Abstract
Background
Unlike conventional β-blockers, nebivolol, a third-generation β-adrenergic receptor blocker with vasodilator properties, promotes insulin sensitivity. Objective: The objective of this study was to determine whether nebivolol regulates overnutrition-induced activation of cardiac nutrient sensor kinases and inflammatory signaling.
Methods
Young Zucker obese (ZO) rats, a rodent model for overnutrition, and age-matched Zucker lean rats were treated with nebivolol (10 mg/kg/day; 21 days) and cardiac function was monitored by echocardiography and pressure volume loop analysis. Activation status of nutrient sensor serine/threonine kinases mammalian target for rapamycin (mTOR), and p70 S6kinase (S6K1) and S6K1-substrate RPS6, inflammatory marker Janus kinase 2 (Jak2) and its substrate STAT1, and energy sensor AMP-dependent kinase (AMPK) were monitored by determining phosphorylation status of pSer2448 of mTOR, pThr389 of S6K1, pSer235/236 of RPS6, pTyr1007/1008 of Jak2, pTyr701 of STAT1, and pThr172 of AMPK, respectively.
Results
Nebivolol reduced weight and improved cardiac function of ZO rats as shown by improvements in the myocardial performance index and a decrease in the diastolic parameter tau (τ), the time constant of isovolumic relaxation. Nebivolol also attenuated excessive activation of the nutrient sensor kinases mTOR and S6K1 and their substrate RPS6 as well as the inflammatory marker Jak2 and substrate STAT1 in ZO myocardium (p < 0.05). Moreover, nebivolol reversed suppression of the energy sensor kinase AMPK in ZO hearts (p < 0.05).
Conclusion
We report for the first time that nebivolol regulates overnutrition-induced activation of cardiac mTOR and Jak/STAT signaling and reverses suppression of AMPK. Since it also suppresses weight gain, nebivolol appears effective in the treatment of overnutrition-related cardiac inflammation and diastolic dysfunction.
Key Words: Nebivolol, Zucker obese, AMP kinase, mTORC1, Jak/STAT
Introduction
In conditions of obesity and the cardiorenal metabolic syndrome (CRS), a complex interplay between nutrients, the β-adrenergic receptor (β-AR) signaling pathway, the renin-angiotensin system, and insulin metabolic signaling underlies progression of cardiorenal pathology. Data emerging from randomized clinical trials and subsequent meta-analyses indicate that conventional β1 adrenergic receptor (β1-AR) blockers have a propensity to increase weight and promote an increased incidence of new-onset diabetes [1, 2, 3]. In this context, nebivolol is a third-generation β1-AR blocker that is structurally different from previous β-blockers and possesses the highest selectivity for β1-AR (up to 320-fold vs. β2-AR). Further, nebivolol is unique in that it activates endothelial nitric oxide synthase (eNOS) and promotes vasodilatation [4, 5]. We and others reported that nebivolol improves cardiac and renal protection in both rodent models and patients with hypertension and insulin resistance [6, 7, 8, 9, 10]. These beneficial effects of nebivolol are attributable to nebivolol-mediated dose-dependent nitric oxide (NO) production that can promote vasodilatation and improve insulin sensitivity. However, it remains unclear if nebivolol attenuates overnutrition-associated excessive activation of cardiac nutrient sensor kinases and inflammatory signaling and promotes cardiac health in conditions of overnutrition.
Nebivolol induces dose-dependent NO production, an action that is not inhibited by other β1-AR blockers, but attenuated by the β3-AR antagonist SR59230A [11, 12, 13]. Therefore, β3-AR-induced myocardial NOS upregulation and NO bioavailability underlies the beneficial pleiotropic effects of nebivolol in the heart. In this regard, the cardioprotective serine (Ser) kinase AMP activated kinase (AMPK) that serves as an energy sensor phosphorylates Ser633 of eNOS and activates eNOS [14], is a potential target of nebivolol. A recent report suggests that AMPK serves as a mediator for the β3-AR agonist BRL-37344-induced NO production [15]. However, AMPK-mediated phosphorylation of the scaffolding protein Raptor and tubular sclerosis complex 2 (TSC2) inhibits the formation of the nutrient sensor kinase mTOR complex 1 (mTORC1) and subsequent phosphorylation of p70 S6 kinase (S6K1). We showed that nebivolol treatment activated AMPK in the transgenic mRen2 rat heart [6]. In conditions of overnutrition, mTORC1/S6K1 activation acts as a nexus for converging signaling from nutrients, cytokines, insulin and angiotensin II, and becomes excessively activated in cardiovascular tissues and this likely contributes to structural and functional abnormalities [16, 17]. Activated S6K1 induces inhibitory phosphorylation of Ser residues in insulin receptor substrate 1 (IRS-1) and thus attenuates normal insulin metabolic signaling. It is conceivable that if nebivolol can activate AMPK, it would attenuate mTORC1/S6K1 signaling in the heart. Thereby, we posited that in addition to activating eNOS and promoting vasodilatation, nebivolol would modulate nutrient sensor kinases (mTOR/S6K1).
In the heart, pro-apoptotic pathways are activated by the signal transducer and activator of transcription-1 (STAT1)-dependent pathway [18]. Janus kinase 2 (Jak2), the inflammatory tyrosine (Tyr) kinase that phosphorylates pTyr701 of STAT1, is the upstream activator of STAT1. Interestingly, studies show that mTOR also can regulate STAT1 [19]. Since overnutrition can activate both mTOR and Jak2, we posited that STAT1 would be activated in cardiac tissue under conditions of overnutrition and nebivolol would attenuate STAT1 activation. This investigation was undertaken to determine whether nebivolol attenuates overnutrition-induced excess activation of cardiac nutrient sensor kinases (mTOR/S6K1) and inflammatory signaling via Jak2/STAT1 and promotes activation of energy sensor kinase AMPK in Zucker obese (ZO) rats. We further posited that nebivolol would improve diastolic function in this rodent model for obesity that exhibits characteristics of the CRS.
Methods
Animal Studies
All animal procedures were approved in advance by the Harry S. Truman Veterans Memorial Hospital Subcommittee for Animal Safety, as well as by the University of Missouri IACUC, and animals were cared for in accordance with Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health publication 85–23). Male Zucker lean (ZL) control and ZO rats (9–12 weeks old) were purchased from Charles River Laboratories (Saint-Constant, Que., Canada) and housed under standard laboratory conditions where room temperature was 21–22°C and light and dark cycles were 12 h each. Animals were divided into four groups: vehicle treated (ZOC and ZLC; n = 6 each) or nebivolol treated (ZON and ZLN; n = 6 each), respectively. ZON and ZLN animals were treated with nebivolol 10 mg/kg/day released via an implanted osmotic mini-pump for 21 days as described previously [7, 9].
Echocardiography and Pressure Volume Loop Analysis
Transthoracic echocardiography (echo), including Doppler examination, was performed on isoflurane anesthetized rats (4% induction and 1.75% maintenance) using a GE Vivid i ultrasound system with a 10.5-MHz phased-array pediatric probe (10S). Echo examinations were performed while rats were in the left lateral decubitus position as explained previously [16]. All parameters were assessed by using an average of three beats, and calculations were made in accordance with published guidelines for echocardiographic studies in rodents [16, 20]. Cardiac pressure volume (PV) loop analysis was performed by PV loop system (Scisense, Inc., Ont., Canada) employing direct catheterization of the left ventricle which yields several load-independent indices of diastolic and systolic function, as we have reported previously [16, 21].
Immunoblotting
Left ventricular (LV) lysates from different treatments (40–60 μg/lane) were separated and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories). All antibodies were from Cell Signaling Technology Inc. (Boston, Mass., USA). Immunoblotting was performed as described previously [6, 21].
Statistics
Values are expressed as means ± SE. Statistical comparisons were performed with Sigma Stat (Aspire Software Intl., Ashburn, Va., USA) using ANOVA followed by the Scheffé's test. A value of p < 0.05 was considered to be significant.
Results
Nebivolol Suppressed Weight Gain and Improved Cardiac Functions in the ZO Heart
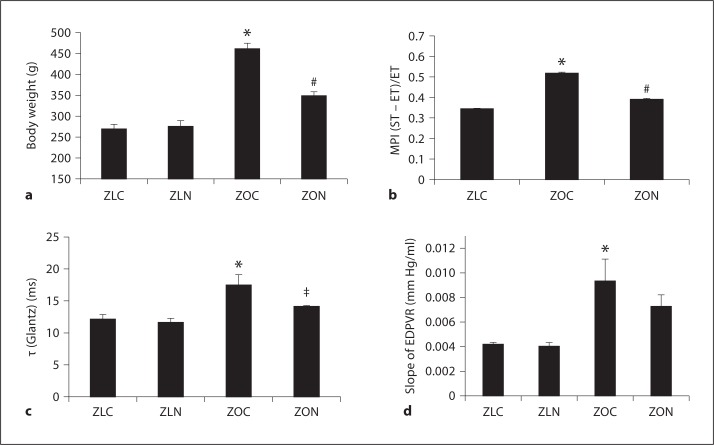
ZO rats have a mutation in the leptin receptor and are hyperphagic and hyperinsulinemic. They exhibited significantly higher body weight at the age of 12 weeks than age-matched ZL controls, and this weight gain was suppressed by nebivolol (fig. 1a; p = 0.05). It has been shown that ZO rats develop obesity-related diastolic and systolic dysfunction [9]. The myocardial performance index (MPI), an echocardiographic index of global cardiac function, tau (τ), the time constant of isovolumic relaxation and an index of the active component of early diastolic relaxation, and the slope of the end-diastolic pressure volume relationship (EDPVR), a marker of LV wall stiffness, were all significantly elevated in 12-week-old ZO rats compared to ZL rats (p < 0.05; fig. 1b–d) indicating impaired diastolic function in ZO rats. Nebivolol treatment normalized MPI (fig. 1b, ZO vs. ZON; p < 0.05) and τ (p = 0.06), but did not significantly reduce EDPVR (p > 0.05). These observations support the concept that nebivolol results in modest attenuation of overnutrition-associated weight gain and cardiac dysfunction in 12-week-old ZO rats.
Fig. 1.
Nebivolol suppressed weight gain and improved cardiac function parameters in ZO rats. a Body weight of ZO and ZL rats with and without nebivolol treatment. ZLC = Untreated ZL rat; ZOC = untreated ZO rat; ZLN = ZL rat treated with nebivolol; ZON = ZO rat treated with nebivolol; * p < 0.05 vs. ZLC; # p < 0.05 vs. ZOC. b Echocardiographic analysis showed MPI was improved (MPI is decreased) in ZO rats treated with nebivolol. c, d PV loop analysis showed that nebivolol partially reversed the changes in tau (τ) (Glantz) and EDPVR in ZOC rats. * p < 0.05 vs. ZLC; # p < 0.05 vs. ZOC;‡ p = 0.06 vs. ZOC. Results are expressed as mean ± SEM of 4 rats per experimental group. ST = Systolic time; ET = ejection time.
Nebivolol Attenuated Overnutrition-Associated Activation of mTOR-S6K1-RPS6 Signaling in the Myocardium of 12-Week-Old ZO Rat Hearts
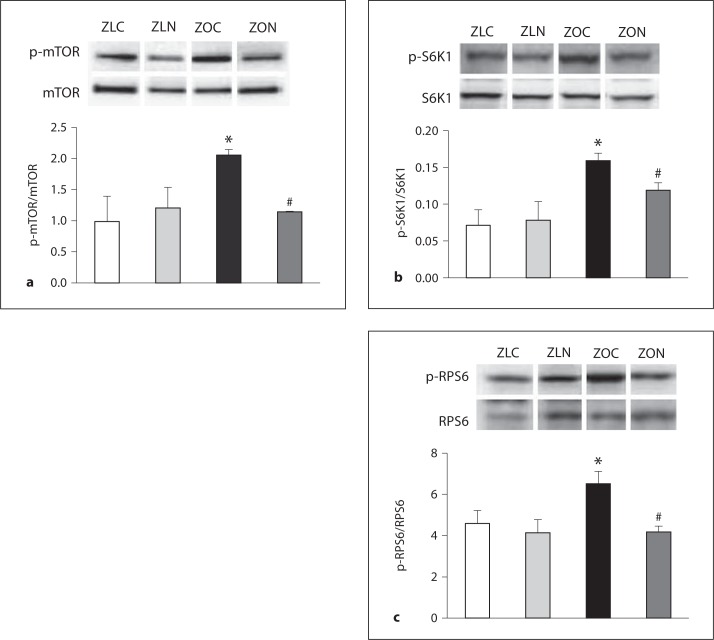
We observed significant reductions in total protein levels of mTOR and S6K1 in LV lysates of ZO rats compared to ZL rats; however, the ratios of phosphoprotein (mTORpSer2448, S6K1pThr389, and RPS6pSer235/236) to total protein were significantly higher in ZO LV lysates compared to ZL LV lysates (fig. 2a–c). Nebivolol treatment restored the ratios of phosphoprotein to total protein for mTOR, S6K1, and RPS6 (fig. 2a–c). To our knowledge, this is the first report that shows that nebivolol regulates overnutrition-related mTORC1 activation.
Fig. 2.
Nebivolol suppressed excessive activation of nutrient sensor kinases in ZO myocardium. Levels of pSer2448 mTOR (p-mTOR) (a), pThr389S6k1 (p-S6K1) (b), and pSer235/236RPS6 (p-RPS6) (c) were elevated in ZOC LV lysates, and nebivolol attenuated these effects. * p < 0.05 vs. control; # p < 0.05 vs. Ang II. Results are expressed as mean ± SEM of 4 rats per experimental group.
Nebivolol Enhanced Stimulatory Phosphorylation of AMPK in the Myocardium of 12-Week-Old ZO Rat Hearts
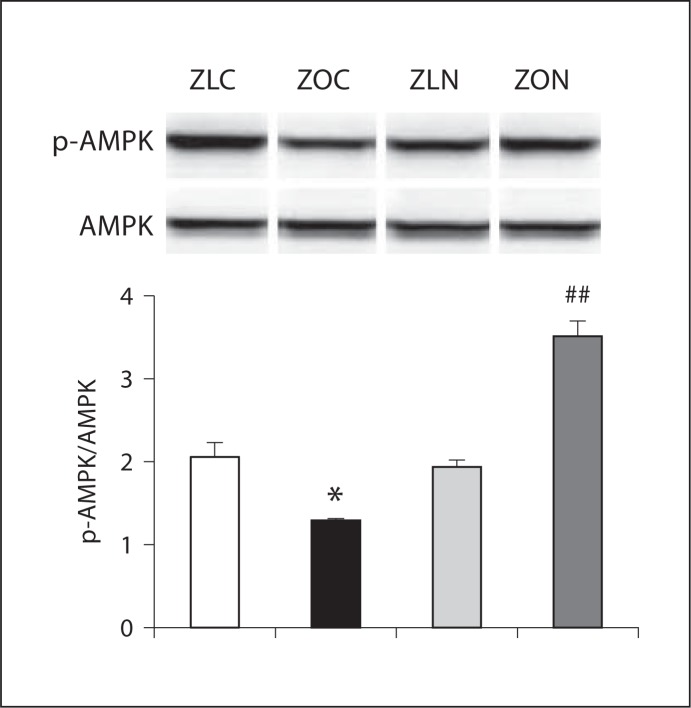
Previous studies demonstrated that AMPK, an energy sensor/metabolic switch [14, 22], responds to energy stress by suppressing cell growth and biosynthetic processes, in part through its inhibition of mTORC1. Since nebivolol attenuated mTORC1 activation, we tested if nebivolol activates AMPK in the myocardium and HL-1 cardiomyocytes. Moreover, β3-AR agonists (such as nebivolol) activate eNOS and increase NO production, in part, via AMPK activation [15]. We observed a significant decrease in pThr172AMPK in LV lysates of ZO rats, and this effect was reversed in nebivolol-treated ZO rats (fig. 3). This observation is consistent with nebivolol's ability to activate β3-AR that signals through AMPK.
Fig. 3.
Nebivolol enhanced AMPK phosphorylation in ZO myocardium. Level of pThr172 AMPK was reduced in ZO rats, and nebivolol reversed this effect. * p < 0.05 vs. ZLC; # p < 0.01 vs. ZOC. Results are expressed as mean ± SEM of 4 rats per experimental group.
Nebivolol Attenuated Stimulatory Phosphorylation of Tyr Kinase Jak2 and Its Substrate STAT1 in the Myocardium of 12-Week-Old ZO Rat Hearts
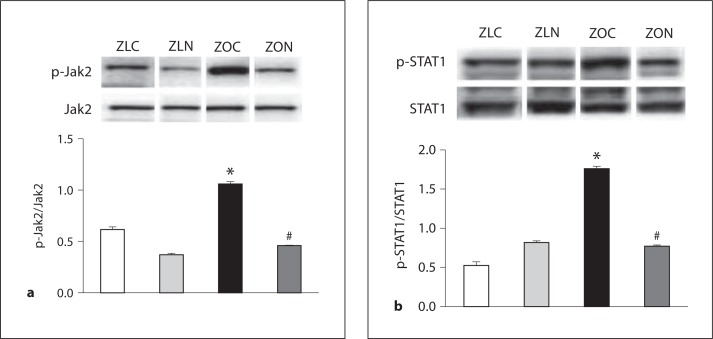
In conditions of overnutrition, many factors (hyperinsulinemia, activation of the renin-angiotensin-aldosterone system, oxidative stress, and other cytokines) can activate Jak2, an inflammatory kinase implicated in hypertension and ischemic injury [23]. While Jak2 activates STAT1 by phosphorylation of Tyr701, mTOR is implicated in associating with STAT1 and increasing STAT1-dependent gene expression [19]. Therefore, we tested if Jak2 and STAT1 are activated in cardiac tissues of hyperinsulinemic, insulin-resistant obese ZO rats and whether their activation is suppressed by nebivolol. Significant increases in phosphorylation of Tyr1007/1008 of Jak2 and Tyr701 of STAT1 were observed in ZO LV lysates compared to those of ZL (fig. 4a, b). Nebivolol treatment significantly reduced stimulatory phosphorylation of both Jak2 and STAT1. This is the first report that shows activation of the Jak2/STAT1 pathway in cardiac tissues of the obese, insulin-resistant ZO rat and that nebivolol attenuates this inflammatory signaling.
Fig. 4.
Nebivolol attenuated activation of inflammatory, pro-apoptotic Jak2/STAT1 signaling in ZO myocardium. Levels of pTyr1007/1008 Jak2 (a) and pTyr701STAT1 (b) were elevated in ZOC LV lysates, and nebivolol attenuated these effects. * p < 0.05 vs. ZLC; # p < 0.05 vs. Ang II. Results are expressed as mean ± SEM of 4 rats per experimental group.
Discussion
Nebivolol is a third-generation β1-adrenoceptor antagonist approved for treatment of hypertension and heart failure. In this context, we previously showed that nebivolol improved insulin sensitivity, myocardial ultrastructure, and diastolic function, as well as reduced myocardial fibrosis and weight gain in younger (9-week-old) ZO rats [9]. This study confirms the efficacy of nebivolol in an older cohort of ZO rats and extends those results by demonstrating that nebivolol possesses novel pleiotropic effects that confer cardioprotection by blunting the excessive pro-growth and pro-inflammatory signaling that occurs in the setting of obesity.
Previous studies demonstrate that mTORC1 inhibition by rapamycin suppresses myocardial hypertrophy [24, 25]. Moreover, endogenous mTOR expression is significantly elevated in patients with advanced heart failure and in mice with pathological hypertrophy and cardiac dysfunction [25]. Activation of mTORC1 signaling leads to insulin resistance in high fat-fed obese rats [26]. In addition, studies with S6K1 knockout mice demonstrate that S6K1 plays a central role in the development of obesity and insulin resistance [27]. Consistent with these studies, our data show increases in phosphorylation of mTOR (pSer2448), S6K1 (pThr389), and RPS6 (p Ser235/236) in ZO myocardium, all of which were significantly reduced by nebivolol treatment. Because mTORC1 activation impairs insulin-stimulated vasodilatation by inhibiting eNOS activation [28], we posit that the nebivolol-mediated increase in eNOS activation and increased vasodilation in ZO rats may be partly due to its inhibitory effect on the mTORC1 signaling pathway.
Another salient point emerging from this study is that nebivolol activates the energy sensor AMPK (fig. 3) in concert with improvements in LV function. AMPK, a stress-activated protein kinase, plays a cardioprotective role by improving LV function and cardiomyocyte survival in heart failure [29, 30]. Deficiency in AMPK exacerbates cardiac hypertrophy and contractile dysfunction [31]. AMPK enhances eNOS activity by direct phosphorylation at Ser1177 and increases the production of endothelium-derived NO. Consistent with our results from transgenic Ren2 rats [6], we observed a decrease in phosphorylation of AMPK (pThr172) in ZO myocardium, which was significantly enhanced by nebivolol treatment. The ability of nebivolol to activate AMPK in ZO myocardium is also consistent with the concept that nebivolol can promote β3-AR agonism that leads to AMPK activation and subsequent eNOS activation.
Activation of the Jak/STAT pathway is implicated in promoting cardiac inflammation [18, 19]. The observation that nebivolol, a β3-AR agonist, suppresses cardiac Jak2 and STAT1 in the ZO rat is also surprising. It was reported previously that activation of β3-AR stimulates Jak2/STAT1 signaling in adipocytes [32]. Therefore, it is conceivable that nebivolol-mediated inhibition of inflammatory and pro-apoptotic Jak2/STAT1 pathway might not be mediated via β3-AR. To this point, it should be noted that, in fact, leptin is an activator of Jak2. Further studies are required to decipher how nebivolol suppresses cardiac Jak2/STAT1 signaling in the leptin-resistant ZO rat.
Emerging evidence strongly supports the notion that diet-induced obesity can lead to leptin resistance, whereas leptin resistance predisposes individuals to hyperphagia which further exacerbates diet-induced obesity and CRS [33]. In this context, the ZO rat serves as an interesting model since its obesity is a result of its leptin resistance. Data presented here suggest that nebivolol can help to overcome this vicious cycle leading to weight gain by a leptin-independent mechanism since a 3-week nebivolol treatment significantly reduced weight gain (fig. 1a) and improved cardiac function (fig. 1b–d) in a leptin-resistant animal (ZO rat).
In conclusion, data presented here demonstrate that nebivolol regulates overnutrition-induced activation of cardiac mTOR and Jak/STAT signaling and reverses suppression of AMPK. Moreover, nebivolol also suppresses weight gain by a leptin-independent mechanism and improves cardiac function. These novel pleotropic effects of nebivolol imply that nebivolol could be an effective cardioprotective drug in conditions of overnutrition and leptin resistance.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by University of Missouri Mission Enhancement Fund (L.P.), the Forest Research Institute grant (L.P.) and NIH (R01 HL73101-08 and R01 HL107910-03) (J.R.S.) and Veterans Affairs Merit System 0018 (J.R.S.). We thank N. Rehmer, M. Garro, R. Schneider, and S. Arnold for their technical support and B. Hunter for editorial assistance.
References
- 1.Bielecka-Dabrowa A, Aronow WS. Rysz J. Banach M. Current place of beta-blockers in the treatment of hypertension. Curr Vasc Pharmacol. 2010;8:733–741. doi: 10.2174/157016110793563861. [DOI] [PubMed] [Google Scholar]
- 2.Bangalore S, Wild D, Parkar S, Kukin M, Messerli FH. Beta-blockers for primary prevention of heart failure in patients with hypertension: insights from a meta-analysis. J Am Coll Cardiol. 2008;52:1062–1072. doi: 10.1016/j.jacc.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomized trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Münzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol. 2009;54:1491–1499. doi: 10.1016/j.jacc.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Wright HM. Nebivolol: a highly selective beta1-adrenergic receptor blocker that causes vasodilation by increasing nitric oxide. Cardiovasc Ther. 2008;26:189–202. doi: 10.1111/j.1755-5922.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Gul R, Habibi J, Yang M, Pulakat L, Whaley-Connell AT, Ferrario CM, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.01126.2011. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habibi J, Hayden MR, Sowers JR, Pulakat L, Tilmon RD, Manrique C, Lastra G, Demarco VG, Whaley-Connell A. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2011;152:659–668. doi: 10.1210/en.2010-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manrique C, Lastra G, Habibi J, Pulakat L, Schneider R, Durante W, Tilmon R, Rehmer J, Hayden MR, Ferrario CM, Whaley-Connell A, Sowers JR. Nebivolol improves insulin sensitivity in the TGR(Ren2)27 rat. Metabolism. 2011;60:1757–1766. doi: 10.1016/j.metabol.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, DeMarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavazzi L. Nebivolol for heart failure in the elderly. Expert Rev Cardiovasc Ther. 2007;5:423–433. doi: 10.1586/14779072.5.3.423. [DOI] [PubMed] [Google Scholar]
- 11.Maffei A, Di Pardo A, Carangi R, Carullo P, Poulet R, Gentile MT, Vecchione C, Lembo G. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–656. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- 12.Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 2011;58:2683–2691. doi: 10.1016/j.jacc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozec B, Erfanian M, Laurent K, Trochu JN, Gauthier C. Nebivolol, a vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the nonfailing transplanted human heart. J Am Coll Cardiol. 2009;53:1532–1538. doi: 10.1016/j.jacc.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodis J, Vaclavíková R, Farghali H. Beta-3 agonist-induced lipolysis and nitric oxide production: relationship to PPARgamma agonist/antagonist and AMP kinase modulation. Gen Physiol Biophys. 2011;30:90–99. doi: 10.4149/gpb_2011_01_90. [DOI] [PubMed] [Google Scholar]
- 16.Pulakat L, DeMarco VG, Ardhanari S, Chockalingam A, Gul R, Whaley-Connell A, Sowers JR. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol. 2011;301:R885–R895. doi: 10.1152/ajpregu.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 19.Kristof AS, Marks-Konczalik J, Billings E, Moss J. Stimulation of signal transducer and activator of transcription-1 (STAT1)-dependent gene transcription by lipopolysaccharide and interferon-gamma is regulated by mammalian target of rapamycin. J Biol Chem. 2003;278:33637–33644. doi: 10.1074/jbc.M301053200. [DOI] [PubMed] [Google Scholar]
- 20.Hoit BD. Echocardiographic characterization of the cardiovascular phenotype in rodent models. Toxicol Pathol. 2006;34:105–110. doi: 10.1080/01926230500369535. [DOI] [PubMed] [Google Scholar]
- 21.DeMarco VG, Johnson MS, Ma L, Pulakat L, Mugerfeld I, Hayden MR, Garro M, Knight W, Britton SL, Koch LG, Sowers JR. Overweight female rats selectively breed for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2012;302:H1667–H1682. doi: 10.1152/ajpheart.01027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananthakrishnan R, Hallam K, Li Q, Ramasamy R. JAK-STAT pathway in cardiac ischemic stress. Vascul Pharmacol. 2005;43:353–356. doi: 10.1016/j.vph.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 25.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 27.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 28.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302:E201–E208. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 30.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9:592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westphal S, Perwitz N, Iwen KAH. Kraus D. Schick R. Fasshauer M. Klein J. Expression of ATRAP in adipocytes and negative regulation by β-adrenergic stimulation of JAK/STAT. Horm Metab Res. 2008;40:165–171. doi: 10.1055/s-2007-1022547. [DOI] [PubMed] [Google Scholar]
- 33.Scarpace PJ, Zhang Y. Leptin resistance: a predisposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]