Abstract
Prostate carcinoma is among the most common causes of cancer-related death in men, representing 15% of all male malignancies in developed countries. Neuroendocrine differentiation has been associated with tumor progression, poor prognosis and with the androgen-independent status. Currently, no successful therapy exists for advanced, castration-resistant disease. Because hypoxia has been linked to prostate cancer progression and unfavourable outcome, we sought to determine whether hypoxia would impact the degree of neuroendocrine differentiation of prostate cancer cells, in vitro.
Results
exposure of LNCaP cells to low oxygen tension induced a neuroendocrine phenotype, associated with an increased expression of the transcription factor neurogenin3 and neuroendocrine markers, such as neuron-specific enolase, chromogranin A and β3-tubulin. Moreover, hypoxia triggered a significant decrease of Notch 1 and Notch 2 mRNA and protein expression, with subsequent down regulation of Notch-mediated signalling, as demonstrated by reduced levels of the Notch target genes, Hes1 and Hey1. Neuroendocrine differentiation was promoted by attenuation of Hes1 transcription, as cells expressing a dominant negative form of Hes1 displayed increased levels of neuroendocrine markers under normoxic conditions. Although hypoxia down regulated Notch 1 and Notch 2 mRNA transcription and receptor activation also in the androgen independent cell lines, PC3 and Du145, it did not change the extent of NE differentiation in these cultures, suggesting that androgen sensitivity may be required for transdifferentiation to occur.
Conclusions
hypoxia induces neuroendocrine differentiation of LNCaP cells in vitro, which appears to be driven by the inhibition of Notch signalling with subsequent down-regulation of Hes1 transcription.
Keywords: prostate cancer, hypoxia, notch, Hes, LNCaP, neuroendocrine differentiation
INTRODUCTION
Carcinoma of the prostate is the second leading cause of cancer-related death in men, representing 15% of all male malignancies in developed countries (1). Although the incidence of prostate cancer varies according to race, about 85% of patients are diagnosed after the age of 65 years (2). However, little is known about the molecular mechanisms that underline its development and progression.
Neuroendocrine differentiation (NED) of prostate cancer leads to a worst prognosis and is associated with a lack of response to androgen-deprivation therapy, which is the only effective treatment for advanced metastatic disease. Indeed, it has been suggested that NE differentiated prostate cancer cells present within the tumor mass sustain proliferation, invasion and metastasis through the production and release of peptide hormones (3). Moreover, NED has been associated with chemotherapy resistance (4).
NE cells exist in normal adult prostate, where they regulate growth, differentiation and secretory properties of the gland. Malignant NE cells have been identified in all human prostate cancer tissues, with a prevalence that varies among different studies (5, 6). This may be due, at least in part, to inconsistency in techniques used for the identification of the most widely employed markers of prostate NED, neuron-specific enolase (NSE) and chromogranin A (CGA) (7).
How prostate cancer shifts towards the NE phenotype is still debated. Androgen deprivation therapy seems to favour the appearance of NE cells through the activation of PI3K-AKT-mTOR intracellular signalling pathway (8).
In a tumor mass, oxygen tension is constantly changing, following changes in microvascular supply, with periods of acute and chronic hypoxic conditions (9). Hypoxia is involved in cancer progression and has been linked to modulation of Notch signalling in solid tumors (10, 11). Notch is an evolutionary conserved receptor/ligand system that mediates several biological processes: cell fate specification, differentiation, proliferation, apoptosis, migration, and angiogenesis (12, 13). Dysregulation of Notch signal occurs in several types of tumors (14–16), including prostate cancer (17). Notch signalling is also required for normal prostate development (18, 19). When Notch receptor interacts with its ligands, it undergoes a series of proteolytic cleavages that result in the production of an intracellular domain (NICD), which translocates to the nucleus where it binds to the transcription factor CBF1 (13). The formation of the CBF1-NICD complex leads to the recruitment of the nuclear protein MAML1 that functions as a transcriptional activator of CBF1-dependent genes, such as transcriptional repressors belonging to the Hes and Hey families (20).
This study was aimed at investigating whether hypoxia influences the degree of NED of prostate cancer cells, in vitro, and whether NE trans-differentiation is supported by Notch signalling.
MATERIALS AND METHODS
Antibodies and chemicals
Mouse monoclonal antibody against β3-tubulin (TUJ-1) (1:2500) and rabbit polyclonal antibody against human Notch 1 (sc-6014-R) (1:1000) were obtained from Santa Cruz Biotechnology Inc. (DBA, Italy); mouse monoclonal antibody against HIF- 1α (1:1000) was obtained from Novus Biologicals (DBA, Italy); mouse monoclonal antibody against human NSE (1:1500) was obtained from Dako Cytomation (Glostrup, Denmark); rabbit monoclonal antibody against GAPDH (1:1000), mouse monoclonal antibody against HA epitope tag (1:1000), and rabbit monoclonal antibody against human Notch 2 (D67C8) (1:1000) were purchased from Cell Signaling Technology (Euroclone, Milan, Italy). Mouse monoclonal antibody against V5 epitope tag (1:5000) was purchased from Invitrogen SRL (Milan, Italy). Horseradish peroxidase (HRP)-conjugated secondary anti-mouse and anti-rabbit antibodies were purchased from GE Healthcare Italia (Milan, Italy) and Pierce Biotechnology Inc. (Euroclone, Milan, Italy), respectively. All other reagents were obtained from Sigma (Sigma-Aldrich S.r.l., St. Louis, USA). The γ-secretase inhibitor DAPT (20 mM stock solution) was dissolved in DMSO. Dihydrotestosterone (DHT) was dissolved in ethanol.
Cell cultures
LNCaP (androgen-dependent human prostate cancer cell line), PC-3 and Du145 (androgen-independent human prostate cancer cell lines) were obtained from ATCC (Manassas, VA, USA), maintained in liquid nitrogen and used within few weeks after thawing. Cells were grown in RPMI-1640 (LNCaP) or DMEM (PC-3 and Du145) (Euroclone), supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Euroclone), L-glutamine (Euroclone) and 1% (vol/vol) antibiotic/antimycotic solution (Gibco, Invitrogen S.r.l.). Hypoxia was achieved by maintaining the cells at 2% oxygen, in a CO2 incubator (Forma Series II, Thermo Scientific) with oxygen sensor control, and with CO2 and N2 gas regulators, for up to 14 days. The cells were split every 4 days.
Cell growth analysis
To assess cell growth under normoxia and hypoxia, cultures were plated at 40.000 cells per well, in a 6 well-tissue culture plate. At day three and five after plating, cells were detached by trypsin and counted using a haemocytometer. Four wells were counted for each point. Growth response to DHT stimulation was studied by plating 80.000 cells per well, in a 6 well-tissue culture plate. Forty-eight hours later, culture medium was changed with medium containing 1% FBS and 4% charcoal-stripped FBS, and the cells were put in normoxic or hypoxic environment. After five days, cells were stimulated with DHT [10 nM]; seven days after treatment, cells were detached by trypsin and counted using a haemocytometer. Four wells were counted for each point. Student’s t test was used for statistical analysis.
Phase contrast microscopy
Phase contrast photographs of the cells, under normoxic and hypoxic conditions were taken with a phase contrast Zeiss Axiovert 25 inverted microscope, equipped with an AxioCam MR camera and Axiovision software.
Immunocytochemistry
Mouse monoclonal antibody against NSE (pre-diluted) was obtained from CellMarque (Ventana, Italy). The immunocytochemical analysis was performed by Benchmark XT Ventana System. Cells, grown on poly-L-lysine treated glass slides, were rinsed with PBS, and fixed in 50% ethanol. Immunostaining was performed using the HRP multimer system and specific mouse monoclonal antibodies; the 3,3′-diaminobenzidine (DAB, Ventana) was used as a substrate chromogen solution for the development of the peroxidase activity (UltraView Universal DAB Detection Kit. Ventana, Italy).
RNA extraction and quantitative Real Time RT-PCR
Total RNA was isolated using the RNEasy Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. During RNA purification, the sample was treated with Rnase-free Dnase I (Qiagen) to eliminate any genomic DNA contamination. The concentration of total RNA was determined spectrophotometrically with Nanodrop® ND-1000 (National Instruments Corporation, Texas, USA). Total RNA (1–2 μg) was reverse transcribed into cDNA by using TaqMan reverse transcription reagents, with random hexamers (Applied Biosystem Inc., Italy). The profile of the reverse transcription reaction was 10 min at 25° C, 30 min at 48° C, and 5 min at 95° C. Each reverse transcription was carried out in triplicate. Expression of Notch (N)1, N2, N3, N4, Jagged (J)1, J2, Delta-like (Dll)-1, Dll-3, Dll-4, Hes1, Hey1, androgen receptor (AR), neurogenin (Ngn)3 and chromogranin A (CGA) mRNA was determined by quantitative real-time polymerase chain reaction RT-PCR (qPCR), performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems), according to the manufacturer’s instructions. All PCR amplifications were performed using MicroAmp optical 96-well reaction plate with TaqMan Fast Universal PCR Master Mix and with TaqMan Gene Expression Assay (Applied Biosystems).
Adenoviral transduction
Dominant negative Hes1 (dnHes1:HA) (21), constitutively active Notch 1 (caN1:V5) consisting of Notch 1 intracellular domain (22), and LacZ (control gene), cloned in a pAdlox adenoviral construct, were used to transduce LNCaP cells. Recombinant adenoviruses were produced, purified and titrated as described (23). Briefly, CRE8 cells were transfected with SfiI-digested pAdlox-derived constructs, and infected with the ψ5 virus. Lysates were prepared 4 days after infection. Viruses were passed twice through CRE8 cells, and purified from the second passage using a caesium density gradient. The viruses were quantified by optical density at 260 nm, and the bioactivity was determined by the plaque forming unit assay. Adenoviral transduction was performed in serum-free DMEM with approximately 103 viral particles/cell in the presence of poly-D-lysine hydrobromide (Sigma Aldrich, USA), for 2 hours at 37° C. Then the adenovirus-containing medium was removed and replaced with serum-containing medium.
Western blot analysis
Cells grown under normoxic and hypoxic conditions were scraped in cold PBS, centrifuged and resuspended in cold lysis buffer (10 mM TrisHCl pH7.4, 25 mM MgCl2, 1% Triton X-100, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 1mM Na3VO4). Lysates were obtained by sonication on ice, followed by centrifugation to collect supernatants. After measurement of total protein content (Comassie Plus-Bradford Assay kit, Pierce), equal amounts of cell lysates (50 μg) were resolved by 10% (w/v) sodium dodecyl sulphate (SDS) – polyacrylamide gel electrophoresis (PAGE), transferred to a nitrocellulose membrane (Hybond C, Amersham Pharmacia Biothec) and immunoblotted using primary antibodies. Immunoreactive bands were visualized by chemioluminescence assay (ECL) (Amersham Pharmacia Biotech), following manufacturer’s instructions. Densitometric analysis was conducted using GAPDH immunoreactive bands for normalization. Each experiment was performed at least three times.
RESULTS
LNCaP cells exposed to hypoxia adopt a neuroendocrine (NE) phenotype
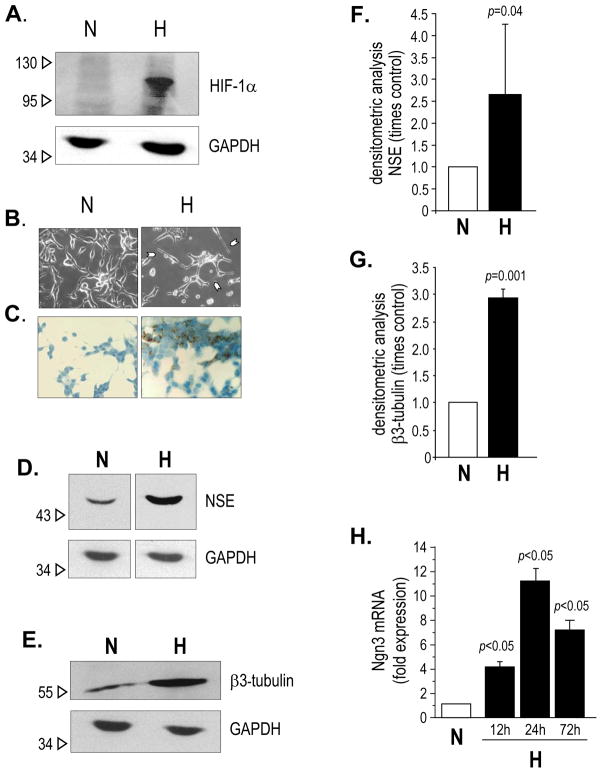
LNCaP cells were exposed to 2% oxygen (hypoxia) for up to 14 days. Similar to other reports (24), we found that transcription of HIF-1α mRNA was constitutive in LNCaP, and was nearly unchanged after 24 hours of exposure to hypoxia (data not shown). However, the expression of HIF-1α protein was enhanced under reduced oxygen tension, indicating stabilization of the protein (25) (Figure 1A). Cells grown at 2% oxygen for at least three days changed their morphology and assumed a neuronal-like phenotype, with the appearance of long dendritic-like processes in numerous cells, suggesting NED. The phenotypic change was more striking after seven days of hypoxia (Figure 1B) and still evident at 14 days. To confirm that the change in phenotype was due to NED, we analyzed the expression of two NE markers: neuron-specific enolase (NSE) (Figure 1C, 1D and 1F) and class III beta (β3)-tubulin (26, 27) (Figure 1E and 1G): both markers were significantly up-regulated after 7 days of exposure to hypoxia. Because neurogenin3 (Ngn3), a pro-neurogenic bHLH transcription factor, was demonstrated to be expressed in the NE prostate cancer mouse model, 12T-10 (28), we analyzed the expression of Ngn3 by qPCR, under normoxic and hypoxic conditions. As demonstrated in Figure 1H, Ngn3 transcription was significantly up-regulated by hypoxia. Neither PC-3 nor Du145 cells showed any change in morphology suggestive of NED when grown under hypoxic conditions. According to this observation, the level of expression of NED markers did not rise when the cells were exposed to reduced oxygen tension (data not shown).
Figure 1.
A. Western blotting for the detection of HIF-1α in LNCaP cells exposed to normoxia (N) or hypoxia (H) for 24 hours. Detection of GAPDH was used for normalization of protein loading; B–C. LNCaP cells exposed to hypoxia (H) for 7 days, compared to cells exposed to normoxia (N), assessed by phase contrast microscopy (B) and by immunoperoxidase staining (brown) for the detection of NSE (C); D–E. Representative immunoblot for the expression of NSE (D) and β3-tubulin (E) in LNCaP cells exposed to normoxia (N) and hypoxia (H) for 7 days. Detection of GAPDH was used for normalization of protein loading. F–G. Densitometric analysis of three independent experiments for the detection of NSE (F) and β3-tubulin (G); values are expressed as mean ± SE. H. Neurogenin (Ngn)3 mRNA expression in LNCaP cells exposed to normoxia (N) and hypoxia (H), at different time points, assessed by quantitative real time PCR.
Hypoxia modulates the expression of Notch receptors and ligands
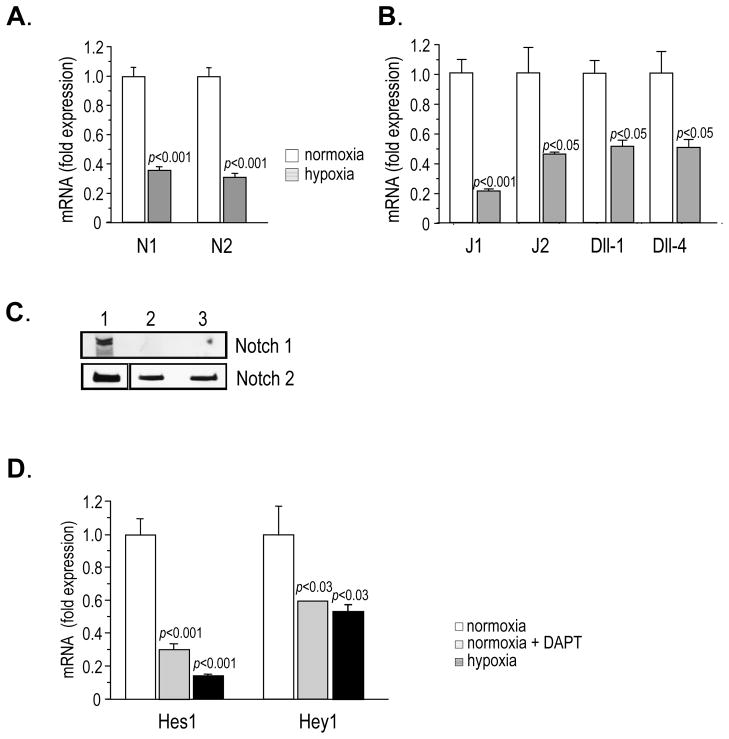
Because Notch signalling has a key role in cell fate and differentiation, and hypoxia has been demonstrated to modulate Notch signalling in some tumors (10), we sought to determine whether Notch was involved in the hypoxia-mediated NED of LNCaP cells. Notch 1–4 (N1-4), Jagged 1 (J1) and J2, Delta-like (Dll)-1, 3 and 4 mRNA levels were assessed by qPCR, under normoxic and hypoxic conditions. N4 and Dll-3 were undetectable under both conditions. N1 and N2 mRNA were abundant under normoxia and decreased significantly under hypoxia (Figure 2A). N3 mRNA was less represented and slightly decreased under hypoxic conditions (Supplementary Figure 1A). We also determined the expression of N1 and N2 proteins by Western blotting. As shown in Figure 2C, compared to normoxia (lane 1), the expression of the transmembrane (TM) form of N1 was undetectable and TM N2 was significantly reduced under hypoxia (lane 2). The decrease of N1 and N2 receptor proteins was not modified by treatment of the cells with γ-secretase inhibitor DAPT (lane 3), indicating that it was related to reduced protein translation, rather than receptor activation. The decreased expression of Notch receptors was maintained up to 14 days (data not shown). J1 and J2 were the main Notch ligands expressed in LNCaP; J1, J2, Dll-1 and Dll-4 mRNA levels decreased significantly under hypoxic conditions (Figure 2B). Similarly to Notch receptors, hypoxia-induced modification of ligand expression was still maintained after 14 days of exposure to hypoxic conditions (data not shown). In order to determine whether the modulation of Notch receptor/ligand system under hypoxia was a common feature of prostate cancer cells, we examined PC-3 and Du145 for the transcription levels of Notch 1–4 and their ligands, under normoxic and hypoxic conditions: PC-3 and Du145 expressed abundant levels of N1, N2 and N3; J1 and J2 were the main ligands (Supplementary Figure 1B). N4 and Dll-3 were undetectable. Under hypoxia, N1 and N2 were significantly down regulated in both cell lines (Supplementary Figure 2A). With the exception of J2 in PC-3 cells, ligands were also significantly down regulated under hypoxic conditions (Supplementary Figure 2B).
Figure 2.
A–B. Notch 1 (N1), N2, Jagged (J)1, J2, Delta-like (Dll)-1, Dll-4 mRNA expression in LNCaP cells exposed to normoxia and hypoxia for 7 days, assessed by quantitative real time PCR. Data are expressed as fold expression of mRNA levels under hypoxia, normalized to normoxia level (equal 1). C. Western blotting for the detection of transmembrane N1 and N2 proteins in LNCaP cells exposed to normoxia (1), hypoxia (2) and hypoxia + DAPT [20 μM] (3), for 7 days. D. Expression of Notch target genes, Hes1 and Hey1, mRNA in LNCaP cells exposed to normoxia and hypoxia for 7 days, assessed by quantitative real time PCR. Cells treated with the γ-secretase inhibitor DAPT [20 μM] were used as control for inhibition of Notch signal.
Hypoxia modulates Notch-mediated signalling
We next studied Notch signalling under normoxic and hypoxic conditions and found that the transcription of Notch target genes, Hes1 and Hey1, was significantly down-regulated when LNCaP cells were exposed to 2% oxygen, in comparison to normoxia, indicating that Notch-mediated signalling was turned down. Cells treated with the γ-secretase inhibitor DAPT were used as controls for inhibition of Notch activation (Figure 2D). As in LNCaP cells, Hes1 and Hey1 expression was significantly down regulated when PC-3 and Du145 were grown at 2% oxygen (Supplementary Figure 3).
Notch signalling modulates the extent of NED in LNCaP cells
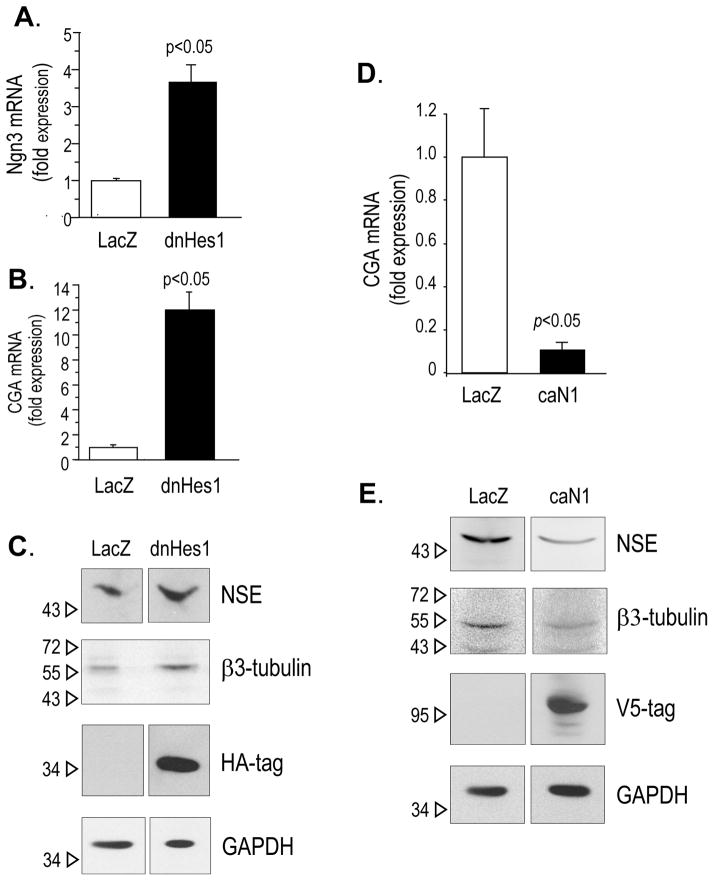
Because Notch signalling was down regulated under hypoxia and because in the developing neuroblast, Notch signalling and transcription of Hes genes are repressed (29), we sought to determine whether hypoxia-mediated NED was supported by the down-regulation of Notch signal. To do so, we transduced LNCaP cells with a dominant negative form of Hes1 (dnHes1) (21). Cells transduced with LacZ were used as controls. Ninety-six hours after infection, the cells were analyzed for the pro-neurogenic transcription factor Ngn3 and chromogranin A (CGA) mRNA expression, using qPCR (Figure 3A and 3B), and for NSE and β3-tubulin protein expression, by Western blotting (Figure 3C). Cells expressing dnHes1 up-regulated Ngn3, CGA, NSE andβ3-tubulin, compared to cells infected with the LacZ construct. These data suggest that hypoxia-induced NED may be facilitated by the attenuation of Notch receptor activity, through the down-regulation of Hes1 transcription. To confirm that the extent of NE features correlated to the level of Notch receptor activation, we next transduced LNCaP cells with a construct expressing a constitutively active form of Notch 1 (caN1) (22). Cells transduced with LacZ were used as controls. Ninety-six hours after infection, we analyzed the level of NE markers: GCA by qPCR (Figure 3D), and NSE and β3-tubulin by Western blotting (Figure 3E). In cells virally transduced with caN1, the expression of all three NE markers was significantly reduced.
Figure 3.
A–C. LNCaP cells virally transduced with LacZ or with a dominant negative form of Hes1 (dnHes1), in frame with a HA-tag, were analyzed for Ngn3 and CGA mRNA expression 4 days after transduction, by quantitative real time PCR (A–B) and for NSE and β3-tubulin protein expression, by Western blotting (C). Detection of HA-tag was used to determine the expression of the transduced dnHes1 construct. Detection of GAPDH was used for normalization of protein loading. D–E. LNCaP cells virally transduced with LacZ or with a constitutively active form of N1 (caN1), in frame with a V5-tag, were analyzed for CGA mRNA expression 3 days after transduction, by quantitative real time PCR (D), and for NSE and β3-tubulin protein expression, by Western blotting (E). Detection of V5-tag was used to determine the expression of the transduced caN1 construct. Detection of GAPDH was used for normalization of protein loading.
Hypoxia modulates the expression and function of androgen receptor (AR)
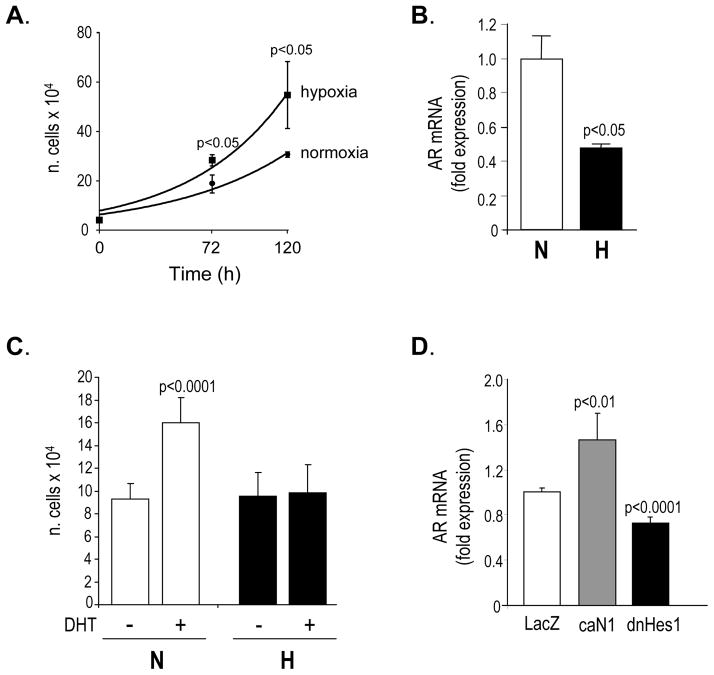
Because it has been reported that malignant prostatic NE cells are androgen-independent (30), we analyzed the growth of the cells and the expression of AR mRNA by qPCR, under normoxic and hypoxic conditions. As demonstrated in Figure 4A, cell growth was not inhibited under hypoxia, but rather enhanced. However, transcription of AR significantly decreased when LNCaP cells were exposed to reduced oxygen tension (Figure 4B). Moreover, stimulation of the cells with 5α-dihydrotestosterone (DHT) for 7 days was able to sustain cell growth under normoxia, but not under hypoxic conditions (Figure 4C), indicating that at 2% oxygen cells were unresponsive to exogenous hormone stimulation. To test whether there was a correlation between the expression of AR and the level of Notch signal, we analyzed cells transduced with caN1 and dnHes1 for AR mRNA expression, by qPCR. Cells transduced with LacZ were used as controls. As reported in Figure 4D, caN1 potentiated, while dnHes1 attenuated, the transcription of the receptor. These data suggest that down regulation of Notch signal may contribute to the down regulation of AR when the cells are grown under reduced oxygen tension.
Figure 4.
A. Exponential growth curve of LNCaP cells grown under normoxic and hypoxic conditions; B. AR mRNA expression in LNCaP cells exposed to normoxia (N) and hypoxia (H) for 7 days, assessed by quantitative real time PCR; C. Growth of LNCaP cells under normoxic (N) and hypoxic (H) conditions, in the absence or presence of DHT [10 nM]. Cells were counted at day 7. Data are shown as mean ± standard deviation; D. Expression of AR mRNA in LNCaP cells virally transduced with LacZ, caN1 and dnHES1, 4 days after transduction, assessed by quantitative real time PCR.
DISCUSSION
Progression of prostate cancer to a hormone-independent state is associated with resistance to androgen-deprivation therapy and poor prognosis. A growing body of evidence suggests that NED plays a role in the development of androgen-independency. Moreover, a recent report demonstrated that focal NED in prostate cancer is a powerful independent predictor of outcome (31). NED can range from the presence of scattered clusters of neuronal-like cells within the adenocarcinoma tumor mass, to a small cell carcinoma or carcinoid of the prostate, which is composed of 100% NE cells (32, 33). A recent report showed that a xenograft of NE differentiated prostate cancer cells implanted into a castrated mouse, enabled the growth of a xenograft of androgen-dependent tumor cells implanted into the opposing flank. This result suggests that NE differentiated cells, within the tumor mass, may support the growth of androgen-dependent cancer cells, even in the absence of androgenic stimulation (3), likely favouring the selection of hormone-independent clones. For this reason, understanding the molecular mechanisms that underline NE transdifferentiation should be of great interest.
Like many solid tumors, hypoxia develops in prostate cancerous tissues and has a role in tumor development and progression (34). We demonstrated that exposure of LNCaP cells to hypoxia induced NED. In a tumor mass, changes in microvascular supply lead to the development of acute and chronic hypoxic conditions (9). Pathological hypoxia has been established under 5% oxygen; however, compromised blood flow in specific areas may result in oxygen tension less than 1% (35). We chose to grow the cells at 2% oxygen to be able to study the “long term”, chronic effect of oxygen reduction. Exposure of LNCaP cells to hypoxia induced a profound modification of Notch receptor-ligand expression profile.
Notch signalling is central in cell fate specification, in embryonic and adult tissues (12, 13). Notch mediated signal has been linked increasingly to carcinogenesis; however, its role in cancer is highly context-dependent. In lung and breast cancer, hypoxia appears to increase Notch signaling (10, 11); in breast cancer cells hypoxia up regulates the expression of Notch receptors and ligands, as well as of Notch target genes, Hes1 and Hey1 (11). In LNCaP cells, low oxygen tension produced the opposite effect on Notch expression and activity, suggesting a further tissue-specific regulation of the Notch system. Hypoxia down regulated N1 and N2 mRNA transcription also in the androgen independent cell lines, PC-3 and Du145, although it did not change the extent of NE trans-differentiation in these models. It is possible that androgen sensitivity may be required for trans-differentiation to occur, as demonstrated by a number of studies that pointed out how NED progresses more rapidly if androgen deprivation is more intense (36, 37).
Moreover, as suggested by Marchiani (38), there is variability in the pathways leading to NED among different cell lines, in vitro, which may mirror tumor variability of prostate cancer cells, in vivo.
Notch is a major regulator of neuronal development, through the well-known mechanism of lateral inhibition: in a cell differentiating towards the neuronal lineage, Notch is not activated and, consequently, transcription of Hes genes is repressed; the cell expresses the transcription factor Ngn, which leads to the expression of Notch ligands. The expression of ligands on the developing neuroblast activates Notch receptors on adjacent cells, leading to the transcription of Hes genes that function as repressors of neuronal differentiation (39). This mechanism prevents the cells surrounding the newly formed neuroblast to differentiate towards the neuronal lineage as well. Hes1 has been demonstrated to be expressed by almost all undifferentiated cells, and it is crucial for suppressing differentiation of neural stem cells. Interestingly, Hes1 deficient mice exhibit premature differentiation and severe defects in the brain, eye and pancreas (39). Recently, several reports have suggested the involvement of Notch signalling in pancreatic exocrine and endocrine cell fate, through a mechanism similar to lateral inhibition: in the developing pancreas, a cell committed to the endocrine lineage expresses Ngn3, forcing neighbouring cells to adopt a non-endocrine phenotype. The existence of a cross-talk between Ngn and Hes transcription factors in the developing pancreas has been demonstrated by several lines of evidence: overexpression of Ngn3 or disruption of Hes1 gene results in an increased number of endocrine progenitor cells, with depletion of precursors bearing the capacity to differentiate into exocrine pancreas (40, 41). It has been demonstrated that Hes1 is able to halt Ngn3 expression by binding to several silencer sites near the transcription initiation site of the gene (40).
We report that, under hypoxic conditions, LNCaP cells down regulate Notch signal and Hes1 mRNA transcription, and up-regulate the expression of Ngn3 and the extent of NE features. Because a dominant negative Hes1 construct is also able to increase the level of Ngn3 transcription and NED, we propose that the loss of Notch signalling may determine NED in a manner similar to what has been described during pancreas development, with down regulation of Hes1 allowing neuronal associated proteins to be synthesized. In a previous study, immunohistochemical analysis of 12T-10 transgenic NE prostate cancer mouse model demonstrated the presence of pro-neuronal transcription factors, such as Foxa2, Ngn3 and Nkx2.2, associated with a loss of Hes1 transcript (28). Moreover, in 80 samples of human prostate cancers, grouped according to the level of CGA expression, human achaete-scute homolog 1 (hASH1), which is negatively regulated by Notch signal, was expressed and co-localized with CGA, in NE differentiated samples, and its expression correlated positively to the extension of NE features (42). In addition, during the preparation of this manuscript, a paper was published reporting that the formation of NE prostate tumors in the TRAMP mouse model is regulated by HIF-1α availability. The authors also demonstrated that HIF-1α cooperates with the pro-neuronal transcription factor Foxa2 to initiate the transcriptional program required for the NE phenotype to develop (43). Our data confirm that hypoxia can trigger the appearance of NED in prostate cancer cells and suggest a further level of complexity, with modulation of Notch signalling as an additional contributor. Interestingly, using osteoblastic skeletal prostate metastatic cancer cells, Zayzafoon and colleagues demonstrated that expression of N1 and Hes1 was increased, when compared to the primary tumor, indicating that Notch signalling was activated, and likely participated to the acquisition of osteoblastic properties (44). Therefore, modulation of Notch signalling appears to be crucial for prostate cancer progression by determining cancer cell differentiation and controlling the acquisition of specific phenotypes.
Several reports have indicated that malignant prostatic NE cells are AR negative (30). When LNCaP cells were grown under hypoxic conditions, AR mRNA transcription was significantly down-regulated, although not completely absent. We also found that the level of AR transcription correlated to the level of Notch signal. Therefore, we propose that the attenuation of Notch mediated signal, detected under hypoxia, promotes not only NED but also a reduction of AR expression.
Some authors have reported an increased sensitivity of prostate cancer cells to hormone stimulation, under hypoxia (45). When we assessed cell growth in our experimental system, we found that LNCaP cells exposed to hypoxia were able to grow even faster than cells exposed to normoxia. At the same time, at 2% oxygen, the cells did not respond to androgen stimulation. These results are in accordance with Suzuki et al. that reported that DHT-dependent growth and ARE-mediated transcriptional activity of LNCaP cells were depressed under reduced oxygen tension (46). Although this behavior seems to suggest the acquisition of an androgen-independent phenotype, when we passaged the cells with charcoal-stripped serum, which is considered a model of androgen-deprivation, their growth was hampered equally under normoxia and hypoxia (data not shown). One possible explanation is that charcoal-stripped serum is deprived not only of androgens, but also of other mitogens that may be necessary to sustain the growth of prostate cancer cells, under hypoxia, in our experimental system. Further studies will be necessary to establish whether chronic hypoxia favors the appearance of an androgen-independent phenotype and to elucidate which mitogenic stimuli sustain the growth of the cells under reduced oxygen tension. One likely candidate is the prototype member of the Fibroblast Growth Factor family, FGF-1. Indeed FGF-1 is a well-known mitogenic stimulus for prostate cancer cells (47) and its secretion has been linked to a down regulation of N1 receptor activity (48).
Several lines of evidence indicate that Notch signalling may have a facilitating role in the progression of prostate cancer; indeed, J1 is highly expressed in metastatic tumors and in clinically localized tumors with higher risk of recurrence (49). Moreover, down regulation of N1 and J1 inhibits cancer cell growth, migration and invasion (50, 51). For all these reasons, strategies aimed at inhibiting Notch pathway have been proposed as alternative anti-cancer therapies for metastatic prostate cancer, refractory to endocrine therapy, whose treatment is currently based on docetaxel (52). However, based on our results, great caution should be exerted before proposing such strategy as single therapy in patients with advanced prostate cancer, as it may favour the appearance of a NE-mediated, hormone-independent phenotype with self-sustained proliferative capacity and aggressive clinical behaviour. At the same time, characterizing the down-regulation of Notch signal as a mechanism involved in NED may open new perspectives for treatment of hormone-refractory tumors. Indeed, while somatostatin analogs have been employed with quite elusive results in prostate cancers characterized by NE features (53), Notch signalling may be regarded as a novel molecular target to prevent/revert NE transdifferentiation.
In conclusion, hypoxia induces down regulation of Notch-mediated signal, sustaining NED of prostate cancer cells in vitro. Further studies are needed to understand whether the modulation of Notch, in combination with strategies aimed at inhibiting signalling pathways responsible for the self-sustained proliferative capacity (54), may slow down the progression of prostate cancer toward a NE-mediated, androgen-independent phenotype.
Supplementary Material
Supplementary Figure 1. A. Notch 1 (N1), N2, N3, Jagged (J)1, J2, Delta-like (Dll)-1, Dll-4 mRNA expression in LNCaP exposed to normoxia or hypoxia for 7 days. Data are expressed as Δct. B. N1, N2, N3, J1, J2, Dll-1, Dll-4 mRNA expression in PC-3 and Du145. Data are expressed as Δct.
Supplementary Figure 2. A. N1 and N2 mRNA expression in PC-3 and Du145, assessed by quantitative real time PCR. Data are expressed as fold expression of mRNA levels under hypoxia (for 7 days), normalized to normoxia level (equal 1). B. J1. J2 and Dll-4 mRNA expression in PC-3 and Du145, assessed by quantitative real time PCR. Data are expressed as fold expression of mRNA levels under hypoxia (for 7 days), normalized to normoxia level (equal 1).
Supplementary Figure 3. Expression of Notch target genes, Hes1 and Hey1, mRNA in PC-3 and Du145 exposed to normoxia or hypoxia for 7 days, assessed by quantitative real time PCR. Cells treated with the γ-secretase inhibitor DAPT [20 μM] were used as controls for inhibition of Notch signal.
Acknowledgments
This work was supported by Maine Cancer Foundation grant and NIH ARRA grant R01 HL035627 to IP, by a grant from Fondazione Ente Cassa di Risparmio di Firenze to GD, and by grants IG8780 from the Associazione Italiana per la Ricerca sul Cancro (AIRC) and from Ministero dell’Istruzione dell’Università e della Ricerca (PRIN 2008) to ML.
This paper is dedicated to the memory of Prof. Mario Serio. We thank Dr. Anders Strom (Karolinska Institute, Sweden) for the dnHes1 DNA. dnHes1 adenovirus suspension was a generous gift of Dr. Lucy Liaw (MMCRI, Scarborough, ME, USA).
Footnotes
The Authors have no conflict of interest to declare.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 3.Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB, et al. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64:5489–95. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Chen HQ, Chen MF, Liu HZ, Dai YQ, Lv H, et al. Neuroendocrine differentiation is involved in chemoresistance induced by EGF in prostate cancer cells. Life Science. 2009;84:822–7. doi: 10.1016/j.lfs.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–19. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya N, Suzuki H, Kawamura K, Imamoto T, Naya Y, Tochigi N, et al. Neuroendocrine differentiation in stage D2 prostate cancers. Int J Urol. 2008;15:423–8. doi: 10.1111/j.1442-2042.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 7.Komiya A, Suzuki H, Imamoto T, Kamiya N, Nihei N, Naya Y, et al. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol. 2009;16:37–44. doi: 10.1111/j.1442-2042.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Huang J. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem. 2007;282:3571–83. doi: 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]
- 9.Janssen HL, Haustermans KM, Balm AJ, Begg AC. Hypoxia in head and neck cancer: how much, how important? Head Neck. 2005;27:622–38. doi: 10.1002/hed.20223. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, et al. Oxygen concentration determines the biological effects of Notch-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007;67:7954–9. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Imanaka N, Chen J, Griffin JD. Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br J Cancer. 2010;102:351–60. doi: 10.1038/sj.bjc.6605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–18. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 13.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–47. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–31. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 15.Villaronga MA, Bevan CL, Belandia B. Notch signaling: a potential therapeutic target in prostate cancer. Curr Cancer Drug Targets. 2008;8:566–80. doi: 10.2174/156800908786241096. [DOI] [PubMed] [Google Scholar]
- 16.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang Z, Ahmed F, Banerjee S, Li Y, Sarkar FH. Down-regulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int J Cancer. 2006;119:2071–7. doi: 10.1002/ijc.22077. [DOI] [PubMed] [Google Scholar]
- 18.Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, Radtke F, et al. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Developmental Biology. 2006;290:66–80. doi: 10.1016/j.ydbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- 20.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–33. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 21.Hartman J, Müller P, Foster JS, Wimalasena J, Gustafsson JA, Ström A. HES-1 inhibits 17beta-estradiol and heregulin-beta1-mediated upregulation of E2F-1. Oncogene. 2004;23:8826–33. doi: 10.1038/sj.onc.1208139. [DOI] [PubMed] [Google Scholar]
- 22.Small D, Kovalenko D, Soldi R, Mandinova A, Kolev V, Trifonova R, et al. Notch activation suppresses fibroblast growth factor-dependent cellular transformation. J Biol Chem. 2003;278:16405–13. doi: 10.1074/jbc.M300464200. [DOI] [PubMed] [Google Scholar]
- 23.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–9. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghafar MA, Anastasiadis AG, Chen MW, Burchardt M, Olsson LE, Xie H, et al. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate. 2003;54:58–67. doi: 10.1002/pros.10162. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 26.Katsetos CD, Herman MM, Mörk SJ. Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton. 2003;55:77–96. doi: 10.1002/cm.10116. [DOI] [PubMed] [Google Scholar]
- 27.Terry S, Ploussard G, Allory Y, Nicolaiew N, Boissiére-Michot F, Maillé P, et al. Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br J Cancer. 2009;101:951–6. doi: 10.1038/sj.bjc.6605245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Wang Y, Browne C, Kim S, Case T, Paul M, et al. Neuroendocrine differentiation in the 12T-10 transgenic prostate mouse model mimics endocrine differentiation of pancreatic beta cells. Prostate. 2008;68:50–60. doi: 10.1002/pros.20650. [DOI] [PubMed] [Google Scholar]
- 29.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–8. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Bonkhoff H, Stein U, Remberger K. Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic, and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol. 1993;423:291–4. doi: 10.1007/BF01606893. [DOI] [PubMed] [Google Scholar]
- 31.Tarján M. Prognostic significance of focal neuroendocrine differentiation in prostate cancer: Cases with autopsy-verified cause of death. Indian J Urol. 2010;26:41–5. doi: 10.4103/0970-1591.60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetu B, Ro JY, Ayala AG, Ordonez NG, Logothetis CJ, von Eschenbach AC. Small cell carcinoma of prostate associated with myasthenic (Eaton-Lambert) syndrome. Urology. 1989;33:148–52. doi: 10.1016/0090-4295(89)90017-4. [DOI] [PubMed] [Google Scholar]
- 33.di Sant’Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992;23:287–96. doi: 10.1016/0046-8177(92)90110-o. [DOI] [PubMed] [Google Scholar]
- 34.Stewart GJ, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010;105:8–13. doi: 10.1111/j.1464-410X.2009.08921.x. [DOI] [PubMed] [Google Scholar]
- 35.Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Pinover WH, Greenberg RE, et al. Hypoxia in human prostate carcinoma: an Eppendorf PO2 study. Am J Clin Oncol. 2001;24:458–61. doi: 10.1097/00000421-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Tarle M, Ahel MZ, Kovacic K. Acquired neuroendocrine positivity during maximal androgen blockade in prostate cancer patients. Anticancer Res. 2002;22:2525–9. [PubMed] [Google Scholar]
- 37.Sasaki T, Komiya A, Suzuki H, Shimbo M, Ueda T, Akakura K, et al. Changes in chromogranin A serum levels during endocrine therapy in metastatic prostate cancer patients. Eur Urol. 2005;48:224–9. doi: 10.1016/j.eururo.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Marchiani S, Tamburrino L, Nesi G, Paglierani M, Gelmini S, Orlando C, et al. Androgen-responsive and –unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int J Androl. 2010;33:784–93. doi: 10.1111/j.1365-2605.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 39.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–8. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–34. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- 41.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 42.Rapa I, Ceppi P, Bollito E, Rosas R, Cappia S, Bacillo E, et al. Human ASH1 expression in prostate cancer with neuroendocrine differentiation. Mod Pathol. 2008;21:700–7. doi: 10.1038/modpathol.2008.39. [DOI] [PubMed] [Google Scholar]
- 43.Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R, et al. Siah-2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18:23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zayzafoon M, Abdulkadir SA, McDonald JM. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem. 2004;279:3662–3670. doi: 10.1074/jbc.M308158200. [DOI] [PubMed] [Google Scholar]
- 45.Mitani T, Yamaji R, Higashimura Y, Harada N, Nakano Y, Inui H. Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1α in a low androgen environment. J Steroid Biochem Mol Biol. 2011;123:58–64. doi: 10.1016/j.jsbmb.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki Y, Kondo Y, Hara S, Kimata R, Nishimura T. Effect of the hsp90 inhibitor geldanamycin on androgen response of prostate cancer under hypoxic conditions. Int J Urol. 2010;17:281–5. doi: 10.1111/j.1442-2042.2010.02450.x. [DOI] [PubMed] [Google Scholar]
- 47.Dorkin TJ, Robinson MC, Marsh C, Neal DE, Leung HY. aFGF immunoreactivity in prostate cancer and its colocalization with bFGF and FGF8. Journal of Pathology. 1999;189:564–9. doi: 10.1002/(SICI)1096-9896(199912)189:4<564::AID-PATH480>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Kacer D, McIntire C, Kirov A, Kany E, Roth J, Liaw L, et al. Regulation of non-classical FGF1 release and FGF-dependent cell transformation by CBF1-mediated notch signaling. J Cell Physiol. 2011;226:3064–75. doi: 10.1002/jcp.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–7. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 50.Bin Hafeez B, Adhami VM, Asim M, Siddiqui IA, Bhat KM, Zhong W, et al. Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin Cancer Res. 2009;15:452–9. doi: 10.1158/1078-0432.CCR-08-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, et al. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109:726–36. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- 52.Clarke NW. Management of the spectrum of hormone refractory prostate cancer. Eur Urol. 2006;50:428–38. doi: 10.1016/j.eururo.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Vainas G, Pasaitou V, Galaktidou G, Maris K, Christodoulou K, Constantinidis C, et al. The role of somatostatin analogues in complete antiandrogen treatment in patients with prostatic carcinoma. J ExpClin Cancer Res. 1997;16:119–26. [PubMed] [Google Scholar]
- 54.Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237–49. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. A. Notch 1 (N1), N2, N3, Jagged (J)1, J2, Delta-like (Dll)-1, Dll-4 mRNA expression in LNCaP exposed to normoxia or hypoxia for 7 days. Data are expressed as Δct. B. N1, N2, N3, J1, J2, Dll-1, Dll-4 mRNA expression in PC-3 and Du145. Data are expressed as Δct.
Supplementary Figure 2. A. N1 and N2 mRNA expression in PC-3 and Du145, assessed by quantitative real time PCR. Data are expressed as fold expression of mRNA levels under hypoxia (for 7 days), normalized to normoxia level (equal 1). B. J1. J2 and Dll-4 mRNA expression in PC-3 and Du145, assessed by quantitative real time PCR. Data are expressed as fold expression of mRNA levels under hypoxia (for 7 days), normalized to normoxia level (equal 1).
Supplementary Figure 3. Expression of Notch target genes, Hes1 and Hey1, mRNA in PC-3 and Du145 exposed to normoxia or hypoxia for 7 days, assessed by quantitative real time PCR. Cells treated with the γ-secretase inhibitor DAPT [20 μM] were used as controls for inhibition of Notch signal.