adrenocortical carcinoma, National Cancer Database, cancer screening, stage migration
Introduction
ACC is remarkably uncommon. Its etiology is unknown, early symptoms are rare, and risk factors remain unidentified. While the exact incidence of the disease is unclear, it has been approximated at 0.5 to 2.0 per million or 300 cases per year in the United States.1–3 Unless impinging on adjacent organs or metabolically active, ACC is unfortunately asymptomatic. The primary data source for ACC historically has been individual institutional case series.1, 4–6 More recently, the Surveillance Epidemiology and End Results (SEER) database has provided population-based data on patients with ACC. Unfortunately even this registry contains less than 1000 records of individuals with the disease from 1973 to 2004.3, 7, 8 To date, the National Cancer Database maintains the largest cohort of patients with ACC.9
The adrenal gland is located in the retroperitoneum immediately adjacent to the kidney with little anatomic variability. It is universally imaged on abdominal computed tomography and magnetic resonance imaging scans of the kidney and often seen during cross sectional imaging of the lung. Hence, the adrenal is incidentally screened in patients with non localized complaints as well as those being staged or surveyed for multiple non-adrenal tumors. As such the incidence of adrenal incidentalomas has increased significantly overtime.10 Increased utilization of radiographic screening has also resulted in diagnostic shifts over time such as incremental early detection, as well as stage and size migration of tumors of the kidney – retroperitoneal organs adjacent to the adrenals.11, 12 Since ACC is uncommon, asymptomatic, without known risk factors and most commonly detected by incidental imaging, we sought to evaluate whether the presentation of ACC has changed regarding tumor size, stage at presentation and relative survival over the last 2 decades.
Methods
The National Cancer Data Base (NCDB), a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons, was established in 1989 and serves as a comprehensive clinical surveillance resource for cancer care in the United States. It has been described in detail elsewhere.13 The NCDB currently captures approximately 75% of all newly diagnosed cancer cases from over 1,400 facility-based cancer registries annually and holds information on over 25 million cases of cancer diagnosed between 1985 and 2007.
We used data from the NCDB to establish a cohort of patients with of adrenal cortical carcinoma (ACC) diagnosed between 1985 and 2007. Cases were limited to patients aged 18 and older with International Disease for Oncology (ICD-O-3)14 histology code 8370, and primary site codes C740 and C749.
Since American Joint Committee on Cancer (AJCC) staging rules for ACC were not adopted until 2010 (AJCC Version 7), SEER summary staging, which is available for the years 1985–2000, was used in the analysis. Mean tumor size was compared across diagnosis years using a p-value of 0.05, and trends in the distribution of grouped tumor sizes were assessed with the Cochran Armitage Chi-square test for trend. Relative five-year survival rates and corresponding 95% confidence intervals were calculated by stage, tumor size, race and sex for cases diagnosed through 2002. Relative survival is the ratio of the observed survival rate to the expected survival rate adjusted for age, sex, race, and Hispanic origin. Expected survival rates are based on 1990 life expectancy tables from the National Cancer Institute.15 Multivariate Cox regression was used to assess the risk of mortality according to age, race, sex, diagnosis years, tumor size, stage and hospital type, with all variables included in the model. All cause mortality was used in the analyses whereas cause specific mortality was not available. Median survival was assessed using Kaplan Meier estimates. Analyses were conducted using IBM SPSS Statistics version 18.16 This project was approved by the Fox Chase Cancer Center Institutional Review Board.
Results
We identified 4,275 cases of ACC from a total of approximately 25 million registered cases of solid malignancies in the NCDB. The demographics and clinicopathological variables for the cohort of 4,275 patients are provided in Table 1. Data regarding symptoms at presentation nor metabolic function of adrenal tumors are not currently captured through the NCDB. The majority of the sample were female (58.1%), white (88.8%), and were treated at a teaching/research institution (46.8%). Over a third of hospitals (36%) saw only one case of ACC, and 71.5% of hospitals saw 3 or less cases of the disease during the time period (1985–2007). The median age at diagnosis was 54.5 years (mean 54.6, range 18–100). More than a quarter of the sample (27.1%) was diagnosed between 2003 and 2007 with 34 % diagnosed between 1996 and 2002 and 38.8% between 1985 and 1995. Kaplan Meier results show a median survival of 24 months for cases diagnosed between 1985–2002 (Figure 1). Alive cases had a median follow-up of 67.0 months. At last contact, for cases diagnosed through 2002 (i.e. those with at least 5-years of follow-up), 29% were censored, while 71% were documented as deceased.
Table 1.
Clinicopathologic characteristics of 4,275 patients registered as having adrenocortical carcinoma in the NCBD database from 1985 to 2007.
| Diagnosis Years | |||||
|---|---|---|---|---|---|
| 1985–1990 | 1991–1995 | 1996–2002 | 2003–2007 | Total | |
| Number of cases | 757 | 903 | 1,455 | 1,160 | 4,275 |
| Mean Age, 95% Confidence Intervals | 53.5 (52.4–54.6) | 54.5 (53.5–55.5) | 54.9 (54.0–55.7) | 54.8 (54.0–55.7) | 54.5 (54.1–55.0) |
| Age Group | |||||
| 19–34 | 13.9 % | 11.5 % | 11.3 % | 10.0 % | 11.4 % |
| 35–44 | 16.8 % | 18.1 % | 15.3 % | 16.0 % | 16.4 % |
| 45–54 | 17.6 % | 17.7 % | 22.2 % | 22.0 % | 20.4 % |
| 55–64 | 23.6 % | 21.6 % | 20.9 % | 22.8 % | 22.1 % |
| 65–74 | 20.5 % | 21.8 % | 19.2 % | 19.2 % | 20.0 % |
| >=75 | 7.7 % | 9.3 % | 11.1 % | 9.9 % | 9.8 % |
| Sex | |||||
| Male | 42.7% | 43.5 % | 40.3 % | 41.7 % | 41.8 % |
| Female | 57.3% | 56.5 % | 59.7 % | 58.2 % | 58.2 % |
| Race | |||||
| White | 90.0% | 89.5 % | 88.8 % | 87.5 % | 88.8 % |
| Black | 6.5% | 7.0 % | 7.2 % | 8.2 % | 7.3 % |
| Other, Unknown | 3.6% | 3.5 % | 4.0 % | 4.3 % | 3.9 % |
| Hospital Type | |||||
| Community | 10.4 % | 12.6 % | 11.2 % | 10.9 % | 11.3 % |
| Comprehensive | |||||
| Community | 40.4 % | 38.9 % | 40.1 % | 39.1 % | 39.6 % |
| Teaching/Research | 42.7 % | 42.0 % | 45.3 % | 47.7 % | 44.8 % |
| Other | 6.5 % | 6.5 % | 3.4 % | 2.2 % | 4.3 % |
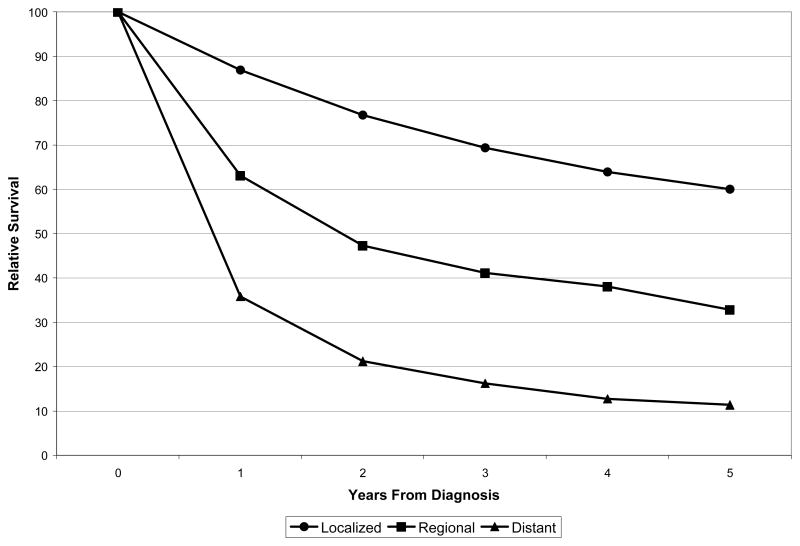
Figure 1.
Adrenocortical carcinoma relative survival by SEER Summary Stage (1985–2000)
SEER summary staging was available for the years 1985–2000 (n= 2,251). Localized ACC accounted for 43.9% (n=988) of cases, regional disease comprised 21.7% (n=488) of the cohort, and distant disease was documented in 34.4% (n=755). No stage migration over 5 year intervals was noted (Table 2). Primary tumor size was available for all years of the cohort in 3,360 cases (78.6%). Mean tumor size for the cohort was 11.5 cm (median=10.5, range=0–98.9 (Supplementary Materials). No statistical trends were noted in changes of tumors size over the years in these patients who underwent resection for localized tumors (p= 0.32).
Table 2.
ACC Mean Tumor Size by Seer Summary Stage, Cases Diagnosed in (1985–2000).
| Mean tumor size, 95% Confidence Intervals, N, by SEER Stage | ||||||
|---|---|---|---|---|---|---|
| Local | N | Regional | N | Distant | N | |
| Diagnosis Years1 | ||||||
| 1985–1990 | 10.9 (9.5–12.3) cm | 192 | 10.0 (8.8–11.1) cm | 95 | 11.6 (9.6–13.5) cm | 117 |
| 1991–1995 | 10.6 (9.9–11.4) cm | 288 | 12.2 (11.3–13.1) cm | 152 | 12.7 (11.1–14.2) cm | 165 |
| 1996–2000 | 11.2 (10.4–12.0) cm | 336 | 13.0 (11.6–14.4) cm | 151 | 11.9 (11.0–12.7) cm | 218 |
| Total | 10.9 (10.4–11.5) cm | 816 | 12.0 (11.3–12.6) cm | 398 | 12.1 (11.3–12.8) cm | 500 |
Seer Stage available for diagnosis years 1985–2000 only
Over three quarters of patients in the cohort underwent surgical intervention (n=3,241, 76.7%). Meanwhile, 22.8% (n=964) did not have surgical intervention for ACC, while a handful of patients were classified as having undergone “tumor destruction” (n=18) or did not contain information regarding whether or not surgical intervention was undertaken (n=52).
Relative survival by SEER Summary Stage is presented in Figure 1. Multivariate analysis examining factors that influence survival and adjusting for age, race, sex, diagnosis years, tumor size, stage and hospital type is shown in Supplementary Materials. Five-year relative survival for the entire cohort was 38.1% (95% CI: 36.0–40.1). Patients with localized disease had a 5-year relative survival of 60%, while patients with regional and distant disease exhibited a relative survival of 33% and 11%, respectively. Overall relative survival data by year of diagnosis is summarized in Table 3. Across all patients with ACC, no improvement in 5-year relative survival was noted over the time period of the cohort (p>0.32 for all comparisons). Table 4 presents relative survival for patients with localized tumors only stratified by tumor size, revealing no significant change in survival based on tumor size.
Table 3.
ACC 5-Year Relative Survival (%), All Stages, All Tumor Sizes by Diagnosis Years
| Relative Survival | 95% Confidence Intervals | N | |
|---|---|---|---|
| 1985–1990 | 40.2 % | 36.3–44.0 % | 753 |
| 1991–1995 | 39.4 % | 35.8–43.1 % | 900 |
| 1996–2002 | 35.0 % | 32.2–37.9 % | 1,454 |
1985–1990 compared to 1991–1995, p=0.87;
1985–1990 compared to 1996–2002, p=0.32;
1991–1995 compared to 1996–2002, p=0.52.
Table 4.
ACC Five-Year Relative Survival, Localized Tumors Only (1985–2000)
| < 6cm | 6.0–9.9 cm | >= 10 cm | |
|---|---|---|---|
| 1985–1990 | 77.9 (62.5–93.2) % | 56.4 (42.6–70.1) % | 58.8 (47.5–70.1) % |
| N=40 | N=61 | N=92 | |
| 1991–1995 | 66.6 (51.5–81.8) % | 50.9 (39.1–62.7) % | 59.7 (50.3–69.0) % |
| N=55 | N=92 | N=143 | |
| 1996–2000 | 69.5 (53.9–85.0) % | 64.8 (52.8–76.7) % | 58.4 (50.0–66.9) % |
| N=54 | N=100 | N=181 | |
| All years | |||
| 1985–2000 | 70.9 (62.0–79.8) % | 57.4 (50.2–64.6) % | 58.9 (53.4–64.4) % |
| N=149 | N=253 | N=416 |
none of the survival differences across time periods were significant at p<0.05
Discussion
Despite its rarity, ACC represents an important clinical entity. The malignancy is aggressive and treatment options for regional or systemic disease are currently extremely limited and ineffective.17 In fact, complete surgical resection of localized lesions offers the only chance for durable cure.18 Given the extremely poor outcomes of patients with metastatic ACC, all patients with adrenal masses in whom ACC cannot be ruled out are currently considered for surgical resection.10, 19, 20 However, since the prevalence of incidentally-discovered adrenal lesions is estimated at 4 to 6% of the population,10 clinical protocols that affect this large group of patients have a significant impact on both the quality and cost of healthcare delivery. Hence, a better understanding of the clinical behavior of ACC impacts not only the patients with this rare malignancy, but also the large number of individuals who are found to have adrenal incidentaloma in whom this diagnosis must be considered. As such, we performed an analysis of data from the NCDB – the largest cohort of ACC patients that has been accumulated to date – in order to better understand the historical trends in diagnosis and outcome of patients with ACC.
Modern adrenal imaging characteristics help guide clinical decisions regarding the risk of malignancy17, 18; however, radiographic size of the lesion continues to play a pivotal role in the recommendation of whether surgery is warranted.10, 19 Generally, primary adrenal masses ≥6cm are resected, since malignancy rates in this group of patients are reported to exceed 25%.1, 21 Even though only approximately 6% of lesions between 4 and 6 cm in size are malignant, adrenalectomy is often recommended for individuals who are at an acceptable risk for surgery.10, 22–25 With the advent of frequent cross-sectional imaging in the past 2 decades, one would expect the average size of ACC at presentation to show a downward trend. Indeed, tumors of the kidney – an organ that is adjacent to the adrenal in the retroperitoneum – have shown a significant trend to smaller size at presentation over the past 20 years. Both population-based11 and hospital-based12 cohorts clearly document this phenomenon. For instance, an analysis of the NCDB revealed that mean size of Stage I renal tumors decreased some 12% between 1993 and 2004 (p<0.001).12 Similar data have been published using SEER data.11
In distinct contrast to renal masses, decreasing tumor size at presentation was not seen for ACC over the 22 year time period of this cohort. Specifically, no statistically significant change in tumor size was noted in patients who presented with localized ACC and underwent surgical resection from 1985 to 2000 (p= 0.32). Furthermore, unlike in the case for renal cell carcinoma (RCC),26 there was no stage migration identified for ACC with 56.1 % of patients presenting with malignancy that had already spread beyond the adrenal. These hospital-based data coincide with the data from the smaller population-based cohort previously reported from the SEER dataset (n=602).3 More rapid growth kinetics of ACC compared to the relatively slow growth kinetics of localized RCC 27 may explain the discrepancy in the trends of size/stage migration in the tumors of these two neighboring organs. In fact, patients with both localized and systemic ACC present with very large masses. Mean overall tumor size at presentation in the NCDB cohort was 11.5 cm. Patients who presented with localized disease (43.9%) had a mean tumor size of 10.9 cm. Indeed, only 16% of all masses and 18.2 % of localized masses were <6cm in diameter, while 91.1% of all masses and 89.5% of localized masses were larger than 4cm. These data are in agreement with reports from large series of incidental adrenal masses where the 4 cm adrenal mass cutoff as a trigger for resection afforded approximately 90% sensitivity in identifying ACC.25, 28 The large size of ACC at presentation is consistent with the anatomical seclusion of the retroperitoneum where symptoms do not manifest until tumors are of extreme size or have metastasized.
The absence of an identifiable size and stage migration at presentation for ACC corresponded to a lack of any notable improvement in survival trends for this malignancy. In the overall NCDB cohort, overall relative survival was 38%. Relative survival was stage dependent with patients who presented with localized disease exhibiting significantly better overall survival than patients with regional or metastatic disease (Figure 1). On a multivariate analysis, lower SEER stage, younger age, and smaller tumors size significantly correlated with improved survival. Conversely, neither sex, race, hospital type, or year of diagnosis were associated with survival in patients with ACC. Overall there was no improvement in relative survival over the time period of the cohort or in patients with localized disease. Furthermore, no improvement in survival over time was seen in patients with localized disease when these patients were stratified by tumor size. As such, these data suggest that neither the lead time effects of screening or improved treatments of patients with ACC have altered prognosis over the last two decades.
Conclusion
In summary, in this cohort of ACC patients (n=4262) – the largest reported to date – fewer than half presented with localized disease (43.9%). Moreover, we could not identify any shift toward lower stage or smaller tumor size over a more than two decade period in which non-invasive abdominal imaging became routine. These data are in contrast to the well-documented stage migration for tumors of the kidney – a neighboring retroperitoneal organ – that coincided with the advent of cross-sectional imaging. Rapid growth kinetics of asymptomatic ACC might explain these findings. Furthermore, no significant improvement in survival was noted over time even when controlling for age, year of diagnosis, size at diagnosis and stage at diagnosis. Incidental screening resulting from scans conducted for other purposes has not been associated with earlier detection nor better outcomes in patients with ACC. As such, developing strategies to minimize over-treatment of patients with adrenal incidentalomas, while optimizing treatment of patients with proven ACC remains an essential clinical and epidemiological task.
Supplementary Material
Acknowledgments
The authors were supported in part through P30 CA006927 Comprehensive Cancer Center Program at Fox Chase (AK, YNW, RGU) and Department of Defense, Physician Research Training Award (AK).
Footnotes
Reprints will not be available from the authors.
Supplementary Material can be found at the following URL: http://www.fccc.edu/docs/healthProfessionals/KutikovSupplementaryMaterial02.pdf
References
- 1.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Paton BL, Novitsky YW, Zerey M, et al. Outcomes of adrenal cortical carcinoma in the United States. Surgery. 2006;140:914. doi: 10.1016/j.surg.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Ng L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol. 2003;169:5. doi: 10.1016/S0022-5347(05)64023-2. [DOI] [PubMed] [Google Scholar]
- 5.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 6.Kendrick ML, Lloyd R, Erickson L, et al. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg. 2001;136:543. doi: 10.1001/archsurg.136.5.543. [DOI] [PubMed] [Google Scholar]
- 7.Zini L, Capitanio U, Jeldres C, et al. External validation of a nomogram predicting mortality in patients with adrenocortical carcinoma. BJU Int. 2009;104:1661. doi: 10.1111/j.1464-410X.2009.08660.x. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 10.Young WF., Jr The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Mallin K, Ritchey J, et al. Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. J Urol. 2008;179:2131. doi: 10.1016/j.juro.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 13.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3. Lyon: Geneva Health Organization; 2000. [Google Scholar]
- 15.Shambaugh E, Young JL, Zippin C, et al. Statistics and Epidemiology for Cancer Registrars, Self-instruction Manual for Cancer Registrars, Book 7: Surveillance Epidemiology and End Results Program. Bethesda: National Institutes of Health; 1995. [Google Scholar]
- 16.SPSS Inc: 233 S. Wacker Drive, 11th floor. Chicago, Illinois 60606
- 17.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 18.Phan AT. Adrenal cortical carcinoma--review of current knowledge and treatment practices. Hematol Oncol Clin North Am. 2007;21:489. doi: 10.1016/j.hoc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the Clinically Inapparent Adrenal Mass (“Incidentaloma”) Ann Intern Med. 2003;138:424. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 20.Kutikov A, Crispen PL, Uzzo RG. Pathophysiology, Evaluation, and Medical Management of Adrenal Disorders. In: Wein AJ, Kavoussi LR, Partin AW, et al., editors. Campbell-Walsh Urology. 10. Philadelphia: Elsevier; 2010. in press. [Google Scholar]
- 21.Barzon L, Sonino N, Fallo F, et al. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 22.Barry MK, van Heerden JA, Farley DR, et al. Can Adrenal Incidentalomas Be Safely Observed? World Journal of Surgery. 1998;22:599. doi: 10.1007/s002689900441. [DOI] [PubMed] [Google Scholar]
- 23.Young WF., Jr Management approaches to adrenal incidentalomas. A view from Rochester Minnesota. Endocrinol Metab Clin North Am. 2000;29:159. doi: 10.1016/s0889-8529(05)70122-5. [DOI] [PubMed] [Google Scholar]
- 24.Thompson GB, Young WF., Jr Adrenal incidentaloma. Current Opinion in Oncology. 2003;15:84. doi: 10.1097/00001622-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Mantero F, Terzolo M, Arnaldi G, et al. A Survey on Adrenal Incidentaloma in Italy. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 26.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 27.Kunkle DA, Crispen PL, Chen DY, et al. Enhancing renal masses with zero net growth during active surveillance. J Urol. 2007;177:849. doi: 10.1016/j.juro.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 28.Angeli A, Osella G, Ali A, et al. Adrenal incidentaloma: an overview of clinical and epidemiological data from the National Italian Study Group. Horm Res. 1997;47:279. doi: 10.1159/000185477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.