Abstract
Objective
There has been recent interest in characterizing potential abnormalities of pain processing in patients with sleep disorders such as Restless Legs Syndrome (RLS). The aim of this study was to evaluate psychophysical responses to noxious heat and pressure stimuli in both treated and untreated RLS patients, compared to matched controls.
Methods
This study is a cross-sectional group comparison of RLS patients with matched controls. A total of 31 patients (15 treated, 16 untreated) with a confirmed diagnosis of RLS were compared to 18 controls with no history of RLS or related sleep disorders.
Results
RLS patients (both treated and untreated) demonstrated reduced pain thresholds and reported greater clinical pain relative to controls. Moreover, RLS patients demonstrated enhanced temporal summation of heat pain (p< .05), which may reflect aberrant central nervous system facilitation of pain transmission. Both treated and untreated RLS patients reported disrupted sleep relative to controls, and mediation analyses suggested that the reduced pain thresholds in RLS were attributable to sleep disturbance. However, the effect of RLS on the magnitude of temporal summation of heat pain was independent of sleep disturbance.
Conclusions
These findings suggest that central nervous system pain processing may be amplified in RLS, perhaps partially as a consequence of sleep disruption. RLS patients, even those whose symptoms are managed pharmacologically, may be at elevated long-term risk for the development or maintenance of persistent pain conditions. Further studies in larger samples could help to improve the prospects for pain management in RLS patients.
Introduction
Restless Legs Syndrome (RLS) is a neurological disorder that affects up to 10% of the population (1). Its symptoms show circadian variability, with a worsening in the evening, and include dysesthetic sensations in the legs along with an urge to move (2). Although the pathophysiology of RLS remains incompletely characterized, strong evidence suggests a central role for dopaminergic (3) and opioidergic systems (4). Given that opioids and dopamine play leading roles in central pain-modulatory processes (5–7), it is natural to inquire whether the symptomatology of RLS may include alterations in the perception and experience of pain.
There appears to be significant comorbidity among RLS, fibromyalgia (8), and headache (9), and recent surveys of RLS patients have revealed high rates of moderate to severe pain (10–13). Other work has indicated that the severity of core RLS symptoms correlates with pain severity (12;14), suggesting overlap between manifestations of the disease and pain. Moreover, effective treatment with dopaminergic agonists reduces daily pain complaints among RLS patients (15). While these clinical findings hint that the perception of pain may be amplified in RLS, few laboratory studies have examined responses to standardized stimuli in a controlled environment.
In one such report, RLS patients exhibited profound mechanical hyperalgesia at multiple body sites, which normalized after long-term treatment with dopamine agonists (16). The study’s authors noted that RLS should perhaps be categorized as a disorder of central pain processing as well as a motor and sleep disorder (16), a suggestion echoed by other RLS researchers (3;10;17). In addition, one functional neuroimaging study revealed a dysfunctional pattern of cerebral endogenous opioid binding in RLS patients. These findings, in concert with indications of enhanced spinal reflexes in RLS (18) indicate that RLS may be associated with central sensitization of spinal neurons or reduction in supraspinally-generated pain-inhibition.
In the present investigation, we used quantitative sensory testing (19) to evaluate the pain responses of both treated and untreated RLS patients to a variety of noxious stimuli, compared to matched controls. Prior studies of pain responses in RLS patients have not generally evaluated both treated and untreated participants in order to assess the putative effects of pharmacologic management of RLS symptoms on pain perception. In addition, we assessed whether the qualitative severity of sleep disruption, which is often severe in RLS patients (1;12;20), accounted for any observed group differences in pain responses. Since previous studies have suggested that naturally-occurring sleep disturbance (21;22), or experimental sleep disruption (23;24), results in enhanced pain perception and pain report, this constitutes one apparent mechanism by which RLS may impact the perception of pain.
Materials and Methods
Subject Recruitment and Screening
All subjects provided verbal and written informed consent, and all procedures were approved by the Institutional Review Board. All subjects were screened using medical history and RLS diagnostic questionnaires, as well as the validated Hopkins diagnostic interview for RLS (25), performed by an RLS specialist (RPA). Controls subjects had to have no positive responses to any of the four defining RLS features in order to have a definite NOT-RLS diagnosis. RLS subjects had to have all four defining features of RLS (20) and not have other symptoms or conditions that might mimic RLS in order to have a “definite” RLS diagnosis (25). Any subjects who had chronically painful conditions such as arthritis, neuropathy, or muscle pain were excluded, as were subjects who reported being on analgesic medications (e.g., opiates).
For RLS subjects who were off medication for this study, all centrally active medications, including RLS medications, were withdrawn at least 11 days or 6 drug half-lives (whichever length of time was greater) prior to the study. All but three of the subjects in this group were on dopaminergic agonists for at least 3 months prior to withdrawal. The other three subjects were taking clonazepam (one subject) and gabapentin (two subjects). For RLS subjects who remained on medications, the individual had to have been on the current dose of medication at least 3 months and report satisfaction with treatment of their RLS of 85% or better. The two RLS groups did not differ in their RLS severity based on the Johns Hopkins RLS Severity Scale (26) (see Table 1). This scale queries respondents about RLS symptoms at the time of onset/diagnosis; the fact that no group differences were observed suggests that the treated and untreated patients experienced approximately equivalent levels of initial RLS symptomatology. Control subjects were age-, and gender-matched to RLS cohort and were not on any centrally acting medications.
Table 1.
Laboratory pain and questionnaire response data by participant group.
| Variable | Controls | RLS-Treated | RLS-Untreated |
|---|---|---|---|
| Age | 60.5 ± 8.8 | 63.3 ± 9.4 | 58.0 ± 9.5 |
| % female | 44% | 53% | 50% |
| % white | 89% | 93% | 94% |
| HPTh (arm) (°C) | 46.5 ± 3.9a | 43.7 ± 4.4b | 45.0 ± 2.9ab |
| PPTh-Leg (kPa) | 898.6 ± 326.9a | 687.3 ± 274.5b | 670.0 ± 257.0b |
| PPTh-Thumb (kPa) | 444.0 ± 150.9 | 374.7 ± 164.2 | 358.7 ± 113.8 |
| PPTh-Trapezius (kPa) | 608.4 ± 252.1a | 442.0 ± 149.6b | 468.9 ± 164.7b |
| Cold Pain Ratings (0–100) | 65.4 ± 21.3 | 77.1 ± 17.1 | 70.7 ± 14.8 |
| DNIC Index | 134.4 ± 44.3 | 121.8 ± 20.2 | 123.3 ± 30.2 |
| JHRLSSS | 0.0 ± 0a | 2.1 ± 0.6b | 2.0 ± 0.8b |
| SF-36 BP | 89.7 ± 12.0a | 65.5 ± 21.1b | 73.6 ± 19.8b |
| PSQI | 6.0 ± 3.0a | 12.7 ± 3.9b | 12.9 ± 4.5b |
| BDI | 2.6 ± 3.0a | 9.7 ± 11.1b | 6.5 ± 6.3ab |
Groups with like letters do not differ; groups with differing letters differ at p< .05.
Note: HPTh= Heat Pain Threshold; PPTh= Pressure Pain Threshold, in Kilopascales; DNIC= Diffuse Noxious Inhibitory Controls; JHRLSSS= Johns Hopkins Restless Legs Syndrome Severity Scale; SF-36 BP= Short Form 36, Bodily Pain; PSQI= Pittsburgh Sleep Quality Index; BDI= Beck Depression Inventory;
Session Protocol
The setting for the study was a Clinical Research Center based within a university hospital. Participants arrived between 12:00 and 12:30 pm. Standardized questionnaires included a medical history form, the Beck Depression Inventory (BDI) (27), the Pittsburgh Sleep Quality Index (PSQI) (28), and the SF-36 (29). After a 15-min period of rest, participants underwent the psychophysical pain testing procedures described below.
Psychophysical Pain Testing
Mechanical pain thresholds were assessed first using a digital pressure algometer (Somedic; Sollentuna, Sweden). Pressure pain thresholds (PPThs) were determined twice, bilaterally: the trapezius muscle, the metacarpophalangeal joint of the thumb, and the quadriceps muscle, near the insertion of the proximal patellar tendon. At each site, mechanical force was applied using a 0.5-cm2 probe covered with polypropylene pressure-transducing material; pressure was increased at a steady rate of 30 kPA/s until the subject indicated that the pressure was “first perceived as painful”.
Next, contact heat stimuli were delivered using a Medoc Thermal Sensory Analyzer (TSA-II, Ramat Yishai, Israel) with a 9 cm2 thermode. We first tested heat pain thresholds (HPTh) on the ventral forearm using an ascending method of limits paradigm with a rate of rise of 0.5°C/Sec. Three trials of HPTh were performed, followed by several trials of suprathreshold heat stimulation to assess temporal summation of heat pain. Temporal summation of pain (i.e., the human analog to “wind-up”) is a frequently-used index of central pain facilitation (30–32) which involves rapidly applying a series of identical noxious stimuli and determining the increase in pain across trials. In brief, sequences of 10 rapid heat pulses were applied to the forearm, as in prior studies (33). Within each sequence, the procedure was as follows: from a 38°C baseline temperature, 10 successive heat pulses were delivered. The rate of rise and fall of the thermode temperature was 10°C/sec, and target temperatures were delivered for approximately 0.5 sec each. The thermode remained in a fixed position during administration of the 10 pulses and was then repositioned between sequences, with inter-sequence intervals of 2 min. Two different target temperatures (49°C and 51°C) were used. Subjects verbally rated the painfulness of each heat pulse on a 0–100 (0= “no pain”, 100= “most intense pain imaginable”) numeric rating scale, and then verbally rated the painfulness of after-sensations 15 seconds after the stimuli had ceased (34).
Finally, responses to noxious cold were evaluated using a repeated cold pressor task (CPT), involving immersion of the right hand in a circulating cold water bath maintained at 4°C. The CPT is the most commonly-used method of pain induction in the laboratory and has demonstrated clinical relevance (19). In the present protocol, participants underwent a series of five cold pressor tasks, with the first 4 consisting of serial immersions of the right hand for 30 sec, with 2 min between immersions. The 5th and final CPT involved an immersion of the right hand lasting until a participant reached pain tolerance (or a 3 min maximum). Participants rated the intensity of the cold pain on a 0–100 scale (“no pain” to “most intense pain imaginable”) at the midpoint of each CPT.
During the first 4 cold pressor tasks we also assessed diffuse noxious inhibitory controls (DNIC), a non-invasive test of endogenous pain-inhibitory systems using a heterotopic noxious conditioning stimulation paradigm (35;36). During each CPT, PPTh was assessed on the contralateral trapezius. DNIC was quantified as percent change in PPTh during the cold pressor tasks relative to baseline PPTh, with an increase in PPTh being expected.
Data Analysis
No laterality effects were found for any of the pressure pain thresholds examined. Moreover, heat and pressure pain thresholds were highly correlated (rs ≥ .40). In order to minimize Type I error rates, we derived a Pain Threshold Composite Score by computing and averaging standardized (z) scores for all heat and pressure pain threshold values. This composite value was used in primary and mediational analyses. We used the Bodily Pain subscale of the SF-36 as our index of clinical pain.
The data analysis proceeded in two phases. First, we examined group differences in Pressure and Heat Pain Threshold values, Cold Pain Ratings, and DNIC, as well as SF-36 Bodily Pain, PSQI global sleep quality, and BDI scores, using univariate between-groups (Control, RLS-Untreated, RLS-Treated) Analysis of Variance (ANOVA). Significant omnibus ANOVAs were followed up with planned single-df LSD comparisons. Group differences in the temporal summation of heat pain at 49°C and 51°C were examined using mixed-model ANOVAs with Group as the between-groups factor and Pulse (1–10) as the repeated measures factor. Pain ratings obtained for each pulse served as the dependent variables.
Second, we conducted mediation analyses to examine direct (i.e., main effects) and indirect (i.e., mediation) effects of RLS on pain variables for which significant group effects were observed in univariate analyses. More specifically, we were interested in determining whether differences between RLS patients and controls were attributable to the sleep disturbances experienced by RLS patients. We applied a bootstrapping technique designed to assess simple mediation as has been recommended (37). Conceptually, bootstrapping is a nonparametric approach that has been recommended as a means of reducing the power-limiting effects of asymmetries and non-normality in relatively small samples (37–39). Bootstrapping appears to lower type I error rates relative to other mediation analyses, such as the regression analyses recommended by Baron and Kenny (40).
The bootstrapping approach is completed through taking a large number of samples of size n (where n is the original sample size) from the data, sampling with replacement (and therefore an observation that appears only once in the original data set can appear multiple times in a bootstrapped dataset), and computing the “indirect effect” (i.e., the effect of RLS on pain response via the pathway of sleep disturbance) in each sample. The resulting mean indirect effect was calculated over the bootstrap samples, with an accompanying estimated standard error. For estimation of 95% confidence intervals, we took 1,000 bootstrap samples, as is customary.
The Pain Threshold Composite score served as a dependent variable in mediation analyses. We also computed an index of temporal summation for the 49°C and 51°C heat stimuli; this was done by calculating a standardized residualized change index for each temperature by regressing a subject’s initial pulse rating on their peak rating. These indices represented the degree of summation (controlling for initial ratings) from the initial to the peak pain ratings across the 10 pulses.
Results
A total of 49 participants were tested: 18 healthy controls, 15 RLS patients taking dopaminergic agonists (11/15 were taking pramipexole, 3/15 were taking roprinirole, and 1 patient was taking pergolide), and 16 patients not taking medications for their RLS. Table 1 presents demographic, raw heat and pressure pain threshold values, cold pain ratings, DNIC values, and questionnaire data by Group. Importantly, study groups were well-matched on sex, race, and age [all ps > .20].
Heat and Pressure Pain Threshold and Cold Pain Responses
A significant Group effect emerged for the Pain Threshold Composite Score [F(2,46) = 4.3, p < .05]. Comparisons revealed that RLS-untreated and RLS-treated participants had lower pain thresholds (i.e., they were more pain-sensitive) than controls (ps < .05). Cold pain ratings and the magnitude of DNIC responses did not differ significantly across groups (ps > .1); although the RLS patients tended to have higher pain ratings and lower DNIC values compared to the controls, these differences were quite modest.
Temporal Summation of Heat Pain
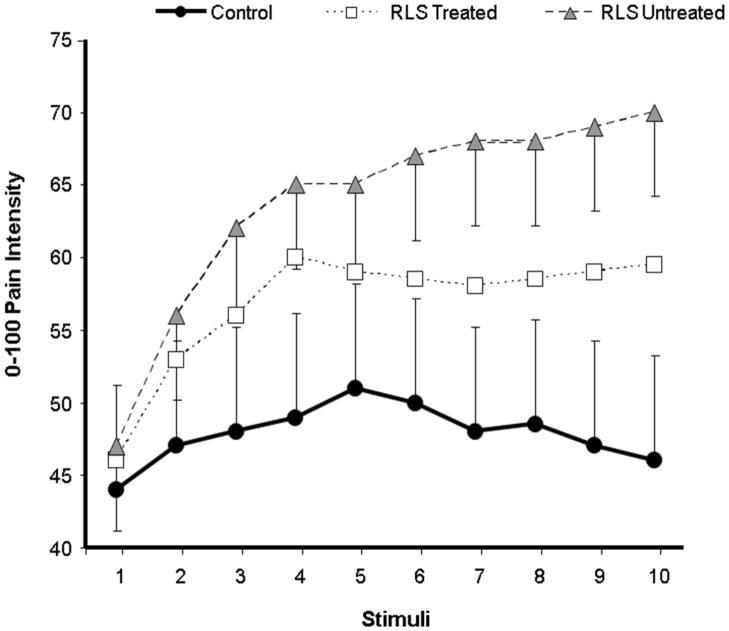
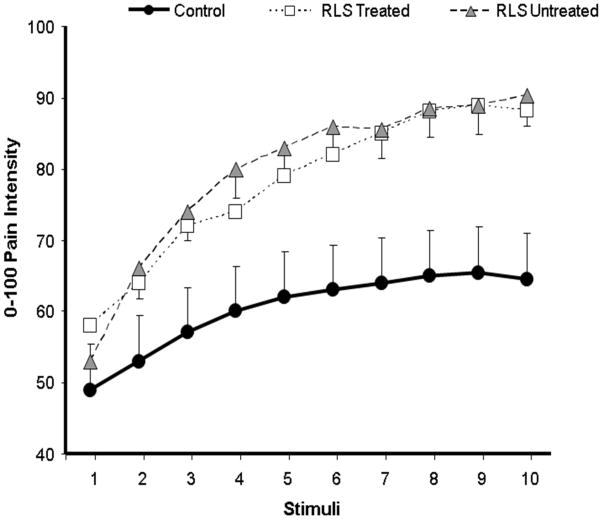
We used mixed-model ANOVAs to evaluate group differences in temporal summation of heat pain. For the sequence of 49°C stimuli, there was a non-significant effect of Group [F(1,48)= 2.2, p= .15] and a significant Group X Pulse interaction effect [F(9,432)=3.6, p= .001], suggesting group differences in the pattern of changes in ratings across the sequence of 10 heat pulses. For the sequence of 51°C stimuli, there was a significant main effect of Group [F(1,48)= 7.2, p= .01] and a Group X Pulse interaction [F(9,432)=3.0, p= .002], suggesting group differences in overall ratings as well as in the summation of pain. See Figures 1 and 2 for a graphical depiction of these data. At 49°C, follow-up simple interaction comparisons indicated that controls differed significantly from RLS-treated patients ( p < .001) but not RLS-untreated patients (p > .10). Although RLS-untreated patients showed greater temporal summation of heat pain at 49°C than RLS-treated patients, this effect did not reach conventional levels of statistical significance (p > .10). Comparisons revealed that the temporal summation of heat pain at 51°C was significantly greater for RLS-untreated and RLS-treated patients relative to controls (ps < .05). RLS-untreated and RLS-treated groups did not differ from one another in temporal summation of heat pain at 51°C (p > .10).
Figure 1.
Ratings of 49°C heat pulses in each group (data presented as Mean ± SEM).
Figure 2.
Ratings of 51°C heat pulses in each group (data presented as Mean ± SEM).
Self-Reported Clinical Pain, Sleep Disturbance and Depressive Symptoms
Omnibus Group effects emerged for SF-36 Pain Subscale, PSQI Global Sleep Disturbance and BDI scores [Fs(2,46) ≥ 3.7, all ps < .05]. Comparisons for the SF-36 Pain Subscale revealed that RLS-untreated and RLS-treated participants reported more clinical pain than controls (ps < .05). On this SF-36 subscale, higher scores reflect less pain, indicating that both groups of RLS patients reported greater day-to-day pain complaints relative to controls. No difference between RLS-untreated and RLS-treated participants was noted (p > .10). Comparisons for the PSQI Global Sleep Disturbance scores revealed that RLS patients had significantly greater sleep disturbance than controls (ps < .001). No difference between RLS-untreated and RLS-treated participants was noted (p > .10). For BDI scores, RLS-untreated and RLS-treated participants reported more depressive symptoms than controls (ps < .05). But RLS groups did not differ from one another(p > .10). Critically, even controlling for BDI scores, PSQI global sleep disturbance scores were greater in RLS patients versus controls [F(2,45) = 13.9, p < .001]. Moreover, group differences in SF-36 Bodily Pain remained significant after adjusting for BDI scores [F(2,45) = 3.3, p < .05].
Mediation Analyses
We conducted three simple bootstrapped mediation analyses. First, we examined whether the RLS effect on pain threshold was direct or indirect via self-reported global sleep disturbance. We then examined whether RLS effects on temporal summation were direct or indirect via self-reported global sleep disturbance. For temporal summation, we analyzed 49°C and 51°C in separate models. Results of the bootstrapped tests of the mediation of RLS on pain responses through self-reported global sleep disturbance are provided in Table 2. With pain threshold composite scores as the DV, the total effect of RLS was significant (p < .05). Moreover, RLS was associated with sleep disturbance (p < .001), which in turn was associated with pain threshold composite scores (p < .05). Of particular note, the direct effect of RLS on pain threshold composite scores was not statistically significant (p = .66). In contrast, there was no evidence for statistical mediation of the RLS-temporal summation relationships by sleep disturbance for either the 49°C or 51°C heat pulses. In both cases, the direct effect of RLS was statistically significant (ps < .05), and the direct effects of sleep disturbance on temporal summation indices were non-significant (ps ≥ .16), precluding evidence for formal statistical mediation (see Table 2). It is important to note that inclusion of BDI scores as a covariate did not alter the pattern of results reported above for the pain threshold composite scores or indices of temporal summation at 49°C and 51°C.
Table 2.
Simple bootstrapped (n=1000) mediation models of direct and indirect (via sleep disruption) effects of Group on pain responses.
| Effect | Estimate | Bootstrap SE |
t | 95% Bias Corrected CI |
|---|---|---|---|---|
| DV: Pain Threshold Composite | ||||
| RLS-to-PSQI (Sleep Disturbance) | 3.5 | .70 | 5.0* | |
| PSQI-to-DV | −.06 | .03 | −2.1* | |
| RLS-to-DV (Total Effect) | −.29 | .14 | −2.0* | |
| RLS-to-DV (Direct Effect) | .08 | .17 | −.45 | |
| RLS-to-DV (Indirect Effect) | −.21 | .10 | a | (LL = −.44; UL = −.04) |
| DV: Temporal Summation (49°C) | ||||
| RLS-to-PSQI (Sleep Disturbance) | 3.5 | .70 | 5.0* | |
| PSQI-to-DV | .03 | .03 | 1.0 | |
| RLS-to-DV (Total Effect) | .57 | .15 | 3.8* | |
| RLS-to-DV (Direct Effect) | .45 | .19 | 2.4* | |
| RLS-to-DV (Indirect Effect) | .11 | .10 | a | (LL = −.07; UL = .34) |
| DV: Temporal Summation (51°C) | ||||
| RLS-to-PSQI (Sleep Disturbance) | 3.5 | .70 | 5.0* | |
| PSQI-to-DV | .05 | .03 | 1.4 | |
| RLS-to-DV (Total Effect) | .57 | .15 | 3.8* | |
| RLS-to-DV (Direct Effect) | .41 | .18 | 2.2* | |
| RLS-to-DV (Indirect Effect) | .16 | .10 | a | (LL = −.01; UL = .38) |
Note. Table shows unstandardized coefficients for the indirect effect of RLS (IV) on pain outcomes (DVs specified within Table) through self-reported global sleep disturbance (PSQI; mediator). PSQI = Pittsburgh Sleep Quality Index; CI = Confidence Interval; LL = lower limit; UL = upper limit.
A p-value for the indirect effect is not provided because this value is depends upon a normal distribution of the indirect effect. Given that indirect effects are positively skewed, interpretation of this p-value is misleading and should thus not be used as a determinant of statistical mediation.
p < .05
Discussion
Alterations in the central processing of pain-related information may be a significant feature of RLS. Patients experience elevated rates of daily pain complaints (10–12), including fibromyalgia (13) and headache (9). In the present study, pain reports on the SF-36 Bodily Pain subscale were higher in both treated and untreated RLS patients compared to controls. In addition, psychophysical testing revealed lower pain thresholds and elevated indices of temporal summation among RLS patients. Temporal summation, an analog of central sensitization, represents an important pathophysiological process that contributes to the development and maintenance of pain states in a number of clinical contexts (41–44). While the temporal summation of pain involves processes at the spinal level, recent functional neuroimaging studies have highlighted the clear contribution of supraspinal processes as well (45–47). Collectively, the modulation of temporal summation appears to involve the activity of descending pain-inhibitory systems, which are known to play crucial roles in pain processing (48). In prior studies, medications such as NMDA antagonists, GABA agonists, and opioids have all been shown to produce analgesic benefits and reduce temporal summation of pain (49–53); future studies may benefit from examination of potential abnormalities in these neurotransmitter systems in RLS patients.
The fact that pain thresholds were reduced in RLS patients at multiple anatomic locations suggests that deficits in central pain inhibition, which have a central etiologic role in persistent pain conditions such as fibromyalgia (54), might be operative among RLS patients. Other psychophysical investigations of pain responses in RLS patients support the conclusion that RLS patients, both primary and secondary, exhibit hyperalgesia to a variety of nociceptive stimuli (55). Interestingly, hypoesthesia to nonpainful thermal stimuli was also observed in the subset of RLS patients with biopsy-confirmed small fiber neuropathy, and, since neuropathic symptoms are prevalent in RLS (56), future studies should consider using a similar methodology in order to subtype patients. Recent studies using electrophysiological methods such as sympathetic skin reflex recording and assessment of cutaneous silent periods have suggested that the functional properties of peripheral nociceptive afferents are normal in RLS patients, indicating that aberrant central nervous system processing of pain may be responsible for the phenomenon of amplified pain sensitivity (57;58). The present finding of generalized hyperalgesia at both lower and upper extremity anatomic sites is also consistent with Stiasny-Kolster and colleagues’ study of eleven untreated RLS subjects (16). That study also demonstrated that long-term (approximately 1 year) treatment with levodopa or cabergoline in a subsample of six subjects was associated with a reversal of hyperalgesia. However, our data suggest that pharmacologically-treated RLS patients demonstrated no observable differences in pain responses relative to the medication-free group. This apparent discrepancy in findings might be explained by differences in type of dopaminergic agents used, variable duration of medication use, or differences in sleep disturbance between the two samples.
The pathophysiology of RLS may include disruption of descending pain-inhibitory dopaminergic and opioidergic pathways (59). Multiple treatment studies have indicated that opioid analgesics are effective in managing RLS symptoms, and one recent neuroimaging study revealed a dysfunction of opioid binding in the brains of RLS patients (10). A still more recent post-mortem immunohistochemistry study identified diminished numbers of beta-endorphin and met-enkephalin positive cells in sensory pathways in RLS patients (60), suggesting that information regarding painful stimuli in RLS patients might be altered at the thalamic level because of a relative deficit in endogenous opioid inhibition of ascending input. Though this explanation is attractive, it remains to be determined why no group differences in DNIC were observed in the present study since previous research has demonstrated that DNIC involves at least partial mediation of its descending inhibitory effects by endogenous opioids (61) and prior work has demonstrated that sleep disruption in both healthy controls (35) and chronic pain patients (62) is associated with diminished pain-inhibitory capacity. It may be that putative deficits in opioid-mediated pain-inhibitory processes in RLS patients are specific to certain pathways or that deficient inhibition and aberrant sensitization take place largely in the spinal cord in RLS, rather than at supraspinal levels. However, the failure to find statistically significant DNIC effects might also be due to inadequate statistical power in this study, and future studies in this area may help to answer some of these open questions. Alternatively, our comparison of DNIC across groups may have been confounded by group differences in blood pressure. Recent evidence suggests that RLS patients demonstrate elevations in blood pressure, and a propensity to hypertension (63;64); since prior studies have related increased cardiovascular reactivity to enhanced DNIC (65), it is possible that any RLS-related decrements in DNIC were “masked” in this study by larger blood pressure increases during the cold pressor tasks. Future work in this area might benefit from alternative assessments of DNIC that do not involve pressor responses.
Interestingly, treated and untreated RLS patients were virtually indistinguishable in their pain responses as well as in their reports of sleep disruption and fatigue. This suggests the possibility that pharmacologically ameliorating core RLS symptoms of lower extremity restlessness may not improve associated symptomatology (e.g., insomnia, pain). Moreover, the present findings suggest that some of the observed hyperalgesia in RLS is attributable to ongoing sleep disturbance. This is an important and novel finding, as prior studies of pain perception in RLS have not evaluated sleep disruption as a potential contributing factor to any observed abnormalities. Recent reports have tied naturally-occurring disturbances of sleep to augmented pain perception and pain report in the general population (22;66), among fibromyalgia patients (21), and in the context of many other persistent pain conditions such as osteoarthritis (67) and orofacial pain (62). We (along with other researchers in this area) have also hypothesized that sleep disruption produces maladaptive effects on central pain-modulatory systems (35;68). The findings of the present study indicate that disturbances of sleep are a contributing factor to the widespread reduction of pain thresholds that have been observed in samples of RLS patients. The enhanced temporal summation of pain displayed by both groups of RLS patients, however, was independent of sleep quality and may represent a core pathophysiologic process of RLS.
Some important limitations of this study will need to be addressed in future research. First, we assessed pain responses at a particular time of day, whereas a circadian disorder such as RLS might be most productively studied by repeating the assessment procedures across a 24-hour period. In addition, in future work, we will test both “affected” (e.g., the lower leg) and “unaffected” sites in RLS patients in order to evaluate whether disease-related peripheral sensitization might play a role in amplifying local pain responses. Second, while we included both a treated and untreated RLS group, we were not able to prospectively examine changes in pain responses over the course of a pharmacologic treatment regimen. Hence, this cross-sectional study does not have the capacity to determine the causal links between RLS disease processes and aberrant central processing of pain. Third, the present study relied on self-report of pain, sleep, and other constructs of interest. In future work, we plan to incorporate objective measures of sleep parameters (e.g., using actigraphy or polysomnography) and pain responses (e.g., functional neuroimaging of brain responses to painful stimulation). In spite of these limitations, this study highlights the potential importance of altered pain responses in patients with RLS. Converging evidence points to pathophysiological processes in RLS (e.g., alterations in central opioid and dopaminergic function and disruption of sleep) that appear to impact the processing of pain-related information in the central nervous system. RLS patients may be at elevated long-term risk for acute and persistent pain syndromes, and further studies in this area may help to improve the prospects for pain management in RLS patients.
Acknowledgments
Funding: This work was supported by National Institutes of Health Grant K23 AR051315 (to RRE), by the Johns Hopkins General Clinical Research Center (M01-RR002719) and National Institutes of Health Grants R01 AR05487, P01 AG21190 (to CJE), and R01 NS42857 (to CJE).
Footnotes
COI: None of the authors have any competing interests, or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gamaldo CE, Earley CJ. Restless legs syndrome: a clinical update. Chest. 2006;130(5):1596–1604. doi: 10.1378/chest.130.5.1596. [DOI] [PubMed] [Google Scholar]
- 2.Lesage S, Earley CJ. Restless Legs Syndrome. Curr Treat Options Neurol. 2004;6(3):209–219. doi: 10.1007/s11940-004-0013-8. [DOI] [PubMed] [Google Scholar]
- 3.Trenkwalder C, Paulus W. Why do restless legs occur at rest?--pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2) Clin Neurophysiol. 2004;115(9):1975–1988. doi: 10.1016/j.clinph.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Zucconi M, Manconi M, Ferini SL. Aetiopathogenesis of restless legs syndrome. Neurol Sci. 2007;28 (Suppl 1):S47–S52. doi: 10.1007/s10072-007-0737-9. [DOI] [PubMed] [Google Scholar]
- 5.Potvin S, Grignon S, Marchand S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse. 2009;63(5):390–402. doi: 10.1002/syn.20616. [DOI] [PubMed] [Google Scholar]
- 6.Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, et al. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol. 2004;500(1–3):187–192. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8(5):781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 8.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21(3):481–497. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 9.d’Onofrio F, Bussone G, Cologno D, Petretta V, Buzzi MG, Tedeschi G, et al. Restless legs syndrome and primary headaches: a clinical study. Neurol Sci. 2008;29 (Suppl 1):S169–S172. doi: 10.1007/s10072-008-0916-3. [DOI] [PubMed] [Google Scholar]
- 10.von Spiczak S, Whone AL, Hammers A, Asselin MC, Turkheimer F, Tings T, et al. The role of opioids in restless legs syndrome: an [11C]diprenorphine PET study. Brain. 2005;128(Pt 4):906–917. doi: 10.1093/brain/awh441. [DOI] [PubMed] [Google Scholar]
- 11.Leutgeb U, Martus P. Regular intake of non-opioid analgesics is associated with an increased risk of restless legs syndrome in patients maintained on antidepressants. Eur J Med Res. 2002;7(8):368–378. [PubMed] [Google Scholar]
- 12.Abetz L, Allen R, Follet A, Washburn T, Earley C, Kirsch J, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26(6):925–935. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 13.Stehlik R, Arvidsson L, Ulfberg J. Restless legs syndrome is common among female patients with fibromyalgia. Eur Neurol. 2009;61(2):107–111. doi: 10.1159/000180313. [DOI] [PubMed] [Google Scholar]
- 14.Bentley AJ, Rosman KD, Mitchell D. Can the sensory symptoms of restless legs syndrome be assessed using a qualitative pain questionnaire? Clin J Pain. 2007;23(1):62–66. doi: 10.1097/01.ajp.0000210948.11805.69. [DOI] [PubMed] [Google Scholar]
- 15.Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Open-label study of the long-term efficacy and safety of pramipexole in patients with Restless Legs Syndrome (extension of the PRELUDE study) Sleep Med. 2008;9(5):537–541. doi: 10.1016/j.sleep.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Stiasny-Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127(Pt 4):773–782. doi: 10.1093/brain/awh079. [DOI] [PubMed] [Google Scholar]
- 17.Schattschneider J, Bode A, Wasner G, Binder A, Deuschl G, Baron R. Idiopathic restless legs syndrome: abnormalities in central somatosensory processing. J Neurol. 2004;251(8):977–982. doi: 10.1007/s00415-004-0475-3. [DOI] [PubMed] [Google Scholar]
- 18.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54(8):1609–1616. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114(3):315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 21.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59(7):961–967. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137(1):202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66(6):932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 24.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1–3):56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Hening WA, Allen RP, Washburn M, Lesage S, Earley CJ. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008;9(3):283–289. doi: 10.1016/j.sleep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Allen RP, Earley CJ. Validation of the Johns Hopkins restless legs severity scale. Sleep Med. 2001;2(3):239–242. doi: 10.1016/s1389-9457(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 27.Beck ATSRAGMG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Reviews. 1988;8:77–100. Ref Type: Journal (Full) [Google Scholar]
- 28.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 30.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13(3 Suppl 5):12–17. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 32.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8(11):893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22(8):730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 34.Staud R, Koo E, Robinson ME, Price DD. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain. 2007;130(1–2):177–187. doi: 10.1016/j.pain.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 36.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(1–2):155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 37.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 38.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- 39.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Personal Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 41.Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000;4(1):5–15. doi: 10.1053/eujp.1999.0154. [DOI] [PubMed] [Google Scholar]
- 42.Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41(9):1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 43.Eide PK, Stubhaug A. Relief of trigeminal neuralgia after percutaneous retrogasserian glycerol rhizolysis is dependent on normalization of abnormal temporal summation of pain, without general impairment of sensory perception. Neurosurgery. 1998;43(3):462–472. doi: 10.1097/00006123-199809000-00036. [DOI] [PubMed] [Google Scholar]
- 44.Bradley LA, McKendree-Smith NL, Alarcon GS, Cianfrini LR. Is fibromyalgia a neurologic disease? Curr Pain Headache Rep. 2002;6(2):106–114. doi: 10.1007/s11916-002-0006-9. [DOI] [PubMed] [Google Scholar]
- 45.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12(8):1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129(1–2):130–142. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran TD, Wang H, Tandon A, Hernandez-Garcia L, Casey KL. Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation. Pain. 2010;150(1):93–102. doi: 10.1016/j.pain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 49.Harding LM, Kristensen JD, Baranowski AP. Differential effects of neuropathic analgesics on wind-up-like pain and somatosensory function in healthy volunteers. Clin J Pain. 2005;21(2):127–132. doi: 10.1097/00002508-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the N-methyl-D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain. 2005;6(5):323–332. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- 51.Lomas LM, Picker MJ. Behavioral assessment of temporal summation in the rat: sensitivity to sex, opioids and modulation by NMDA receptor antagonists. Psychopharmacology (Berl) 2005;180(1):84–94. doi: 10.1007/s00213-005-2153-2. [DOI] [PubMed] [Google Scholar]
- 52.Eichenberger U, Giani C, Petersen-Felix S, Graven-Nielsen T, Arendt-Nielsen L, Curatolo M. Lumbar epidural fentanyl: segmental spread and effect on temporal summation and muscle pain. Br J Anaesth. 2003;90(4):467–473. doi: 10.1093/bja/aeg100. [DOI] [PubMed] [Google Scholar]
- 53.Enggaard TP, Poulsen L, Arendt-Nielsen L, Hansen SH, Bjornsdottir I, Gram LF, et al. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92(1–2):277–282. doi: 10.1016/s0304-3959(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 54.Bradley LA. Pathophysiology of fibromyalgia. Am J Med. 2009;122(12 Suppl):S22–S30. doi: 10.1016/j.amjmed.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachmann CG, Rolke R, Scheidt U, Stadelmann C, Sommer M, Pavlakovic G, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. 2010;133(Pt 3):762–770. doi: 10.1093/brain/awq026. [DOI] [PubMed] [Google Scholar]
- 56.Iannaccone S, Zucconi M, Marchettini P, Ferini-Strambi L, Nemni R, Quattrini A, et al. Evidence of peripheral axonal neuropathy in primary restless legs syndrome. Mov Disord. 1995;10(1):2–9. doi: 10.1002/mds.870100103. [DOI] [PubMed] [Google Scholar]
- 57.Tyvaert L, Laureau E, Hurtevent JP, Hurtevent JF, Derambure P, Monaca C. A-delta and C-fibres function in primary restless legs syndrome. Neurophysiol Clin. 2009;39(6):267–274. doi: 10.1016/j.neucli.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Han JK, Oh K, Kim BJ, Koh SB, Kim JY, Park KW, et al. Cutaneous silent period in patients with restless leg syndrome. Clin Neurophysiol. 2007;118(8):1705–1710. doi: 10.1016/j.clinph.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 59.Paulus W, Dowling P, Rijsman R, Stiasny-Kolster K, Trenkwalder C, de Weerd A. Pathophysiological concepts of restless legs syndrome. Mov Disord. 2007;22(10):1451–1456. doi: 10.1002/mds.21533. [DOI] [PubMed] [Google Scholar]
- 60.Walters AS, Ondo WG, Zhu W, Le W. Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J Neurol Sci. 2009;279(1–2):62–65. doi: 10.1016/j.jns.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 61.Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. Eur J Pharmacol. 1990;182(2):347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 62.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13(10):1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68(15):1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 65.Edwards RR, Ness TJ, Fillingim RB. Endogenous opioids, blood pressure, and diffuse noxious inhibitory controls: a preliminary study. Percept Mot Skills. 2004;99(2):679–687. doi: 10.2466/pms.99.2.679-687. [DOI] [PubMed] [Google Scholar]
- 66.Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford) 2007;46(4):666–671. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 67.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 68.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]