Abstract
Objective
Cardiac and kidney diseases are common, and the impact of acute kidney injury (AKI) on patient outcome is well known. We aimed to investigate the incidence of acute cardiorenal syndrome (CRS) and the risk factors and outcomes associated with the disease.
Methods
We conducted a retrospective cohort study comprising 289 patients with acute coronary syndrome (ACS) and acute decompensated heart failure (ADHF), examining the incidence of AKI defined according to the Acute Kidney Injury Network (AKIN) classification, the factors contributing to AKI, and the impact of AKI on in-hospital mortality and hospital re-admission.
Results
Of 71 patients with AKI, 36 (50.7%) had ACS and 35 (49%) had ADHF. Overall in-hospital mortality was 5.5% (n = 16). Multivariate logistic regression identified the following independent predictors of AKI in male patients with ACS: previous myocardial infarction at age >65 years (OR 5.967, 95% CI 1.16–30.47, p = 0.03), chronic kidney disease (OR 3.72, 95% CI 1.31–16.61, p = 0.01), and decreased hemoglobin levels (OR 0.684, 95% CI 0.53–0.88, p = 0.03). No variable was identified as an independent risk factor in ADHF patients. Kaplan-Meier survival curves indicated that patients with ACS plus AKI had significantly higher in-hospital mortality (log rank = 0.007).
Conclusion
Acute CRS (type 1 CRS) is more frequent in patients with ADHF and can be considered multifactorial. Although CRS is less frequent in ACS patients, it is associated with longer hospital stay and with higher in-hospital mortality. The heart-kidney interaction should be managed collaboratively between cardiologists and nephrologists to increase our knowledge and enhance clinical approaches.
Key Words: Acute renal failure, Chronic kidney disease, Statistics and epidemiology, Acute cardiorenal syndrome, Acute kidney injury, Acute coronary syndrome, Acute decompensated heart failure
Introduction
Prevalent heart and kidney disease is increasing [1, 2, 3, 4], and the complexity of this interactive relationship continues to emerge. Recently, the Acute Dialysis Quality Initiative (ADQI) Working Group proposed a consensus definition for this interaction, cardiorenal syndrome (CRS), providing classifications for five subtypes [5]. The primary term CRS refers to ‘disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other’. Acute CRS (type 1 CRS) is characterized by acute worsening of heart function leading to acute kidney injury (AKI) and/or dysfunction. The spectrum of acute cardiac events that may contribute to AKI includes acute decompensated heart failure (ADHF), acute coronary syndrome (ACS), cardiogenic shock, and surgery-associated low cardiac output syndrome.
Many studies have evaluated the development of AKI in ADHF and ACS [6, 7] and have used the term ‘worsening renal function’ to describe changes in kidney function. Until recently, the inherent problem was the lack of a consensus definition for acute renal dysfunction. Previous epidemiological studies analyzed the incidence and outcome of AKI using a variety of definitions. However, comparisons of studies and patients have been confounded by multiple definitions of AKI. The ADQI group recently established a consensus definition for AKI called the RIFLE criteria, standing for Risk, Injury, Failure, Loss and End-stage kidney disease [8]. The RIFLE classification defines three grades of AKI based on the serum creatinine criterion (CrCr) and urine output criterion (UOCr). The Acute Kidney Injury Network (AKIN) group refined the RIFLE classification further [9]. The most noticeable aspect of the refinement of the AKI criteria is a small change in the serum creatinine (SCr) level (≥0.3 mg/dl). The RIFLE and AKIN classifications can detect AKI with high sensitivity and specificity, while establishing different severity levels to predict the prognosis of affected patients [10].
It is of critical significance to determine the incidence of and outcomes associated with CRS for understanding the overall burden of the disease as well as its natural history, morbidity, and mortality. Incidence estimates for AKI associated with ACS and ADHF range from 9 to 19% and from 20 to 45%, respectively [11, 12, 13, 14]. This wide range may be attributed to the use of different definitions of AKI, differences in the observation period, and/or heterogeneity of the selected populations. In addition, the development of AKI in ACS and ADHF patients has been increasingly recognized as an independent risk factor for morbidity and mortality [11, 12].
To our knowledge, no study has investigated the epidemiology of CRS in Turkey. We conducted the present study to determine the incidence of AKI, its clinical predictors, and its prognostic impact on in-hospital mortality and hospital re-admission in Yeditepe University Hospital, Coronary Intensive Care Unit (CICU), Istanbul, Turkey.
Methods
A retrospective cohort study was conducted in patients with acute cardiac events. The study was approved by the Clinical Research Review Board of Yeditepe University Medical School. Informed consents were waived due to the retrospective design of the study.
Patient Population
The study included 289 patients with ACS (myocardial infarction with or without ST-segment elevation or unstable angina) and with ADHF who were admitted to our five-bed CICU over a 3-year period (2008–2011). Definitions of ACS and ADHF were based on the current guidelines [15, 16]. We excluded patients who had a previous history of end-stage renal disease requiring renal replacement therapy, those who underwent cardiac surgery for emergency coronary revascularization, or those with active infections.
Clinical and Laboratory Data Collection
Data were obtained from our Hospital Information System, a computerized system used for daily patient documentation, and verified with the written records of each patient. Standard demographic, clinical, and physiological data were collected. Demographic information included age, sex, height (cm), weight (kg), body mass index, and length of hospital stay. Clinical data included primary diagnoses, comorbidities (diabetes mellitus, hypertension, chronic kidney disease, history of cerebrovascular accidents, myocardial infarction, or coronary revascularization), and smoking status (table 1). SCr and blood urea nitrogen (BUN) values at admission and discharge were recorded as peak values. We recorded only serum sodium, potassium, hemoglobin (Hb), and white blood cell values at admission and discharge. Hemodynamic parameters (systolic and diastolic blood pressure and heart rate), echocardiography findings (table 2), and medications were also recorded.
Table 1.
Baseline characteristics of the patients with or without acute CRS
| Variables | AKI |
No AKI |
p value |
||||
|---|---|---|---|---|---|---|---|
| ACS (n = 36) | ADHF (n = 35) | ACS (n = 200) | ADHF (n = 18) | p* | p** | p | |
| Demographic characteristics | |||||||
| Age, years | 68.58 ± 10.56 | 75.29 ± 10.71 | 59.86 ± 12.50 | 74.72 ± 10.56 | 0.00 | 0.85 | 0.08 |
| Male sex | 26 (72.2) | 12 (66.7) | 151 (75.5) | 13 (37.1) | 0.67 | 0.49 | 0.023 |
| BMI | 27.05 ± 3.43 | 26.16 ± 5.81 | 27.50 ± 3.46 | 25.93 ± 4.22 | 0.47 | 0.86 | 0.22 |
| Comorbidities | |||||||
| Hypertension | 26 (72.2) | 17 (94.4) | 103 (51.5) | 28 (80.0) | 0.02 | 0.24 | 0.002 |
| Diabetes mellitus | 16 (44.4) | 12 (66.7) | 60 (30.0) | 19 (54.3) | 0.12 | 0.55 | 0.010 |
| Chronic kidney disease | 19 (9.5) | 14 (77.8) | 13 (36.1) | 24 (68.6) | 0.00 | 0.53 | 0.000 |
| Previous CVA | 6 (16.7) | 1 (5.6) | 15 (7.5) | 5 (14.3) | 0.10 | 0.65 | 0.058 |
| Previous MI | 55 (27.5) | 5 (27.8) | 15 (41.7) | 7 (20.0) | 0.11 | 0.73 | 0.650 |
| Previous coronary revascularization | 19 (9.5) | 4 (22.2) | 6 (16.7) | 7 (20.0) | 0.23 | 1.00 | 0.099 |
| Smoking history | 17 (47.2) | 6 (33.3) | 114 (57.0) | 13 (37.1) | 0.28 | 1.00 | 0.075 |
| Selected medications | |||||||
| ACEi | 13 (36.1) | 10 (38.9) | 73 (36.5) | 7 (28.6) | 1.00 | 0.53 | 0.569 |
| ARB | 7 (19.4) | 5 (27.8) | 24 (12.0) | 9 (25.7) | 0.28 | 1.00 | 0.088 |
| β-blocker | 13 (36.1) | 9 (50.0) | 81 (40.5) | 14 (40.0) | 0.71 | 0.56 | 0.678 |
| Furosemide | 4 (11.1) | 6 (33.3) | 11 (5.5) | 13 (37.1) | 0.25 | 1.00 | 0.001 |
| HCTZ | 11 (30.6) | 7 (38.9) | 19 (9.5) | 20 (57.1) | 0.02 | 0.25 | 0.000 |
| Length of hospital stay, days | 6.75 ± 3.16 | 6.71 ± 3.33 | 4.67 ± 2.10 | 6.67 ± 4.22 | 0.00 | 0.96 | 0.002 |
Values are mean ± SD or numbers and percentages in parentheses.
CVA = Cerebrovascular accident;
MI = myocardial infarction;
ACEi = angiotensin converting enzyme inhibitor;
ARB = angiotensin receptor blocker;
HCTZ = hydrochlorothiazide.
p = AKI vs. no-AKI patients;
p* = for ACS patients, with vs. without AKI.
p** = for ADHF patients, with vs. without AKI. B values are statistically significant.
Table 2.
Baseline laboratory and hemodynamic characteristics of the patients
| Variables | AKI |
No AKI |
p value |
||||
|---|---|---|---|---|---|---|---|
| ACS (n = 36) | ADHF (n = 35) | ACS (n = 200) | ADHF (n = 18) | p* | p** | p | |
| BUN, mg/dl | 26.75 ± 15.72 | 48.22 ± 26.53 | 17.73 ± 8.67 | 37.77 ± 15.58 | 0.02 | 0.07 | 0.000 |
| Creatinine, mg/dl | 1.23 ± 0.68 | 1.98 ± 1.41 | 0.89 ± 0.33 | 1.69 ± 0.90 | 0.06 | 0.37 | 0.000 |
| eGFR, ml/min/1.73 m2 | 66.69 ± 26.67 | 37.62 ± 18.35 | 90.96 ± 25.46 | 44.50 ± 21.03 | 0.00 | 0.24 | 0.000 |
| Sodium, mg/dl | 136.36 ± 4.53 | 133.08 ± 6.87 | 137.21 ± 3.86 | 134.94 ± 3.91 | 0.29 | 0.21 | 0.003 |
| Potassium, mg/dl | 4.30 ± 0.49 | 4.78 ± 0.77 | 4.28 ± 0.38 | 4.66 ± 0.49 | 0.89 | 0.47 | 0.012 |
| Hemoglobin, g/dl | 12.39 ± 2.50 | 11.24 ± 2.03 | 13.55 ± 1.82 | 12.06 ± 1.90 | 0.11 | 0.16 | 0.000 |
| White blood cells | 10.01 ± 3.71 | 10.37 ± 4.63 | 9.39 ± 3.25 | 9.37 ± 3.30 | 0.35 | 0.37 | 0.143 |
| Systemic pressure, mm Hg | |||||||
| Systolic | 114.89 ± 16.11 | 102.17 ± 17.70 | 119.02 ± 17.12 | 115.17 ± 17.68 | 0.16 | 0.16 | 0.000 |
| Diastolic | 70.19 ± 10.32 | 61.03 ± 11.15 | 72.6 ± 89.85 | 70.56 ± 10.37 | 0.18 | 0.04 | 0.000 |
| Heart rate, bpm | 74.17 ± 10.49 | 78.86 ± 18.18 | 73.65 ± 11.66 | 82.17 ± 13.17 | 0.79 | 0.45 | 0.276 |
| EF, % | 46.11 ± 9.93 | 37.94 ± 12.00 | 50.43 ± 12.45 | 37.06 ± 13.70 | 0.25 | 0.82 | 0.000 |
EF = Ejection fraction. p = AKI vs. no-AKI patients.
p = for ACS patients, with vs. without AKI.
p = for ADHF patients, with vs. without AKI. Bold values are statistically significant.
Assessment of Kidney Function
AKI was classified based on SCr levels, as proposed by the AKIN criteria that classify AKI into three stages of severity (table 3) [9]. Patients with evidence for an abrupt (within 48 h) absolute increase in SCr (≥0.3 mg/dl) or an increase by up to ≥150–200% (1.5-to 2-fold) from baseline or a urine output of <0.5 ml/kg/h for >6 h were classified as stage 1 AKI. Stage 2 AKI was diagnosed as a ≥2-fold increase in SCr from baseline or a urine output of >0.5 ml/kg/h for >12 h. Patients with a ≥3-fold increase in SCr from baseline with an acute increase of at least 0.5 mg/dl, an increase in SCr ≥4.0 mg/dl, a urine output of <0.3 ml/kg/h for 24 h, or anuria for 12 h were classified as stage 3 AKI.
Table 3.
Classification/staging system for AKI according to AKIN [9]
| Stage | Serum creatinine criteria | Urine output criteria |
|---|---|---|
| 1 | Increase in serum creatinine of ≥0.3 mg/dl (≥26.4 μmol/l) or increase to ≥150–200% (1.5-to 2-fold) from baseline | <0.5 ml/kg/h for >6 h |
| 2 | Increase in serum creatinine to >200–300% (>2-to 3-fold) from baseline | <0.5 ml/kg/h for >12 h |
| 3 | Increase in serum creatinine to >300% (>3-fold) from baseline [or serum creatinine of ≥4.0 mg/dl (≥354 μmol/l) with an acute increase of at least 0.5 mg/dl (44 μmol/l)] | <0.3 ml/kg/h for 24 h or anuria for 12 h |
Baseline SCr was defined as the admission level. For some patients, SCr at discharge was lower than that at admission, and this value was considered to be the basal level. Chronic kidney disease was diagnosed in patients with an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2 for >3 months, based on the guidelines of the National Kidney Foundation [17]. Baseline GFR was estimated using the four-component (age, race, sex, and SCr level) Modification of the Diet in Renal Disease equation: estimated GFR = 186 × (SCr level in mg/dl)−1.154 × (age in years)−0.203. The product of this formula is multiplied by a correction factor of 0.742 for women and 1.21 for African-Americans [8, 10, 18].
We assessed the incidence of and risk factors for AKI after CICU admission. The outcomes considered were in-hospital mortality and re-admission to the CICU.
Statistical Analyses
Continuous variables were presented as mean ± SD, and discrete variables were presented as frequencies and percentages. The independent two-sample t test was used to evaluate the differences in means between the two groups. The χ2 test was used to compare the frequencies of the groups.
A multiple logistic regression analysis was used to evaluate the independent correlates of AKI. Parameters with a p value <0.05 in the univariate analysis were included in the model.
In-hospital survival rate was calculated using the Kaplan-Meier method, and a comparison between AKI stages was performed using the log-rank test. The adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for survival rates were calculated using the Cox proportional hazards model, and adjustments were made for all other variables. The model's fit was evaluated by the Hosmer-Lemeshow goodness of fit test. Two-tailed p values <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS version 19.0 software (SPSS Inc., Chicago, Ill., USA).
Results
Baseline Characteristics
Tables 1 and 2 include the demographic, clinical, and laboratory characteristics of patients with and without AKI. The mean BUN, creatinine, and potassium levels at admission were significantly higher in patients who later developed AKI than in those who did not, and eGFR, sodium, Hb levels, and ejection fraction of patients with AKI were significantly lower, as well as systolic and diastolic blood pressures. These patients also showed higher incidences of hypertension, diabetes, and chronic kidney disease compared to those without AKI. Due to the variable nature of ACS and ADHF as acute cardiac events contributing to AKI in the development of acute CRS (type 1 CRS), we evaluated these events separately and then compared them to identify differences. We found that baseline BUN and eGFR levels as well as history of hypertension remained significant in patients with ACS (p = 0.02, p > 0.00, and p = 0.02, respectively). Patients with ACS and AKI were older (p > 0.00) and had a higher frequency of hydrochlorothiazide use (p = 0.02) compared to non-AKI patients. Among patients with ADHF, only diastolic blood pressure was significantly lower in AKI versus non-AKI patients (p = 0.04). ACS patients with AKI had considerably longer hospital stay than ADHF patients with AKI (p < 0.00 and p = 0.96, respectively).
Incidence of AKI
A total of 71 patients had AKI, of which 36 (50.7%) had ACS, and 35 (49.2%) had ADHF. The characteristics of AKI in ACS and ADHF patients are shown in table 4. In ACS patients, time to peak SCr was 1.19 ± 1.21 days, and time to recover baseline SCr levels was 4.33 ± 2.80 days, whereas ADHF patients exhibited significantly earlier peak SCr levels (0.17 ± 0.56 days), and recovery to baseline levels (1.94 ± 2.78 days; p < 0.00 and p < 0.00, respectively). Of patients with ACS and AKI two patients (5.5%), and of patients with ADHF and AKI 4 patients (11.4%), were on renal replacement therapy, but this was not found significant (p = 0.42).
Table 4.
AKI characteristics
| Characteristics | ACS (n = 36) | ADHF (n = 35) | p value |
|---|---|---|---|
| AKI | |||
| Stage 1 | 26 (11) | 21 (39.6) | 0.322 |
| Stage 2 | 7 (3) | 11 (20.8) | 0.285 |
| Stage 3 | 3 (1.3) | 3 (5.7) | 1.000 |
| No AKI | 200 (84.7) | 18 (34) | 0.000 |
| RRT | 2 (5.6) | 4 (11.4) | 0.429 |
| Time to peak SCr, days | 1.19 ± 1.21 | 0.17 ± 0.56 | 0.000 |
| Recovery to baseline SCr, days | 4.33 ± 2.80 | 1.94 ± 2.78 | 0.001 |
Values are numbers and percentages in parentheses or mean ± SD. RRT = Renal replacement treatment.
Predictors of AKI
In univariate analysis for ACS patients, advanced age (OR 1.067; 95% CI 1.03–1.10), history of hypertension (OR 2.44; 95% CI 1.12–5.34), chronic kidney disease (OR 5.38; 95% CI 2.35–12.32), decreased Hb and eGFR levels (OR 0.751; 95% CI 0.62–0.89 and OR 0.96; 95% CI 0.95–0.98, respectively) were significantly associated with AKI (table 5). After a multivariable adjustment, previous myocardial infarction at age >65 years (OR 5.967; 95% CI 1.16–30.47; p = 0.03), chronic kidney disease (OR 3.72; 95% CI 1.31–16.61; p = 0.01), and decreased Hb levels in male patients (OR 0.684; 95% CI 0.53–0.88; p = 0.03) were determined to be independent predictors of AKI in ACS patients. For patients with ADHF, univariate analysis showed decreased systolic (OR 0.96; 95% CI 0.92–0.99; p = 0.0) and diastolic (OR 0.914; 95% CI 0.85–0.97) blood pressures as risk factors. However, multivariate analysis showed no variable as an independent risk factor for this patient group (table 5).
Table 5.
Univariate analysis for predictors of AKI in patients with ACS and ADHF
| Predictors | ACS |
ADHF |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (years) | 1.067 | 1.031–1.104 | 0.000 | 1.005 | 0.952–1.061 | 0.859 |
| BMI | 0.962 | 0.865–1.070 | 0.476 | 1.009 | 0.904–1.125 | 0.879 |
| Diabetes mellitus | 1.867 | 0.905–3.849 | 0.091 | 0.594 | 0.182–1.941 | 0.388 |
| History of hypertension | 2.449 | 1.122–5.343 | 0.024 | 0.235 | 0.027–2.082 | 0.193 |
| Chronic kidney disease | 5.384 | 2.352–12.326 | 0.000 | 0.623 | 0.166–2.335 | 0.483 |
| Previous CVA | 2.467 | 0.887–6.856 | 0.083 | 2.833 | 0.305–26.296 | 0.360 |
| Previous MI | 1.883 | 0.906–3.914 | 0.090 | 0.650 | 0.173–2.440 | 0.523 |
| Previous coronary revascularization | 1.905 | 0.704–5.158 | 0.205 | 0.875 | 0.219–3.499 | 0.850 |
| Smoking status | 0.675 | 0.331–1.375 | 0.279 | 1.182 | 0.357–3.908 | 0.784 |
| Systolic blood pressure on admission | 0.985 | 0.964–1.007 | 0.181 | 0.960 | 0.926–0.994 | 0.022 |
| Diastolic blood pressure on admission | 0.974 | 0.939–1.011 | 0.168 | 0.914 | 0.854–0.978 | 0.009 |
| Heart rate (bpm) | 1.004 | 0.973–1.036 | 0.803 | 0.988 | 0.954–1.023 | 0.490 |
| Hemoglobin (g/dl) | 0.751 | 0.628–0.897 | 0.002 | 0.813 | 0.605–1.091 | 0.167 |
| EF (%) | 0.972 | 0.944–1.000 | 0.052 | 1.006 | 0.959–1.055 | 0.810 |
| eGFR (ml/kg/1.73 m2) | 0.966 | 0.953–0.980 | 0.000 | 0.981 | 0.952–1.012 | 0.226 |
CVA = Cerebrovascular accident; EF = ejection fraction. Bold values are statistically significant.
Association between AKI and Outcome
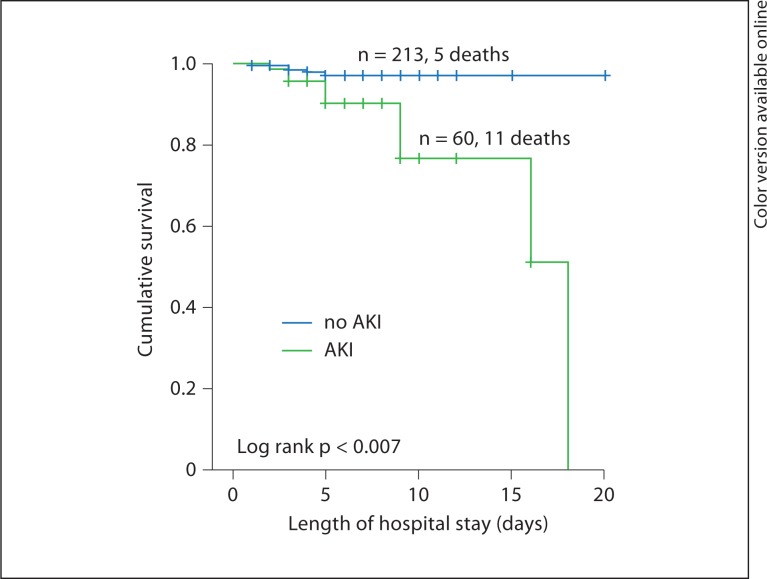
A total of 16 in-hospital deaths (5.5%) occurred. Of these patients, 11 (4.7%) had ACS, and 5 (9.4%) had ADHF. Kaplan-Meier survival curves indicated that patients with both ACS and AKI had a significantly higher in-hospital mortality compared to those without AKI (fig. 1).
Fig. 1.
Kaplan-Meier survival curves for ACS patients with and without AKI.
Ten patients (14.3%) with AKI (5 with ACS and 5 with ADHF) were re-admitted to the hospital within the observation period. Thirty patients without AKI were re-admitted (13.8%). A Cox proportional hazards model showed that AKI was not a significant risk factor for hospital re-admission.
Discussion
In the present study, we found that history of previous myocardial infarction at advanced age (>65 years) and chronic kidney disease and decreased Hb levels in males were independent predictors of AKI in patients with ACS, whereas we did not detect any independent risk factor for AKI in patients with ADHF. Our findings also showed that patients with ACS and AKI had significantly higher in-hospital mortality, but the presence of AKI was not correlated with in-hospital mortality in patients with ADHF. These were the most remarkable differences between the two patient groups regarding AKI predictors and outcomes.
A large number of studies used the term ‘worsening renal function’ to describe changes in renal function occurring after ACS or ADHF, the incidence ranging between 29 and 72% for patients with ADHF [14, 19, 20] and between 11 and 19.5% for patients with ACS [11, 21, 22, 23]. These wide ranges may be attributable to differences in the definitions used to determine worsening renal function and/or ethnic or geographical differences in the selected populations. In the present study, we used the RIFLE and AKIN classifications to detect AKI, which have been reported to have high sensitivity and specificity, while determining different severity levels to predict the prognosis of affected patients [10]. As the reported incidences of worsening renal function in patients with ACS or ADHF lie on relatively broad limits, further studies with a large sample size are needed to clarify what contribution the AKIN criteria for AKI makes to identify patients presenting with CRS. Despite the higher incidence of AKI in patients with ADHF, our results are consistent with those of previous studies.
Many studies have evaluated the association of various predictors with the occurrence of AKI in ACS and ADHF patients, and age, ejection fraction, diabetes, hypertension, and chronic kidney disease have been reported as independent predictors of AKI [19, 22, 24, 25]. Most of these studies are secondary or post hoc analyses from large registry databases or clinical drug therapy trials and include a large number of patients. Our relatively small data set provided consistent results in demonstrating the importance of chronic kidney disease as a predictor of AKI in patients with acute cardiac events.
Several studies have shown that the development of AKI is associated with prolonged hospital stay [14, 20]. Interestingly, we observed that hospital stay was longer in ACS patients than in ADHF patients, a finding that we were unable to compare due to the lack of a similar comparison in the literature. A European prospective heart failure outcome study (POSH) reported that hospital stay was longer in Europe than in the USA [14]. This may result from different management strategies of diverse medical centers.
Observational data from many studies have shown that AKI is associated with an increased risk for a poor outcome. Although a large number of studies focused on the long-term prognosis of AKI patients [22, 23, 25], only a few studies reported on in-hospital mortality [11, 14]. In our retrospective study, we observed that AKI had a marked impact on in-hospital mortality.
Our study has several limitations. The first limitation of the study is that this is a retrospective chart review inevitably involving the limitations of the sample size, unequal distribution, and inability to draw conclusions upon population attributable risk. The second limitation is that the contribution of nephrotoxic drugs to AKI development was not reviewed. Though we adjusted pharmacological treatments according to clinical status, the amount of contrast agent used for coronary angiography was unknown. The third limitation is that this was completely an observational study, and the mechanisms of AKI and the most appropriate treatment options for these high-risk groups were not assessed. Further prospective studies are needed with large patient groups to establish the underlying mechanisms of renal injury and to subsequently analyze whether changes in preventative action and treatment approaches would affect outcomes.
In conclusion, we found that AKI during hospitalization occurred four times more frequently in ADHF patients than in ACS patients, and patients with ACS had increased in-hospital mortality. Although the predominant mechanisms of AKI and its negative effect on the outcome of ADHF and ACS remain to be understood, the syndrome is clearly multifactorial. The presence of preexisting chronic kidney disease may have an impact on the incidence of acute CRS. In the future, the heart-kidney interaction should be managed collaboratively between cardiologists and nephrologists to enhance our knowledge and improve clinical approaches.
References
- 1.Efendigil MC, Harley A, Deegan T, McKendrick CS. Changes in glomerular filtration rate following myocardial infarction. Cardiovasc Res. 1975;9:741–744. doi: 10.1093/cvr/9.6.741. [DOI] [PubMed] [Google Scholar]
- 2.Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, Watnick SG, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 3.Udani SM, Koyner JL. The effects of heart failure on renal function. Cardiol Clin. 2010;28:453–465. doi: 10.1016/j.ccl.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lourenco C, Teixeira R, António N, Monteiro S, Baptista R, Jorge E, et al. Impact of renal function on mortality and incidence of major adverse cardiovascular events following acute coronary syndromes. Rev Port Cardiol. 2010;29:1331–1352. [PubMed] [Google Scholar]
- 5.Ronco C, Ronco F. Cardio-renal syndromes: a systematic approach for consensus definition and classification. Heart Fail Rev. 2011 doi: 10.1007/s10741-010-9224-0. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Cruz DN, Gheorghiade M, Palazzuoli A, Ronco C, Bagshaw SM. Epidemiology and outcome of the cardio-renal syndrome. Heart Fail Rev. 2011;16:531–542. doi: 10.1007/s10741-010-9223-1. [DOI] [PubMed] [Google Scholar]
- 7.Palazzuoli A, Ronco C. Cardio-renal syndrome: an entity cardiologists and nephrologists should be dealing with collegially. Heart Fail Rev. 2011 doi: 10.1007/s10741-011-9267-x. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care; 2004. pp. R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201–208. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, et al. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337. doi: 10.1016/j.ahj.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Marenzi G, Moltrasio M, Assanelli E, Lauri G, Marana I, Grazi M, et al. Impact of cardiac and renal dysfunction on inhospital morbidity and mortality of patients with acute myocardial infarction undergoing primary angioplasty. Am Heart J. 2007;153:755–762. doi: 10.1016/j.ahj.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 14.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B. POSH Investigators: Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction: Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 17.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. National Kidney Foundation. Am J Kidney Dis. 2002;39((2 suppl 1)):S1–S266. [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logeart D, Tabet JY, Hittinger L, Thabut G, Jourdain P, Maison P, Tartiere JM, Solal AC. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–232. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 21.Lazaros G, Tsiachris D, Tousoulis D, Patialiakas A, Dimitriadis K, Roussos D, et al. In-hospital worsening renal function is an independent predictor of one-year mortality in patients with acute myocardial infarction. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.10.024. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, et al. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J. 2010;16:1065–1071. doi: 10.1016/j.ahj.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987–995. doi: 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 24.Mielniczuk LM, Pfeffer MA, Lewis EF, Blazing MA, de Lemos JA, Mohanavelu S, et al. Acute decline in renal function, inflammation, and cardiovascular risk after an acute coronary syndrome. Clin J Am Soc Nephrol. 2009;4:1811–1817. doi: 10.2215/CJN.03510509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]