Abstract
Background
Despite the introduction of blood donor screening, worldwide, children continue to become infected with HCV via un-sterile medical injections, receipt of unscreened blood and isolated hospital contamination outbreaks. It is plausible that the natural history and disease progression in these children might differ from that of their vertically infected counterparts.
Materials and Methods
Vertically and parenterally HCV infected children were prospectively followed within the European Paediatric HCV Network and the UK National HCV Register respectively. Biological profiles were compared.
Results
Vertically and parenterally HCV infected children differed in terms of some key characteristics including the male:female ratio and the proportion of children receiving therapy. Parenterally infected children were more likely to have at least one hepatomegaly event during follow-up, 20% vs. 10%. Parenteral infection did not significantly affect the odds of being consistently viraemic, AOR 1.14 p=0.703 and there was no significant difference in the odds of having consistently elevated ALT levels and mode of acquisition, AOR 0.83 p=0.748. The proportion of children with 2 or more markers of HCV infection did not differ significantly by mode of acquisition, χ21.13 p=0.288.
Conclusions
This analysis does not support substantial differences between vertically and parenterally infected groups but there are specific mechanisms identified requiring further investigation. Given the continued parenteral infection of children worldwide it is vital that knowledge of disease progression in this group is accurate and that the differences in comparison to vertically infected children are clarified to inform more accurate and individualised clinical management.
Keywords: biological markers, children, hepatitis C virus, mode of acquisition
Introduction
Most available information about paediatric Hepatitis C virus (HCV) infection is derived from a limited number of birth cohorts of vertically infected children (1-6). Parenteral acquisition of HCV infection via contact with contaminated blood or blood products is now rare in many countries following the implementation of donor screening in the early 1990s. However, worldwide, children continue to be infected in this way as a result of un-sterile medical injections, receipt of unscreened blood and via isolated hospital contamination outbreaks (7-11).
Information on the impact of mode of acquisition of paediatric HCV infection on biological markers of infection is limited. It is plausible that given the infection of vertically infected children during early immune maturation, or the possible adaptation of the immune system of the vertically infected children following early exposure in utero, the natural history and disease progression in parenterally infected children acquiring HCV later in childhood might differ from that of their vertically infected counterparts. To inform the management of parenterally HCV infected children worldwide, and in the light of continued outbreaks and diagnoses of new cases, it is important to understand any differences between this group and vertically infected children, upon whom the majority of paediatric HCV guidelines are based.
Using databases from two ongoing prospective cohort studies, we compare biological markers of HCV infection in children to clarify whether routinely collected biological data, which may be useful in predicting future disease outcome, differs by mode of acquisition of infection.
Materials and Methods
Children with vertically acquired HCV infection were identified from the European Paediatric HCV Network (EPHN) and those with parenterally acquired HCV infection were identified from the Health Protection Agency’s UK National HCV Register (2;13). Vertically infected children were prospectively followed from birth according to a study protocol described elsewhere with routine follow-up visits at least every six months (2). Data on parenterally infected children were collected every two years from UK clinicians and follow-up began after notification of the child via one of several surveillance/lookback initiatives which often occurred some years after acquisition of infection. Full details of the study protocol are listed elsewhere (13).
For the purposes of the present study, vertically infected children were those with a positive HCV antibody result at or after 18 months of age and/or two consecutive positive HCV RNA PCR test results at any age who were born to mothers with HCV infection confirmed before or during pregnancy. Parenterally infected children were those who tested positive to HCV antibodies with a known parenteral risk factor for infection and follow-up information available before 18 years of age. HIV/HCV coinfected children were not included. Clearance of viraemia was defined as two consecutive HCV RNA PCR test results following confirmed infection. A sustained virological response (SVR) to treatment was defined by consecutive negative HCV RNA PCR tests 6 months after the cessation of treatment (14).
The estimated date of HCV infection in the parenterally infected study population was the date of transfusion or receipt of blood products. Fifty (40%) children were transfused outside the UK or received multiple transfusions during childhood, and thus the date of infection could not be accurately estimated.
Elevated ALT levels were defined as greater than 60U/L in boys and 50U/L in girls younger than 18 months of age and 40U/L in boys and 35U/L in girls older than 18 months (15).
Statistical analyses
ALT SD z-scores were calculated for HCV infected children using LMS software (LMS 1.22, Institute of Child Health, London) and maximum penalised likelihood methods (16), giving a measure of how far from the reference group each ALT level was, accounting from age at measurement. Comparisons between parenterally and vertically infected children were carried out using Chi-squared tests or Mann Whitney ranksum tests (17). Logistic regression identified factors associated with ALT, PCR and hepatomegaly summary variables. Statistical analyses were performed using Stata software, version 9 (Stata Corporation, Texas, USA).
Results
Data on 395 HCV infected children were available and included 269 vertically infected and 126 parenterally infected children. Seventy-six (60%) parenterally infected children had a known date of infection and the median age at infection for this group was 19 months of age. Follow-up in vertically infected children was more intensive; and the larger number of median follow-up visits is likely a reflection of the different study protocols (2;13).
The male to female sex ratio in the vertically infected children was approximately 1:2, reflecting the increased likelihood of vertical HCV infection in girls compared to boys while the male to female sex ration in the parenterally infected children was approximately 2:1, p<0.001 (Table 1) (18). HCV genotype was recorded for 115/269 (43%) vertically infected children and 50/126 (40%) parenterally infected. No significant differences were found in HCV genotype profiles by mode of acquisition when looking at each genotype individually or genotype 1 in comparison to any other genotype (Table 1).
Table 1. Key characteristics and follow-up profiles of vertically and parenterally HCV infected children.
| Mode of acquisition | |||
|---|---|---|---|
| Vertically infected n=269 (%) |
Parenterally infected n=126 (%) |
Comparison (χ2test or Mann Whitney) |
|
| Sex | |||
| Male | 96 (38.9) | 69 (63.9) | χ 2=18.91, p<0.001 |
| Female | 151 (61.1) | 39 (36.1) | |
| Missing | 22 | ||
| Median age at infection | Birth | 19.13 months (n=76) (range 2.1days-13.5 yrs) |
N/A |
| Known date of infection | |||
| Yes | 269* | 76 (60.3) | N/A |
| No | 0 | 50 (39.7) | |
| Median age at start of follow-up | Birth | 12.2 yrs (range 12.0months- 17.9yrs) |
N/A |
|
Median number of follow-up test dates |
4 (range 1-25) | 2 (range 1-10) | Mann Whitney=11.74, p<0.001 |
| median duration of follow-up | 2.8 yrs (range 1month-17.9yrs) |
2.5 yrs (range 1month-10.0yrs) |
Mann Whitney=2.59, p=0.010 |
| HCV Genotype | |||
| 1 | 55 (47.8) | 29 (58) | χ 2=4.845, p=0.304 |
| 2 | 18 (15.7) | 5 (10) | |
| 3 | 31 (27.0) | 13 (26) | |
| 4 | 11 ( 9.6) | 2 ( 4) | |
| 5 | 0 | 1 ( 2) | |
| Missing | 154 | 76 | |
| Treated | |||
| No | 259 (96.3) | 87 (69.0) | χ 2=58.57, p<0.001 |
| Yes | 10 ( 3.7) | 39 (31.0) | |
| Response to treatment | |||
| No response | 5 (50.0) | 15 (38.5) | χ 2=1.037, p=0.595 |
| Sustained virological response | 3 (30.0) | 15 (38.5) | |
| Unknown | 5 (20.0) | 9 (23.1) | |
All vertically HCV infected children were assumed to have been infected at birth
Ten vertically infected children and 39 parenterally infected children received anti-HCV therapy during follow-up. Nine (90%) vertically infected children received IFN-alpha with the remaining child receiving IFN-alpha with Ribavirin. Eighteen (46%) parenterally infected children received IFN-alpha, 12 (30%) IFN-alpha with Ribavirin and nine (23%) Pegylated IFN alpha with Ribavirin. Parenterally infected children were significantly more likely to have received HCV treatment than vertically infected children (Table 1) and the median age at the start of treatment was 11 years (range 1.9-17.3 years) and 5.7 years (range 2.3-11.7 years) respectively. A lower proportion of children with genotype 1 achieved a SVR (18.2% (2/11)) than children with any other genotype (50% (4/8)) but this difference was not statistically significant, Fisher’s exact p-value=0.319.
Parenterally HCV infected children with a known date of infection began follow-up at an earlier age than those with no known infection date (median age at infection 10.5 yrs and 14 years respectively, p<0.001) and had a longer duration of follow-up (median number of follow-up visits 3 and 2 respectively, p<0.001) but otherwise did not differ in terms of their gender, HCV genotype or treatment profiles.
Clinical signs and symptoms of HCV infection
Parenterally infected children were significantly more likely than vertically infected children to have hepatomegaly reported at least once during follow-up, 25/126 (19.8%) and 27/269 (10.0%) respectively (χ2=7.215, p=0.007). Liver biopsy was carried out on a total of 27/269 (10.0%) vertically infected children and 73/126 (57.9%) parenterally infected children. Similar proportions of vertically and parenterally infected children showed signs of chronic hepatitis, 82% and 67% respectively (χ2=1.973, p>0.10).
HCV RNA PCR
Qualitative HCV RNA PCR was measured for 262 (of 269) vertically infected and 116 (of 126) parenterally infected children, of whom 250 and 68 respectively had at least two PCR results recorded overall. A higher proportion of parenterally infected children had PCR results which were all positive than vertically infected children, 34/68 (50.0%) and 101/250 (40.4%) respectively, but this difference did not reach statistical significance, χ2=2.017, p=0.156. Nearly 60% of both vertically and parenterally infected children were consistently viraemic (75% or more HCV RNA PCR test results positive); 145/250 (58.0%) and 37/68 (54.4%) respectively, χ 2=0.281, p=0.596. Clearance of HCV viraemia was seen in similar proportions of vertically (30%, 74/250) and parenterally infected children (34%, 23/68), z=−0.633, p=0.527.
Logistic regression identified factors associated with being consistently HCV viraemic in 300 children (232 vertically infected and 68 parenterally infected) who had information available on all the variables in Table 2. The odds of being consistently HCV viraemic was significantly associated with a 10 times increase in the odds of having consistently elevated ALT z-scores (Table 2). Although not significantly so, being female, parenterally infected or receiving anti-HCV therapy reduced the odds of being consistently HCV viraemic. In contrast, ever having evidence of hepatomegaly increased the odds of being consistently HCV viraemic but again, not significantly so.
Table 2. Logistic regression of factors associated with being consistently HCV viraemic (n=300).
| Univariable logistic regression OR (95% CI) |
p-value | Multivariable logistic regression* AOR (95% CI) |
p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male (n=128) | 1.00 | 1.00 | ||
| Female (n=172) | 0.97 (0.61-1.55) | 0.912 | 0.88 (0.54-1.44) | 0.615 |
| Mode of acquisition | ||||
| Vertical (n=232) | 1.00 | 1.00 | ||
| Parenteral (n=68) | 0.89 (0.52-1.53) | 0.670 | 1.137 (0.59-2.20) | 0.703 |
| Evidence of hepatomegaly | ||||
| No (n=260) | 1.00 | 1.00 | ||
| Yes (n=40) | 1.50 (0.75-3.00) | 0.255 | 1.20 (0.55-2.65) | 0.646 |
| Ever treated | ||||
| No (n=284) | 1.00 | 1.00 | ||
| Yes (n=34) | 0.65 (0.32-1.32) | 0.232 | 0.67 (0.29-1.55) | 0.349 |
|
Proportion of ALT z-scores above 2SD |
||||
| Less than 75% (n=263) | 1.00 | 1.00 | ||
| 75% or greater (n=37) | 10.58 (3.17-35.31) | <0.001 | 10.28 (3.03-34.85) | <0.001 |
Multivariable regression adjusting for all other factors in the table.
Univariable logistic regression was carried out on the subset of 125 children with genotype information available separately; the odds of being consistently viraemic was higher in children with HCV genotype 1 versus any other genotype but this association just failed to reach statistical significance (unadjusted OR=1.94 (95% CI 0.92-4.08, p=0.082).
ALT levels
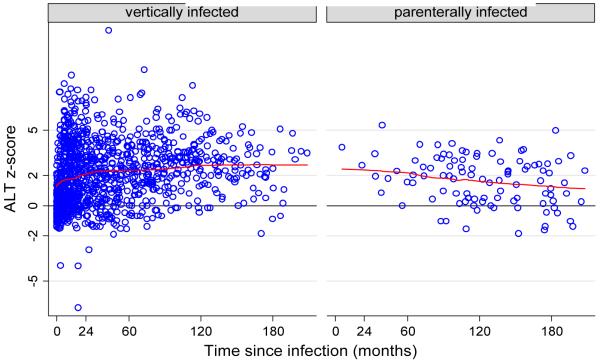
A total of 1404 ALT observations were recorded (range 2-1213 U/l) from 218 vertically infected and 91 parenterally infected children. At least one elevated ALT level was recorded from 137 (62.8%) vertically infected and 53 (58.2%) parenterally infected children (χ2=0.574, p>0.10). In reference to a standard population of HCV uninfected children born to HCV infected mothers, the mean ALT z-score for vertically infected children was 2.049 (SD 2.059) and for parenterally infected children was 1.541 (SD 1.783), t=2.949, p=0.03 (Figure 1). The mean ALT z-score in 247 children infected before 12 months of age (2.033, SD 2.033) was also significantly higher than the mean ALT z-score in the 24 children infected after 12 months of age (1.387 SD 1.652), t=1.887, p=0.030. Among 53 parenterally infected children, there was no difference by age at infection in terms of mean ALT z-score; 1.64 (SD 1.37) in those infected before 12 months of age and 1.63 (SD1.66) in those infected after 12 months of age, t=0.010, p=0.992.
Figure 1. ALT z-score over time since infection in vertically and parenterally infected children.
 Running mean smoother calculating smoothed values for each data point
Running mean smoother calculating smoothed values for each data point
Fifty-one children had consistently elevated ALT z-scores (defined as having 75% or more ALT z-scores 2 SD or above in those children with 2 or more ALT results recorded); 43/204 (21.1%) vertically infected children and 8/39 (20.5%) parenterally infected children. Children who had ever had evidence of hepatomegaly were over three times as likely to have consistently elevated ALT z-scores in multivariable logistic regression and those who were consistently HCV viraemic were over 12 times as likely to also have consistently elevated ALT z-scores (Table 3)
Table 3. Logistic regression of factors associated with having consistently elevated ALT levels (n=205).
| Univariable logistic regression OR (95% CI) |
p-value | Multivariable logistic regression* AOR (95% CI) |
p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male (n=87) | 1.00 | 1.00 | ||
| Female (n=118) | 1.34 (0.68-2.64) | 0.394 | 1.43 (0.67-3.04) | 0.358 |
| Mode of acquisition | ||||
| Vertical (n=175) | 1.00 | 1.00 | ||
| Parenteral (n=30) | 1.31 (0.54-3.18) | 0.614 | 0.83 (0.26-2.61) | 0.748 |
| Evidence of hepatomegaly | ||||
| No (n=174) | 1.00 | 1.00 | ||
| Yes (n=31) | 4.33 (1.94-9.67) | <0.001 | 3.07 (1.25-7.52) | 0.014 |
| Ever treated | ||||
| No (n=187) | 1.00 | 1.00 | ||
| Yes (n=18) | 1.37 (0.46-4.07) | 0.571 | 1.13 (0.28-4.53) | 0.863 |
| Proportion of HCV RNA PCR results positive |
||||
| Less than 75% (n=82) | 1.00 | 1.00 | ||
| 75% or greater (n=123) | 14.15 (4.22-42.51) | <0.001 | 12.69 (3.73-43.17) | <0.001 |
Multivariable logistic regression adjusting for all other variables in table.
In univariable logistic regression only including the 122 children with ALT z-score and genotype information, the odds of having consistently elevated ALT levels was higher in children with HCV genotype 1 versus other genotypes but this association just failed to reach statistical significance (unadjusted OR 2.23, p=0.072).
Fifteen children were consistently HCV viraemic, had consistently elevated ALT z-scores and evidence of hepatomegaly from a total of 295 children with no missing information on any of these variables. The proportion of children with two or more of these three markers of infection was similar in parenterally (15/84, 17.9%) and vertically infected children and also similar in those infected before 12 months of age and after 12 months of age (Table 4). Similar proportions of those parenterally infected before and after 12 months of age were found to have two or more markers of infection. Similar proportions of children receiving anti-HCV and those receiving none had two or more markers of infection and there was no significant difference between the number of children with genotype 1 who had two or more markers of infection and those with any other genotype (Table 4).
Table 4. Characteristics of children with two or more markers of infection (consistently viraemic, consistently elevated ALT z-scores, evidence of hepatomegaly).
| Number of children with two or more markers of infection (%) |
Comparison (χ2, p-value) |
|
|---|---|---|
| Mode of acquisition | ||
| Vertically infected (n=211) | 45 (21.3%) | χ2=-0.67, p=0.504 |
| Parenterally infected (n=84) | 15 (17.9%) | |
| Age at infection | ||
| Younger than 12 months (n=239) | 52 (21.8%) | χ2=1.13, p=0.288 |
| Older than 12 months (n=24) | 3 (14.3%) | |
|
Age at infection – only parenterally infected |
||
| Younger than 12 months (n=28) | 7 (25.0%) | χ2=1.30, p=0.254 |
| Older than 12 months (n=24) | 3 (10.7%) | |
| Treated | ||
| Not treated (n=259) | 51 (19.7%) | χ2=0.55, p=0.458 |
| Treated (n=36) | 9 (25.0%) | |
| Genotype | ||
| Genotype 1 (n=73) | 21 (28.8%) | χ2=2.71, p=0.098 |
| Any other genotype (n=75) | 13 (17.3%) |
Discussion
The impact of mode of acquisition on biological markers of HCV infection in the largest comparison of vertically and parenterally infected children to date was investigated. A significantly higher mean ALT z-score in vertically versus parenterally infected and a significantly higher mean ALT z-score in children infected before 12 months of age, regardless of mode of acquisition, was found. This latter association did not remain when only parenterally infected children were investigated which may be due to small numbers but may also indicate that the differences between groups are not completely explained by age at infection but may be related more directly to the mode of acquisition of infection itself.
Comparing biological markers of HCV infection in vertically and parenterally infected children is problematic in light of the often substantial differences in populations in terms of age at infection, age at study entry, treatment profile, likelihood of clearance of viraemia or genotype. In the children from the cohorts studied here, four times as many parenterally than vertically infected children received HCV treatment; this may be due to more severe disease in parenterally infected children due to their age or mode of acquisition, as suggested by the finding here of a higher proportion with two or more markers of disease progression. Alternatively their older age at diagnosis may make them more eligible for treatment while vertically infected children who were followed from birth, will have started on a regime of clinical monitoring without treatment. Although HCV therapy is well adhered to in the paediatric population the unpleasant side-effects and the potential impact on growth make the decision to treat a complex one (19). There could also be bias in terms of the individual clinic or national treatment policies, especially given the continually evolving nature of information on paediatric HCV treatment and the ongoing debate about when to initiate treatment (20).
This debate on when to initiate treatment is partly fuelled by knowledge that some children spontaneously clear viraemia without treatment. In this study there was no effect of mode of acquisition on clearance of viraemia in contrast to some previous studies (12). However, parenterally infected children in the UK National HCV Register were not followed up from the time of infection and possibly a large number of those who cleared viraemia did so before diagnosis or study entry. Therefore, any estimate of clearance here and elsewhere, likely underestimates the true proportion clearing viraemia in parenterally infected groups. Of note, however, is the high clearance rates in parenterally infected groups reported in this and other studies (9;21;22) which would presumably be even higher if those who cleared viraemia prior to diagnosis could be included. It may therefore be the case that parenterally infected children are more likely to clear the virus than vertically infected children but it is unlikely that this can be substantiated as follow-up from infection in parenterally infected children is rare.
No differences were found in the HCV genotype profiles of vertically and parenterally infected children in contrast to Jara et al who found genotype 1b more frequently in children with transfusion-acquired HCV and genotype 3 more frequently in vertically infected children from seven European countries (23). It is possible that this is due to differences in the years at which infection occurred and may reflect changes in the genotype profile of the HCV epidemic in Europe as suggested recently (24). A higher proportion of children with genotypes other than type 1 achieved a SVR to therapy, as has been reported by other paediatric studies (24). Additionally, in univariable logistic regression, children with genotype 1 were more likely to have consistently elevated ALT levels and consistently positive HCV RNA PCR results, although the associations did not reach statistical significance likely due to small numbers. This finding does however support those of Harris et al who suggested that type 1 infections may be more aggressive than types 2 or 3 (25) and those of a previous EPHN study which found that intrauterine vertical transmission was more likely to occur from mothers with genotype 1 (26). As no differences in the genotype profile of vertically and parenterally infected children were found here, it is unlikely that any differences in biological or clinical markers of HCV infection between groups can be attributed to the possible differences in HCV progression by genotype.
In multivariable logistic regression no association between consistently raised ALT z-scores and mode of acquisition was found, possibly due to a lack of power, although the odds ratio remained below one, indicating higher ALT z-scores in vertically infected children. ALT levels have been shown to peak in the first two years of life in vertically infected children (27-29) and to adjust for this peak and any other differences resulting from age at measurement, ALT z-scores were used. The finding of increased ALT z-scores in vertically infected children adjusted for age is similar to an Australian study in which significantly higher geometric mean ALT levels in 16 vertically versus 15 parenterally infected children in the first five years of life were found, again after accounting for the early peak in ALT levels (12).
Significant positive associations were found between consistently high ALT z-scores and both consistent HCV RNA viraemia and ever having evidence of hepatomegaly. There was also a higher odds of having consistently positive HCV RNA PCRs in children ever having evidence of hepatomegaly but not significantly so. The associations between these three markers of HCV-related disease progression support previous studies indicating that they may define a group of children with evidence of chronic progressive HCV or who are at increased risk of rapid or more severe progression (2). In this analysis, similar proportions of parenterally and vertically infected children had evidence of two or more of these markers of infection. Similarly, no difference was found in the proportion with two or more markers and age at infection, in either all children or just the parenterally infected group. This lack of association with mode of or age at acquisition may have been due to combining the markers of infection into this summary variable and also the small number (15 children) of parenterally and vertically infected children with evidence of two or more markers of infection.
The prevalence of comorbidities in parenterally infected children is high given the nature of their infection during receipt of medical treatment (30) and this may have been influential in terms of the child’s ability to mount an initial or continued immune response to HCV infection. In contrast, vertically infected children, although acquiring infection during immune development may benefit from persistence of maternal antibodies (12;31). These mechanisms require specific investigation and although evidence from this analysis doesn’t support substantial differences between vertically and parenterally infected group, until they can be further defined it is important that the potential differences between are recognised in a clinical setting. This study also highlights the growing need for epidemiological data on parenterally infected children and the difficulties in analysing such data. Recent estimates suggest that 40% of HCV infections worldwide are acquired via unsafe medical injections and a great number of these occur in children. It is therefore vital that knowledge of disease progression in parenterally infected children is accurate and that the differences between vertically and parenterally infected groups is clarified to inform more accurate and individualised clinical management.
Acknowledgements
We would like to thank EPHN collaborators and the HCV National Register Steering Group
EPHN Collaborators: A Amoroso (Università di Trieste, Trieste, Italy), F Asensi-Botet, A Pereda (University Children’s Hospital La Fè, Valencia, Spain), V Balossini, G Bona, M Zaffaroni (Clinica Pediatrica, Università del Piemonte Orientale, Novara, Italy), A Bandelloni, A Coscia, C Fabris, S Aime (Cattedra di Neonatologia, Università di Torino, Torino, Italy), G Bossi (Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy), M Stronati (Neonatal Intensive Care Unit, IRCCS Fondazione Policlinico San Matteo, Pavia, Italy), C Boucher (University Hospital Utrecht, Utrecht, The Netherlands), W Buffolano, G De Luca (Dipartimento di Pediatria, Università Federico II, Napoli, Italy), K Butler (Our Lady Hospital for Sick Children, Crumlin, Dublin, Ireland), L Cabero Roura, JM Bertran Sanges (Hospital Universitari Materno-Infantil, Barcelona, Spain), P Cigna (Centro di Neonatologia, Ospedale Infantile Regina Margherita, Torino, Italy), LM Ciria, C Servera Ginard (Hospital Son Dureta, Palma de Mallorca, Spain), G Claret Teruel, C Fortuny (Hospital Sant Joan de Déu, Barcelona, Spain), O Coll (Hospital Clinic, Barcelona, Spain), A Corrias, R Ledda, S Floris (Servizio di Puericultura, Cagliari, Italy), A De Maria (Dipartimento di Medicina Interna, Università di Genova, Genova, Italy), J Echeverria, G Cilla (Department of Paediatrics and Department of Microbiology, Hospital Donostia, San Sebastian, Spain), G Faldella, E Tridapalli, M Stella, G Capretti (Università di Bologna, Bologna, Italy), B Fischler, A-B Bohlin, S Lindgren, G Lindh (Karolinska University Hospital, Huddinge, Sweden), V Giacomet, V Fabiano, S Stucchi, S Fasan, A Viganò (Ospedale Sacco, Milano, Italy), S Hannam (King’s College Hospital, London, UK), A Hatzakis (National Retrovirus Reference Centre, University of Athens, Athens, Greece), C Inchley, HO Fjaerli (Akershus University Hospital, Norway), A Maccabruni (Department of Infectious Diseases, Università di Pavia, Pavia, Italy), M Marcellini, MR Sartorelli (Ospedale Bambino Gesù, Roma, Italy), P Martin Fontelos (Servicio de Pediatria, Instituto de Salud Carlos III, Madrid, Spain), A Mazza (Ospedale Santa Chiara di Trento, Trento, Italy), JYQ Mok (Royal Hospital for Sick Children, Edinburgh), A Mûr, M Viñolas (Hospital del Mar, Universitat Autonoma de Barcelona, Spain), DM Paternoster, P Grella (Istituto di Ginecologia e Ostetricia, Padova, Italy), S Polywka (Institute for Medical Microbiology and Immunology, University Hospital Eppendorf, Hamburg, Germany), I Quinti, A M Casadei (Università La Sapienza, Roma, Italy), A Rojahn, A Berg (Ullevål University Hospital, Oslo, Norway), R Rosso, E Repetto, C Viscoli (Clinica Malattie Infettive, Università di Genova, Genova, Italy), J Ruiz Contreras, A Manzanares (Hospital 12 de Octubre, Madrid, Spain), A Ruiz Extremera (Hospital Clinico San Ceciliò, Granada, Spain), F Salvini, G V Zuccotti, (Ospedale San Paolo, Milano, Italy), T Schmitz, I Grosch-Wörner, C Feiterna Sperling, T Piening (Charité Virchow-Klinikum, Berlin, Germany), H Souayah, J Levy (Hospital St Pierre, Brussels, Belgium), A Vegnente, R Iorio (Dipartimento di Pediatria, Università Federico II, Napoli, Italy), L Lazier, C Bertaina, E Antonielli d’Oulx, O Delmonte, S Bezzio (Dipartimento di Pediatria, Università di Torino, Torino, Italy), R Wejstal, G Norkrans (Ostra Hospital, Goteborg, Sweden), A Zanetti, E Tanzi (Università di Milano, Milan, Italy)
HCV National Register Steering Group: Graeme Alexander, Brian Gunson, Helen Harris, Julia Heptonstall, Patricia Hewitt, Giorgina Mieli-Vergani, Bernard Portmann, Mary Ramsay, Gerry Robb.
Footnotes
The authors declare no conflicts of interest.
References
- 1.European Paediatric HCV Network. Tovo P-A, Pembrey L, Newell M-L. Persistence rate and progression of vertically acquired hepatitis C infection. J Infect Dis. 2000;181:419–24. doi: 10.1086/315264. [DOI] [PubMed] [Google Scholar]
- 2.European Paediatric HCV Network. Pembrey L, Tovo P-A, Newell M-L. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41(1):45–51. doi: 10.1086/430601. [DOI] [PubMed] [Google Scholar]
- 3.Ferrero S, Lungaro P, Bruzzone BM, Gotta C, Bentivoglio G, Ragni N. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000) Acta Obstet Gynecol Scand. 2003;82(3):229–34. doi: 10.1034/j.1600-0412.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML, Pembrey L. Mother-to-child transmission of hepatitis C virus infection. Drugs Today. 2002;38(5):321–37. doi: 10.1358/dot.2002.38.5.668600. [DOI] [PubMed] [Google Scholar]
- 5.Resti M, Jara P, Hierro L, et al. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. 2003;70(3):373–7. doi: 10.1002/jmv.10405. [DOI] [PubMed] [Google Scholar]
- 6.Seef LB. Natural history of Hepatitis C. Hepatology. 1997;26(3 (suppl 1):21S–8S. doi: 10.1002/hep.510260704. [DOI] [PubMed] [Google Scholar]
- 7.El Raziky MS, El Hawary M, El Koofy N, et al. Hepatitis C virus infection in Egyptian children: single centre experience. J Viral Hepat. 2004;11(5):471–6. doi: 10.1111/j.1365-2893.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 8.Gibb DM, Neave PE, Tookey PA, et al. Active surveillance of hepatitis C infection in the UK and Ireland. Arch Dis Child. 2000;82(4):286–91. doi: 10.1136/adc.82.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Riordan JM, Conroy A, Nourse C, et al. Risk of hepatitis C infection in neonates transfused with blood from donors infected with hepatitis C. Transfus Med. 1998;8(4):303–8. doi: 10.1046/j.1365-3148.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Patel PR, Larson AK, Castel AD, et al. Hepatitis C virus infection from a contaminated radiopharmaceutical used in Myocardial perfusion studies. JAMA. 2006;296(16):2005–11. doi: 10.1001/jama.296.16.2005. [DOI] [PubMed] [Google Scholar]
- 11.Prati D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. J Hepatol. 2006;45(4):607–16. doi: 10.1016/j.jhep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Rerksuppaphol S, Hardikar W, Dore GJ. Long-term outcome of vertically acquired and post-transfusion hepatitis C infection in children. J Gastroenterol Hepatol. 2004;19(12):1357–62. doi: 10.1111/j.1440-1746.2004.03463.x. [DOI] [PubMed] [Google Scholar]
- 13.Harris HE, Ramsay ME, Heptonstall J, Soldan K, Eldridge KP. The HCV National Register: towards informing the natural history of hepatitis C infection in the UK. J Viral Hepat. 2000;7(6):420–7. doi: 10.1046/j.1365-2893.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38(1):75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 15.European Paediatric HCV Network . Age and sex related reference ranges of alanine aminotransferase in children. 2008. In Press ed. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood B, Sterne J. Essential Medical Statistics. 2003. 1992;11(10):1305–19. [Google Scholar]
- 18.European Paediatric HCV Network. Tovo PA, Pembrey L, Newell ML. A significant sex--but not elective cesarean section--effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192(11):1872–9. doi: 10.1086/497695. [DOI] [PubMed] [Google Scholar]
- 19.Fischler B. Hepatitis C virus infection. Semin Fetal Neonatal Med. 2007 doi: 10.1016/j.siny.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Pembrey L, Newell ML, Tovo PA. The management of HCV infected pregnant women and their children European paediatric HCV network. J Hepatol. 2005;43(3):515–25. doi: 10.1016/j.jhep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka S, Tatara K, Hayabuchi Y, Nii M, Mori K, Kuroda Y. Post-transfusion chronic hepatitis C in children. J Paediatr Child Health. 1994;30(6):544–6. doi: 10.1111/j.1440-1754.1994.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 22.Vogt M, Lang T, Frosner G, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341(12):866–70. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 23.Jara P, Resti M, Hierro L, et al. Chronic hepatitis C virus infection in childhood: clinical patterns and evolution in 224 white children. Clin Infect Dis. 2003;36(3):275–80. doi: 10.1086/345908. [DOI] [PubMed] [Google Scholar]
- 24.Bortolotti F, Iorio R, Resti M, et al. Epidemiological profile of 806 Italian children with hepatitis C virus infection over a 15-year period. J Hepatol. 2007;46(5):783–90. doi: 10.1016/j.jhep.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Harris HE, Eldridge KP, Harbour S, Alexander G, Teo CG, Ramsay ME. Does the clinical outcome of hepatitis C infection vary with the infecting hepatitis C virus type? J Viral Hepat. 2007;14(3):213–20. doi: 10.1111/j.1365-2893.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 26.Mok J, Pembrey L, Tovo PA, Newell ML. When does mother to child transmission of hepatitis C virus occur? Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F156–F160. doi: 10.1136/adc.2004.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paccagnini S, Principi N, Massironi E, et al. Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. Pediatr Infect Dis J. 1995;14(3):195–9. doi: 10.1097/00006454-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Palomba E, Manzini P, Fiammengo P, Maderni P, Saracco G, Tovo PA. Natural history of perinatal hepatitis C virus infection. Clin Infect Dis. 1996;23(1):47–50. doi: 10.1093/clinids/23.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Tovo PA, Pembrey LJ, Newell ML. Persistence rate and progression of vertically acquired hepatitis C infection. European Paediatric Hepatitis C Virus Infection. J Infect Dis. 2000;181(2):419–24. doi: 10.1086/315264. [DOI] [PubMed] [Google Scholar]
- 30.Popova IA, Burova NV, Fomin I, Rakhmanova AG, Voronin EE, Galkina MV. The clinical course of HIV infection in children who were parenterally infected. Zh Mikrobiol Epidemiol Immunobiol. 1999;(1):75–8. [PubMed] [Google Scholar]
- 31.Murakami J, Okamoto M, Miyata H, Nagata I, Shiraki K, Hino S. Evolution in the hypervariable region of hepatitis C virus in infants after vertical transmission. Pediatr Res. 2000;48(4):450–6. doi: 10.1203/00006450-200010000-00006. [DOI] [PubMed] [Google Scholar]