Abstract
Objective
To investigate the antibacterial effect of augmenting a biological dressing with polymer films containing silver nanoparticles.
Background
Biological dressings, such as Biobrane, are commonly used for treating partial-thickness wounds and burn injuries. Biological dressings have several advantages over traditional wound dressings. However, as many as 19% of wounds treated with Biobrane become infected, and, once infected, the Biobrane must be removed and a traditional dressing approach should be employed. Silver is a commonly used antimicrobial in wound care products, but current technology uses cytotoxic concentrations of silver in these dressings. We have developed a novel and facile technology that allows immobilization of bioactive molecules on the surfaces of soft materials, demonstrated here by augmentation of Biobrane with nanoparticulate silver. Surfaces modified with nanometer-thick polyelectrolyte multilayers (PEMs) impregnated with silver nanoparticles have been shown previously to result in in vitro antibacterial activity against Staphylococcus epidermidis at loadings of silver that are noncytotoxic.
Methods
We demonstrated that silver-impregnated PEMs can be nondestructively immobilized onto the surface of Biobrane (Biobrane-Ag) and determined the in vitro antibacterial activity of Biobrane-Ag with Staphylococcus aureus. In this study, we used an in vivo wound infection model in mice induced by topical inoculation of S aureus onto full-thickness 6-mm diameter wounds. After 72 hours, bacterial quantification was performed.
Results
Wounds treated with Biobrane-Ag had significantly (P < 0.001) fewer colony-forming units than wounds treated with unmodified Biobrane (more than 4 log10 difference).
Conclusions
The results of our study indicate that immobilizing silver-impregnated PEMs on the wound-contact surface of Biobrane significantly reduces bacterial bioburden in full-thickness murine skin wounds. Further research will investigate whether this construct can be considered for human use.
Keywords: biological dressing, Biobrane, silver nanoparticles, polyelectrolyte multilayers, infection, chronic wound, murine
Approximately 6.5 million people in the United States suffer from chronic wounds,1 whereas an additional 450,000 people each year require medical treatment for burn injuries.2 It is estimated that the financial burden of chronic wound care represents an annual cost of $25 billion in the United States alone.1 Chronic wounds and burn injuries are challenging health care problems, both for the patient and physician. Traditional treatments often involve lengthy hospital stays and prolonged outpatient care with multiple painful dressing changes. The risks of infection and other morbidities are high, and the health care costs are significant.
A wound dressing is considered biological if it has been engineered to provide some component of living tissue to the wound bed, such as growth factors, collagen, or other bioactive molecules. Biological dressings are commonly used to treat both partial-thickness wounds and burn injuries. These dressings have been shown to enhance healing in wounds and substantially reduce the discomfort associated with wound care.3
Biobrane (UDL Labs) is an example of a leading biological dressing. It is commonly used to treat partial-thickness wounds and second-degree burns.4 Biobrane is composed of 2 layers, an outer layer of silicone that provides a protective barrier, and a wound contact layer of nylon fibers embedded in the silicone sheet to which collagen peptides are covalently bonded. Successful application of Biobrane results in a firm bonding of the dressing to the wound bed until epithelialization beneath the dressing leads to its release from the tissues. The advantages of Biobrane in partial-thickness burn injuries are well documented.4–8 Patients whose burns are treated with Biobrane experience less pain, decreased hospital stays, increased comfort, and an earlier return to function compared with patients who receive traditional treatment. Health care costs are also significantly reduced when Biobrane is employed.4–8
One of the major complications with the use of biological dressings is infection of the wound beneath the dressings. Therefore, indications for use of such dressings remain limited when wounds have any significant level of bacterial bioburden. Infection rates of up to 19% have been reported with Biobrane use.9 Once infection occurs, Biobrane must be removed and traditional dressings employed. Therefore, Biobrane can be used only in fresh wounds that have minimal contamination. If the risk of infection under such dressings could be decreased, the proportion converted to traditional treatments could be reduced and the potential applications for biological dressings could be expanded.
Silver has a long history as an effective antimicrobial. The broad-spectrum action of silver has been shown to have effects against methicillin-resistant Staphylococcus aureus10 and fungi,11 in addition to a wide range of bacteria.12–14 Silver sulfadiazine cream (1%) has long been used in clinical practice to prevent and reduce wound infection.15 More recently, many commercial dressings have been introduced that are augmented with silver. However, these dressings contain high loadings of silver (Approximately 100 μg/cm2),16 causing tissue toxicity and impaired wound healing.17–20 In addition, the chemical processing that is required to embed silver ions or nanoparticles into the biological dressings can degrade the biological component of the dressing (eg; collagen) because of the harsh and acidic conditions used during processing.
We have developed a novel method of immobilizing silver nanoparticles into nanometer thick polyelectrolyte multilayers (PEMs), which we have shown previously to result in in vitro antibacterial activity at loadings of silver that are noncytotoxic.19,21 Antibacterial efficacy of silver-nanoparticle PEMs was seen with Staphylococcus epidermidis and Pseudomonas aeruginosa in an in vitro assay where PEMs were transferred to the dermal surface of irradiated human skin (GammaGraft, Promethean LifeSciences, Inc).21 In recent work, we showed that silver-impregnated PEMs transferred onto full-thickness wounds in mice do not impair wound healing in vivo.22
In this article, we investigated the feasibility of transferring silver-nanoparticle PEMs to a biological dressing, using Biobrane as an example (Biobrane-Ag). We then tested the antibacterial efficacy in vitro of Biobrane-Ag against S aureus. To then test the in vivo antibacterial efficacy of Biobrane-Ag, we developed a full-thickness wound infection model in mice using topical application of S aureus. We hypothesized that the use of Biobrane-Ag would significantly decrease the bacterial bioburden present within the wound compared with untreated Biobrane.
METHODS
Fabrication and Characterization of Polymer Films Impregnated With Silver Nanoparticles
Polyelectrolyte multilayers of the oppositely charged polyelectrolytes poly(allylaminehydrochloride) (PAH) (Mw = 70 kDa; Sigma Aldrich, St Louis, MO) and poly(acrylic acid) (PAA) (Mw = 60 kDa; Polysciences, Warrington, PA) were assembled on elastomeric poly(dimethylsiloxane) (PDMS) (Dow Chemical, Midland, MI) sheets by “layer-by-layer” deposition as described in detail elsewhere.21 Briefly, PEMs with the desired number of multilayers were assembled on PDMS sheets using a StratoSequence Robot (nanoStrata Inc, Tallahassee, FL), by sequential incubation in solutions (0.01 M by repeat unit) of PAA (pH 5.5) and PAH (pH 7.5) for 10 minutes each. The formation of the PEMs onto PDMS was initiated by the adsorption of PAH. PDMS sheets were rinsed with deionized water 3 times for 1 minute each after immersion in each polyelectrolyte solution. After assembly, the PEMs were dried in vacuum at 60°C for 1 hour. Number of bilayers (n) fabricated are denoted as (PAH/PAA)n.
Negatively charged, crimson fluorescent (Ext/Em—625/645 nm) carboxylate-modified polystyrene (PS) microspheres, 2 μm in diameter (Cat No. F8816; Invitrogen, Carlsbad, CA), were incorporated into the PEMs as described elsewhere.21 Briefly, PAH(PAA/PAH)10 multilayers deposited on PDMS sheets and terminating in a positively charged PAH layer were incubated with a 1 wt% suspension of microspheres for 2 hours and then were washed 3 times with water. Subsequently, another set of (PAH/PSS)2 multilayers was deposited over the microspheres (PSS = poly(styrenesulfonate), Mw = 70 kDa, Sigma). This was followed by the deposition of a layer of positively charged 20-nm diameter poly(vinylpyrrolidone)-coated silver nanoparticles (NanoAmor Inc, Houston, TX).23–25 Briefly, a 0.2 wt% suspension of nanoparticles in aqueous solution, adjusted to pH 2.0 using dilute nitric acid, was sonicated and vortexed for 30 minutes several times for homogenous suspension. The suspension was then incubated over the PEMs ending with negatively charged PSS for 2 minutes and then rinsed off with water 3 times. To increase the loading of silver in the PEMs, another set of PSS(PAH/PSS)2 multilayer was deposited over the first layer of silver nanoparticles, followed by deposition of the second layer of silver nanoparticles. Finally, the PEMs were capped with a set of (PAA/PAA)2 multilayer.
The final structure of the PEMs is denoted as PAH(PAA/PAH)10(PS-microspheres)(PAH/PSS)2(Ag-NP)PSS (PAH/PSS)2(Ag-NP)(PAA/PAH)2. The incorporation of micro-spheres into the PEMs was quantified by fluorescent imaging. An Olympus IX70 inverted microscope equipped with Chroma (Chroma Technology Corp, Rockingham, VT) fluorescence filter cubes was used to image the fluorescence from fluorescent PS microspheres. Images were captured and analyzed using the Metavue version 7.1.2.0 software package (Molecular Devices, Toronto, Canada).
The loading of silver incorporated into the PEMs was determined by extracting silver from PEMs into diluted nitric acid for 24 hours. The concentration of Ag+ extracted from the PEMs was measured by elemental analysis using an inductively coupled plasma emission spectrometer (ICPES; Perkin Elmer Optima 3000 DV) at the wavelength of 328.068 nm, as described elsewhere.19
Mechanical Transfer of Silver-Nanoparticles–Impregnated PEMs on Biobrane
For transferring the prefabricated PEMs onto Biobrane, PDMS sheets supporting PEMs were placed onto the wound-contact surface of the dressing and pressed with a handheld seam roller (McMaster-Carr, Robbinsville, NJ). A pressure of approximately 200 kPa was applied to the sheets in contact with the dressing for 30 seconds, as described elsewhere.21 Imaging of the fluorescently labeled micro-spheres on the PDMS sheets and the dressing was used to confirm transfer of PEMs onto the dressing quantitatively. Extraction of silver from PEMs loaded onto the dressing was used to quantify the amount of silver transferred to the dressing.
In Vitro Antibacterial Assay
For all in vitro and in vivo studies, a bacterial suspension of S aureus subspecies aureus ATCC 25923 was used. The assay for testing the antibacterial efficacy of silver-nanoparticle PEMs has been described previously.19,21 Briefly, test substrates (Biobrane loaded with PEMs, n = 5; and untreated Biobrane, n = 5) were cut using biopsy punches to fit into the wells of a 96-well PS plate (approximately 6-mm diameter wells), placed at the bottom of the wells, and incubated with 100 μL of phosphate-buffered saline (PBS) buffer containing 107 colony forming units (CFU) of bacteria. The plate was incubated on a shaker at 100 rpm within an incubator at 37°C. After 24 hours of incubation, the supernatants were collected and the wells were rinsed twice using ice-cold PBS and collected with the supernatants for bacterial quantification. Serial dilutions of each sample were plated onto blood agar plates. The plates were incubated overnight at 37°C and colony counts were performed and log-transformed for graphical representation and statistical analysis. Samples from each group were averaged, and statistical analysis was performed using statistical software, SigmaPlot (Systat Software, Inc, San Jose, CA). Groups were compared using a Student t test, and significance was set at a P < 0.05.
In Vivo Studies
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin. Phenotypically normal male mice (heterozygous for Leprdb, Jackson Laboratories, Inc) between the ages of 8 to 12 weeks were used for the studies. Mice were housed in groups during a 1-week acclimation period before the study but housed individually thereafter. Throughout the study period, mice were maintained in a temperature-controlled facility with a standard light/dark cycle. All mice were provided with environmental enrichment and food and water ad libitum. Mice were randomly assigned to either control or experimental groups on the day of surgery, and body weights were recorded.
For wounding, mice were anesthetized with inhaled isoflurane, administered using an induction chamber. The mice were injected subcutaneously with buprenorphine (0.1 mg/kg) for pain control and the cranial thoracodorsal region was shaved and aseptically prepared for surgery. A 6-mm biopsy punch was used to create 2 symmetrical wounds in the cranial dorsal region, one on each side of midline. Mice were recovered from anesthesia on a warming pad. Upon completion of the study, mice were killed by intraperitoneal injection of Beuthanasia-D (Schering-Plough) solution (0.5 mL per mouse) after induction of anesthesia as described earlier.
Wound Infection With S aureus
Ten microliters of either phosphate-buffered saline (controls; n = 4 mice) or the bacterial suspension (2.5 × 105 CFU/cm2; n = 16 mice) were applied topically to the wound and allowed to absorb for 15 minutes. Wounds were left open, and mice were monitored daily for signs of pain and illness. Body weights were obtained on postoperative days 1 and 3, and at the end of the study.
On each of days 1, 2, 3, and 4 postoperatively, 1 control mouse and 4 inoculated mice were killed and the wounds harvested for quantitative bacterial analysis. All wounds were completely excised, to include a deep tissue layer, using aseptic technique with a 6-mm biopsy punch and homogenized for 15 minutes with 1 mL of phosphate-buffered saline. Homogenates were serially diluted and plated on blood agar plates. Plates were incubated overnight at 37ÌC and colony counts were performed.
Bacterial Dose–Response for Wound Infection With Biobrane
A modified splinted full-thickness murine wound model was used for the remainder of the studies (Fig. 1). Silicone O-rings (McMaster-Carr, inner diameter 11 mm, outer diameter 15 mm) were applied to the skin 4-mm caudal to the base of the ears on each side of the dorsal midline and secured with tissue glue (Tissumend II) and six 5–0 interrupted nylon sutures (Fig. 1). O-ring splint application was used to decrease contracture of the wound margins over the course of the study.26 Wounds were centered in the skin circumscribed by the O-ring. Ten microliters of either phosphate-buffered saline (controls; n = 2 mice) or bacterial suspension were applied topically to the wound and allowed to absorb for 15 minutes. Three doses of bacteria were tested (3.7 × 103, 3.7 × 104, and 3.7 × 105 CFU/cm2); each dose was applied to the wounds of 3 mice. Each wound was then covered with an 8-mm disc of Biobrane, which had been sterilized by UV light for 30 minutes. Sterile nonadherent padding was placed on top of the Biobrane, and the entire construct was covered with Tegaderm (3M), secured with tissue glue. Body weights were obtained on postoperative day 1 and at the end of the study. After 72 hours, all mice were killed and all wounds and Biobrane pieces were harvested and processed for bacterial quantification as described earlier.
FIGURE 1.
Murine full-thickness skin wound model. A, O-ring placement on cranial dorsal region; B, Sutures are placed and a 6-mm wound created using a dermal punch, to which a bacterial solution is applied; C, For studies with Biobrane, an 8-mm Biobrane disc was applied to the wound; and D, covered with a nonadherent pad and clear adhesive cover.
Wound Infection With Silver-Impregnated Biobrane
Preparation of silver-nanoparticle PEMs onto the wound contact surface of Biobrane was performed as described earlier. Wounds were created and splinted as described earlier. Ten microliters of bacterial solution (1.8 × 105 CFU/cm2 S aureus) were applied topically to each wound and were allowed to absorb for 15 minutes. Wounds were covered and monitored as described earlier. Control mice (n = 10) received untreated Biobrane and the experimental group (n = 10 mice) received Biobrane-Ag. After 72 hours, all mice were killed and all wounds with the applied Biobrane were harvested and processed for quantitative bacteriology as described earlier.
For all in vivo studies, bacterial quantification (per cm2 wound surface area) was calculated for each wound and log-transformed for graphical presentation and statistical analysis. If a wound yielded zero bacterial growth, a value of 0.1 was assigned to the plate of the highest dilution for analytical purposes. For the initial study, results for the 2 wounds on each mouse were averaged. For the Biobrane studies, wounds from each experimental group were averaged. Statistical analysis was performed for the in vivo Biobrane-Ag study using SigmaPlot and R software (version 2.11.1). Treatment groups were compared using the Mann-Whitney U test and significance was set at P < 0.05. For further analysis, wounds with no bacterial growth were counted in each group and groups were compared using Fisher exact test, with significance set at P < 0.05.
RESULTS
In vitro Studies
PEMs containing fluorescent microspheres and layers of silver nanoparticles were efficiently transferred from the PDMS sheets onto the wound-contact surface of the Biobrane, as shown in fluorescent micrographs (Fig. 2). Multiple applications were used to achieve uniform coating. Quantitatively, the fraction of the fluorescent area transferred from the PDMS sheets onto the Biobrane was 90% ± 3% (averaged over 10 samples).
FIGURE 2.
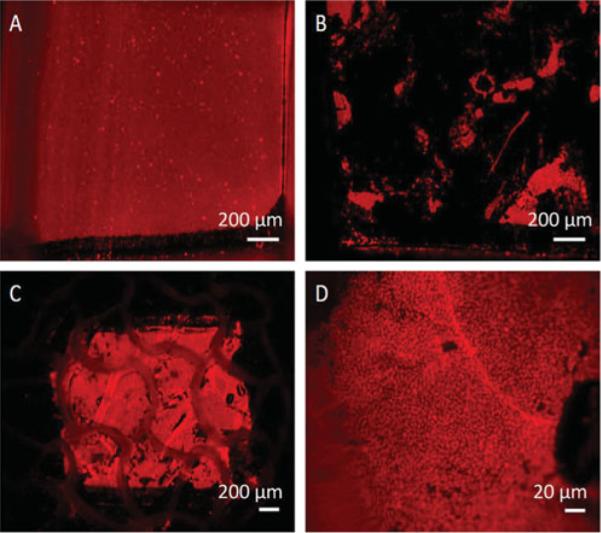
Transfer of fluorescently labeled PEMs from elastomeric sheets onto Biobrane. A, PDMS coated with PEMs containing fluorescently labeled microspheres, scale bar 200 μm; B, PDMS surface after transferring PEMs on to Biobrane, scale bar 200 μm; C, Surface of loaded Biobrane, scale bar 200 μm; D, Biobrane-Ag, magnified (scale bar 20 μm). Note that the pore at the bottom right of the micrograph is a normal feature of Biobrane.
Silver loading in the PEMs was found to be 11.85 ± 2.28 μg/cm2 for Biobrane loaded with PEMs containing one layer of silver nanoparticles, and 20.89 ± 4.57 μg/cm2 for Biobrane loaded with PEMs containing 2 layers of silver nanoparticles (Fig. 7). The modified dressing released less than 1 μg/cm2 of silver each day and provided a sustained release of silver into the aqueous solution for the 10 days that the experiment was performed (Fig. 8). For relevance, it should be noted that commercially available textile-/fabric-based silver dressings such as Acticoat release up to 100 μg/cm2 of silver ions in aqueous solutions in 24 hours.16
FIGURE 7.
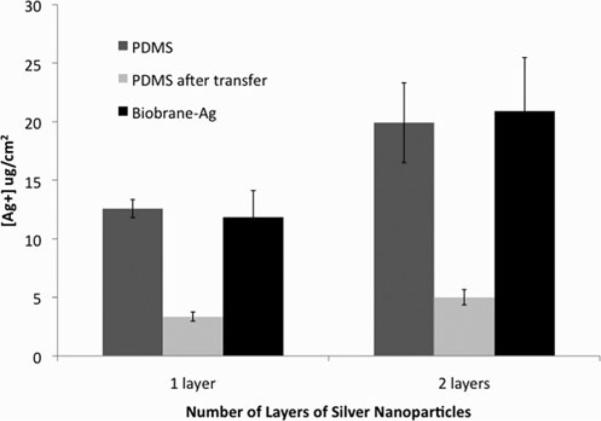
Efficient transfer of silver from PDMS sheets onto the wound-contact surface of the Biobrane was obtained using PEMs containing 1 layer or 2 layers of 20 nm silver nanoparticles, measured by 24 hour extraction silver ions in nitric acid from PDMS sheets before and after transfer, and Biobrane after transfer (Biobrane-Ag).
FIGURE 8.
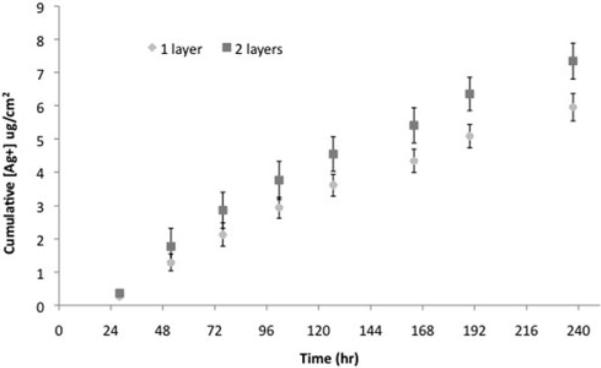
Sustained release of silver ions into milliQ water from the Biobrane loaded with PEMs containing 1 layer or 2 layers of silver nanoparticles, denoted in the graph as 1 layer or 2 layers, respectively. Data are presented as average and standard deviation over 5 samples for each time point.
The in vitro antibacterial assay demonstrated that Biobrane-Ag significantly decreased the concentration of S aureus present, compared to untreated Biobrane (P < 0.001) (Fig. 3). Bacterial numbers were decreased by more than 4 orders of magnitude, equivalent to a 99.99% reduction in total bacteria.
FIGURE 3.
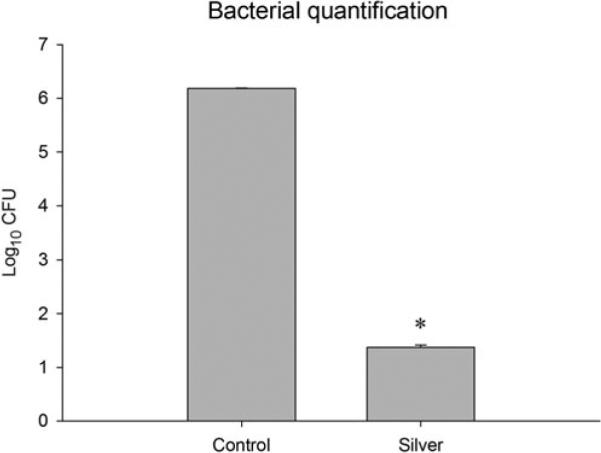
In vitro assay evaluating the effect of silver-nanoparticle PEMs, immobilized on the wound contact surface of Biobrane, on bacterial concentration after 5 hours of incubation. Silver-treated samples had significantly lower concentrations of S aureus compared to controls, P < 0.001.
In Vivo Studies
Wound Infection With S aureus
All mice completed all in vivo studies with no mortality, surgical complications, signs of pain, illness, or significant weight loss. Wounds that were not inoculated with bacteria (control group) had minimal growth of organisms on days 1 and 2 and no growth by days 3 and 4 (Fig. 4). Inoculated wounds yielded an average concentration of 4.7 × 103 CFU/cm2 S aureus on day 1, 7.6 × 104 CFU/cm2 on day 2, 2.1 × 104 CFU/cm2 on day 3, and 1.5 × 102 CFU/cm2 on day 4. Two mice achieved concentrations of bacteria higher than the initial inoculating dose. One inoculated wound evaluated on day 3 had colony-forming units at the highest dilution that were too numerous to count and the wound was not included in data analysis.
FIGURE 4.
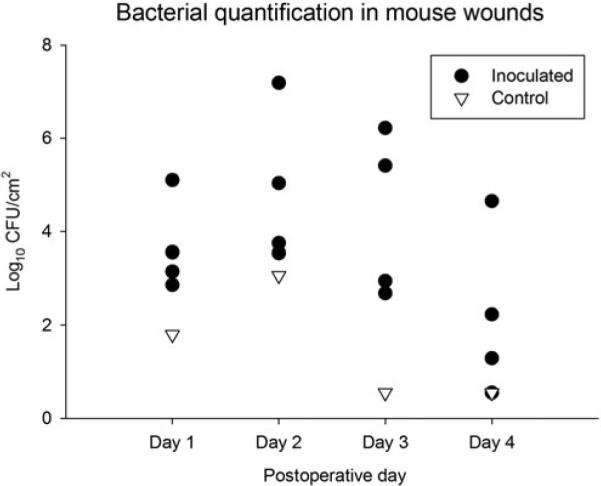
Bacterial colonization of full-thickness wounds after inoculation with S aureus (n = 16 mice). Wounds were inoculated with 2.5 × 105 CFU/cm2 S aureus and left uncovered. Wounds were harvested up to 4 days postinoculation for bacterial quantification. Control mice were inoculated with saline (n = 4 mice). Each data point represents the average of 2 wounds in a single mouse.
Bacterial Dose–Response for Wound Infection With Biobrane
Wounds from all control mice that were not inoculated with bacteria before application of Biobrane yielded zero colony-forming units (data not shown) at the end of 72 hours. The numbers of bacteria recovered from inoculated wounds increased with the inoculating dose and were always higher than the inoculating dose. Wounds inoculated with 3.7 × 103, 3.7 × 104, or 3.7 × 105 CFU/cm2 yielded an average of 1.4 × 105, 2.4 × 106, and 4.7 × 107 CFU/cm2, respectively, at 3 days after inoculation and application of Biobrane (Fig. 5).
FIGURE 5.
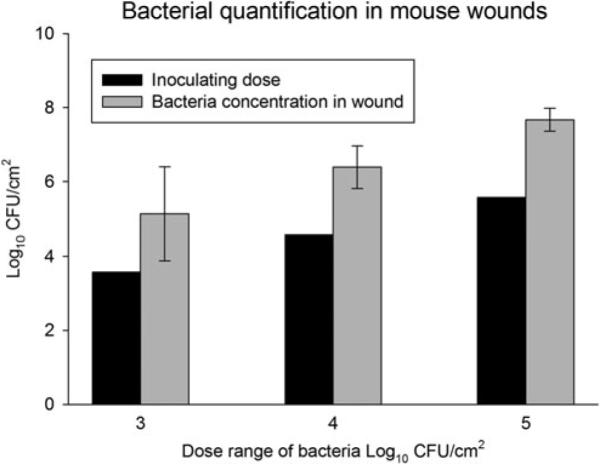
Bacterial dose response for establishing infection under a Biobrane wound dressing in mice. Mouse wounds were inoculated with saline, or 1 of 3 different doses of S aureus followed by covering with Biobrane (n = 11 mice). All wounds were found to have higher numbers of bacteria in the wound at 72 hours than the initial inoculation dose indicating bacterial proliferation under the Biobrane wound dressing. Error bars denote standard error of the mean.
Wound Infection With Silver-Impregnated Biobrane
The Biobrane-Ag used in this experiment was loaded with PEMs containing 2 layers of silver nanoparticles, and released 20.89 ± 4.57 μg/cm2 of silver over 24 hours in dilute nitric acid, as determined by ICPES.
The average number of colony-forming units present in wounds of inoculated mice with untreated Biobrane was 1.3 × 107 CFU/cm2 (Fig. 6). In contrast, treatment of wounds with Biobrane-Ag yielded wounds in which the quantity of bacteria recovered was reduced by approximately 5 orders of magnitude to an average of 7.0 × 102 CFU/cm2 (P < 0.001 vs control wounds).
FIGURE 6.
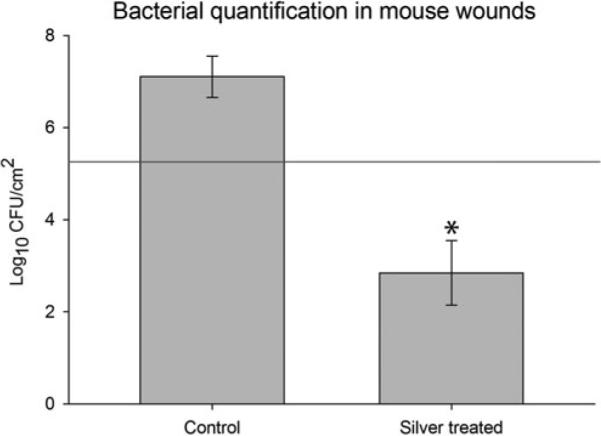
Effect of augmenting Biobrane with silver-nanoparticle PEMs on wound infection in mice inoculated with S aureus. Wounds were inoculated with 1.8 × 105 CFU/cm2 S aureus and covered with either untreated Biobrane (n = 10 mice) or Biobrane augmented with silver-nanoparticle PEMs (n = 10 mice). Augmentation of Biobrane with silver resulted in reductions in bacterial counts to levels significantly less than that of untreated Biobrane, and reaching levels below that considered significant for a clinical wound infection. Error bars denote standard error of the mean. Groups significantly different (Mann-Whitney U test, P < 0.001). Red line indicates initial inoculating dose.
When evaluating elimination of bacteria under the Biobrane wound dressing, only 2 of 20 wounds (10%) in the inoculated control group with untreated Biobrane yielded zero growth upon quantitative bacteriology. In contrast, the number of wounds with zero bacterial growth was significantly higher (P < 0.01) in wounds where Biobrane-Ag was applied (11 of 20 wounds, or 55%, with zero growth).
DISCUSSION
In this study, we have shown that silver-nanoparticle PEMs can be easily transferred to a biological dressing without chemical processing, and without damaging the integrity of the dressing. We have also shown that silver-nanoparticle PEMs exhibit antimicrobial activity in vitro against S aureus when immobilized on the wound contact surface of Biobrane. We have detailed a successful in vivo murine wound infection model, in both uncovered and covered wounds utilizing a biological dressing. When testing silver-nanoparticle PEMs in our in vivo model, we found that immobilization of nanoparticulate silver onto a biological dressing (Biobrane) using a PEM-based technology results in a significant decrease in bacterial bioburden beneath the dressing at 72 hours after application to contaminated wounds.
Utilization of a PEM-based strategy represents a broad technological platform for immobilization of a wide variety of agents onto soft surfaces. Immobilization of agents either directly onto the wound surface or at the interface between a biological dressing and the wound represents a substantial technical advance over current methods. Using contemporary silver-containing wound dressing products as an example, current methods involve deposition of large amounts of silver into the dressing to create a macroscopic reservoir from which silver can diffuse across wound fluids and into the wound bed. These high concentrations of silver in the dressing result in poor control of the amount of silver released into the wound and can cause cellular toxicity.19 In contrast, a PEM-based approach allows precise tuning of the concentration of silver to be released and places it at the site of action, the wound surface, where it can exert its antimicrobial effects at lower nontoxic concentrations. A further advantage of our PEM-based approach for immobilization of silver is that the PEMs can be transferred to a soft surface with no additional chemical processing. Biological dressings have not previously been effectively combined with silver because the harsh chemical processing involved has deleterious effects on the biological components of these dressings. In utilizing PEMs for immobilizing silver on such a dressing, all fabrication of the silver-impregnated PEM film is done separately and then the dressing and PEM are combined into a single construct by gentle pressure. This process does not damage the dressing or its biological properties, while augmenting it with an antibacterial component.
A key observation of this study was that the development of infection in a contaminated wound covered with Biobrane could be suppressed by augmenting the dressing with a silver-impregnated PEM film. This was clearly shown by wounds covered with Biobrane alone that had a quantity of bacteria present in the wound that exceeded the initial inoculum, whereas wounds treated with Biobrane-Ag exhibited a decline in bacterial numbers well below 105 CFU/cm2. In fact, the majority of wounds treated with Biobrane-Ag had no bacteria recoverable within the wound tissue after 72 hours. These outcomes provide strong evidence that support the evaluation of a Biobrane-Ag construct in a clinical situation, where most chronic wounds and many acute partial-thickness wounds would be considered to be “at risk” for nonadherence and infection under a biological dressing.
S aureus is often cited as the most common bacterial species isolated in burns,27–29 chronic wounds,30 and surgical site infections,31 which is why we chose to use it as our inoculating organism. Further studies with Biobrane-Ag need to be conducted using other commonly isolated organisms from burns and chronic wounds, such as Pseudomonas sp.
In this study, 2 wounds of 20 in the control inoculated group had no bacteria recovered. The reason for this could be experimental error, or an indication that the animal's immune system can occasionally clear the infection under the Biobrane. However, this result was in stark contrast to the wounds treated with Biobrane-Ag, where 11 wounds had no bacteria and the remainder had significant reductions in bacterial counts to a level less than what is considered to indicate clinical infection. The difference between the experimental and control groups with respect to the number of wounds with no bacterial growth was significant.
The model used for this study is relevant to clinical use of Biobrane, where recommendations indicate that it takes approximately 48 to 72 hours for Biobrane to adhere to the wound bed.32 If Biobrane does not adhere to the wound bed, infection is likely present.4 The indications for Biobrane, similar to other biologic dressings, are that it should not be applied to wounds colonized with bacteria. Typically, a wound with a significant microbial bioburden would have a concentration of bacteria at the wound surface of more than 105 CFU/g tissue.30,33–36 For Biobrane, concentrations of bacteria at that level will result in nonadherence and clinical infection.37,38 We chose our inoculating dosages of bacteria on the basis of this knowledge and our confirmatory dose–response studies. Our bacterial dose–response experiments showed that the dose of 105 CFU/cm2 resulted in the most consistent infection (least variance) compared to lower dosages.
Although the initial studies in which wounds were left uncovered established colonization of the wound surface, the addition of Biobrane as a wound cover potentiated the infection of contaminated wounds. This was indicated by a decrease in bacterial concentration on the surface of uncovered wounds starting after day 2, which was in contrast to wounds covered with Biobrane where the concentration of bacteria increased over the same time period. This observation in our model mimics problems seen in the clinical situation where it is well known that covering wounds can result in significant wound contamination that negates the benefits seen with use of such dressings.
There are limitations with the experimental model used in this study, compared with wounds generally encountered in the human population. Human wounds exhibit differences in healing characteristics compared with mice, where contraction is a much more significant component of wound healing.39 Thus, we utilized a splinted wound model to minimize the contraction, as has been shown previously,26 and to reduce potential difficulties that could arise with adherence of Biobrane to an aggressively contracting wound. Furthermore, Biobrane is not indicated for full-thickness wounds as was done here. However, the difficulty in consistently creating partial-thickness wounds in mice, combined with our goal to study the impact of a silver-impregnated PEM dressing construct in reducing wound bioburden, led us to conclude that the murine full-thickness skin wound model was warranted for these first proof-of-principle studies. Future studies examining silver nanoparticle PEM augmented dressings in a more clinically relevant partial-thickness wound model, such as a porcine wound model, would be a valuable next step in investigating the use of this technology.
In conclusion, we have shown that infection can be induced in full-thickness wounds in mice with topical application of S aureus and that covering moderately contaminated wounds with a biological dressing increases the severity and duration of the infection. Nanoparticulate silver-impregnated PEMs can be immobilized on the wound contact surface of Biobrane, creating a clinically applicable construct dressing that allows silver nanoparticles' direct contact with the wound bed at concentrations that are not cytotoxic. In our murine wound infection model, covering wounds with this new silver-augmented dressing resulted in a decrease in bacteria to levels below that considered to indicate infection in a clinical situation, and significantly less than that seen with untreated Biobrane. The antibacterial efficacy of Biobrane-Ag, combined with its significantly lower loadings of silver than conventional dressings, makes it a promising potential treatment for use in humans. Further studies are needed to examine the use of Biobrane-Ag in partial-thickness burns and chronic wounds, and to assess the use of this technology of nanoparticulate silver-impregnated PEMs in other biological dressings.
ACKNOWLEDGMENTS
The authors thank Diego F. Calderon, DMV, Nancy G. Faith, BS, and Tyler B. Nelson, BS, for their assistance with this research.
Disclosure: Funding for all experiments was provided by the National Institutes of Health (RC-2 grant). A.A., N.L.A., C.J.M., C.J.C., M.J.S., and J.F.M. are founding members of Imbed Biosciences, Inc.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Burn Association . American Burn Association Fact Sheet. American Burn Association; Chicago IL: 2011. [Google Scholar]
- 3.Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–2424. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood J, Clausen J, Kavanagh S. Experience with biobrane: uses and caveats for success. Eplasty. 2009;9:e25. [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker I, Prowse S, Potokar T. A critical evaluation of the use of Biobrane as a biologic skin substitute: a versatile tool for the plastic and reconstructive surgeon. Ann Plast Surg. 2008;60:333–337. doi: 10.1097/SAP.0b013e31806bf446. [DOI] [PubMed] [Google Scholar]
- 6.Barret J, Dziewulski P, Ramzy P, et al. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg. 2000;105:62–65. doi: 10.1097/00006534-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Bishop J. Pediatric considerations in the use of Biobrane in burn wound management. J Burn Care Res. 1995;16:331–333. doi: 10.1097/00004630-199505000-00022. discussion 333–334. [DOI] [PubMed] [Google Scholar]
- 8.Gerding R, Imbembo A, Fratianne R. Biosynthetic skin substitute vs. 1% silver sulfadiazine for treatment of inpatient partial-thickness thermal burns. J Trauma. 1988;28(8):1265–1269. doi: 10.1097/00005373-198808000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Nichols K, Moaveni Z, Mcewan C, et al. BA01 Audit of Waikato Regional Burns Centre: biobrane infection rates. ANZ J Surg. 2009;79:A7. [Google Scholar]
- 10.Maple PAC, Hamilton-Miller JMT, Brumfitt W. Comparison of the in-vitro activities of the topical antimicrobials azelaic acid, nitrofurazone, silver sulphadiazine and mupirocin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1992;29:661–668. doi: 10.1093/jac/29.6.661. [DOI] [PubMed] [Google Scholar]
- 11.Wright JB, Lam K, Hansen D, et al. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control. 1999;27:344–350. doi: 10.1016/s0196-6553(99)70055-6. [DOI] [PubMed] [Google Scholar]
- 12.Furr JR, Russell AD, Turner TD, et al. Antibacterial activity of Actisorb Plus, Actisorb and silver nitrate. J Hosp Infect. 1994;27:201–208. doi: 10.1016/0195-6701(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 13.Balogh L, Swanson DR, Tomalia DA, et al. Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001;1:18–21. [Google Scholar]
- 14.Ip M, Lui SL, Poon VKM, et al. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol. 2006;55:59–63. doi: 10.1099/jmm.0.46124-0. [DOI] [PubMed] [Google Scholar]
- 15.Zachary L, Heggers J, Robson M, et al. The use of topical antimicrobials combined with Biobrane in burn wound infections. J Trauma. 1982;22:833–836. [PubMed] [Google Scholar]
- 16.Taylor PL, Ussher AL, Burrell RE. Impact of heat on nanocrystalline silver dressings: part I: chemical and biological properties. Biomaterials. 2005;26:7221–7229. doi: 10.1016/j.biomaterials.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 17.Atiyeh BS, Costagliola M, Hayek SN, et al. Effect of silver on burn wound infection control and healing: Review of the literature. Burns. 2007;33:139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Poon VKM, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns. 2004;30:140–147. doi: 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal A, Weis TL, Schurr MJ, et al. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials. 2010;31:680–690. doi: 10.1016/j.biomaterials.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innes ME, Umraw N, Fish JS, et al. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns. 2001;27:621–627. doi: 10.1016/s0305-4179(01)00015-8. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Guthrie KM, Czuprynski CJ, et al. Polymeric multilayers that contain silver nanoparticles can be stamped onto biological tissues to provide antibacterial activity. Adv Funct Mater. 2011;21:1863–1873. doi: 10.1002/adfm.201002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guthrie KMAA, Tackes DS, Johnson KW, et al. Antibacterial Efficacy of Silver-Impregnated Polyelectrolyte Multilayers Immobilized on Biobrane in a Murine Wound Infection Model. The Symposium on Advanced Wound Care and Wound Healing Society; Dallas, TX: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podsiadlo P, Paternel S, Rouillard JM, et al. Layer-by-layer assembly of nacre-like nanostructured composites with antimicrobial properties. Langmuir. 2005;21:11915–11921. doi: 10.1021/la051284+. [DOI] [PubMed] [Google Scholar]
- 24.Sambhy V, MacBride MM, Peterson BR, et al. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. J Am Chem Soc. 2006;128:9798–9808. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]
- 25.Yu DG, Lin WC, Yang MC. Surface modification of poly(L-lactic acid) membrane via layer-by-layer assembly of silver nanoparticle-embedded polyelectrolyte multilayer. Bioconjugate Chem. 2007;18:1521–1529. doi: 10.1021/bc060098s. [DOI] [PubMed] [Google Scholar]
- 26.Galiano R, Michaels V. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 27.Taylor G, Kibsey P, Kirkland T, et al. Predominance of staphylococcal organisms in infections occurring in a burns intensive care unit. Burns. 1992;18:332–335. doi: 10.1016/0305-4179(92)90158-q. [DOI] [PubMed] [Google Scholar]
- 28.Adeniran A, Shakespeare P, Patrick S, et al. Influence of a changed care environment on bacterial colonization of burn wounds. Burns. 1995;21:521–525. doi: 10.1016/0305-4179(95)00034-9. [DOI] [PubMed] [Google Scholar]
- 29.Lesseva M, Hadjiiski O. Staphylococcal infections in the Sofia Burn centre, Bulgaria. Burns. 1996;22:279–282. doi: 10.1016/0305-4179(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 30.Frank DN, Wysocki A, Specht Glick DD, et al. Microbial diversity in chronic open wounds. Wound Repair Regen. 2009;17:163–172. doi: 10.1111/j.1524-475X.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 31.Giacometti A, Cirioni O, Schimizzi A, et al. Epidemiology and microbiology of surgical wound infections. J Clin Microbiol. 2000;38:918–922. doi: 10.1128/jcm.38.2.918-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang E, Eiberg C, Brandis M, et al. Biobrane in the treatment of burn and scald injuries in children. Ann Plast Surg. 2005;55:485–489. doi: 10.1097/01.sap.0000182652.88669.a6. [DOI] [PubMed] [Google Scholar]
- 33.Fonder MA, Lazarus GS, Cowan DA, et al. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 34.Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg. 2006;117:42S–58S. doi: 10.1097/01.prs.0000225432.17501.6c. [DOI] [PubMed] [Google Scholar]
- 35.O'Meara S, Nelson E, Golder S, et al. Systematic review of methods to diagnose infection in foot ulcers in diabetes. Diabet Med. 2006;23:341–347. doi: 10.1111/j.1464-5491.2006.01830.x. [DOI] [PubMed] [Google Scholar]
- 36.Robson M, Mannari R, Smith P, et al. Maintenance of wound bacterial balance. Am J Surg. 1999;178:399–402. doi: 10.1016/s0002-9610(99)00208-1. [DOI] [PubMed] [Google Scholar]
- 37.Lal S, Barrow R, Wolf S, et al. Biobrane (R) improves wound healing in burned children without increased risk of infection. Shock. 2000;14:314–318. doi: 10.1097/00024382-200014030-00013. discussion 318–319. [DOI] [PubMed] [Google Scholar]
- 38.Gonce S, Miskell P, Waymack J. A comparison of Biobrane vs. homograft for coverage of contaminated burn wounds. Burns Incl Therm Inj. 1988;14:409–412. doi: 10.1016/0305-4179(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 39.Greenhalgh D. Models of wound healing. J Burn Care Rehabil. 2005;26(4):293–305. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]



