Abstract
Background/Aims
There are controversial data about renal function following off-pump coronary artery bypass grafting (CABG). The present study aimed to evaluate renal function changes 24 h after on- and off-pump CABG, as well as renal function correlated with high-sensitivity C-reactive protein (hs-CRP) and tumor necrosis factor-α (TNF-α).
Methods
Ninety patients with coronary artery disease referred to our center for CABG from July 2006 to November 2007 were enrolled in the study. Patients were equally and randomly divided in two groups, on- and off-pump. Serum levels of creatinine (Cr), blood urea nitrogen, creatinine clearance (CrCl), hs-CRP, and TNF-α were determined immediately before and 24 h after surgery.
Results
Cr and CrCl changes after surgery were not significantly different between the two groups; however, blood urea nitrogen levels after surgery were significantly higher in the on-pump group (p = 0.035). No statistically significant difference was noted between the two groups in terms of changes in levels of hs-CRP and TNF-α (p = 0.350 and 0.805, respectively). The changes in CrCl levels had no significant correlation with hs-CRP and TNF-α.
Conclusions
The early Cr and CrCl levels after surgery are not significantly different in on- and off-pump groups. The early renal function after on- or off-pump CABG is not correlated with the levels of inflammatory markers including hs-CRP and TNF-α.
Key Words: Coronary artery bypass grafting, Renal function, C-reactive protein, Tumor necrosis factor-α, On-pump, Off-pump, Cardiopulmonary bypass, Cardiac surgery
Introduction
Coronary artery bypass grafting (CABG) is one of the most frequently performed operations worldwide. Since the incidence of coronary artery disease and, subsequently, the number of CABGs performed continue to rise, any improvement in the safety and efficacy of the procedure would have a major impact on the outcomes [1, 2].
Despite advances in methods and technologies, postoperative acute renal insufficiency continues to be a significant cause of morbidity and mortality after cardiac operations performed through the on-pump method using cardiopulmonary bypass (CPB). The etiology of this condition is multifactorial, including the factors related to conduct and management of CABG such as bypass time, use of vasopressors before CABG, systemic inflammatory response, hypoperfusion, and loss of pulsatile perfusion [3]. As CPB has pathophysiologic sequels, there has been a revival of interest in performing CABG on a beating heart (off-pump method) [4]. Proponents of off-pump CABG emphasize decreased incidences of postoperative neurological dysfunction, myocardial infarction, bleeding, renal failure, and respiratory failure [5]; however, controversies exist regarding the changes on renal function following off-pump CABG.
Furthermore, it is known that the CPB and pulmonary-myocardial reperfusion during on-pump CABG activate the innate and adaptive immune systems. The extent of this immune reaction has been linked to postoperative complications seen in this type of surgery [6]. Therefore, the avoidance of CPB and subsequent myocardial ischemia reperfusion has been proposed to significantly reduce postoperative complications, one of which is renal dysfunction [7]. It is known that systemic inflammatory response plays a major role in perioperative complications. Interleukin (IL)-1b, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) are produced in the acute phase of the inflammatory response, and high-sensitivity C-reactive protein (hs-CRP) has been shown to correlate with multi-organ failure [8, 9, 10]. We aimed to investigate the changes in renal function after on- and off-pump CABG and their association with hs-CRP and TNF-α for the first time.
Materials and Methods
Patients and Study Design
This is a prospective case-control study which was conducted in Shahid Madani Hospital of Tabriz University of Medical Sciences in Tabriz, Iran. Data were collected from July 2008 to July 2009.
Different blinded researchers conducted each part of the study including random assignment of the patients to each group (blocked randomization by varying the size of the blocks from 2 to 8), collecting samples, laboratory evaluations, and data analysis. Ninety patients with coronary artery disease referred to our center for CABG were enrolled in the study. All the patients met the criteria for CABG based on the American College of Cardiology/American Heart Association guideline [11]. Afterwards, patients were divided randomly into the on- and off-pump CABG groups, 45 cases in each group. All the surgeries were conducted by the same surgical team.
The study was approved by the Institutional Review Board of Tabriz University of Medical Sciences and was in compliance of the Helsinki Declaration. All patients gave informed consent for participation in the study.
Inclusion criteria included age between 45 and 65 years; no history of neoplastic diseases including benign and malignant tumors; no recent history of steroid or non-steroidal anti-inflammatory drug therapy in the last 2 weeks before surgery; no history of respiratory failure including asthma, adult respiratory distress syndrome, prolonged mechanical ventilation, and acute lung injury; no history of rheumatologic diseases including gout, lupus, dermatomyositis, polymyositis, sclerodermia, rheumatoid arthritis, and osteoarthritis; no history of autoimmune neurologic diseases including myasthenia gravis and multiple sclerosis; no history of chronic hematologic and oncologic disorders including hemolytic anemia and idiopathic and thrombotic thrombocytopenic purpura; no insulin-dependent diabetes; no acute/chronic renal failure, determined by creatinine clearance (CrCl) using Cockroft-Gault equation [12] ≤50 ml/min, and no recent active infectious diseases. Presence of any major complications during 24 h after surgery including cardiac arrest and inability to obtain serum samples were considered as exclusion criteria.
Surgical Management
On-Pump CABG Method
Cefazoline (Kefzol; 1 g) was given for preoperative antibiotic prophylaxis. Anesthesia was induced with etomidate (Amidate; 200–300 mg/kg) and fentanyl (Sublimaze; 20–30 mg/kg). After muscle relaxation with cisatrocurium (Nimbex; 0.15 mg/kg) and endotracheal intubation, anesthesia was maintained using fentanyl (Sublimaze), midazolam (Versed; http://en.wikipedia.org/wiki/Midazolam) and isoflurane (0.4–1.5%).
The CPB equipment included nonpulsatile roller pumps (Stoeckert, Munich, Germany) and membrane oxygenators (Affinity, Avecor Cardiovascular, Plymouth, Mass., USA). The pump was primed with a standard electrolyte solution containing 5,000 IU heparin, 1,000 ml Ringer's lactate, 500 ml NaCl 0.9%, and 250 ml of a 15% mannitol solution (Osmofundin 15% N; Braun Melsungen, Melsungen, Germany). Heparin (300 IU/kg) was administered immediately before vascular cannulation. After the institution of CPB at a flow rate of 2.4–3 l/m2/min, the aorta was cross clamped and a bloody cardioplegic solution was injected. After CPB, protamine sulfate was infused.
Off-Pump CABG Method
The same perioperative management steps described for the on-pump CABG method were applied here; this includes induction of anesthesia, endotracheal intubation, and the maintenance of anesthesia.
Once the pericardium was opened, an initial heparin dose of 2 mg/kg was administered. Intravenous heparin was then used to maintain an activated clotting time of >350 s until the anastomoses were created. An Octopus stabilizer system (Medtronic Inc., Minneapolis, Minn., USA) was used during the operation. After incision of the coronary artery, an intracoronary shunt was inserted and an anastomosis was performed.
Following revascularization, the heparin effect was reversed with protamine sulfate (at a ratio of 1.5:1).
Blood Sampling
Venous blood samples were taken immediately before operation and 24 h after operation. Samples were collected in sterile tubes, centrifuged at 3,000 r.p.m. for 10 min at 4°C, and then stored at −79°C until assayed.
General Parameters
General parameters studied included age; sex; weight; history of disease including hypertension, diabetes, and hyperlipidemia; history of smoking and drug history; family history; the number of involved coronary arteries; graft type, and ejection fraction.
Biochemical Parameters
Serum levels of creatinine (Cr) and blood urea nitrogen (BUN) were measured using an automated chemical analyzer (Abbott analyzer, Abbott laboratories, Abbott Park, North Chicago, Ill., USA) with commercial reagents obtained from Pars Azmoon Laboratories Ltd. (Tehran, Iran). CrCl was determined by using the standard formula – Cockroft-Gault equation [12].
Inflammatory Parameters
Levels of hs-CRP and TNF-α were measured by the ELISA method using commercial kits (hs-CRP by Monobind Inc., Calif., USA, Lot No. EIA-1K2L7, and TNF-α by Human TNF-α ELISA, Bender MedSystems, Vienna, Austria, Lot No. 41881009).
Statistical Analyses
Statistical analyses were performed using the SPSS statistical package version 13.0 (SPSS Inc., Chicago, Ill., USA). The quantitative data are presented as means ± SD, and the qualitative data are expressed as numbers and percentages. The Kolmogorov-Smirnov test was used to assess the normality. Independent sample/paired t tests, and Mann-Whitney U and Wilcoxon signed rank tests were used to assess the differences between stages when applied. Comparison of qualitative data was also performed by χ2 test and correlation assessed using Pearson's correlation test. Also, multivariable analysis using a multiple regression test was carried out to assess effects of the type of surgery and of the changes in levels of hs-CRP and TNF-α on the CrCl changes. A p value <0.05 was considered significant.
Results
The demographic characteristics of patients are shown in table 1. There was no significant difference between the two groups in terms of age, weight, history of smoking, uncontrolled hypertension, diabetes mellitus, hyperlipidemia, left ventricular ejection fraction, number of bypassed arteries, type of graft, and blood/Rho groups. Also, as shown in table 2, there were no significant differences between the two groups in terms of the medications they were on before surgery.
Table 1.
Demographic characteristics of studied population and comparison between the groups
| Type of CABG surgery |
p | ||
|---|---|---|---|
| off-pump (n = 45) | on-pump (n = 45) | ||
| Age, years | 58.08 ± 5.10 | 59.24 ± 4.24 | 0.267 |
| Weight, kg | 77.54 ± 8.74 | 73.17 ± 8.98 | 0.087 |
| Ejection fraction, % | 43.85 ± 9.32 | 45.45 ± 8.27 | 0.423 |
| Smoking | 28 (62.22) | 27 (60) | 0.470 |
| Uncontrolled hypertension | 11 (24.44) | 15 (33.34) | 0.144 |
| Diabetes mellitus | 9 (20) | 9 (20) | 1 |
| Hyperlipidemia | 10 (22.22) | 15 (33.34) | 0.391 |
| Number of grafts one/two/three vessels | 2/15/28 | 0/14/31 | 0.201 |
| Type of graft LIMA/SVG/both | 10/3/32 | 6/0/39 | 0.125 |
| Surgery duration, min | 352.66 ± 37.94 | 331.85 ± 20.11 | 0.176 |
Values are means ± SD or numbers with percentages in parentheses unless otherwise indicated.
LIMA = Left internal mammary artery; SVG = saphenous vein graft.
Table 2.
Medication history of participants
| Drug type | Drug subgroup | Type of CABG surgery |
p | |
|---|---|---|---|---|
| off-pump (n = 45) | on-pump (n = 45) | |||
| ACE-I/ARBs | Captopril (Capoten)/enalapril (Vasotec) Losartan (Cozaar) | 15 (33.34) 3 (6.66) | 18 (40) 5 (11.11) | 0.207 |
| β-Blocker | 36 (80) | 39 (86.66) | 0.248 | |
| Ca channel blocker | Dihydropyridin Non-dihydropyridin | 6 (13.33) 2 (4.44) | 9 (20) 2 (4.44) | 0.729 |
| Digoxin (Lanoxin) | 2 (4.44) | 4 (8.88) | 0.691 | |
| Anticoagulant | Aspirin (USAN) | 30 (66.66) | 32 (71.11) | 0.874 |
| Enoxaparin (Xaparin) | 1 (2.22) | 1 (2.22) | ||
| Diuretic | Thyazids | 0 | 1 (2.22) | 0.486 |
| Furosemide (Lasix) | 0 | 2 (4.44) | ||
| Spironolactone (Aldactone) | 1 (2.22) | 1 (2.22) | ||
| Statins | 38 (84.44) | 30 (66.66) | 0.099 | |
| Nitrates | Nitrocontin | 25 (55.55) | 2 (4.44) | 0.798 |
| Nitroglycerin | 26 (57.77) | 3 (6.66) | ||
Values are numbers with percentages in parentheses.
ACE-I = Angiotensin-converting enzyme inhibitor; Ca = calcium.
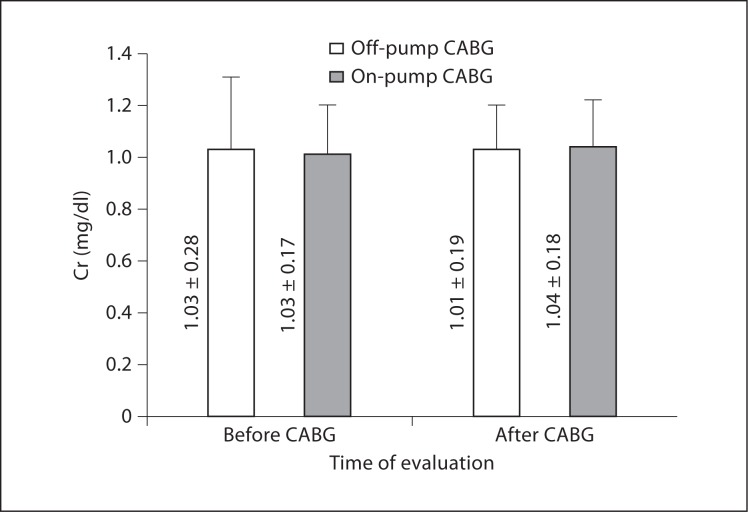
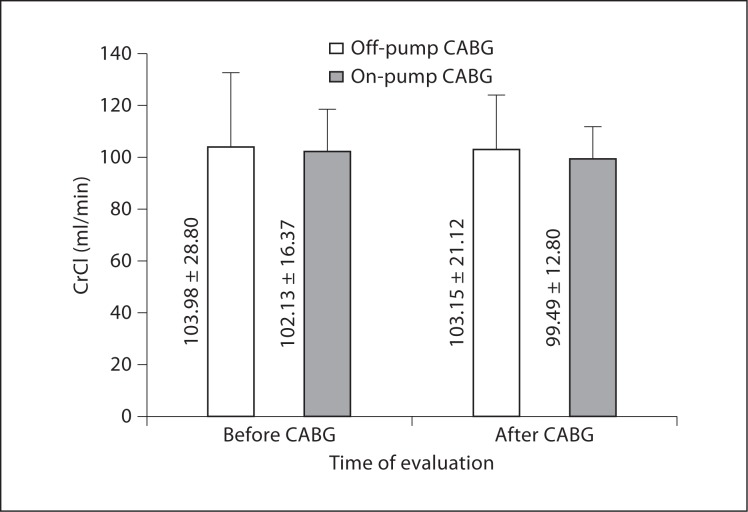
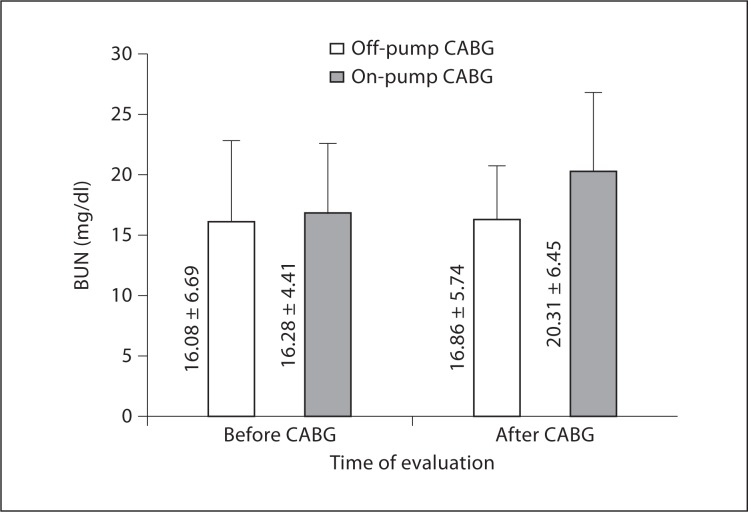
As demonstrated in table 3 and figures 1, 2, and 3, preoperative levels of serum Cr, BUN, and CrCl were not significantly different between off- and on-pump groups. Postoperative levels of Cr and CrCl between the two groups were not significantly different either; however, postoperative BUN levels were significantly higher in the on-pump group compared to the off-pump group (p = 0.035). Intragroup comparison of the off-pump group showed no significant changes in levels of Cr, BUN, and CrCl before and after surgery (p = 0.537, 0.497, and 0.932, respectively). In the on-pump group, although Cr and CrCl levels were not significantly changed (p = 0.934 and 0.480, respectively), BUN levels were significantly increased after surgery compared to before surgery (p = 0.020).
Table 3.
Compression of renal function parameters between on-pump and off-pump methods, and before and after operation
| Type of CABG surgery |
p | |||
|---|---|---|---|---|
| off-pump (n = 45) | on-pump (n = 45) | |||
| Cr, mg/dl | Before | 1.03 ± 0.28 | 1.03 ± 0.17 | 0.918 |
| After | 1.01 ± 0.19 | 1.04 ± 0.18 | 0.772 | |
| ΔCr, mg/dl | −0.02 ± 0.18 | 0.00 ± 0.19 | 0.631 | |
| CrCl, ml/min | Before | 103.98 ± 28.80 | 102.13 ± 16.37 | 0.536 |
| After | 103.15 ± 21.12 | 99.49 ± 12.80 | 0.351 | |
| ΔCrCl, ml/min | −0.35 ± 18.66 | −3.69 ± 19.74 | 0.608 | |
| BUN, mg/dl | Before | 16.08 ± 6.69 | 16.28 ± 4.41 | 0.609 |
| After | 16.86 ± 5.74 | 20.31 ± 6.45 | 0.035 | |
| ΔBUN, mg/dl | 1.00 ± 0.15 | 3.45 ± 8.33 | 0.136 | |
Values are means ± SD.
ΔCr = Cr level after surgery − Cr level before surgery;
ΔCrCl = CrCl level after surgery − CrCl level before surgery;
ΔBUN = BUN level after surgery − BUN level before surgery.
Fig. 1.
Cr levels following CABG.
Fig. 2.
CrCl levels following CABG.
Fig. 3.
BUN levels following CABG.
In both off- and on-pump groups, hs-CRP levels after CABG surgery were significantly increased (p < 0.001 for both); however, the amount of increase (Δhs-CRP = postsurgical hs-CRP – presurgical hs-CRP) was not significantly different in the off-pump group compared to the on-pump group (table 4). Postsurgical levels of TNF-α did not differ significantly in either group compared to the presurgical levels (p = 0.171 and 0.710, respectively); the amount of change in TNF-α level (ΔTNF-α = postsurgical TNF-α – presurgical TNF-α) was not significantly different comparing the two groups either (table 4).
Table 4.
Comparison of hs-CRP and TNF-α levels between on-pump and off-pump groups
| Type of CABG surgery |
p | ||
|---|---|---|---|
| off-pu mp (n = 45) | on-pump (n = 45) | ||
| hs-CRP before surgery, mg/dl | 10.91 ± 14.92 | 10.77 ± 14.77 | 0.967 |
| hs-CRP after surgery, mg/dl | 136.06 ± 88.76 | 116.07 ± 92.97 | 0.338 |
| TNF-α before surgery, pg/ml | 12.63 ± 12.11 | 12.69 ± 11.77 | 0.790 |
| TNF-α after surgery, pg/ml | 14.50 ± 12.50 | 14.25 ± 11.33 | 0.953 |
| Δhs-CRP, mg/dl | 105.15 ± 95.58 | 125.28 ± 91.92 | 0.350 |
| ΔTNF-α, pg/ml | 0.86 ± 5.24 | 1.55 ± 6.58 | 0.805 |
Values are means ± SD.
Also, there was no significant correlation between the level of Cr, BUN, and CrCl changes and Δhs-CRP (p = 0.910, 0.335, and 0.947, respectively) or ΔTNF-α (p = 0.193, 0.450, and 0.081, respectively) in either group.
Hierarchical multiple regression was used to assess the effect of the type of surgery and the amount of change in hs-CRP and TNF-α levels on CrCl after controlling for the influence of demographic characters and lipid profiles. After entry of the type of surgery and changes of hs-CRP and TNF-α levels, the total variance explained by the model as a whole was 51.5% [F (10, 25) = 2.653, p = 0.023]. These factors explained an additional 10.45 of the variance in CrCl changes after controlling for demographic characters and lipid profiles [R2 change = 0.104, F change (3, 25) = 1.789, p = 0.175]. In the final model, only the two control variables significantly affected CrCl changes, with the hypertension history recoding a higher β value (β = −0.545, p = 0.001) than the weight of patients (β = −0.383, p = 0.017).
Discussion
Results of the present study demonstrated that renal function – measured by Cr and CrCl – following CABG was not significantly different in patients undergoing on-pump or off-pump methods. The changes in renal function following both methods were related neither to the levels of hs-CRP nor TNF-α. We were able to successfully control for major confounding factors which could potentially affect the postsurgical renal function including demographic characteristics, concomitant diseases, number of bypassed arteries, duration of surgery, medication history, and preoperative renal function. Furthermore, none of the patients had CrCl <50 ml/min before or after surgery.
Previous studies have compared the degree of renal injury after on- and off-pump procedures. Some of these studies have emphasized biochemical markers of renal injury while others have primarily focused on clinical parameters. In one randomized trial, it was shown that CrCl was significantly higher in the on-pump group intraoperatively, but it deteriorated during the first 48 h postoperatively compared to off-pump patients. In the same study, urinary N-acetyl-b-glucosaminidase (NAG), a marker for renal tubular damage, remained significantly lower in the off-pump group, both during and after surgery [13]. Another study reported increased signs of oxidative stress – as measured by urinary concentrations of hypoxanthine, xanthine, and malondialdehyde – in the on-pump group, while only minimal changes were reported in the off-pump group [4]. A randomized study found that off-pump CABG induced fewer biochemical markers of renal injury including microalbuminuria, free water clearance, NAG levels, and free hemoglobin [14]; however, these parameters were not found to be different between on- and off-pump groups in a different randomized study [15]. Another case-controlled trial found that serum Cr levels were significantly lower and CrCl was significantly higher in the off-pump group compared to the on-pump group [16]. Karthik et al. [17] constructed a propensity score for risk-adjusted comparison of patients undergoing nonelective on- and off-pump CABG. The incidence of renal impairment, as assessed by an increase in Cr level >1.5 mg/ml, was significantly higher in the on-pump group. In a similar analysis of propensity-matched pairs of patients, the rate of renal failure requiring dialysis was 1.5% in the on-pump group versus none in the off-pump group [7]. The incidence of postoperative dialysis in a large cohort of diabetic patients undergoing off-pump CABG was 0.87 versus 2.75% in the on-pump group, while the rate of renal failure not requiring dialysis was similar between the two groups [18]. In a recent meta-analysis, the rate of renal failure was lower in off-pump patients [19]. A recent meta-analysis of randomized and observational studies carried out by Nigwekar et al. [1]showed that patients undergoing off-pump CABG surgery were prone to renal injury and failure despite the different acute kidney injury definitions used in the enrolled studies. As it is evident, the literature reflects controversial evidence regarding renal impairment following on-pump and off-pump surgeries. The results of our study showed no difference in Cr and CrCl levels following on-pump and off-pump techniques; however, BUN levels were higher in the on-pump group, which was associated with using CPB.
It is well recognized that CABG may lead to major organ dysfunction including, but not limited to, renal injury. However, the role of an extracorporeal circulation device in the development of renal injury is not entirely clear. CPB is associated with the development of systemic inflammatory response, endothelial damage, and subsequent tissue edema and organ dysfunction. Interestingly, systemic inflammation is also observed in patients undergoing off-CPB cardiac surgery, albeit on a smaller scale [20]. Although hs-CRP and TNF-α levels were increased after both on- and off-pump surgery in our study, it was statistically significant only in terms of hs-CRP level. More importantly, we found that renal function was related neither to hs-CRP nor TNF-α.
The results of the present study did not show a consistent association between the inflammatory biomarkers and renal function, while prior cross-sectional studies have shown such association. Some studies found associations between renal function and CRP [21], while others revealed a direct relationship between cystatin C and CRP, IL-6, and TNF-α and its two soluble receptors [22]. Considering a significant increase in CRP levels following on-pump CABG, some consequences could be expected including activation of coagulation, down-regulation of physiological anticoagulant mechanisms, and inhibition of fibrinolysis [23, 24], all of which predispose CABG patients to major organ dysfunction such as renal injury. However, during the last decade, the practice of the CPB has benefited from major refinements and improvements in the field of biocompatibility, such as the introduction of warm heart surgery, coated circuits, anti-fibrinolytic drugs, and the elimination of cardiotomy suckers. Warm cardioplegia seems to reduce the oxidative stress [25, 26, 27] and inflammatory response [28, 29].
We acknowledge the limitations inherent in our study including single-sex patient population, small sample size, and short duration of follow-up. We continue to gather data from a larger group of patients for our future studies. Additionally, better renal function biomarkers (e.g. cystatin C) have since been introduced which were not available at the time we conducted our study.
In conclusion, none of our patients in either group developed renal failure, and renal function, determined by early postoperative Cr and CrCl levels, was not different between the on- and off-pump groups. Also, on-pump CABG patients developed mild decrease in renal dysfunction, although within a normal range, and the use of CPB did not result in postoperative CrCl of <50 ml/min after on-pump CABG. These insignificant changes in renal function were independent of inflammatory markers.
Disclosure Statement
The authors have no proprietary interest in any aspect of this study.
Acknowledgements
The present study was supported by the Drug Applied Research Center of Tabriz University of Medical Sciences. The authors thank Ms. Roghaye Rastghoo, Dr. Ali Akbar Abolfathi, Dr. Hassan Argani, Dr. Hossein Babaie, Dr. Behzad Salari, Dr. Alireza Sadighi, Dr. Aimaz Afrough, Dr. Arash Tafrishinejad, Ms. Azra Nezamizadeh, and Ms. Nastaran Ghodratnejad.
References
- 1.Nigwekar SU, Kandula P, Hix JK, Thakar CV. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413–423. doi: 10.1053/j.ajkd.2009.01.267. [DOI] [PubMed] [Google Scholar]
- 2.Yacoub M. Off-pump coronary bypass surgery: in search of an identity. Circulation. 2001;104:1743–1745. [PubMed] [Google Scholar]
- 3.Regragui IA, Izzat MB, Birdi I, Lapsley M, Bryan AJ, Angelini GD. Cardiopulmonary bypass perfusion temperature does not influence perioperative renal function. Ann Thorac Surg. 1995;60:160–164. [PubMed] [Google Scholar]
- 4.Ascione R, Lloyd CT, Underwood MJ, Gomes WJ, Angelini GD. On-pump versus off-pump coronary revascularization: evaluation of renal function. Ann Thorac Surg. 1999;68:493–498. doi: 10.1016/s0003-4975(99)00566-4. [DOI] [PubMed] [Google Scholar]
- 5.Kavarana MN, Asher AS, Barbone A, Williams MR, Faber JM, Weinberg AD, Isidro AB, Oz MC, Esrig BC. A comparison of consecutive off-pump versus conventional coronary artery bypass. Heart Surg Forum. 2001;4:160–165. [PubMed] [Google Scholar]
- 6.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–S720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 7.Sabik JF, Gillinov AM, Blackstone EH, Vacha C, Houghtaling PL, Navia J, Smedira NG, McCarthy PM, Cosgrove DM, Lytle BW. Does off-pump coronary surgery reduce morbidity and mortality? J Thorac Cardiovasc Surg. 2002;124:698–707. doi: 10.1067/mtc.2002.121975. [DOI] [PubMed] [Google Scholar]
- 8.McBride WT, Armstrong MA, Crockard AD, McMurray TJ, Rea JM. Cytokine balance and immunosuppressive changes at cardiac surgery: contrasting response between patients and isolated CPB circuits. Br J Anaesth. 1995;75:724–733. doi: 10.1093/bja/75.6.724. [DOI] [PubMed] [Google Scholar]
- 9.Wei M, Kuukasjarvi P, Laurikka J, Kaukinen S, Iisalo P, Laine S, Laippala P, Metsanoja R, Tarkka M. Cytokine responses and myocardial injury in coronary artery bypass grafting. Scand J Clin Lab Invest. 2001;61:161–166. doi: 10.1080/00365510151097700. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara T, Seo N, Murayama T, Hirata S, Kawahito K, Kawakami M. Transient rise in serum cytokines during coronary artery bypass graft surgery. Eur Cytokine Netw. 1997;8:61–66. [PubMed] [Google Scholar]
- 11.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, Gott JP, Herrmann HC, Marlow RA, Nugent W, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1991 guidelines for coronary artery bypass graft surgery). Circulation. 1999;100:1464–1480. doi: 10.1161/01.cir.100.13.1464. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Potapov EV, Loebe M, Anker S, Stein J, Bondy S, Nasseri BA, Sodian R, Hausmann H, Hetzer R. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart J. 2003;24:1933–1941. doi: 10.1016/j.ehj.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Gerritsen WB, van Boven WJ, Driessen AH, Haas FJ, Aarts LP. Off-pump versus on-pump coronary artery bypass grafting: oxidative stress and renal function. Eur J Cardiothorac Surg. 2001;20:923–929. doi: 10.1016/s1010-7940(01)00941-1. [DOI] [PubMed] [Google Scholar]
- 15.Loef BG, Epema AH, Navis G, Ebels T, van Oeveren W, Henning RH. Off-pump coronary revascularization attenuates transient renal damage compared with on-pump coronary revascularization. Chest. 2002;121:1190–1194. doi: 10.1378/chest.121.4.1190. [DOI] [PubMed] [Google Scholar]
- 16.Tang AT, Knott J, Nanson J, Hsu J, Haw MP, Ohri SK. A prospective randomized study to evaluate the renoprotective action of beating heart coronary surgery in low risk patients. Eur J Cardiothorac Surg. 2002;22:118–123. doi: 10.1016/s1010-7940(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 17.Karthik S, Musleh G, Grayson AD, Keenan DJ, Hasan R, Pullan DM, Dihmis WC, Fabri BM. Effect of avoiding cardiopulmonary bypass in non-elective coronary artery bypass surgery: a propensity score analysis. Eur J Cardiothorac Surg. 2003;24:66–71. doi: 10.1016/s1010-7940(03)00255-0. [DOI] [PubMed] [Google Scholar]
- 18.Magee MJ, Dewey TM, Acuff T, Edgerton JR, Hebeler JF, Prince SL, Mack MJ. Influence of diabetes on mortality and morbidity: off-pump coronary artery bypass grafting versus coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2001;72:776–780. doi: 10.1016/s0003-4975(01)02840-5. discussion 780-771. [DOI] [PubMed] [Google Scholar]
- 19.Reston JT, Tregear SJ, Turkelson CM. Meta-analysis of short-term and mid-term outcomes following off-pump coronary artery bypass grafting. Ann Thorac Surg. 2003;76:1510–1515. doi: 10.1016/s0003-4975(03)01195-0. [DOI] [PubMed] [Google Scholar]
- 20.Asimakopoulos G. Systemic inflammation and cardiac surgery: an update. Perfusion. 2001;16:353–360. doi: 10.1177/026765910101600505. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Katz R, Cushman M, Sarnak MJ, Stehman-Breen C, Psaty BM, Siscovick D, Tracy RP, Newman A, Fried L. Cystatin-C and inflammatory markers in the ambulatory elderly. Am J Med. 2005;118:1416. doi: 10.1016/j.amjmed.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 22.Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, Shlipak MG. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int. 2007;71:239–244. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 23.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 24.Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 25.Morishige N, Tashiro T, Yamada T, Kimura M. Retrograde continuous warm blood cardioplegia reduces oxidative stress during coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2002;8:31–37. [PubMed] [Google Scholar]
- 26.Biagioli B, Borrelli E, Maccherini M, Bellomo G, Lisi G, Giomarelli P, Sani G, Toscano M. Reduction of oxidative stress does not affect recovery of myocardial function: warm continuous versus cold intermittent blood cardioplegia. Heart. 1997;77:465–473. doi: 10.1136/hrt.77.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezzetti A, Calafiore AM, Lapenna D, Deslauriers R, Tian G, Salerno TA, Verna AM, Bosco G, Pierdomenico SD, Caccurullo F. Intermittent antegrade warm cardioplegia reduces oxidative stress and improves metabolism of the ischemic-reperfused human myocardium. J Thorac Cardiovasc Surg. 1995;109:787–795. doi: 10.1016/S0022-5223(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 28.Wan S, Yim AP, Arifi AA, Lee TW, Huynh CH, DeSmet JM, LeClerc JL, Vincent JL. Can cardioplegia management influence cytokine responses during clinical cardiopulmonary bypass? Ann Thorac Cardiovasc Surg. 1999;5:81–85. [PubMed] [Google Scholar]
- 29.Ohata T, Sawa Y, Kadoba K, Taniguchi K, Ichikawa H, Masai T, Shimazaki Y, Matsuda H. Normothermia has beneficial effects in cardiopulmonary bypass attenuating inflammatory reactions. ASAIO J. 1995;41:M288–M291. doi: 10.1097/00002480-199507000-00014. [DOI] [PubMed] [Google Scholar]